CHAPTER 1 Embryologic Basis and Segmental Approach to Imaging of Congenital Heart Disease
There is incredible diversity in the types of human hearts. The first step to accurate diagnosis and management of congenital heart disease is a standardized and logical approach to not only understanding, but also describing, the disease process. This is called the segmental approach to heart disease,1–3 first proposed by Richard Van Praagh in 1972, and later modified by others.4 It is strongly rooted in embryologic principles, and follows a logical sequence from evaluation of morphology and physiology of the heart to decision making regarding treatment. This chapter will summarize the key events of cardiac development and link them to the developmental pathology of the common congenital heart diseases. The discussion of embryology will follow a morphologic approach, and will lay the groundwork for understanding the segmental approach to heart disease by cross-sectional imaging. The molecular genetic basis of cardiac development and congenital heart disease is beyond the scope of this chapter.
CARDIAC EMBRYOLOGY
Formation of the Heart Tube
During the first 2 weeks of embryonic life, there is no heart or vascular system. Cell-to-cell diffusion provides nutrient and oxygen supply to the fetus. As the fetus grows, the stored food supply in the yolk sac is unable to support fetal life, and the cardiovascular system must develop to transfer nutrition from the maternal umbilical cord. The heart develops from two simple epithelial tubes that fuse to form a single-chambered heart that is efficiently pumping blood by the fourth week of embryonic development. The origins of the heart tube are clusters of angiogenic cells that are located in the cardiogenic crescent. The cardiogenic crescent is derived from splanchnopleuric mesoderm, and is located cranial and lateral to the neural plate. These angiogenic cell clusters coalesce to form right and left endocardial tubes.5 Each tube is continuous cranially with a dorsal aorta, its outflow tract, and caudally with a vitello-umbilical vein, its inflow tract. The lateral and cranial folding of the embryo forces the tubes into the thoracic cavity. As a result, these tubes come to lie closer to each other and begin to fuse in a cranial to caudal direction. At approximately day 21, they are completely fused. The splanchnic mesoderm around the heart tube thickens and forms the myoepicardial mantle (future myocardium and epicardium).
Components of the Heart Tube
From caudal to cranial, the following components of the newly formed heart tube (Fig. 1-1) are:
Bulboventricular Looping and Formation of the Ventricles
Whereas the two ends of the heart tube remain relatively fixed, rapid growth of the middle section results in the development of a large S-shaped curve called the bulboventricular loop (Fig. 1-2). As the heart tube grows and becomes longer, it usually bends to the right, termed by Van Praagh as D-looping.1,3 D-looping is responsible for the proximal bulbus cordis (RV) lying anterior and to the right of the primitive ventricle (LV). If the heart tube loops to the left, termed L-looping, the RV will lie anterior and to the left of the LV. The mechanisms underlying normal looping are still being studied, but they are under strong genetic control.6–8 Failure of normal looping (i.e., looping of the heart tube to the left) is a very early embryologic defect,9 and it is not surprising that associated defects of septation and valve formation are the rule rather than the exception.
Formation of the Ventricles and Interventricular Septum
In the newly formed bulboventricular loop, the primitive right and left ventricles appear as expansions in the heart tube. The interventricular sulcus/ridge separates the right and left ventricles (Fig. 1-3). Both ventricles will continue to expand until the late 7th/early 8th week. The growth of the ventricles is due to the centrifugal growth of the myocardium and the diverticulation of the internal walls, which gives the ventricle its trabeculated appearance. The development of the interventricular septum begins around the 27th day of gestation. It develops from three embryonic components: the endocardial cushions, the conus cushions, and the muscular septum (Fig. 1-4). The fusion of the opposing ventricular walls gives rise to the muscular interventricular septum. The endocardial cushions divide the inflow tracts from the ventricles. The conus cushions divide the outflow region of the ventricles.
Division of the Atrioventricular Canal
Because the proximal bulbus cordis gives rise to the RV, blood flows in series from the primitive atrium to the left ventricle, and then to the RV (see Fig. 1-1). There is no direct communication between the atria and the RV—even after the formation of the bulboventricular loop. The atrioventricular canal must shift to the right to achieve communication to the right ventricle in addition to the left ventricle (see Fig. 1-3). Swellings of mesenchymal tissue, the endocardial cushions, appear on the borders of the atrioventricular canal. There are four cushions: inferior and superior (ventral and dorsal), left and right. These are the precursors of the atrioventricular valves and they function during this early development as primitive valves. The endocardial cushions grow toward each other and fuse at approximately day 42, separating the atrioventricular canal into two openings which will eventually become the tricuspid and mitral valves (Fig. 1-4). The fused endocardial cushions are also responsible for the closure of the ostium primum by fusing with the free edge of the septum primum.
Developmental Pathology
Failure of the endocardial cushions to form will produce an AV canal defect (also known as an endocardial cushion defect). Incomplete endocardial cushion formation leads to variations of primum atrial septal defect, AV valve malformations, and/or inlet ventricular septal defect. Failure of the endocardial cushion tissue to shift over both ventricles can result in both AV valves entering the primitive left ventricle, forming a double inlet left ventricle. Failure of the endocardial cushions to shift to their normal position over the ventricles, combined with problems in ventricular septation, can also produce an “unbalanced AV canal” with a large single valve situated primarily over one ventricle.10
The tricuspid and mitral valves are formed by the endocardial cushion tissue. Stenosis of the atrioventricular valves is probably the result of partial fusion, whereas atresia of the atrioventricular valves probably results from complete fusion of the tissue.5
Formation of the Ventricular Outflow Tracts and Septation of the Truncus Arteriosus
In the heart tube stage, the primitive LV and the proximal bulbus cordis (primitive RV) are separated from the truncus arteriosus (which gives rise to both great arteries) by the conus or infundibulum (Fig. 1-5). This fact is fundamental to the understanding of the conotruncal malformations, which are due to abnormal development of the conus. The conus consists of the subpulmonary and subaortic conus cushions. Normally, there is expansile growth of the subpulmonary conus, causing it to protrude anteriorly on the left, carrying the pulmonary valve anteriorly, superiorly, and to the left of the aortic valve. There is resorption of the subaortic conus. Hence, the aortic valve lies posterior, inferior, and right-sided, in direct fibrous contiguity with the mitral valve (Fig. 1-6). Anterior protrusion of the pulmonary conus also twists the developing great arteries because they are fixed distally by the arterial arches. Spiral twisting of the growing tissue and a shift of the conal base to the middle causes the outflow tracts to align with the nearest ventricle (see Fig. 1-5). The anterior pulmonary artery arises above the anterior ventricle (RV) and leads to the posterior sixth arterial arch, which forms the branch pulmonary arteries. The posterior aorta originates above the posterior LV, and leads to the anterior fourth arterial arch (which forms the aortic arch). Recent investigation has shown that this normal partitioning of the conus involves migration of mesenchymal cells from the neural crest.11
By the 28th day of development, the truncus arteriosus divides by means of a bisecting spur that protrudes from both the aorta and pulmonary artery. The resultant aorticopulmonary septum continues in a spiral fashion toward the conus cordis until the two vessels are completely separated, giving rise to the aortic and pulmonary channels (see Fig. 1-5). The truncal cushions meet the conal cushions to complete the closure of the base of the respective outflow tracts.5 At the level of the conus cordis, the truncal swellings develop into pulmonary and aortic valve cusps and form two of the three cusps in each valve. A third swelling, the intercalated swelling, forms the third cusp.
Developmental Pathology
Abnormal conal development can result in tetralogy of Fallot or abnormal connections between the great vessels and the ventricles such as truncus arteriosus, transposition of the great arteries, double outlet RV, or double outlet LV (see Fig. 1-6).
Tetralogy of Fallot occurs when anterior displacement of the conal septum results in a narrowed pulmonary outflow tract, subsequent RV hypertrophy, an inability of the ventricular septum to close, and an abnormally placed aorta, overriding the VSD.5
Truncus arteriosus occurs when there is atresia of the subpulmonary conus, with absence of the pulmonary valve, resulting in a common vessel through which blood flows out of the heart to both pulmonary and systemic circulations.12 Because the absent or hypoplastic truncal cushions cannot meet the conal tissue, an obligatory VSD is present.
Transposition of great arteries was previously thought to be related to a failure of spiral twisting of the aorticopulmonary septum. But now, it is believed to result from variations in differential growth of the aortic and pulmonary conus.13 For instance, the most common form, D-transposition, is due to persistence and overgrowth of the subaortic conus and resorption of the subpulmonary conus. This elevates the aortic valve superiorly, protrudes it anteriorly above the RV, and causes aortic-tricuspid valve fibrous discontiguity. The pulmonary valve stays posterior and inferior, above the posterior LV, and in direct fibrous contiguity with the mitral valve.
The double outlet right ventricle results from variable development of both the subaortic and subpulmonary conus, and failure of the conus to shift to the center, with both great vessels arising from the primitive bulbus cordis (eventually the RV).5
The semilunar valves are formed from three small tubercles of tissue. A failure to develop one of the tubercles causes bicuspid aortic or pulmonary valves. Fusion of two or all three of the valve leaflets produces stenosis or atresia of the valve.5
Formation of the Interatrial Septum
By the time the heart tube has formed the bulboventricular loop, the primitive right and left atria have fused to form a common atrium, which lies cranial to the primitive ventricle and dorsal to the bulbus cordis (see Fig. 1-1D). The truncus arteriosus lies on the roof of the common atrium causing a depression and indicates where septation of the atrium will occur (see Fig. 1-5A). The interatrial septum forms between the 27th and the 37th day of development and occurs in conjunction with the development of the ventricular septum and separation of the truncus arteriosus.
A crest of tissue known as the septum primum grows from the dorsal wall of the atrium toward the endocardial cushions (Fig. 1-7). The ostium (opening) formed by the free edge of septum primum is the ostium primum. Before the septum primum fuses with the endocardial cushions, perforations appear in the upper portion of the septum primum. These perforations will coalesce to form the ostium secundum. Another muscular ridge of tissue known as the septum secundum arises from infolding of the dorsal wall of the atrium, to the right of the septum primum, and covers the ostium secundum. Its free edge forms the foramen ovale. The left venous valve and the septum spurium, located on the dorsal wall of the right atrium, fuse with the septum secundum as it grows.
Developmental Pathology
If the septum secundum fails to form and cover the perforations in the septum primum, the result is a secundum atrial septal defect (ASD). If the very last portion of the septum primum fails to meet with endocardial cushion tissue (coming from lower in the heart), a primum ASD is formed. Embryologists also speculate that if the septum primum perforations fail to form or close prematurely, the diminished right-to-left flow and diminished left-sided volume in utero result in underdevelopment of the left-sided structures, producing hypoplastic left heart syndrome.14
Formation of the Right Atrium, Coronary Sinus, and Systemic Veins
Unlike the atria, the sinus venosus remains a paired structure with right and left horns. Each sinus venosus receives blood from the yolk sac via the vitelline veins, from the chorionic villi via the umbilical veins, and from the cranial region of the embryo and body via the anterior and posterior cardinal veins. The fate of each structure is as follows (Fig. 1-8):
Formation of the Left Atrium and Pulmonary Veins
Development of the left atrium occurs concurrently with that of the right atrium. During the early part of the fourth week, a single pulmonary vein develops as an outgrowth from the back of the left atrium (Fig. 1-9), grows toward the developing lungs, and connects to the blood vessels growing out of the lungs to form pulmonary venous drainage.10 As the left atrium expands, it incorporates both the vein and, eventually, the four connecting pulmonary vessels. As the atrial wall expands, the smooth tissue of the pulmonary veins is incorporated into the wall of the atrium and displaces the trabeculated tissue anteriorly and laterally which will then form the left atrial appendage.
Developmental Pathology
Anomalous pulmonary venous return occurs when the single pulmonary vein fails to fuse with the developing intrapulmonary vessels, either because of lack of development or because of early involution.5 If the intrapulmonary vessels do not connect to the pulmonary vein, they can connect to other “alternative” vessels, such as the superior vena cava, inferior vena cava, the right atrium, the coronary sinus, or other vascular structures.15
Development of the Aortic Arches
In embryonic vascular development, ventral and dorsal aortae are connected by six pairs of aortic/branchial arches. As normal cardiovascular development proceeds, a patterned regression and persistence of the various arches and right-sided dorsal aorta occur,16 ultimately resulting in the mature configuration of the thoracic aorta and its branches (Fig. 1-10). Normally, the first, second, and fifth arches involute, the left fourth arch becomes the aortic arch, the proximal right fourth arch contributes to the innominate artery, the distal left sixth arch becomes the ductus arteriosus, the proximal sixth arches bilaterally contribute to the proximal branch pulmonary arteries, the left dorsal aorta becomes the descending thoracic aorta, and the seventh dorsal intersegmental arteries bilaterally become the subclavian arteries.
Developmental Pathology
Vascular rings are formed when this process of regression and persistence does not occur normally, and the resulting vascular anatomy completely encircles the trachea and esophagus.16,17 If bilateral fourth arches persist, a double aortic arch is formed. If the right fourth arch persists, and the proximal left fourth arch involutes, a right aortic arch with an aberrant left subclavian artery results. The ductus/ligamentum arteriosus travels from the origin of the left subclavian artery to the pulmonary artery, completing the ring. A number of normal variants may occur without forming a complete vascular ring. If the proximal right fourth arch regresses, a left arch with an aberrant right subclavian artery is formed. If the right fourth arch persists, and the distal left fourth arch involutes, a right aortic arch with mirror image branching is formed. In this setting, the ligamentum usually travels from the left innominate artery to the left pulmonary artery without forming a vascular ring. If the left ligamentum travels from the pulmonary artery to the descending thoracic aorta, a vascular ring results.
The Segmental Approach to Diagnosis and Management of Congenital Heart Disease
One can think of the heart as a three-level house (Fig. 1-11). The first level is the visceroatrial situs, the middle level is the ventricular loop, and the third level is the conotruncus. To describe it simply, the three levels are the atria, ventricles, and great arteries. There are two separating walls with doors: the atrioventricular junction and the ventriculoarterial junction. There are two entrances: the systemic veins and the pulmonary veins. The levels represent the major cardiac segments. The connecting doors represent the connecting segments.
The segmental approach to heart disease comprises the following steps:18
Identification of the Major Cardiac Segments
Atrial Identification
The defining features of the morphologic right atrium (systemic venous atrium) and left atrium (pulmonary venous atrium) are based on their venous connections as well as their appendage and pectinate muscle morphology. Use of venoatrial connections for atrial identification is based on the fact that the sinus venosus, which carries the systemic venous return, is an integral part of the morphologic right atrium.19 Hence, the morphologic right atrium receives the inferior and superior vena cavae, and orifice of coronary sinus. However, the SVC and coronary sinus have a high incidence of variation, which can be a source of diagnostic confusion. These variations include left SVC to an unroofed coronary sinus, and bilateral SVC with the left SVC draining to an unroofed coronary sinus. In these cases, the SVC would appear to drain into the left atrium. In rare instances, even the IVC may drain into the coronary sinus, which may be unroofed, or the coronary sinus septum may be absent. In spite of this rare exception, the most reliable means of identifying the morphologic right atrium by cross-sectional imaging is by recognizing its connection to the IVC (Fig. 1-12). Even in the setting of an interrupted IVC, a suprahepatic segment of the IVC is present entering the right atrium, allowing accurate identification.
Anderson has described the morphologic right atrium (systemic atrium) as being characterized by the presence of a triangular appendage with a broad junction (Fig. 1-13), and by the recognition of pectinate muscles extending to the atrioventricular junction. The morphologic left atrium is characterized by a tubular narrow-based appendage, and lack of pectinate muscle extension to the atrioventricular junction. Because determination of pectinate muscle morphology is beyond the resolution of MRI or CT, atrial identification is performed by recognition of venoatrial connections and morphology of the appendages. If this analysis fails to yield a confident identification of the right and left atrium, then a diagnosis of atrial situs ambiguous is made. Even in the setting of visceral situs ambiguous, reliable identification of atrial situs may be made in more than 80% of the cases.
Ventricular Identification
The morphologic LV is identified by the following features:
Great Arterial Identification
The pulmonary artery gives rise to branches to the lungs, and no branches to the body. The aorta gives rise to branches to the body as well as the coronary arteries. A common vessel arising from the ventricles that gives rise to the coronaries, branches to the body as well the lungs and is termed a common arterial trunk or truncus, and a segmental relationship is not assigned (labeled ‘X’ for undetermined). On transverse cross-sectional imaging at the level of the outflow tract, the coronary artery origin is used to identify the aortic annulus. The intercoronary commissure of the aortic valve is pointed toward the right-left commissure of the pulmonary valve and is an important landmark for determining great arterial relationship (Fig. 1-16). In solitus relationship of the great arteries, the aortic valve annulus lies posterior and to the right of the pulmonary valve annulus.
Analysis of the Three Major Cardiac Segments
First Major Segment: Visceroatrial Situs
Situs refers to the position of the atria and viscera relative to the midline. There are three types of situs: solitus (S), inversus (I), and ambiguous (A) (Fig. 1-17). Heterotaxy is synonymous with situs ambiguous and is simply defined as ‘situs other than solitus or inversus’.
Because heterotaxy is defined as “situs other than solitus or inversus”, it is not a specific disease, but a constellation of cardiac, vascular, and visceral abnormalities, including situs ambiguous of the viscera, lung symmetry, atrial appendage symmetry, anomalous systemic venous return, anomalous pulmonary venous return, and associated intracardiac defects. No single finding is pathognomonic. Although there are three syndromes, there is a tendency toward clustering of defects into two syndromes based on the predominance of right-sided or left-sided structures (Fig. 1-18):
In an autopsy series of 109 cases of heterotaxy syndrome by Van Praagh, 53% were asplenia, 42% polysplenia, and 5% single right-sided spleen with levocardia.20 There is a relatively high incidence of discordance between the different components of visceral situs as well atrial situs. Hence, the syndromic approach to heterotaxy has been discarded in favor of a segmental approach in which there is an independent assessment of every involved organ system without any preconceived notions.21 Determining visceral situs by chest radiography is challenging and can be achieved by recognizing the bronchial branching pattern (Fig. 1-19). A symmetric branching pattern is a common manifestation of heterotaxy. The cardiac apex and stomach position are not used for determining visceral situs because of the high incidence of discordance.22 For accurate determination of visceral situs, cross-sectional imaging by ultrasound, CT, or MRI is required for evaluating the status of the liver and spleen.
Second Major Segment: Ventricular Loop
Depending on the direction of ventricular looping during development, the right ventricle may be located spatially on the right or left side of the heart (Fig. 1-20). If the bulboventricular loop occurs to the right, it is termed a D-loop, and the morphologic RV lies anterior and to the right of the morphologic LV. If the bulboventricular loop occurs to the left, it is termed an L-loop, and the morphologic RV lies anterior and to the left of the morphologic LV.
Third Major Segment: Great Arterial Relationship
Normally, the aortic annulus lies posterior, inferior, and to the right of the pulmonary valve annulus. This position is called solitus (S) (Fig. 1-21A). In situs inversus, the aorta lies posterior and to the left of the pulmonary valve, termed inversus (I) (see Fig. 1-21B). Any other position of the aorta and pulmonary artery other than solitus or inversus is termed malposition. If the aorta lies to the right of the PA, it is called D-malposition (see Fig. 1-21C). If the aorta lies to the left of the PA, it is termed L-malposition (see Fig. 1-21D). In rare cases, the aorta lies straight—anterior to the pulmonary artery, and is referred to as A according to segmental nomenclature. If the aorta lies straight—posterior to the pulmonary artery, it is termed P.
Different Types of Human Hearts
The segmental possibilities at each level are as follows:
Based on the various permutations and combinations of atrial, ventricular, and great arterial relationships, as well as on the anatomy of the conus, Van Praagh provided an overview of the diversity that exists in human hearts (Fig. 1-22).18 It becomes immediately apparent that a pattern-based approach or an approach based on connections or the direction of flow of blood will not do justice to the complexity of the disease process. The segmental approach not only takes into account the morphologic variations at each level, but it also provides structural landmarks to distinguish the variations from each other and to determine their impact on physiology, thereby allowing informed decision-making regarding management.
Analysis of the Connecting Segments
First Connecting Segment: Atrioventricular Junction
The types of biventricular AV connections (Fig. 1-23) include the following:
The types of univentricular AV connections (Figs. 1-24 and 1-25) include the following:
In the setting of univentricular AV connections, one of the AV valves is atretic, or both AV valves open partly or wholly into the same ventricle. The second ventricular chamber is subsequently very small or just an outflow chamber, and is of little functional use, apart from serving as a conduit for one of the great arteries. The segmental approach may be applied even in the setting of such functional single ventricles (see Fig. 1-25). Single ventricles may be of a left ventricular, right ventricular, or indeterminate morphology.23 The functional single LV is characterized by the presence of a bulboventricular foramen, which connects the LV chamber to the small anteriorly located infundibular outlet chamber of the RV. The RV sinus (inflow portion) is typically absent. A functional single RV is characterized by the presence of a septal band, and in some cases, by a rudimentary posterolaterally located LV chamber.
Twisted atrioventricular connections: Van Praagh24 introduced the concept of atrioventricular alignment as an independent entity from atrioventricular connections mainly to describe the spectrum of conditions with twisted or crossed atrioventricular connections, variably described as criss-cross, or topsy-turvy hearts.25 These are hearts with discordance between atrioventricular alignment and atrioventricular connection. For instance, Figure 1-26 shows a 3-D volume rendered image of a patient with situs solitus of the atria and viscera, criss-cross atrioventricular relationship, and L-malposition of the great arteries. The RV lies superior and slightly to the left of the LV. But, because of the crossed atrioventricular connections, this patient has concordant atrioventricular connection, with the right atrium connecting to the left-sided RV. The base to apex axis of the heart spirals almost 180 degrees, with the two atrioventricular blood streams crossing each other. The physiology is that of uncorrected transposition. Because of the presence of associated straddling, AV valve, and severe pulmonary stenosis, a single ventricle repair was performed with a Fontan procedure.
Second Connecting Segment: Conotruncus or Ventriculoarterial Junction
The development of the conotruncus is the most important variable in the genesis of outflow tract anomalies. The differential growth of the subpulmonary and subaortic conus cushions largely determines the relationship between the semilunar valves, between the semilunar valves and the ventricles, and between the semilunar valves and the atrioventricular valves. It also determines the presence of distal infundibular stenosis and the location of the VSD in outflow tract anomalies. Based on conal development, the following anatomic types of conus (Fig. 1-27) may be recognized26:
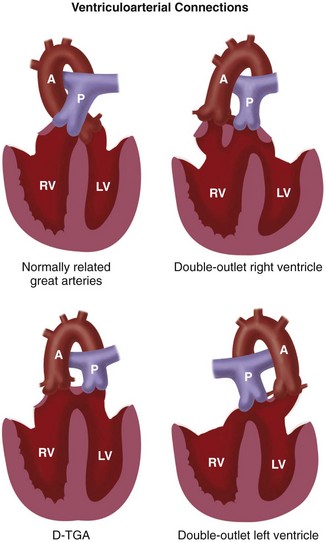
 FIGURE 1-27 Types of ventriculoarterial connections based on conal development.
FIGURE 1-27 Types of ventriculoarterial connections based on conal development.
(Adapted from The segmental and sequential approach to congenital heart disease. In Freedom RM, Mawson JB, Yoo SJ, Benson LN [eds]: Congenital Heart Disease: Textbook of Angiocardiography. Armonk, NY, 1997, Futura, p 111.)
A rare form of conal maldevelopment is anatomically corrected malposition of the great arteries in which there is origin of the malposed aorta above the LV, and origin of the malposed pulmonary artery above the RV.27 Malalignment of the conal septum is also the cause of infundibular obstruction in tetralogy of Fallot (anterior malalignment) and subaortic stenosis with interrupted aortic arch (posterior malalignment).
Evaluation of Associated Anomalies
The presence of associated anomalies involving the atrial and ventricular septum, the atrioventricular and semilunar valves, aorta, pulmonary arteries, pulmonary veins, and systemic veins (Fig. 1-29) plays an important part in the functional outcome of the patient, and screening for these conditions is an integral part of the segmental approach to heart disease.
Evaluation of Function
The final step in the segmental approach to heart disease is to determine how the segmental combinations and connections, with the associated anomalies, function (Case Study 1, Fig. 1-30). Broadly, abnormal function of the congenitally malformed heart may be classified into the following subgroups:
KEY POINTS
 The segmental approach to heart disease provides an elegantly simple method to break down the complexity of congenital heart disease on cross-sectional imaging.
The segmental approach to heart disease provides an elegantly simple method to break down the complexity of congenital heart disease on cross-sectional imaging. It creates a template for standardizing nomenclature in CHD, thereby ensuring that caregivers from different specialties understand each other.
It creates a template for standardizing nomenclature in CHD, thereby ensuring that caregivers from different specialties understand each other.Clark EB, Van Mierop LHS. Development of the cardiovascular system. In: Adams FH, Emmanouilides GC, Riemenschneider TA, editors. Moss’ Heart Disease in Infants, Children, and Adolescents. Baltimore: Williams & Wilkins; 1989:2-15.
Freedom RM, Yoo SJ, Mikalian H, Williams WG, editors. The Natural and Modified History of Congenital Heart Disease. New York: Futura, 2004.
Krishnamurthy R, Chung T. Current imaging status: pediatric MRI. In Lucaya J, Strife JL, editors: Pediatric Chest Imaging, 2nd ed, Berlin: Springer, 2007.
The segmental and sequential approach to congenital heart disease. In: Freedom RM, Mawson JB, Yoo SJ, Benson LN, editors. Congenital Heart Disease: Textbook of Angiocardiography. Armonk, NY: Futura; 1997:95-120.
Van Mierop LHS, Kutsche LM. Embryology of the heart. In Hurst JW, editor: The Heart, 7th ed, New York: McGraw-Hill, 1990.
Van Praagh R. The segmental approach to understanding complex cardiac lesions. In: Eldridge WJ, Gberg H, Lemole GM, editors. Current Problems in Congenital Heart Disease. New York: SP Medical and Scientific Books; 1979:1-18.
1 Van Praagh R. The segmental approach to diagnosis in congenital heart disease. Birth Defects: Original Article Series. 1972;8:4-23.
2 Van Praagh R. The segmental approach to understanding complex cardiac lesions. In: Eldredge WJ, Gberg H, Lemole GM, editors. Current Problems in Congenital Heart Disease. New York: Medical and Scientific Books; 1979:1-18.
3 Van Praagh R. Diagnosis of complex congenital heart disease: morphologic-anatomic method and terminology. Cardiovasc Intervent Radiol. 1984;7:115-120.
4 Shinebourne EA, Macartney FJ, Anderson RH. Sequential chamber localization-logical approach to diagnosis in congenital heart disease. Br Heart J. 1976;38:327-340.
5 Van Mierop LHS, Kutsche LM. Embryology of the heart. In Hurst JW, editor: The heart, 7th ed, New York: McGraw-Hill Information Services Company, 1990.
6 Brueckner M, D’Eustachio P, Horwich AL. Linkage mapping of a mouse gene, iv, that controls left-right asymmetry of the heart and viscera. Proc Natl Acad Sci USA. 1989;86:5035-5038.
7 Burn J. Disturbance of morphological laterality in humans. Ciba Foundation Symposium. 1992:282-300.
8 Hanzlik AJ, Binder M, Layton WM, et al. The murine situs inversus viscerum (iv) gene responsible for visceral asymmetry is linked tightly to the Igh-C cluster on chromosome 12. Genomics. 1990;7:389-393.
9 Layton WMJr, Manasek FJ. Cardiac looping of early iv/iv mouse embryos. In: Van Praagh R, Takao A, editors. Etiology and Morphogenesis of Congenital Heart Disease. Mount Kisco, NY: Futura; 1980:109-126.
10 Anderson RH. Another look at cardiac embryology. In: Yu PN, Goodwin JF, editors. Progress in Cardiology. Philadelphia: Lea & Febiger; 1978:1-53.
11 Clark EB, Van Mierop LHS. Development of the cardiovascular system. In: Adams FH, Emmanouilides GC, Riemenschneider TA, editors. Moss’ Heart Disease in Infants, Children, and Adolescents. Baltimore: Williams and Wilkins; 1989:2-15.
12 Calder L, Van Praagh R, Van Praagh S, et al. Truncus arteriosus communis. Clinical, angiocardiographic, and pathologic findings in 100 patients. American Heart J. 1976;92(1):22-38.
13 Van Praagh R, Perez-Trevino C, Lopez-Cuellar M, et al. Transposition of the great arteries with posterior aorta, subpulmonary conus and fibrous continuity between aortic and atrioventricular valves. Am J Cardiol. 1971;28(6):894-904.
14 Reller MD, McDonald RW, Gerlis LM, Thornburg KL. Cardiac embryology: Basic review and clinical correlations. J Am Soc Echocardiog. 1991;4:519-532.
15 Neill CA. Development of the pulmonary veins. With reference to the embryology of anomalies of pulmonary venous return. Pediatrics. 1956;18:880-887.
16 Edwards JE. Anomalies of derivatives of aortic arch system. Med Clin North Am. 1948;32:925-949.
17 Stewart JR, Kincaid OW, Edwards JE. An atlas of vascular rings and related malformation of the aortic arch system. Springfield: Charles C. Thomas; 1964.
18 Van Praagh R. Terminology of congenital heart disease. Glossary and commentary. Circulation. 1977;56:139-143.
19 Huhta JC, Smallhorn JF, Macartney FJ, et al. Cross-sectional echocardiographic diagnosis of systemic venous return. Br Heart J. 1982;48:388-403.
20 Van Praagh S, Kreutzer J, Alday L, Van Praagh R. Systemic and pulmonary venous connections in visceral heterotaxy, with emphasis on the diagnosis of the atrial situs: a study of 109 postmortem cases. In: Clark E, Takao A, editors. Developmental Cardiology, Morphogenesis and Function. Mt. Kisco, NY: Futura; 1990:671-721.
21 Machado-Atias I, Anselmi G, Machado-Hernandez I, Febres C. Discordances between the different types of atrial arrangement and the positions of the thoraco-abdominal organs. Cardiol Young. 2001;11(5):543-550.
22 Van Praagh R, Vlad P. Dextrocardia, mesocardia, and levocardia: the segmental approach to diagnosis in congenital heart disease. In: Keith JD, Rowe RD, Vlad P, editors. Heart disease in Infancy and Childhood. New York, NY: Macmillan; 1978:638-695.
23 Colvin EV. Single ventricle. In: Garson AJr, Bricker JT, McNamara DG, editors. The Science and Practice of Pediatric Cardiology. Philadelphia: Lea and Febiger; 1990:1246-1279.
24 Van Praagh R. When concordant or discordant atrioventricular alignments predict the ventricular situs wrongly. I. Solitus atria, concordant alignments, and L-loop ventricles. II. Solitus atria, discordant alignments, and D-loop ventricles. J Am Coll Cardiol. 1987;10:1278-1279.
25 Geva T, Van Praagh S, Sanders SP, et al. Straddling mitral valve with hypoplastic right ventricle, criss-cross atrioventricular relations, double outlet right ventricle and dextrocardia: Morphologic, diagnostic and surgical considerations. J Am Coll Cardiol. 1991;17:1603-1612.
26 Van Praagh S, Layton WM, Van Praagh R. The morphogenesis of normal and abnormal relationships between the great arteries and the ventricles: pathologic and experimental data. In: Van Praagh R, Takao A, editors. Etiology and Morphogenesis of Congenital Heart Disease. Mt. Kisco, NY: Futura; 1980:271-316.
27 Van Praagh R, Weinberg PM, Smith SD, et al. Malpositions of the heart. In: Adams FH, Emmanouilides GC, Riemenschneider TA, editors. Moss’ Heart Disease in Infants, Children, and Adolescents. Baltimore: Williams and Wilkins; 1989:530-580.

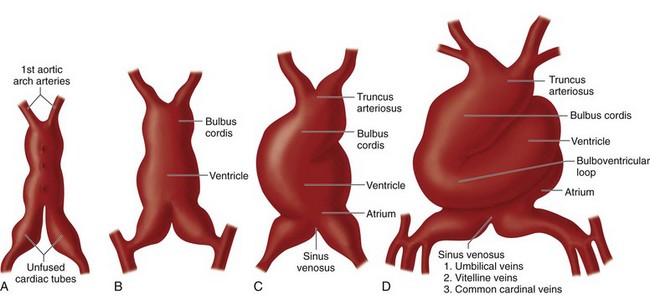
 FIGURE 1-1
FIGURE 1-1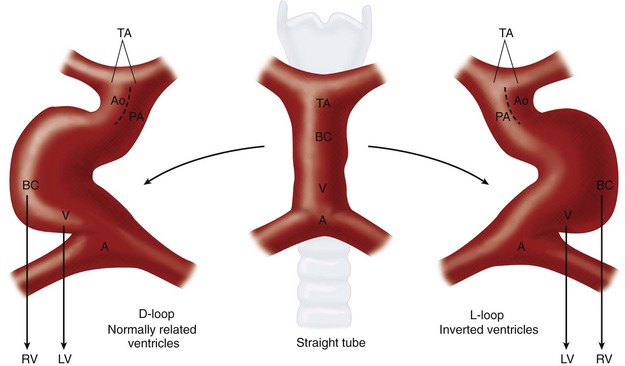
 FIGURE 1-2
FIGURE 1-2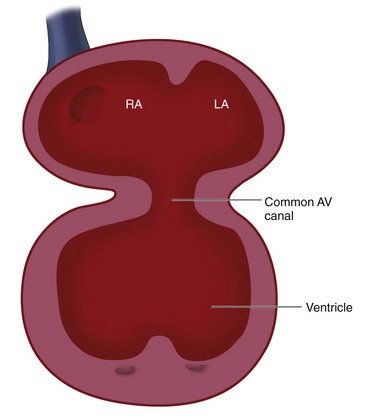
 FIGURE 1-3
FIGURE 1-3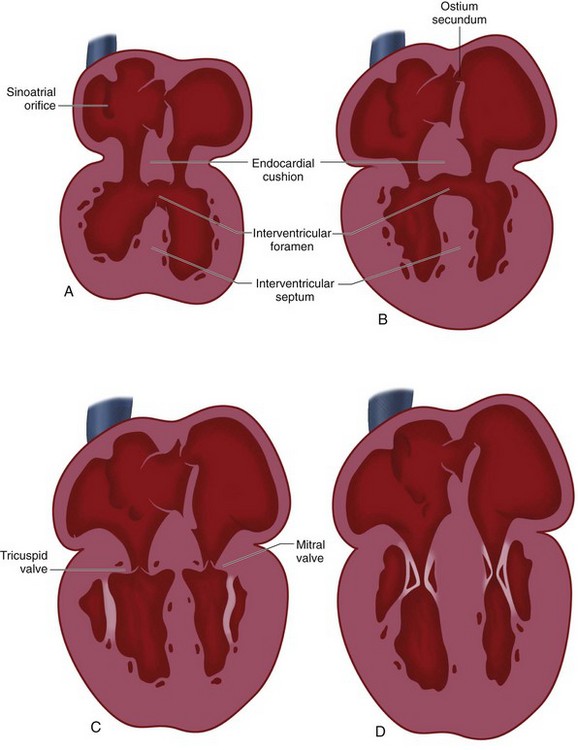
 FIGURE 1-4
FIGURE 1-4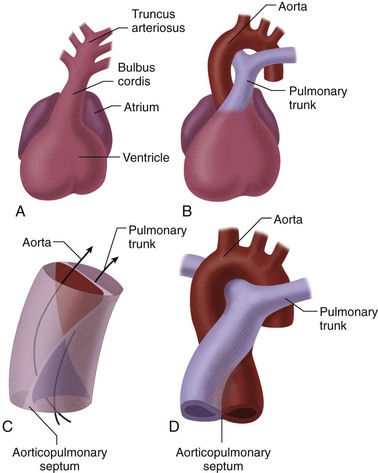
 FIGURE 1-5
FIGURE 1-5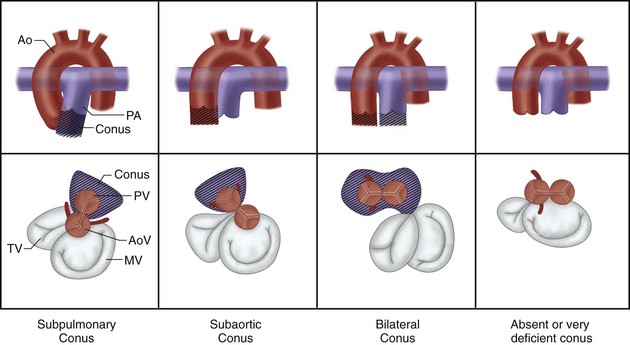
 FIGURE 1-6
FIGURE 1-6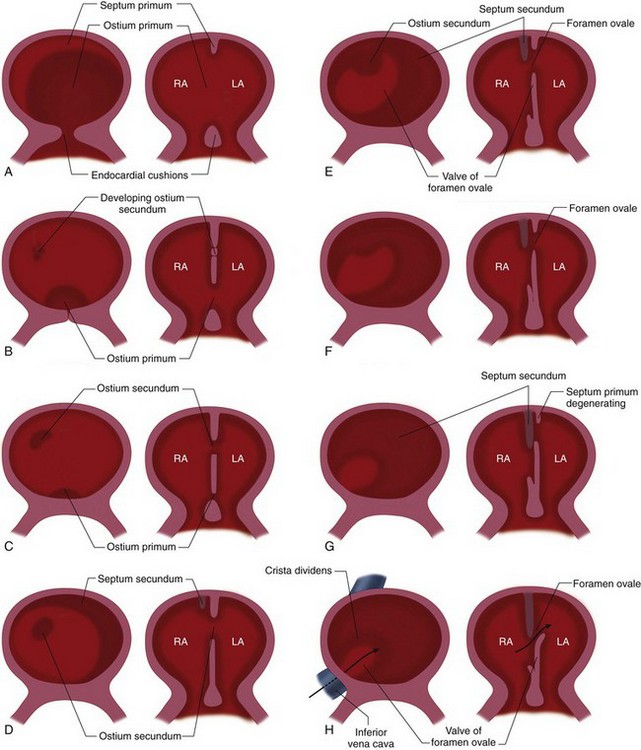
 FIGURE 1-7
FIGURE 1-7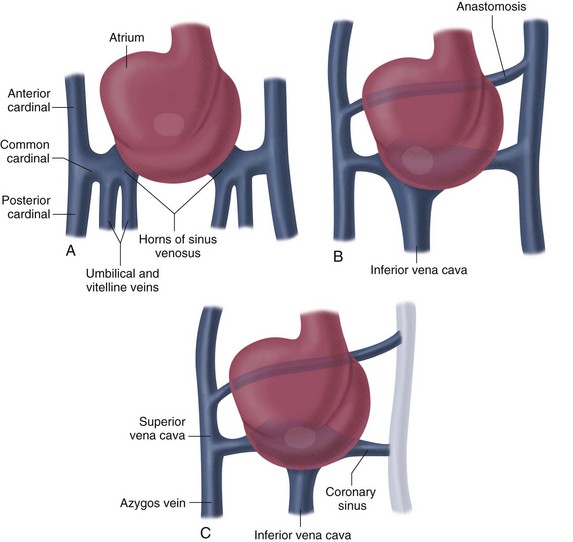
 FIGURE 1-8
FIGURE 1-8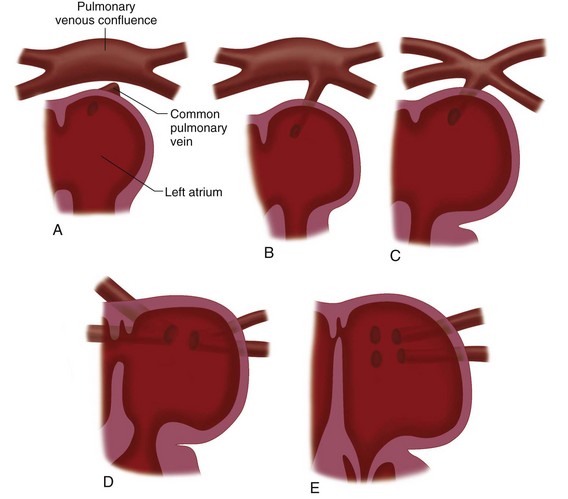
 FIGURE 1-9
FIGURE 1-9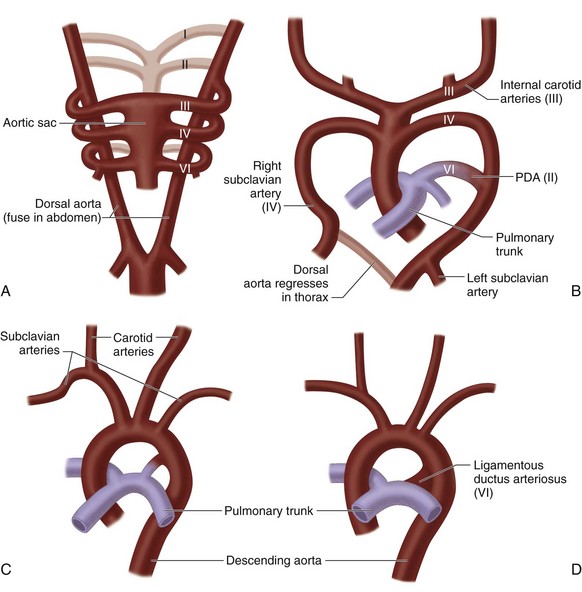
 FIGURE 1-10
FIGURE 1-10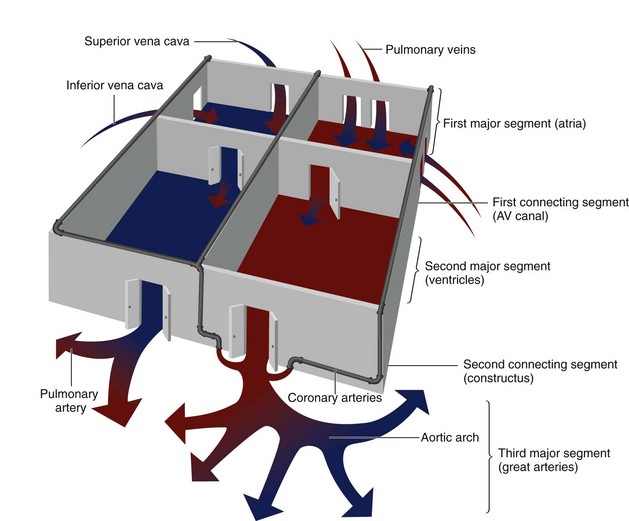
 FIGURE 1-11
FIGURE 1-11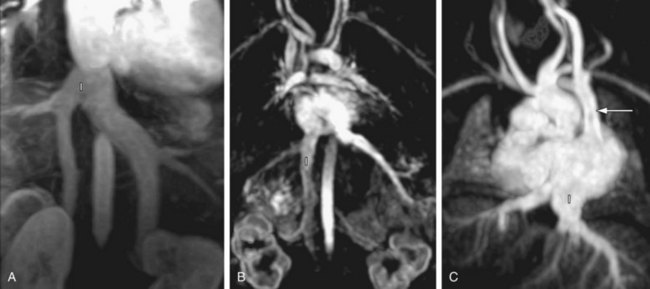
 FIGURE 1-12
FIGURE 1-12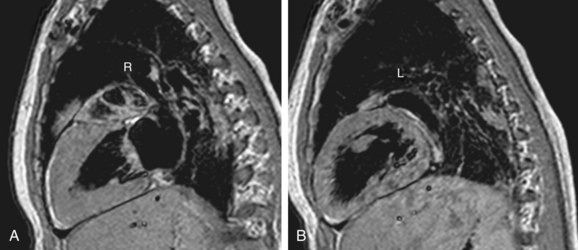
 FIGURE 1-13
FIGURE 1-13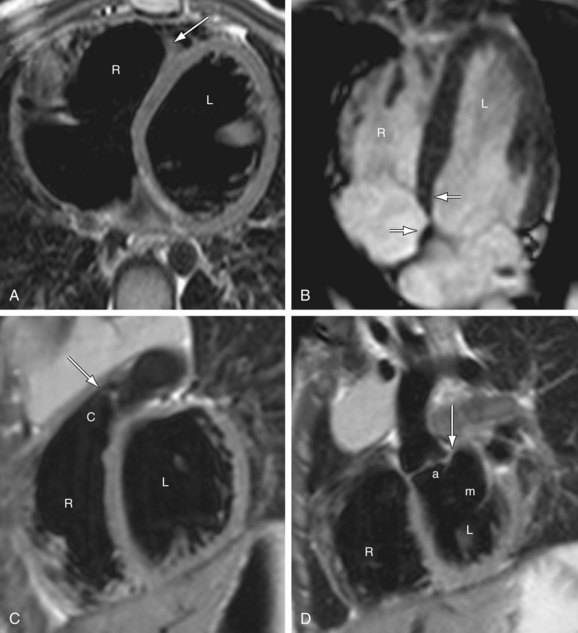
 FIGURE 1-14
FIGURE 1-14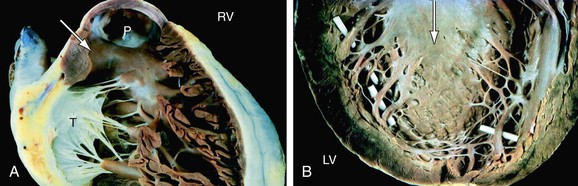
 FIGURE 1-15
FIGURE 1-15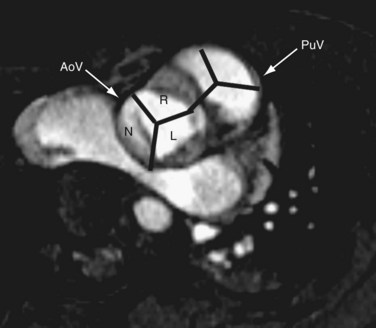
 FIGURE 1-16
FIGURE 1-16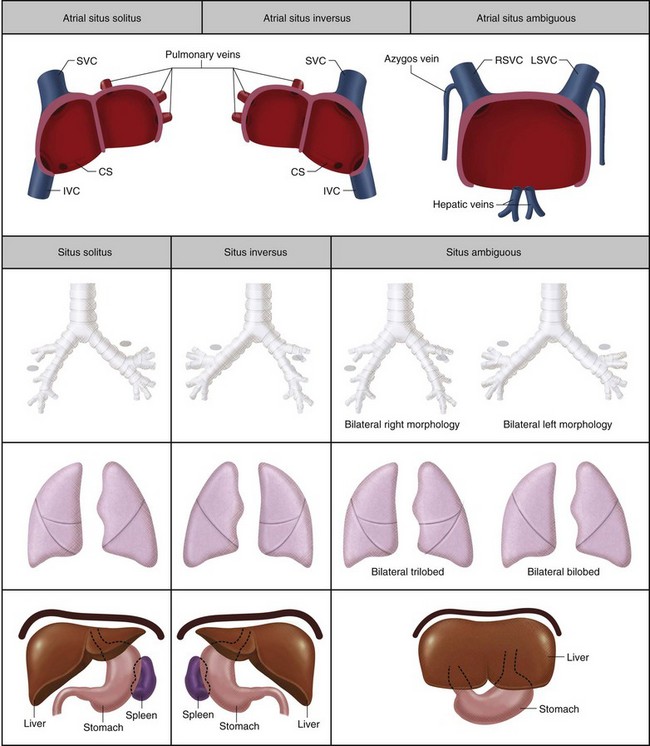
 FIGURE 1-17
FIGURE 1-17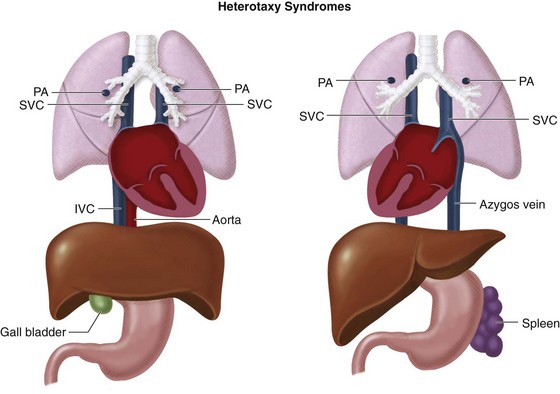
 FIGURE 1-18
FIGURE 1-18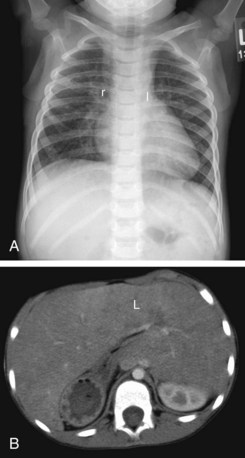
 FIGURE 1-19
FIGURE 1-19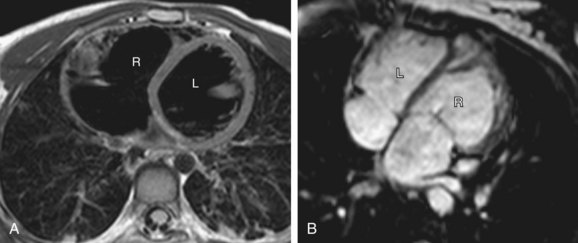
 FIGURE 1-20
FIGURE 1-20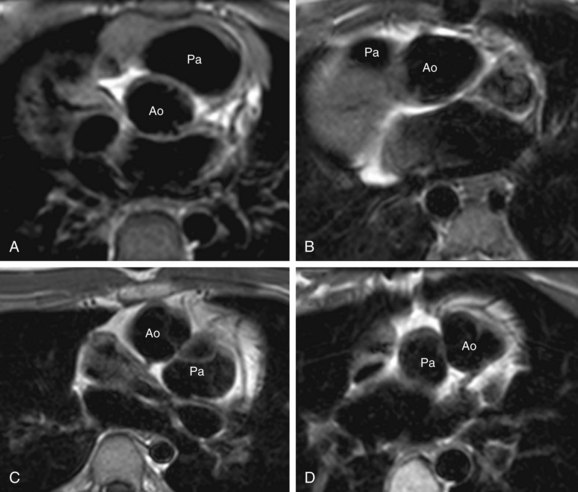
 FIGURE 1-21
FIGURE 1-21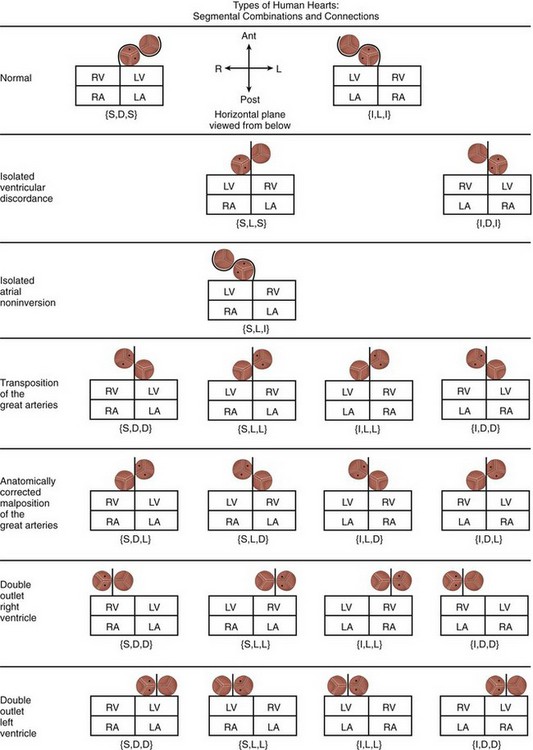
 FIGURE 1-22
FIGURE 1-22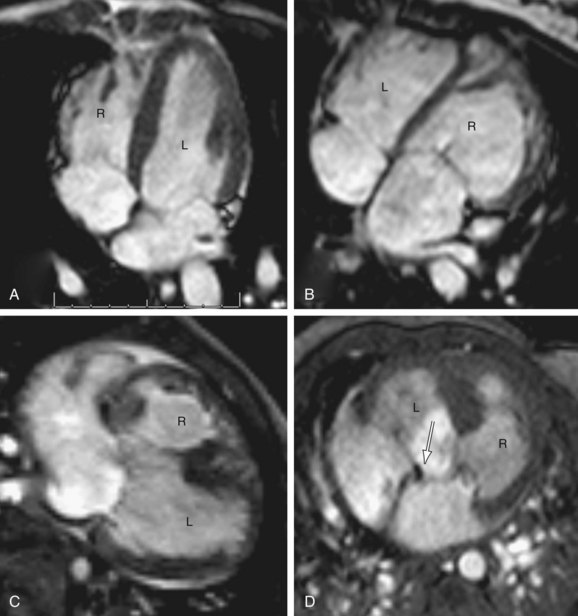
 FIGURE 1-23
FIGURE 1-23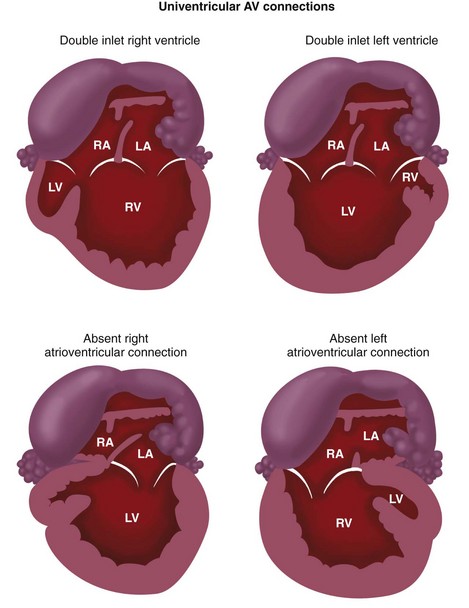
 FIGURE 1-24
FIGURE 1-24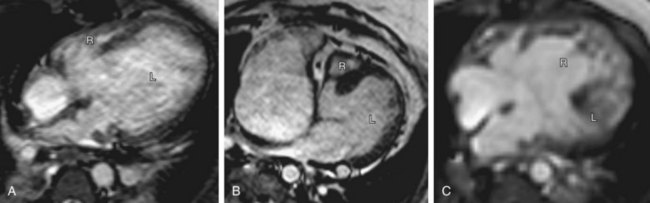
 FIGURE 1-25
FIGURE 1-25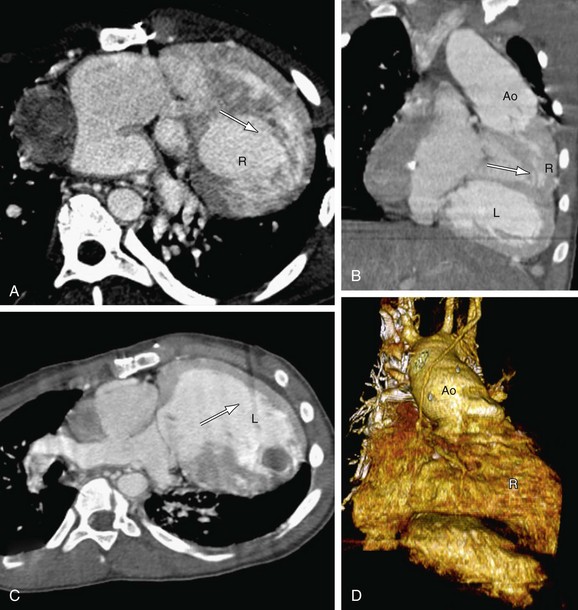
 FIGURE 1-26
FIGURE 1-26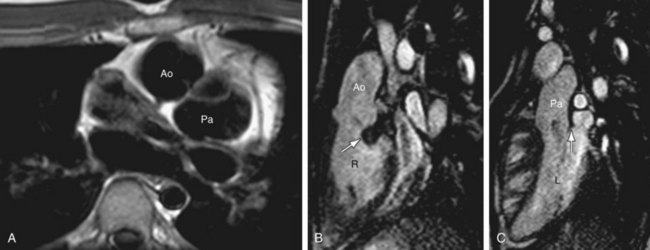
 FIGURE 1-28
FIGURE 1-28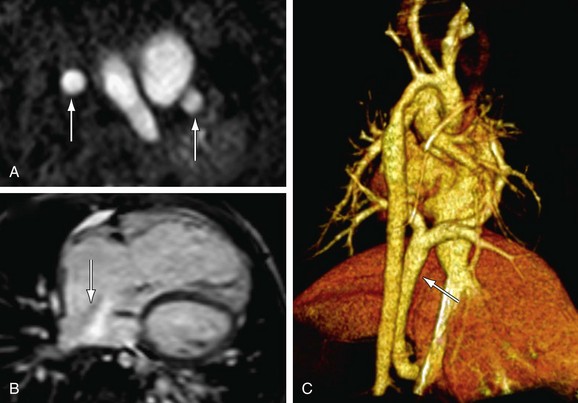
 FIGURE 1-29
FIGURE 1-29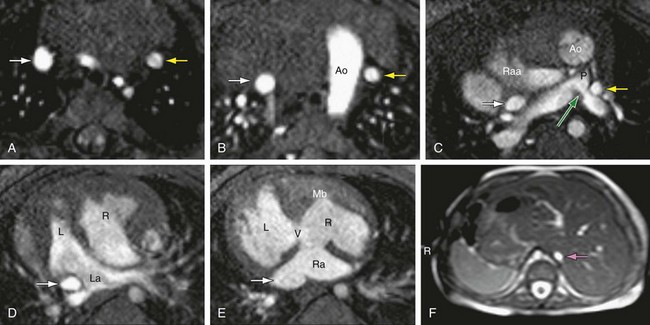
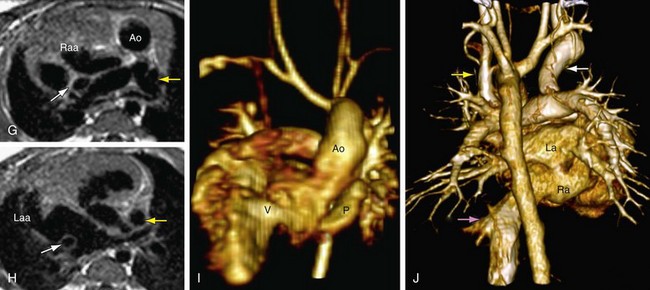
 FIGURE 1-30
FIGURE 1-30

