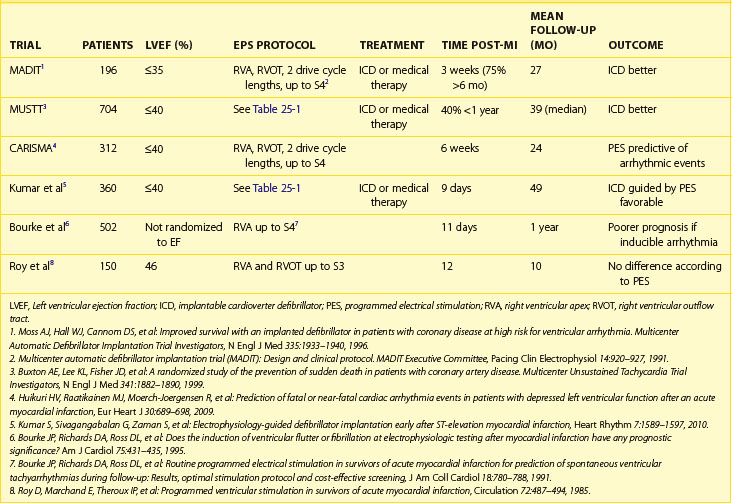
Chapter 25 Electrophysiological Evaluation of Ventricular Fibrillation
Introduction
Sudden cardiac arrest accounts for between 300,000 and 400,000 deaths in the United States alone each year. Although the underlying pathophysiology for the majority of these deaths is coronary artery disease (CAD), in autopsy studies, evidence of recent occlusive coronary thrombus is present in only up to 64% of patients.1 Thus up to one third of all cases of unexplained sudden cardiac arrest may be primarily caused by cardiac arrhythmias, with ventricular fibrillation (VF) being the culprit arrhythmia in the majority of patients. Although secondary and primary prevention trials have demonstrated the superiority of implantable cardioverter defibrillators (ICDs) compared with antiarrhythmic drugs (AADs) in preventing death, which represents ICD therapy as the gold standard treatment for this condition, current methods of risk stratification are primarily based on the assessment of left ventricular ejection fraction (LVEF).2,3
Programmed Ventricular Stimulation
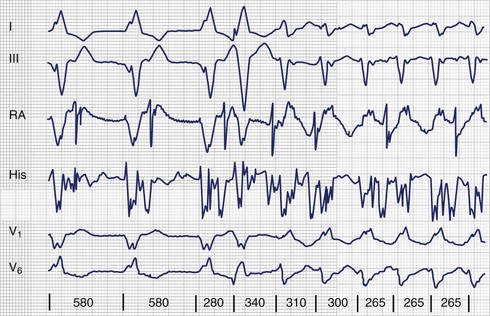
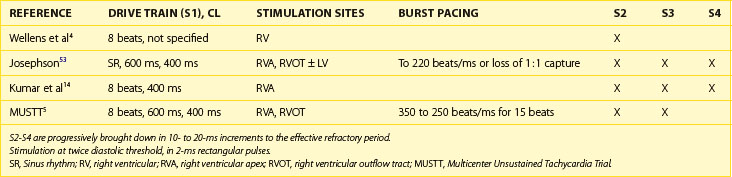
The use of invasive electrophysiological study (EPS) to risk stratify patients goes back almost 40 years to the observation by Wellens et al that in patients with prior myocardial infarction (MI) and ventricular tachycardia (VT), programmed ventricular stimulation could be used to induce the same VT (Figure 25-1).4 Although the sensitivity of programmed ventricular stimulation has been refined since its original description, a nonclinical ventricular arrhythmia is induced in approximately one third of patients.5 Although protocols vary among institutions, stimulation is normally performed from both the right ventricular apex and the right ventricular outflow tract (RVOT). Initially, single, double, and then triple extrastimuli are applied to a sensed ventricular beat, reducing the last extra stimulus by 20 ms each time until the effective refractory period (ERP) is reached. An eight-beat drive train is then followed by a single extrastimulus, then two and finally three extrastimuli, with the coupling interval of the last extrastimulus being decreased by 10 or 20 ms until the ERP is reached for the first extrastimulus. Typically, 600-ms and 400-ms drive trains are used. Most operators stop extrastimuli at a coupling interval of 180 to 200 ms because with shorter coupling intervals, the specificity of the test is reduced and polymorphic VT and VF occur more frequently.6 However, recent work suggests that patients with VT of a cycle length 200 to 250 ms are at high risk of a subsequent event and that this should not be taken as a nonsignificant event in patients with ischemic cardiomyopathy (Table 25-1).7
Ischemic Heart Disease
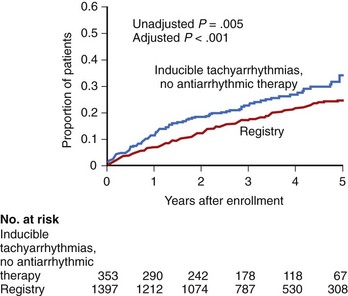
The Multicenter Unsustained Tachycardia Trial (MUSTT) was a multi-center prospective study that enrolled 2202 patients with significant CAD, reduced LVEF (<40%), and nonsustained VT and subjected patients to programmed ventricular stimulation.8 VT was induced in just over one third of patients. These patients were then randomized to either no AAD therapy or AAD therapy guided by repeated stimulation. If VT remained inducible despite AAD therapy, an ICD was implanted. Patients in whom sustained monomorphic VT was induced were monitored by a registry. Although this study confirmed that patients with inducible VT off AADs had a higher rate of arrhythmic events over the following 5 years compared with those without inducible VT (36% vs. 24%; P = .005), the event rate in patients without inducible VT at the time of invasive EPS was not insignificant (Figure 25-2).9
As just stated, it is not uncommon to induce polymorphic VT and VF at shorter extrastimulus coupling intervals and with multiple extrastimuli. In the MUSTT trial, induction of polymorphic VT or VF was considered positive only if this occurred with up to two extrastimuli. In a retrospective analysis of the Multicentre Automatic Defibrillator Trial II (MADIT-II), for which patients had to have an LVEF less than 30% and a prior MI, programmed ventricular stimulation was prognostic only when a positive result was taken—that is, not when polymorphic VT or VF had been induced with aggressive pacing.9,10 Although many studies were performed on acute arrhythmia suppression with AADs, the long-term use of AADs to prevent VT and VF has been disappointing, and it is now common to implant ICDs in patients at risk. However, this is mainly based on the patient’s LVEF, not on invasive testing.8,9,11,12
Current guidance for the use of programmed ventricular stimulation is based largely on the MADIT-I and MADIT-II trials and the results of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).11–13 In the United Kingdom, the National Institute for Health and Clinical Excellence updated the guidelines for ICD implantation, which are used in other health care systems throughout the world. These evidence-based guidelines suggest that the role of programmed ventricular stimulation in the decision to implant an ICD is restricted to patients with a previous MI (>6 weeks), an LVEF of 30% to 35%, a lack of New York Heart Association (NYHA) class IV symptoms, and nonsustained VT on monitoring. These criteria were derived from the enrollment criteria to the MADIT-I study. Patients who were enrolled in the MADIT-II study with an LVEF less than 30% did not need to undergo invasive EPS. Although it appears that programmed ventricular stimulation has quite a narrow indication in the decision of when to implant an ICD, it still remains a useful tool. The absolute benefit from ICD implantation in the MADIT trial was 12% per year in which patients had to have a “positive” test result compared with a more modest absolute benefit of approximately 3% and 1.5% per year, respectively, in the MADIT-II and SCD-HeFT populations, in whom programmed ventricular stimulation was not used to determine ICD implantation.
On the basis of the MUSTT trial results, patients with clinically significant CAD and an LVEF of 30% to 40% and nonsustained VT would also benefit from ICDs if they had a positive programmed ventricular stimulation test result.8,9 Again, a large absolute benefit was demonstrated in this patient population, although for most patients the clinically significant CAD was a prior MI.
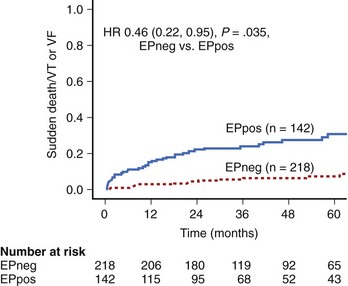
A recent study has shown that performing programmed ventricular stimulation may be beneficial when patients presented within the first few weeks after an MI and with an LVEF of less than 40% (Figure 25-3).14 ICD implantation is not routinely considered in these patients as a result of the Defibrillator in Acute Myocardial Infarction Trial (DINAMIT), which demonstrated that patients with an ICD implanted within the first month of an MI with an LVEF of less than 35% and depressed autonomic function, as assessed by heart rate variability, actually had a worse outcome.15 Although fewer patients died of arrhythmic events, the rate of nonarrhythmic death was substantially high. These data, as well as those from the Immediate Risk Stratification Improves Survival (IRIS) trial, which also investigated early implantation of an ICD in high-risk patients (as assessed by reduced LVEF <40%, depressed autonomic function, or nonsustained VT on Holter monitoring), demonstrated no absolute mortality benefit from implantation.14 This has led to the current situation in which high-risk patients do not receive ICDs after an MI.16,17
The results of MUSTT, taken together with the more recent work on early programmed ventricular stimulation after successful revascularization, suggest that programmed ventricular stimulation is a useful tool in risk stratifying patients with a reduced ejection fraction after MI (Table 25-2). Compared with signal-averaged electrocardiograms (ECGs) and LVEF, programmed ventricular stimulation is the best predictor of spontaneous ventricular arrhythmia late after MI in patients with reduced ejection fraction.18,19
Idiopathic Dilated Cardiomyopathy
The role of programmed ventricular stimulation in the risk assessment of patients with idiopathic dilated cardiomyopathy (IDCM) is not well studied. Approximately 50% of patients with IDCM will have SCD caused by ventricular arrhythmia as opposed to progressive heart failure.20 Impairment of left ventricular function alone does not appear to be a specific marker for risk.11,21,22 In the Cardiomyopathy Trial (CAT), 104 patients with IDCM and an LVEF less than 30% were randomized to either medical or ICD therapy.23 This trial had a very low rate of β-blocker use, which makes interpretation more difficult in the contemporary era. Despite this, overall mortality was very low, and the trial was stopped early because of futility.
In the Amiodarone Versus Implantable Cardioverter-Defibrillator: Randomized Trial in Patients With Nonischemic Dilated Cardiomyopathy and Asymptomatic Nonsustained Ventricular Tachycardia (AMIOVIRT), 103 patients were enrolled, but this trial was also stopped early after just over 2 years because of a failure to show any benefit of an ICD. Again, there was a relatively low mortality rate of 12%.21 Although the use of β-blockers was better, only 53% of patients were taking them at the time of the study. Invasive EPS was again not used to risk stratify patients. In the Defibrillators in Non-Ischaemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial, a much larger number of patients were enrolled compared with either CAT or AMIOVIRT, with similar inclusion criteria.22 In addition, a greater population was on optimal medical therapy, with 84% of the population on β-blockers and 86% on angiotensin-converting enzyme (ACE) inhibitors, although spironolactone was not then part of the standard therapy. However, patients were excluded if they had undergone invasive EPS within 3 months. Although implantation of an ICD led to a significant reduction in the rate of SCD (HR, 0.2), it did not lead to an overall reduction in death from any cause.
A smaller study did investigate the use of programmed ventricular stimulation in this patient population as well as the presence of nonsustained VT on ambulatory monitoring.24 Although more than 80% of patients enrolled in the study were taking β-blockers, fewer than one fourth of patients were taking β-blockers at the start of the study. With a stimulation protocol of a six-beat drive train with two or three extrastimuli from a single site, monomorphic VT was inducible in 7% of patients. The addition of the third extrastimulus increased overall inducibility, but this was caused by the induction of polymorphic VT, ventricular flutter, and VF. During a mean follow-up of just over 2 years, the positive predictive value (PPV) was poor at 29%. Although the negative predictive value (NPV) of programmed ventricular stimulation was high, there was a very low event rate in the overall study population.
A more recent study looked at inducibility of ventricular tachyarrhythmias and subsequent therapy from an ICD implanted for secondary prevention.25 By using a different stimulation protocol from the previous study, with four basic drive train cycle lengths, up to two ventricular stimulation sites, and up to three extrastimuli, the investigators found that induction of polymorphic VT or VF was more predictive of subsequent fast VT or VF events than if sustained monomorphic VT was induced. They also found that these patients had a worse overall mortality rate despite having a similar left ventricular systolic function.26 However, this was a retrospective study, and the data collection took place over a long period, from 1994 to 2007, limiting the clinical application of this study.
Hypertrophic Cardiomyopathy
Although patients with hypertrophic cardiomyopathy (HCM) are at high risk for ventricular arrhythmia, programmed ventricular stimulation is rarely performed in this patient population. Compared with ischemic cardiomyopathy, in which the substrate for ventricular arrhythmia is defined and relatively fixed, the substrate in HCM is more diffuse, and the mechanism underlying arrhythmic death may be modified by abnormal vascular responses or preceding the ischemia.27 Although a study using programmed ventricular stimulation in a high-risk group of patients did induce sustained ventricular arrhythmia in 43% of patients, sustained monomorphic VT was only induced in 10% of patients.28 However, programmed ventricular stimulation has poor PPV and NPV in the risk stratification of patients with HCM. Other markers of risk, namely a history of syncope, nonsustained VT on Holter monitoring, family history, and left ventricular wall thickness, are used in the decision-making process relating to the need to implant an ICD.3 Cardiac magnetic resonance imaging (MRI) to assess myocardial fibrosis, with late gadolinium enhancement to risk stratify these patients, has created some interest, but this is not routine clinical practice.29,30
Congenital Heart Disease
Although patients with corrected congenital heart disease most frequently die from ventricular arrhythmia, the overall incidence is low.31,32 This leads to the diagnostic dilemma of how to risk stratify patients. Although programmed ventricular stimulation has been used in various studies in an attempt to identify high-risk individuals, most studies have focused on patients with repaired tetralogy of Fallot. In a retrospective, multi-center study of 252 patients with repaired tetralogy of Fallot, the PPV of inducible ventricular arrhythmia with programmed ventricular stimulation was 55%, and the presence of sustained monomorphic or polymorphic VT was a strong predictor of future ventricular arrhythmia or SCD (HR, 5.0, P = .0002 vs. HR, 12.9, P < .0001, respectively).33 Unfortunately, the clinical use of programmed ventricular stimulation is limited because of a poor NPV.33,34
Arrhythmogenic Right Ventricular Cardiomyopathy
In ARVC, the myocardium in the right ventricle, and sometimes in the left ventricle, is replaced by fibro-fatty tissue; this substrate can give rise to ventricular arrhythmias, leading to death.35,36 Although it is generally agreed that patients who present after having survived an episode of VF or VT should be implanted with an ICD, the treatment of asymptomatic patients with arrhythmogenic right ventricular dysplasia (ARVD) is more difficult.3,37 Although programmed ventricular stimulation has been used in this population of patients to aid risk stratification, a recent multi-center study has clearly shown that no benefit exists.38,39 In this multi-center study, 106 patients with ARVC were implanted with ICDs on the basis of the presence of a single risk factor for SCD, including programmed stimulation. Although only 67 patients underwent invasive EPS (the reasons that patients did not undergo programmed ventricular stimulation were not discussed), the PPV of the test was only 35% for VF or VT and the NPV was 74%.39 These data do not support the use of programmed ventricular stimulation in the risk assessment of patients with ARVD.
Brugada Syndrome
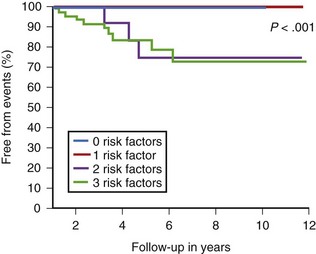
The use of programmed ventricular stimulation in Brugada syndrome is vigorously debated between groups that strongly believe in its role in risk stratification and those that do not.40–47 Although sustained ventricular arrhythmia is easier to induce in patients with Brugada syndrome than in healthy control subjects and is reproducible within the same patient, its prognostic value is debated, with the Brugada researchers strongly supporting its use in risk stratification.48 This is based on a large prospective multi-center trial that enrolled 408 asymptomatic patients, with inducibility at EPS being the strongest predictor of subsequent SCD or resuscitated VF.41 The same group also reported that provocative stimulation was useful in a mixed population of symptomatic and asymptomatic patients, with inducible VF being more frequent in symptomatic patients.42,49 More recently, a combination approach in which inducibility of VF at EPS is one of a number of risk factors taken into account, suggests at least some role for programmed stimulation.50 The highest risk patients had a type 1 ECG at baseline, syncope, a family history of SCD, and a positive EPS result. In patients with only one of three criteria—family history of SCD, syncope, or a positive EPS—no events were noted (Figure 25-4).
However, these conclusions have been contested by a number of groups and studies. In a study in which 86 patients underwent provocative testing were compared with 114 who did not, prior syncope and a Brugada type 1 ECG predicted future events but programmed ventricular stimulation did not.46 Similar results were found in a further study, with inducible VF being more frequent in symptomatic patients than in asymptomatic patients. However, programmed stimulation had no clinical use in asymptomatic patients, who had a very low event rate over the duration of the trial.51 Although the mean follow-up was 40 months, this relatively young group of patients could be expected to live for many years, so a longer follow-up period is required.
A large meta-analysis of the use of programmed stimulation that included 15 studies did not support a role for invasive EPS in Brugada syndrome.52 However, the contentious nature of this issue is reflected in the consensus guidelines for sudden cardiac arrest, with an overall conclusion that the role of programmed ventricular stimulation in Brugada syndrome will be resolved only when a large prospective study is performed.52 Until then, however, the guidelines limit the role of invasive testing to patients with spontaneous ST elevation and assign it as only level C evidence.
Key References
Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289-1300.
Borggrefe M, Trampisch HJ, Breithardt G. Reappraisal of criteria for assessing drug efficacy in patients with ventricular tachyarrhythmias: Complete versus partial suppression of inducible arrhythmias. J Am Coll Cardiol. 1988;12:140-149.
Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887.
Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003;108:3092-3096.
Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937-1945.
Corrado D, Calkins H, Link MS, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144-1152.
Daubert JP, Zareba W, Hall WJ, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47:98-107.
Delise P, Allocca G, Marras E, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: Usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169-176.
Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1-e62.
Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-2488.
Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: A multicenter study. Circulation. 2004;109:1994-2000.
Kumar S, Sivagangabalan G, Choi MC, et al. Long-term outcomes of inducible very fast ventricular tachycardia (cycle length 200–250 ms) in patients with ischaemic cardiomyopathy. J Cardiovasc Electrophysiol. 2010;21:262-269.
Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806-814.
Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation. 2002;105:1342-1347.
Sarkozy A, Boussy T, Kourgiannides G, et al. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334-344.
Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Europace. 2006;8:746-837.
1 Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334-2351.
2 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1-e62.
3 Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. Europace. 2006;8:746-837.
4 Wellens HJ, Schuilenburg RM, Durrer D. Electrical stimulation of the heart in patients with ventricular tachycardia. Circulation. 1972;46:216-226.
5 Buxton AE, Fisher JD, Josephson ME, et al. Prevention of sudden death in patients with coronary artery disease: The Multicenter Unsustained Tachycardia Trial (MUSTT). Prog Cardiovasc Dis. 1993;36:215-226.
6 Morady F, DiCarlo LAJ, Baerman JM, et al. Comparison of coupling intervals that induce clinical and nonclinical forms of ventricular tachycardia during programmed stimulation. Am J Cardiol. 1986;57:1269-1273.
7 Kumar S, Sivagangabalan G, Choi MC, et al. Long-term outcomes of inducible very fast ventricular tachycardia (cycle length 200–250 ms) in patients with ischaemic cardiomyopathy. J Cardiovasc Electrophysiol. 2010;21:262-269.
8 Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
9 Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 2000;342:1937-1945.
10 Daubert JP, Zareba W, Hall WJ, et al. Predictive value of ventricular arrhythmia inducibility for subsequent ventricular tachycardia or ventricular fibrillation in Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2006;47:98-107.
11 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
12 Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883.
13 Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
14 Kumar S, Sivagangabalan G, Zaman S, et al. Electrophysiology-guided defibrillator implantation early after ST-elevation myocardial infarction. Heart Rhythm. 2010;7:1589-1597.
15 Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481-2488.
16 Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427-1436.
17 Steinbeck G, Andresen D, Senges J, et al. Immediate Risk-Stratification Improves Survival (IRIS): Study protocol. Europace. 2004;6:392-399.
18 Richards DA, Byth K, Ross DL, et al. What is the best predictor of spontaneous ventricular tachycardia and sudden death after myocardial infarction? Circulation. 1991;83:756-763.
19 Bourke JP, Richards DA, Ross DL, et al. Routine programmed electrical stimulation in survivors of acute myocardial infarction for prediction of spontaneous ventricular tachyarrhythmias during follow-up: Results, optimal stimulation protocol and cost-effective screening. J Am Coll Cardiol. 1991;18:780-788.
20 Tamburro P, Wilber D. Sudden death in idiopathic dilated cardiomyopathy. Am Heart J. 1992;124:1035-1045.
21 Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with non ischaemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
22 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischaemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
23 Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458.
24 Becker R, Haass M, Ick D, et al. Role of nonsustained ventricular tachycardia and programmed ventricular stimulation for risk stratification in patients with idiopathic dilated cardiomyopathy. Basic Res Cardiol. 2003;98:259-266.
25 Rolf S, Haverkamp W, Borggrefe M, et al. Induction of ventricular fibrillation rather than ventricular tachycardia predicts tachyarrhythmia recurrences in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillator for secondary prophylaxis. Europace. 2009;11:289-296.
26 Borggrefe M, Trampisch HJ, Breithardt G. Reappraisal of criteria for assessing drug efficacy in patients with ventricular tachyarrhythmias: Complete versus partial suppression of inducible arrhythmias. J Am Coll Cardiol. 1988;12:140-149.
27 Nicod P, Polikar R, Peterson KL. Hypertrophic cardiomyopathy and sudden death. N Engl J Med. 1988;318:1255-1257.
28 Fananapazir L, Tracy CM, Leon MB, et al. Electrophysiologic abnormalities in patients with hypertrophic cardiomyopathy. A consecutive analysis in 155 patients. Circulation. 1989;80:1259-1268.
29 Fluechter S, Kuschyk J, Wolpert C, et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2010;12:30.
30 Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887.
31 Khairy P, Dore A, Poirier N, et al. Risk stratification in surgically repaired tetralogy of Fallot. Expert Rev Cardiovasc Ther. 2009;7:755-762.
32 Yap SC, Harris L. Sudden cardiac death in adults with congenital heart disease. Expert Rev Cardiovasc Ther. 2009;7:1605-1620.
33 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of Fallot repair: A multicenter study. Circulation. 2009;204(109):1994-2000.
34 Alexander ME, Walsh EP, Saul JP, et al. Value of programmed ventricular stimulation in patients with congenital heart disease. J Cardiovasc Electrophysiol. 1999;10:1033-1044.
35 Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289-1300.
36 Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806-814.
37 Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: An update. Heart. 2009;95:766-773.
38 Pezawas T, Stix G, Kastner J, et al. Ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Clinical presentation, risk stratification and results of long-term follow-up. Int J Cardiol. 2006;107:360-368.
39 Corrado D, Calkins H, Link MS, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144-1152.
40 Brugada P, Brugada R, Brugada J. Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation. 2005;112:279-292. discussion 279–292
41 Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003;108:3092-3096.
42 Brugada P, Brugada R, Mont L, et al. Natural history of Brugada syndrome: The prognostic value of programmed electrical stimulation of the heart. J Cardiovasc Electrophysiol. 2003;14:455-457.
43 Sarkozy A, Boussy T, Kourgiannides G, et al. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334-344.
44 Priori SG, Napolitano C. Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation. 2005;112:279-292. discussion 279–292
45 Sacher F, Probst V, Iesaka Y, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: A multicenter study. Circulation. 2006;114:2317-2324.
46 Priori SG, Napolitano C, Gasparini M, et al. Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation. 2002;105:1342-1347.
47 Rossenbacker T, Priori SG. The Brugada syndrome. Curr Opin Cardiol. 2007;22:163-170.
48 Gasparini M, Priori SG, Mantica M, et al. Programmed electrical stimulation in Brugada syndrome: How reproducible are the results? J Cardiovasc Electrophysiol. 2002;13:880-887.
49 Brugada P, Geelen P, Brugada R, et al. Prognostic value of electrophysiologic investigations in Brugada syndrome. J Cardiovasc Electrophysiol. 2001;12:1004-1007.
50 Delise P, Allocca G, Marras E, et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: Usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169-176.
51 Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263.
52 Paul M, Gerss J, Schulze-Bahr E, et al. Role of programmed ventricular stimulation in patients with Brugada syndrome: A meta-analysis of worldwide published data. Eur Heart J. 2007;28:2126-2133.
53 Josephson ME, editor. Clinical cardiac electrophysiology, ed 3, Philadelphia: Lippincott, Williams & Wilkins, 2002.