Glucose Elevations and Outcome in Critically Injured Trauma Patients
Stress hyperglycemia, defined as a transient plasma glucose level above 200 mg/dL, is associated with adverse outcomes among the critically ill, including increased mortality [1–7]. Since the landmark study conducted by Van den Berghe and colleagues [8] in Leuven, Belgium, first demonstrated improved survival in ICU patients treated with intensive insulin therapy, there has been considerable attention dedicated toward defining the ideal therapy required to optimize outcome for critically ill patients with hyperglycemia. Although subsequent studies have failed to replicate the findings of the Leuven group, these investigations lacked the methodologic rigor of the initial studies and have provided few data that can be effectively extrapolated to the care of ICU populations, including victims of trauma. The largest body of work examining the risks and treatment of hyperglycemia after injury has been conducted at the University of Maryland R Adams Cowley Shock Trauma Center [1,8–11]. Data from the authors’ group have demonstrated that hyperglycemia has a significant association with adverse outcomes after trauma and that intervention with insulin therapy may significantly improve outcomes for these patients.
Pathophysiology of stress hyperglycemia
Although the precise cause of these glucose elevations has not been comprehensively defined, it has been postulated that they are the result of increased levels of cortisol, glucagons, and epinephrine associated with critical illness [12,13]. The action of these hormones results in increased gluconeogenesis in vivo. As a result of these hormones’ actions, there is also a decrease in peripheral uptake of glucose to insure substrate availability. These combined effects result in high circulating levels of glucose during the physiologic response to critical illness or trauma.
There are several effects of hyperglycemia that have the potential to contribute to associated adverse outcomes observed after acute illness or trauma [7,14–17]. It has been suggested that hyperglycemia may be acutely toxic in critically ill patients because of accentuated cellular glucose overload and associated pronounced side effects of glycolysis and oxidative phosphorylation [18]. Additionally, it has been hypothesized that after trauma or critical illness, the expression of glucose transporters on the membranes of several cell types may be up-regulated. During reperfusion after ischemia, this up-regulation may allow high circulating glucose levels to overload cellular metabolism and cause irreversible damage to cellular function and structure. Other proposed mechanisms of injury include increased generation and deficient scavenging systems for reactive oxygen species produced by the activated glycolysis and oxidative phosphorylation associated with glucose toxicity [17]. All of these proposed mechanisms may contribute to the observed dysfunctions of liver, renal, cardiac, endothelial, and cellular immune functions associated with hyperglycemia in the setting of critical illness [19].
Insulin therapy and strict glucose control in critical illness
In 2001, the first of 2 landmark randomized trials examining the effects of insulin therapy on outcome was reported by Van den Berghe and colleagues [8,20]. In the initial examination, reported in 2001, the investigators enrolled 1548 patients requiring surgical ICU admission and mechanical ventilation. On admission, these patients were randomly assigned to receive intensive insulin therapy (maintenance blood glucose goal 80–110 mg/dL) or conventional glucose control therapy (infusion of insulin only if blood glucose exceeded 215 mg/dL). At 12 months, they found that intensive insulin therapy was associated with reduced overall mortality (4.6% vs 8.0% for the conventional therapy group; P <.04). The researchers also found that the benefit of intensive insulin therapy was most attributable to its effect on mortality in patients who remained in an ICU for more than 5 days. The greatest reduction in mortality involved deaths due to multiple-organ failure with a proved septic focus, with associated overall reductions in infections and renal failure requiring dialysis [20].
A subsequent study conducted by the Leuven group examined the impact of intensive glucose therapy in a population of medical ICU patients using the same glucose control cohort arms [8]. In this study of 1200 patients, the investigators found that the use of intensive insulin therapy significantly reduced blood glucose levels but did not significantly reduce in-hospital mortality (37.3% in the intensive therapy group and 40.0% in the conventional therapy group; P = .33). They did find, however, that for those patients staying in an ICU for more than 3 days, there was a reduction of in-hospital mortality (52.5% to 43.0%; P = .009), with an associated reduction in all-cause morbidity. For those patients who required less than 3 days of admission, however, there was an increased mortality associated with intensive insulin therapy use. Based on these and subsequent post hoc analyses, the Leuven group concluded that intensive insulin therapy was beneficial for ICU patients, with the maximal benefit appreciated by surgical patients. The results of these 2 studies prompted a significant shift in emphasis toward tight glucose control practices among ICU practitioners and were widely promoted as a standard of care practice by such groups as the Institute for Healthcare Improvement.
In the wake of the Leuven group findings, subsequent randomized controlled trials were conducted in heterogeneous populations of ICU patients. The studies failed to achieve the same degree of glucose control as Van den Berghe and colleagues and also failed to support the subsequent benefit of these intensive glucose control practices in this environment [21–24]. One of the largest studies reported was conducted by the Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study group [23].
Meta-analyses of available prospective randomized controlled trials examining intensive insulin therapy in the critical care environment have attempted to provide answers regarding the role of this intervention in the setting of critical illness [25,26]. In the largest meta-analysis of available data to date, Greisdale and colleagues [26] evaluated 26 randomized controlled trials comparing intensive insulin therapy to conventional glucose control therapies, including the NICE-SUGAR study. They found that patients treated in a surgical ICU were the only patients who seemed to benefit from intensive insulin therapy compared with those in the control group of patients undergoing conventional insulin therapy (P = .02). Among all of these studies, however, there has been limited examination of the effect of glycemic control specifically on patients who have required hospitalization or ICU admission after trauma.
Hyperglycemia risk and treatment among trauma populations
The largest studies of hyperglycemic effects and glucose control specifically for dedicated populations of critically ill trauma patients have been conducted at the University of Maryland R Adams Cowley Shock Trauma Center. The earliest of these investigations demonstrated that early hyperglycemia might contribute significantly to adverse outcome after trauma. In 2005, Sung and colleagues [1] conducted a prospective examination of 1003 consecutive trauma patients admitted to the ICU at the Shock Trauma Center over a 2-year period. After excluding those patients with pre-existing diabetes, patients were stratified by serum glucose level (<200 mg/dL vs ≥200 mg/dL), demographics, severity of injury, and other pre-existing risk factors. The investigators found that 25% of patients were admitted with hyperglycemia over the study period and that patients with hyperglycemia had an overall greater infection rate and hospital length of stay. Additionally, the hyperglycemic group had a 2.2-times greater risk of mortality after adjustment for age and Injury Severity Score, with hyperglycemia proving an independent predictor of outcome and infection after trauma.
An additional examination conducted by Bochicchio and colleagues [10] prospectively examined the effects of hyperglycemia on patients requiring immediate operative intervention after trauma. They evaluated 252 consecutive nondiabetic trauma patients who went directly to the operating room from the resuscitation area, stratifying individuals by preoperative serum glucose level (<200 mg/dL vs ≥200 mg/dL), demographic data, severity of injury, and other pre-existing risk factors. Multiple linear regression analysis revealed patients with elevated serum glucose had a significantly higher incidence of infection, longer hospital and ICU lengths of stay, and mortality when matched for age and Injury Severity Score. The investigators concluded that elevated serum glucose on admission is an accurate predictor of postoperative morbidity and mortality when found in the early phases after trauma.
Although the studies (discussed previously) at the authors’ institution established the risks of hyperglycemia in the early phases after trauma, subsequent study was required to better establish the potential adverse effects associated with persistent hyperglycemia after trauma. In 2005, Bochicchio and colleagues [11] collected prospective data on 942 consecutive trauma patients admitted to an ICU during a 2-year period. Patients were stratified by serum glucose level from day 1 to day 7 of ICU stay using 3 different glucose levels (low = 139 mg/dL; medium = 140–219 mg/dL; and high >220 mg/dL). Patients with medium, high, worsening, and highly variable hyperglycemia were found to have increased ICU length of stay, hospital length of stay, and ventilator day requirements. Additionally, univariate analysis revealed higher infection and mortality rates in these same groups of patients. After controlling for age, Injury Severity Score, and glucose pattern, patients with high, worsening, and highly variable hyperglycemia were most predictive of increased ventilator days, ICU and hospital lengths of stay, infection, and mortality (P <.01) [10].
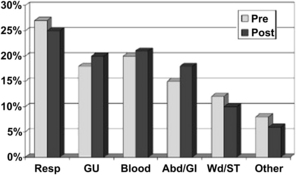
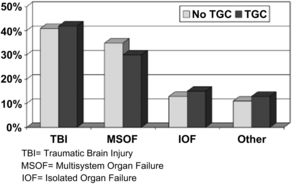
Finally, a study conducted by Scalea and colleagues [9] examined the impact of a tight glucose control policy (goal target 100 to 150 mg/dL) on outcomes associated with hyperglycemia after trauma. The investigators performed a quasiexperimental interrupted time-series design study to evaluate the impact of tight glucose control on a population of critically injured trauma patients requiring ICU admission. They compared outcomes from a 24-month period before implementation of the tight glucose control protocol to a 24-month postintervention phase. After comparing the more than 1000 patients in each arm, they found no significant difference in mechanism of injury, gender, age, or Injury Severity Score. They did find, however, that the tight glucose control group was more likely in the all low or improving pattern of glucose control (P <.001). They also noted that the incidence of early infection (over the first 2 weeks) was decreased from 29% to 21% after the introduction of their tight glucose control protocol (P <.001) (Fig. 1). After controlling for age, Injury Severity Score, obesity, and pre-existing diabetes, the investigators discovered that the non–tight glucose control group required more ventilator days (odds ratio [OR] 3.9, class interval [CI] 1.8–8.1), longer ICU stays (OR 4.3, CI 2.1–7.5), and more hospital days (OR 5.5, CI 2.2–11.0) and were at higher risk for in-hospital mortality (OR 1.4, CI 1.1–10.0) (Fig. 2) [8].
Controversies and future directions
The studies (discussed previously) as well as those of other investigators [27,28] have demonstrated both the adverse outcomes associated with hyperglycemia after trauma and the impact of intervention through effective glucose control. Several important questions still remain regarding the implementation of tight glucose control policies for these patients. Issues that require additional clarification include the timing and mode of implementation for glucose control and the appropriate goal range that should be achieved. Additionally, the impact of increased workload in the ICU that may be associated with tight glucose control policies must be better evaluated. Finally, potential complications of insulin administration, in particular severe hypoglycemia, must be further studied.
Timing of glucose control initiation
The question of the optimal time to initiate tight glucose control protocols remains largely unanswered. The authors’ experiences at the University of Maryland have demonstrated that hyperglycemia may have significant adverse impact in the earliest phases after injury [1,10]. It is unknown, however, if the implementation of intensive glucose control measures in emergency departments or trauma resuscitation bays would significantly mitigate the subsequent associated adverse outcomes. These early elevations in blood glucose may be more reflective of the burden of injury response and, subsequently, may prove less amenable to effective manipulation during the most acute phases after injury. Further study of early intervention for posttraumatic hyperglycemia is required to better elucidate the approach required to optimize outcome.
What is the ideal goal range?
The ideal range of glucose control that should be used in the treatment of hyperglycemia among the critically ill remains a matter of controversy. The initial clinical trials conducted by Van Den Berghe and colleagues [8,20] demonstrated a mortality benefit when a goal of 80 to 110 mg/dL was achieved in a population of predominantly surgical patients. None of the randomized controlled trials completed after these investigations has succeeded in achieving such tight ranges of control. No subsequent randomized trial in any ICU population achieved a median or mean blood glucose level in the intervention group below the upper normal target of blood glucose [17].
The largest meta-analysis on the topic suggests that studies that managed to achieve their stated blood glucose target showed a reduced mortality, whereas randomized studies that did not succeed in reaching the target reported no benefit or even increased mortality [26]. The investigation of glucose control for trauma patients in the ICU conducted at the authors’ facility [9] demonstrated that the use of a tight glucose control protocol with goal range of 100 to 150 mg/dL was associated with improved outcomes, including mortality. Whether this is the optimal goal glucose level for all trauma patients has not been established. Dedicated prospective randomized investigations in this unique population are required to better determine the ideal range required to optimize outcome.
Increased ICU workload associated with tight glycemic control
There remain several potential challenges to the effective implementation of tight glycemic control policies in the ICU environment. Strict glycemic control mandates frequent blood glucose measurements, which may be considered labor intensive among the other demands of the ICU staff. It has been suggested that the ability of nursing staff to obtain tight glycemic control might be hindered by various limitations, including time constraints and deficiencies in training [29]. These concerns can, however, be ameliorated by the effective incorporation and education of nursing staff as part of protocol development and implementation strategy [30]. The degree of training required to effectively train staff for tight glycemic control policies as well as the workload associated with the active use of these policies in the conduct of care have not been well studied and require additional examination.
The risks of tight glycemic control
Hypoglycemia remains the most significant concern regarding implementation of strict glucose control policies. Reported incidences of severe hypoglycemia (blood glucose level <40 mg/dL) have been shown to rise by 5-fold to 10-fold compared with conventional blood glucose control in previously conducted randomized controlled trials [17]. The true impact of these hypoglycemic events, however, has not been definitively elucidated. It has been suggested that hypoglycopenia may cause cerebral damage, epileptic insults, or even coma [31]. The duration and intensity of hypoglycemia required to cause these effects, however, is unknown [32]. Additionally, potential subsets of patients at greatest risk for these potential consequences of hypoglycemia have not been defined.
Currently available data preclude the definitive establishment of a correlation between severe hypoglycemia and harm in critically ill patients subjected to strict glucose control. Two retrospective studies have previously identified severe hypoglycemia as an independent predictor of mortality in the ICU environment [14,33]. In the larger of these 2 studies, however, 30% of patients were not on insulin therapy in the 12 hours preceding severe hypoglycemic events and only a minority of patients were even receiving intravenous insulin therapy [33]. Therefore, this study fails to answer the question of whether severe hypoglycemia with strict glucose control therapy actually influences outcome.
In both of these examinations, strict glycemic control practices were not routinely used in the patient populations studied. In at least 1 initial study suggesting the benefits of glycemic control for critically ill patients, however, severe hypoglycemia was independently associated with mortality and may have diminished the benefit of intervention [8]. Although attempts to minimize the occurrence of severe hypoglycemia during the employment of strict glucose control should remain a concern, the true impact of these occurrences requires additional study.
Future directions
There remains a need for a prospective randomized trial on the effects of tight glucose control practices on outcome in critically ill trauma patients. To date, none of the conducted prospective randomized controlled trials have effectively examined these patients. Currently available evidence from these studies does not permit practitioners to make conclusive recommendations on best practice after trauma. Although evidence from the University of Maryland suggests that tight glucose control practices are beneficial to outcome after trauma, a well-designed prospective randomized study is still required to substantiate the authors’ findings. The development of such a study remains problematic, however, because the standard of care regarding glucose control in the ICU environment has certainly changed in the wake of the Van den Berghe studies [8,20]. As a result of these investigations, and their demonstrated mortality benefit, practice in most ICU environments has already been considerably altered. It is difficult to explain a trial design that requires deliberate exposure of critically ill patients to the hyperglycemia associated with conventional therapy, given this evidence and subsequent new standards of practice.