Chapter 24 Electrophysiological Evaluation of Supraventricular Tachycardia
Paroxysmal supraventricular tachycardia (PSVT) is a common condition that affects a wide range of age groups. The clinical syndrome of PSVT and its presentation and management are discussed in detail in Chapter 41. Electrophysiologic study (EPS) of PSVT is routinely performed in clinical laboratories as a component of the diagnostic and therapeutic approach to management of this condition. The clinical presentation of a patient with PSVT includes a variety of tachyarrhythmias, as detailed in Chapter 41 and summarized in Table 24-1. The purpose of EPS is to differentiate the mechanisms of PSVT in an individual patient and help guide appropriate therapy for patient management. These therapeutic approaches can include catheter ablation procedures (see Chapter 93), drug therapy (see Chapters 79 and 80) and, rarely, device therapy (see Chapter 41). For special consideration of drug therapy or ablation in pediatric or pregnant patients, see Chapters 72, 74, and 75, respectively. The focus of this chapter is to delineate the diagnostic information elicited at clinical EPS in two common forms of PSVT—namely, atrioventricular (AV) nodal reentrant tachycardia (AVNRT) and AV reentrant tachycardia (AVRT). EPS of atrial tachycardia (AT) is examined in Chapter 29.
Table 24-1 Paroxysmal Supraventricular Tachycardias with Narrow and Wide QRS Complexes
| SINUS NODE DISORDERS |
| AV NODAL RE-ENTRANT TACHYCARDIAS |
| RE-ENTRANT AND ECTOPIC ATRIAL TACHYCARDIAS |
| PRE-EXCITATION SYNDROME: WOLFF-PARKINSON-WHITE SYNDROME |
| OTHER PRE-EXCITATION SYNDROMES: MAHAIM CONDUCTION |
| AUTOMATIC AV JUNCTIONAL TACHYCARDIAS |
AV, Atrioventricular.
Diagnostic Approach to the Patient with Supraventricular Tachycardia
The initial diagnostic approach to the patient with PSVT, as mentioned in Chapter 41, is documentation of the arrhythmia by electrocardiogram (ECG).
Atrioventricular Re-entrant Tachycardia and Atrioventricular Node Re-entrant Tachycardia
The methods of catheter placement and programmed electrical stimulation are discussed in detail in Chapter 20 and are not revisited here in detail. In brief, multipolar electrode catheters are placed in the right atrium, His bundle region, coronary sinus, and right ventricle. Recordings are obtained from these regions as well as the right and left AV ring for bypass tract mapping or more detailed septal pathway localization. Programmed atrial stimulation is usually performed from the high right atrium, coronary sinus, right ventricular apex, and the atrial or ventricular insertion site of a bypass tract as needed. In some instances, alternate pacing sites can include the left atrium, right ventricular outflow tract, right ventricular septum, or left ventricle. For the study of propagation and induction of tachycardia, the extrastimulus technique is widely used, although burst pacing is an alternative. These techniques are discussed in Chapter 20. Facilitiation of induction may require the administration of pharmacologic agents such as isoproterenol or atropine (see Chapter 72). Other pharmacologic agents such as adenosine may be used for diagnostic purposes to evaluate the presence of accessory pathway conduction or termination of PSVT involving the AV node (see Chapter 72).
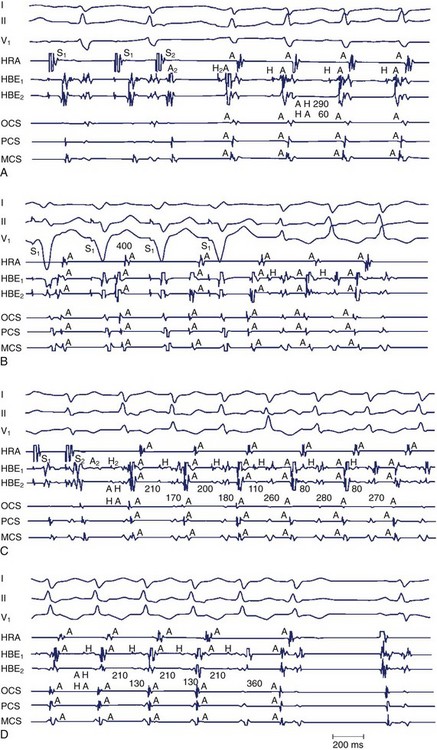
Most SVTs are caused by a re-entrant mechanism and may be induced in the laboratory by using programmed electrical stimulation. In A-V junctional tachycardias, electrophysiological study permits induction of tachycardia in more than 90% of patients with AVRT or AVNRT. In patients with AVNRT, premature atrial stimulation can demonstrate dual AV nodal conduction and induce the tachycardia to determine its mechanism. The arrhythmias associated with WPW syndrome include reciprocating or circus movement tachycardias and atrial arrhythmias. In some instances of AVNRT, concomitant pre-excited QRS complexes may exist because of passive conduction over an accessory pathway that may serve as a passive bystander. Tachycardias related to Mahaim fibers have a particular electrocardiographic presentation with LBBB and left-axis deviation. The ECG in sinus rhythm shows the features of WPW syndrome (Figure 24-1). The electrophysiological study often demonstrates decremental conduction over the accessory pathway.
Atrioventricular Nodal Re-Entrant Tachycardia
Dual Atrioventricular Nodal Pathway
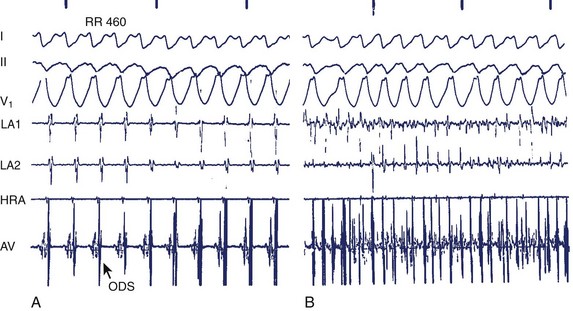
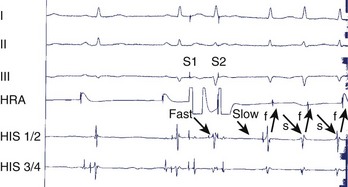
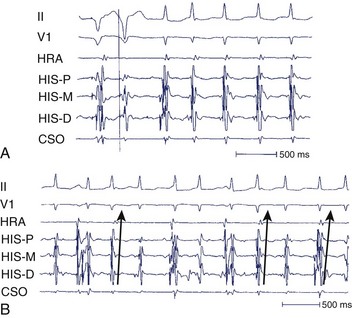
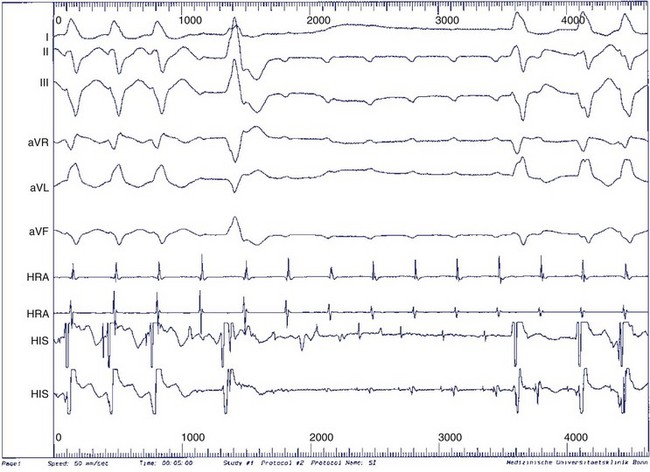
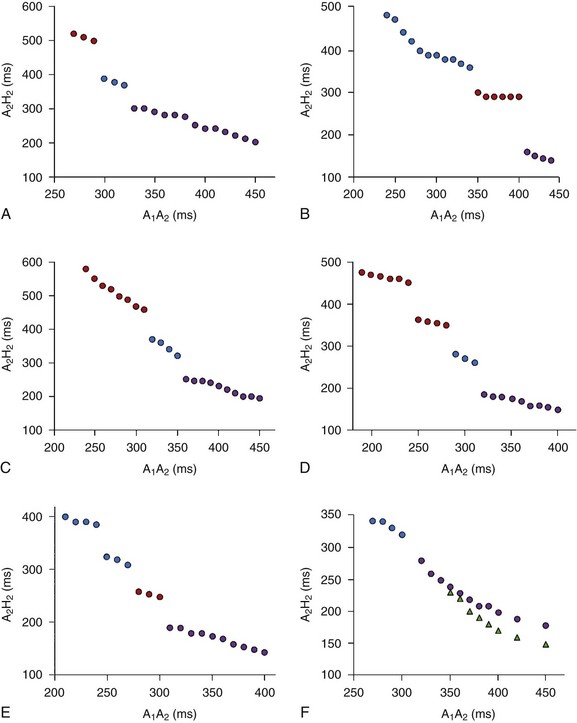
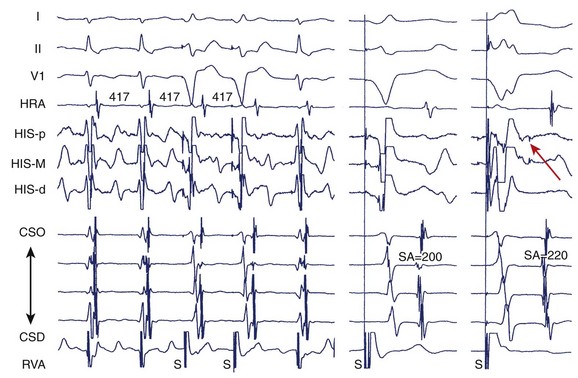
During electrophysiological evaluation, typical AVNRT manifests antegrade conduction via a “slow” pathway and retrograde conduction via a “fast” pathway.1–10 The electrophysiological characteristic is the presence of dual AV nodal pathway physiology demonstrated by a discontinuous AV node functional curve. Discontinuous AV nodal conduction is defined as a sudden increment of 50 ms or greater in the A-H or H-A interval (“jump”), with a decrement in prematurity of the extrastimulus by 10 to 20 ms. In some patients, a jump (>50 ms) of any consecutive A-H intervals during incremental atrial pacing is found, which might be a manifestation of dual AV nodal pathways. Typically, the jump phenomenon is followed by induction of a slow-fast AVNRT (Figure 24-2). During tachycardia in typical slow-fast AVNRT, a long A-H interval with a short V-A interval can be documented (Figure 24-3). When performing endocardial mapping, the earliest atrial activation during tachycardia can be detected at the slow pathway area around the coronary sinus ostium. In contrast, atypical AVNRT has antegrade conduction through a “fast” or “slow” pathway and retrograde conduction through a “slow” pathway.2,3
FIGURE 24-3 Typical slow-fast atrioventricular nodal re-entrant tachycardia with a short V-A interval.
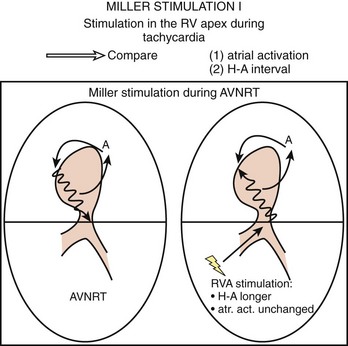
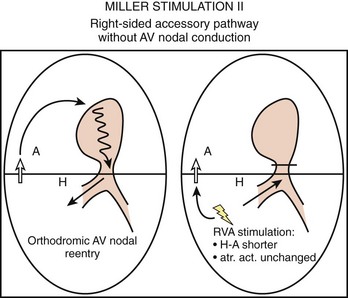
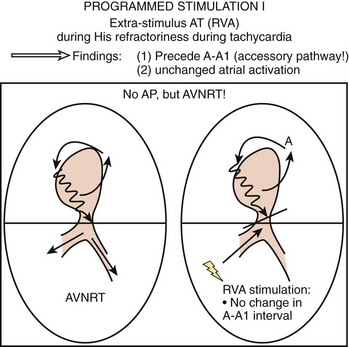
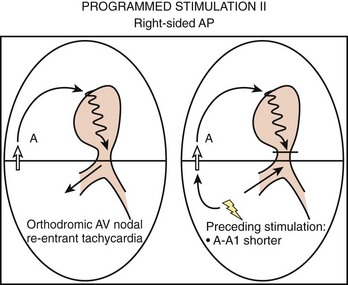
To differentiate AVNRT from AVRT, the following maneuvers can be used4–6:
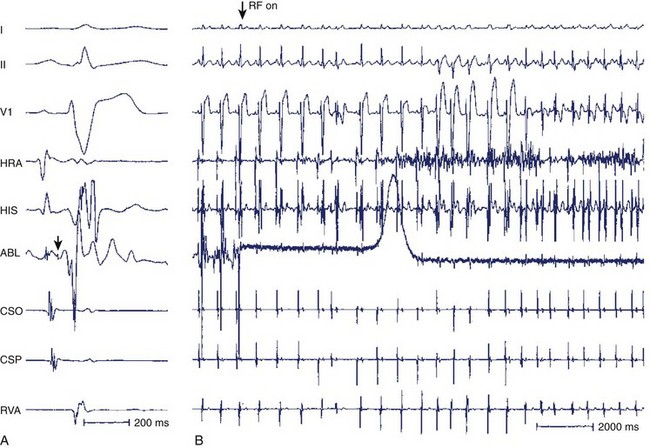
Two important concepts of dual AV nodal pathways have been further clarified by provocative pharmacologic testing and catheter ablation in patients with AVNRT. One major question has been whether dual AV nodal pathways are fully intranodal and caused by longitudinal dissociation of AV nodal tissue or extranodal, involving separate atrial inputs into the AV node. From clinical studies of slow pathway potentials, LH (low followed by high) frequency potentials are observed during asynchronous activation of muscle bundles above and below the coronary sinus orifice. HL (high followed by low) frequency potentials are caused by asynchronous activation of atrial cells and a band of nodal-type cells that may represent the substrate of the slow pathway.7–10 Thus the slow and fast pathways are likely to be atrionodal approaches or connections rather than discrete intranodal pathways. The results of catheter ablation indicate that the fast and slow pathways have their origins outside the limits of the compact AV node and that the tissues targeted during successful ablation are composed of ordinary working atrial myocardium surrounding the AV node itself.11–14 Furthermore, AV or VA conduction block during AVNRT favors the concept that atrial and ventricular tissues are not involved in the maintenance of this tachycardia (Figures 24-8 and 24-9).15,16
Unusual Physiology of Dual Atrioventricular Nodal Pathways
Some patients with AVNRT have multiple antegrade and retrograde AV nodal pathways with multiple discontinuities in the AV node function curve or dual AV nodal pathways with a continuous curve during programmed electrical stimulation.13,17 Furthermore, variant forms (slow-slow, slow-intermediate, fast-intermediate) of AVNRT have been noted (Figures 24-10 and 24-11).11–13 Whether multiple antegrade and retrograde AV nodal pathways originate from anatomically different pathways or represent anisotropic conduction–induced functional pathways is still being debated. Several investigators have demonstrated the marked heterogeneity of the transitional cells surrounding the compact AV nodal pathway. The non-uniform properties of the AV node can produce anisotropic conduction and suggest that the antegrade and retrograde fast pathways are anatomically distant from the multiple “antegrade slow” and “retrograde slow” or intermediate pathways, respectively. Clinical studies have demonstrated that successful ablation or modification of retrograde slow and intermediate pathways occurs at different sites from the antegrade fast or slow pathway, and the possibility of anatomically different antegrade or retrograde multiple pathways should be considered. Furthermore, in the patients who have successful ablation of multiple antegrade slow pathways or retrograde slow and intermediate pathways at a single site, anisotropic conduction over the low septal area of the right atrium is a possible explanation for the presence of multiple antegrade and retrograde AV nodal pathways.11–1317
Patients with AVNRT can have continuous AV node conduction curves. These patients do not exhibit an A-H jump with two extrastimuli and two drive cycle lengths during atrial pacing from the high right atrium and the coronary sinus ostium. The possible mechanisms of the continuous AV node function curves in AVNRT include the following: (1) the functional refractory period of the atrium limits the prematurity with which atrial premature depolarization will encounter the refractoriness in the AV node, which, in turn, produces inability to dissociate the fast and slow AV node pathways; and (2) fast and slow AV nodal pathways have similar refractory periods and conduction times.17
Atrioventricular Re-entrant Tachycardia
Anatomy and Electrophysiology of Accessory Pathways
The oblique orientation of most accessory pathways has been demonstrated by detailed endocardial and epicardial mapping techniques.18–20 The locations of atrial and ventricular insertion sites of accessory pathways can differ by up to 2 cm; furthermore, some accessory pathways have antegrade and retrograde conduction fibers at different locations. This finding has been proven by different ablation sites for antegrade as well as retrograde conduction.21 Thus the anatomic and functional dissociation of the accessory pathway into atrial and ventricular insertions and antegrade and retrograde components is possible. Approximately 90% of AV accessory pathways have fast conduction properties, and the other accessory pathways (including Mahaim fibers) show decremental conduction properties during atrial or ventricular stimuli with shorter coupling intervals.22–25 These pathways with decremental conduction may be sensitive to several antiarrhythmic drugs, including verapamil and adenosine. Accessory pathways in the right free wall and posteroseptal areas have a higher incidence of decremental conduction properties. When decremental conduction is present, the possibility of Mahaim fibers, such as atriofascicular or nodoventricular pathways, must be considered. Several studies have demonstrated that most of the ventricular insertion sites of these particular bypass tracts are close to the right bundle branch and that the typical Mahaim fiber potential can be recorded along the tricuspid annulus in patients with the atriofascicular pathways (Figure 24-12).26–29
Electrophysiological Findings in Atrioventricular Re-entry Tachycardia
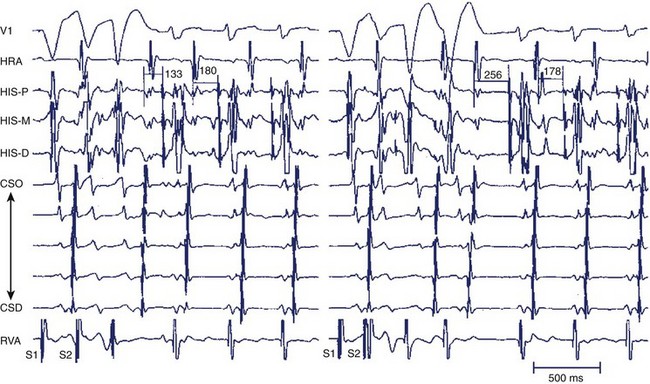
For the diagnosis of accessory pathway–mediated AVRT, a premature ventricular depolarization can be delivered during the tachycardia when the bundle of His is refractory and the impulse still conducts to the atrium; this indicates that retrograde propagation conducts to the atrium over a pathway other than the normal AV conduction system. The definition of AVRT involves re-entry over one or more AV accessory pathways and the AV node, and the classic classification of AVRT includes orthodromic and antidromic tachycardias.29–31 For the initiation of orthodromic tachycardia, a critical degree of delay in the A-V or V-A interval, which can be in the AV node or His-Purkinje system, is usually necessary. However, dual AV nodal pathway physiology, with or without AVNRT, can be noted in some patients (Figure 24-13). Ventricular pacing from different sites can provide valuable information about retrograde conduction through the AV node or via a septal pathway (Figure 24-14). The incidence of antidromic AVRT is much lower than orthodromic AVRT. Comparison with sinus rhythm shows a fully pre-excited QRS complex. Rapid conduction in retrograde AV nodal–His axis is necessary for the initiation and maintenance of antidromic tachycardia. The atrial premature beat usually can advance the next pre-excited ventricular complex through the antegrade accessory pathway, or it can terminate the AVRT through collision with the previous retrograde wavefront (Figure 24-15). The incidence of multiple accessory pathways is approximately 5% to 20%, and antidromic tachycardia is common in this situation.
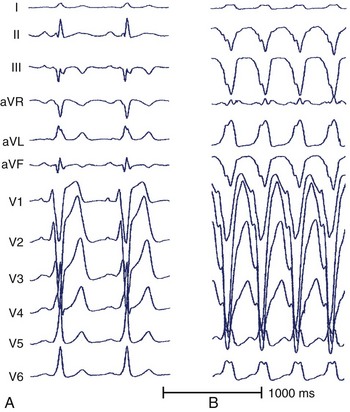
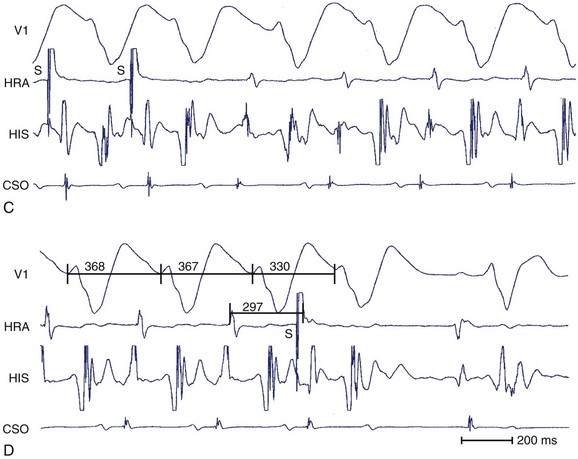
FIGURE 24-15 A, A 12-lead electrocardiogram (ECG) during sinus rhythm. B, Surface 12-lead ECG of antidromic tachycardia.
The most difficult situation for the differential diagnosis of AVRT is the so-called Mahaim tachycardia, which includes atriofascicular or nodofascicular (or nodoventricular) re-entry tachycardia and AVNRT with an “innocent bystander” bypass tract. These arrhythmias often appear as wide-complex tachycardias with LBBB and left-axis deviation. However, the presence of ventriculoatrial dissociation favors nodofascicular tachycardia. Sternick et al recently described a simple parameter to distinguish between decremental or rapidly conducting pathways during pre-excited tachycardia: An AV interval of more than 150 ms during pre-excited tachycardia is reliable for detecting a decrementally conducting accessory pathway.32
Key References
Chen SA, Tai CT, Chiang CE, et al. Electrophysiologic characteristics, electropharmacologic responses and radiofrequency ablation in patients with decremental accessory pathway. J Am Coll Cardiol. 1996;28:732-737.
Denes P, Wu D, Dhingra RC, et al. Demonstration of dual AV nodal pathways in patients with paroxysmal supraventricular tachycardia. Circulation. 1973;48:549-555.
Gallagher JJ, Kasell J, Sealy WC, et al. Epicardial mapping in the Wolff-Parkinson-White syndrome. Circulation. 1978;57:854-866.
Gallagher JJ, Sealy WC. The permanent form of junctional reciprocating tachycardia: Further elucidation of the underlying mechanism. Eur Heart J. 1978;8:413-420.
Goldberger J, Wang Y, Scheinman M. Stimulation of the summit of the right ventricular aspect of the ventricular septum during orthodromic atrioventricular reentrant tachycardia. Am J Cardiol. 1992;70(1):78-85.
Haissaguerre M, Gaita F, Fischer B, et al. Elimination of atrio ventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation. 1992;85:2162-2175.
Huang JL, Chen SA, Tai CT, et al. Long-term results of radiofrequency catheter ablation in patients with multiple accessory pathways. Am J Cardiol. 1996;78:1375-1379.
Jackman WM, Beckman KJ, McClelland JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency ablation of slow-pathway conduction. N Engl J Med. 1992;32:313-316.
Klein LS, Hackett FK, Zipes DP, et al. Radiofrequency catheter ablation of Mahaim fibers at the tricuspid annulus. Circulation. 1993;87:738-747.
Lee SH, Chen SA, Tai CT, et al. Electrophysiologic characteristics and radiofrequency catheter ablation in atrioventricular node reentrant tachycardia with second-degree atrioventricular block. J Cardiovasc Electrophysiol. 1997;8:502-511.
McClelland JH, Wang X, Beckman KJ, et al. Radiofrequency catheter ablation of right atriofascicular (Mahaim) accessory pathways guided by accessory pathway activation potentials. Circulation. 1994;89:2655-2666.
McGuire MA, Lau KC, Johnson DC, et al. Patients with two types of atrioventricular junctional (AV nodal) reentrant tachycardia: Evidence that a common pathway of nodal tissue is not present above the reentrant circuit. Circulation. 1991;83:1232-1246.
Ross DL, Johnson DC, Denniss AR, et al. Curative surgery for atrioventricular junctional (“AV nodal”) reentrant tachycardia. J Am Coll Cardiol. 1985;6:1383-1392.
Selle JG, Sealy WC, Gallagher JJ, et al. Technical considerations in the surgical approach to multiple accessory pathways in the Wolff-Parkinson-white syndrome. Ann Thorac Surg. 1987;43:579-584.
Sternick EB, Lokhandwala Y, Timmermans C, et al. The atrioventricular interval during pre-excited tachycardia: A simple way to distinguish between decrementally or rapidly conducting accessory pathways. Heart Rhythm. 2009;6(9):1351-1358.
Sung RJ, Styperek JL, Myerburg RJ, et al. Initiation of two distinct forms of atrioventricular nodal reentrant tachycardia during programmed ventricular stimulation in man. Am J Cardiol. 1978;43:404-415.
Tai CT, Chen SA, Chiang CE, et al. Complex electrophysiological characteristics in atrioventricular nodal reentrant tachycardia with continuous atrioventricular node function curves. Circulation. 1997;95:2541-2547.
Tai CT, Chen SA, Chiang CE, et al. Electrophysiologic characteristics and radiofrequency catheter ablation in patients with multiple atrioventricular nodal reentry tachycardias. Am J Cardiol. 1996;77:52-58.
Tai CT, Chen SA, Chiang CE, et al. Multiple anterograde atrioventricular node pathways in patients with atrioventricular node reentrant tachycardia. J Am Coll Cardiol. 1996;28:725-731.
Tchou P, Lehmann MH, Jazayeri M, et al. Atriofascicular connection or a nodoventricular Mahaim fiber? Electrophysiologic elucidation of the pathway and associated reentrant circuit. Circulation. 1988;77:837-848.
Wu D, Denes P, Amat-y-Leon F, et al. An unusual variety of atrio-ventricular node reentry due to dual atrioventricular nodal pathways. Circulation. 1977;56:50-59.
References
1 Denes P, Wu D, Dhingra RC, et al. Demonstration of dual AV nodal pathways in patients with paroxysmal supraventricular tachycardia. Circulation. 1973;48:549-555.
2 Wu D, Denes P, Amat-y-Leon F, et al. An unusual variety of atrio- ventricular node reentry due to dual atrioventricular nodal pathways. Circulation. 1977;56:50-59.
3 Sung RJ, Styperek JL, Myerburg RJ, et al. Initiation of two distinct forms of atrioventricular nodal reentrant tachycardia during programmed ventricular stimulation in man. Am J Cardiol. 1978;43:404-415.
4 Miller JM, Rosenthal ME, Gottlieb CD, et al. Usefulness of the delta HA interval to accurately distinguish atrioventricular nodal reentry from orthodromic septal bypass tract tachycardias. Am J Cardiol. 1991;68(10):1037-1044.
5 Chien WW, Wang YS, Epstein LM, et al. Ventricular septal summit stimulation in atrioventricular nodal reentrant tachycardia. Am J Cardiol. 1993;72(17):1268-1273.
6 Goldberger J, Wang Y, Scheinman M. Stimulation of the summit of the right ventricular aspect of the ventricular septum during orthodromic atrioventricular reentrant tachycardia. Am J Cardiol. 1992;70(1):78-85.
7 Ross DL, Johnson DC, Denniss AR, et al. Curative surgery for atrioventricular junctional (“AV nodal”) reentrant tachycardia. J Am Coll Cardiol. 1985;6:1383-1392.
8 Jackman WM, Beckman KJ, McClelland JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency ablation of slow-pathway conduction. N Engl J Med. 1992;32:313-316.
9 Haissaguerre M, Gaita F, Fischer B, et al. Elimination of atrio ventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation. 1992;85:2162-2175.
10 McGuire MA, Lau KC, Johnson DC, et al. Patients with two types of atrioventricular junctional (AV nodal) reentrant tachycardia: Evidence that a common pathway of nodal tissue is not present above the reentrant circuit. Circulation. 1991;83:1232-1246.
11 Yeh SJ, Wang CC, Wen MS, et al. Radiofrequency ablation therapy in atypical or multiple atrioventricular node reentry tachycardias. Am Heart J. 1994;128:742-758.
12 Tai CT, Chen SA, Chiang CE, et al. Complex electrophysiological characteristics in atrioventricular nodal reentrant tachycardia with continuous atrioventricular node function curves. Circulation. 1997;95:2541-2547.
13 Tai CT, Chen SA, Chiang CE, et al. Multiple anterograde atrioventricular node pathways in patients with atrioventricular node reentrant tachycardia. J Am Coll Cardiol. 1996;28:725-731.
14 McGuire MA, Bourke JP, Robinson MC, et al. High resolution mapping of Koch’s triangle using sixty electrodes in humans with atrioventricular junctional (AV nodal) tachycardia. Circulation. 1981;88:2315-2328.
15 Wellens HJJ, Wesdorp JC, Duren DR, et al. Second degree block during reciprocal atrioventricular nodal tachycardia. Circulation. 1976;53:595-599.
16 Lee SH, Chen SA, Tai CT, et al. Electrophysiologic characteristics and radiofrequency catheter ablation in atrioventricular node reentrant tachycardia with second-degree atrioventricular block. J Cardiovasc Electrophysiol. 1997;8:502-511.
17 Tai CT, Chen SA, Chiang CE, et al. Electrophysiologic characteristics and radiofrequency catheter ablation in patients with multiple atrioventricular nodal reentry tachycardias. Am J Cardiol. 1996;77:52-58.
18 Gallagher JJ, Kasell J, Sealy WC, et al. Epicardial mapping in the Wolff-Parkinson-White syndrome. Circulation. 1978;57:854-866.
19 Jackman WM, Friday KJ, Yeung LW, et al. New catheter technique for recording left free-wall accessory atrioventricular pathway activation: Identification of pathway fiber orientation. Circulation. 1988;78:598-610.
20 Tai CT, Chen SA, Chiang CE, et al. Identification of fiber orientation in left free-wall accessory pathways: Implication for radiofrequency ablation. J Interv Card Electrophysiol. 1997;1:235-241.
21 Chen SA, Tai CT, Lee SH, et al. Electrophysiologic characteristics and anatomical complexities of accessory atrioventricular pathways with successful ablation of anterograde and retrograde conduction at different sites. J Cardiovasc Electrophysiol. 1996;7:907-915.
22 Gallagher JJ, Sealy WC. The permanent form of junctional reciprocating tachycardia: Further elucidation of the underlying mechanism. Eur Heart J. 1978;8:413-420.
23 Farre J, Ross D, Wiener I, et al. Reciprocal tachycardia using accessory pathways with long conduction time. Am J Cardiol. 1979;44:1099-1109.
24 Tchou P, Lehmann MH, Jazayeri M, et al. Atriofascicular connection or a nodoventricular Mahaim fiber? Electrophysiologic elucidation of the pathway and associated reentrant circuit. Circulation. 1988;77:837-848.
25 Chen SA, Tai CT, Chiang CE, et al. Electrophysiologic characteristics, electropharmacologic responses and radiofrequency ablation in patients with decremental accessory pathway. J Am Coll Cardiol. 1996;28:732-737.
26 Klein LS, Hackett FK, Zipes DP, et al. Radiofrequency catheter ablation of Mahaim fibers at the tricuspid annulus. Circulation. 1993;87:738-747.
27 McClelland JH, Wang X, Beckman KJ, et al. Radiofrequency catheter ablation of right atriofascicular (Mahaim) accessory pathways guided by accessory pathway activation potentials. Circulation. 1994;89:2655-2666.
28 Cappato R, Schluter M, Weiss C, et al. Catheter-induced mechanical conduction block of right-sided accessory fibers with Mahaim-type preexcitation to guide radiofrequency ablation. Circulation. 1994;90:282-290.
29 Selle JG, Sealy WC, Gallagher JJ, et al. Technical considerations in the surgical approach to multiple accessory pathways in the Wolff-Parkinson-white syndrome. Ann Thorac Surg. 1987;43:579-584.
30 Yeh SJ, Wang CC, Wen MS, et al. Catheter ablation using radiofrequency current in Wolff-Parkinson-White syndrome with multiple accessory pathways. Am J Cardiol. 1992;71:1174-1180.
31 Huang JL, Chen SA, Tai CT, et al. Long-term results of radiofrequency catheter ablation in patients with multiple accessory pathways. Am J Cardiol. 1996;78:1375-1379.
32 Sternick EB, Lokhandwala Y, Timmermans C, et al. The atrioventricular interval during pre-excited tachycardia: A simple way to distinguish between decrementally or rapidly conducting accessory pathways. Heart Rhythm. 2009;6(9):1351-1358.