Chapter 178 Electrodiagnostic Studies
Key Anatomy for Electrodiagnosis
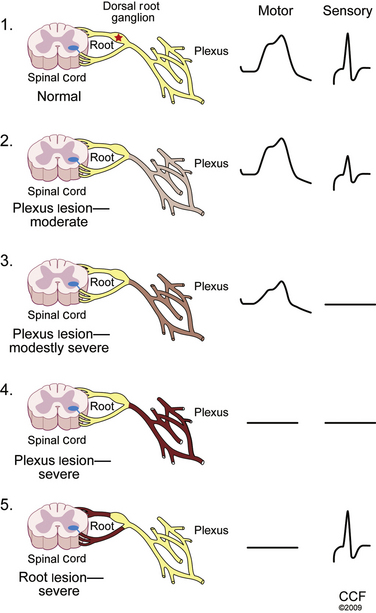
An understanding of key aspects of peripheral nervous system (PNS) anatomy is required for planning and interpreting the results of an EDX study. The motor division of the PNS begins at the anterior horn cell within the spinal cord, whose axon projects outward into the ventral root. The sensory division begins distally along the dorsal root at the dorsal root ganglion, which is typically found within the ostium of the intervertebral foramen.1 Thus, the dorsal (sensory) roots within the spinal canal are preganglionic. For this reason a lesion within the intraspinal canal (e.g., compression by an intervertebral disc) may damage the sensory fibers and cause clinical sensory symptoms, but the postganglionic fibers remain intact and unaffected for the purposes of sensory NCSs. By contrast, an intraspinal canal injury causing ventral (motor) root axon loss is postganglionic, and thus loss of sufficient motor fibers is measurable by motor NCSs and NEEs. This arrangement is of considerable value in localizing intraspinal canal injury electrodiagnostically.
The dorsal and ventral roots merge segmentally to form mixed spinal nerves, including 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal.2 Once the spinal nerves emerge from the intervertebral foramen, they divide into the posterior primary rami, which supply the skin and intrinsic muscles of the posterior neck and back (including the paraspinal muscles) and the anterior primary rami. The anterior primary rami supply the brachial plexus and peripheral nerves of the arm at the cervical segment (Fig. 178-1), the anterolateral aspect of the trunk and the thoracic level, and the lumbosacral plexus and nerves of the leg at the lumbosacral level. Muscles supplied by a given spinal segment are referred to as a myotome, and sensory regions supplied by each spinal segment are referred to as dermatomes.
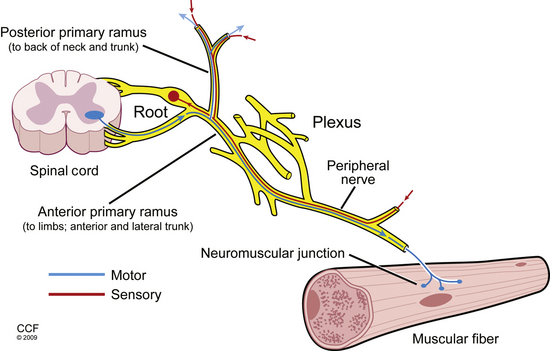
FIGURE 178-1 Basic anatomy of the peripheral nervous system.
(Illustration by David Schumick, BS, CMI. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2009. All rights reserved.)
The motor unit is the basic functional element of the motor PNS. It consists of the anterior horn cell, its axon, and all of the muscle fibers it innervates via neuromuscular junctions. The number of muscles fibers innervated by one motor neuron varies (e.g., 10:1 in the extraocular muscles and 2000:1 in the gastrocnemius muscle) and depends on the degree of fine-motor control required. Muscle is organized into fascicles, with the fibers of a single motor unit distributed randomly over as many as 100 different fascicles. Thus, a skeletal muscle consists of a mosaic fiber from 20 to 50 overlapping motor units.3 Analysis of the electrical activity of the motor units and their muscle fibers is the objective of the NEE.
Nerve Fiber Injury Types and Relevance
The EDX examination tests large myelinated nerve fibers, which can be subject to a range of injury. Regardless of whether nerve fibers are compressed, stretched, mechanically deformed, or lacerated, the damage manifests as either axon loss or demyelination, or a combination of the two. Axon loss produces conduction failure along the affected fibers. Of note, for several days after a focal axon loss lesion (2–3 days for motor fibers and 5–6 days for sensory fibers), the nerve fibers distal to the lesion can conduct an action potential when tested by NCSs, after which wallerian degeneration ultimately results in failure of the entire axon segment distal to the lesion site (5–7 days for motor fibers and 10–11 days for sensory fibers).4 In contrast, focal demyelination remains restricted to the injury site and only affects the largest myelinated nerve fibers, including motor fibers, and sensory fibers mediating vibration and proprioception. Focal demyelinating conduction block thus can only be demonstrated with NCS by stimulation proximal to the area of injury.5
When nerves are injured, the type and degree of axon loss or demyelination affects prognosis. Clinically useful scales rating the degree of nerve injury have been developed by Seddon6 and Sunderland.7 Seddon divides focal nerve injuries into three broad categories by severity: neurapraxia, axonotmesis, and neurotmesis. Neurapraxia, the mildest injury type with the best prognosis, is transient and does not involve loss of nerve continuity. Underlying pathophysiology may include functional conduction block (without demyelination), mild demyelinating lesions, and demyelinating conduction block. Axonotmesis occurs when the nerve axon and myelin sheath are completely interrupted, while the surrounding endoneurium, perineurium, and epineurium remain intact. Prognosis in axonotmesis is better than in neurotmesis (loss of the axon and severance of its supporting connective tissue). Axon regeneration in neurotmesis is limited due to disruption of the conduit through which regenerating axons might extend to reinnervate their target organ. Sunderland’s classification system further divides these injury types, particularly with respect to the degree of supporting structure damage in neurotmesis. EDX testing can differentiate between neurapraxia and axonotmesis, providing useful prognostic information. However, axonotmesis and neurotmesis cannot be differentiated, because they appear identical electrodiagnostically.3
Components of the Electrodiagnostic Examination
Nerve Conduction Studies
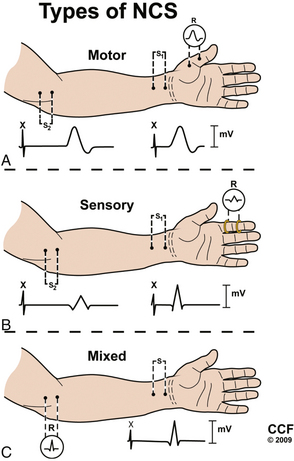
NCSs can be divided into motor, sensory, and mixed studies (Fig. 178-2). Motor and sensory NCSs are considered part of a routine EDX evaluation, whereas mixed studies are typically used only in special situations (e.g., median, ulnar, or tibial nerve entrapment). Although both surface and needle electrodes can be used for stimulating and recording, most EDX laboratories select noninvasive surface electrodes for NCSs.
Motor Conduction Studies
Motor NCSs are typically performed using an “active” recording electrode over the center of a muscle belly and reference electrode distally over the tendon of the muscle (“the belly-tendon montage”). A two-pronged (anode-cathode) stimulator is placed over the nerve supplying the muscle, with the cathode oriented closer to the recording electrode. Current is applied over the nerve to induce an action potential in the underlying nerve fibers. As the current is gradually increased with each subsequent stimulation, action potentials are triggered in more nerve fibers, and in turn more muscle fiber action potentials are generated and recorded distally as a single compound muscle action potential (CMAP). When increases in amperage no longer augment the size of the CMAP (i.e., all axons within the nerve are being stimulated), one final increase in current by 20% is performed to achieve a “supramaximal stimulation,” and the resulting CMAP is recorded for analysis.8
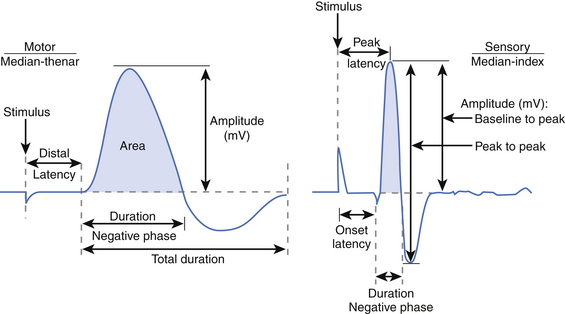
Several key aspects of the CMAP waveform are analyzed (Fig. 178-3). First, the distal latency is measured in milliseconds from the stimulus artifact to the initial baseline deflection. This measurement combines the action potential travel time from the stimulus site to the neuromuscular junction (NMJ), time across the NMJ, and time required for the muscle fibers to depolarize. Note that this measurement only reflects the fastest conducting fibers (i.e., those that reach the recording electrode earliest and produce the initial baseline deflection). Second, the duration of the negative phase is assessed as a measure of muscle fiber discharge synchrony in response to the stimulus. Demyelinating lesions not severe enough to cause conduction block may slow motor fiber conduction to varying degrees within a stimulated nerve, resulting in dyssynchrony or “temporal dispersion,” increasing the CMAP duration. Third, the amplitude and area of the negative phase are measured, providing an index of the number of muscle fibers depolarizing within the range of the recording electrode. Although a reduction in CMAP amplitude (and area) is most often attributed to axon loss, demyelinating conduction block between the stimulus and recording site has the same effect.
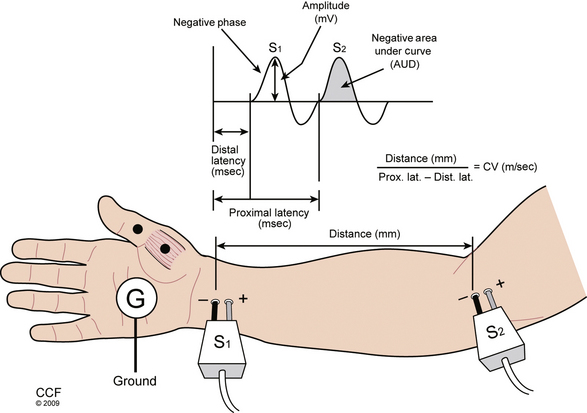
Further analysis of motor nerve conduction requires a second, more proximal stimulus over the nerve recording at the same location, using the technique just described. The more proximal stimulation is performed at a measured distance from the first. The difference between the proximal latency (PL) and distal latency (DL) in milliseconds reflects the conduction time along the fastest nerve fibers between the sites, eliminating the travel time from the distal site and across the NMJ, as well as muscle fiber depolarization time. The distance between the sites in millimeters divided by the nerve conduction time (i.e., PL minus DL) is known as the conduction velocity (Fig. 178-4). The configuration of the CMAP generated by the proximal stimulation is also compared with that of the distal CMAP. Assuming supramaximal stimulus at both sites, the CMAPs should be essentially identical. A proximal stimulation CMAP amplitude less than 50% of the distal CMAP amplitude is consistent with conduction block, or a very early focal axon loss lesion in which wallerian degeneration has not yet occurred.
In most electrodiagnostic laboratories, routine motor NCSs of the arm include median nerve (recording the abductor pollicis brevis muscle) and ulnar nerve (recording the abductor digiti minimi muscle), and in the leg, the tibial nerve (recording the abductor hallucis muscle) and peroneal nerve (recording the extensor digitorum brevis muscle). Proximal stimulation sites can be added to address specific clinical questions (e.g., stimulating the ulnar nerve above and below the elbow, or common peroneal nerve above and below the fibular head to assess for evidence of demyelination). In many cases these routine studies must be supplemented to adequately assess an area of potential injury. In the arm, motor studies of the radial nerve (recording the brachioradialis, extensor digitorum communis, or extensor indicis proprius muscles), ulnar nerve (recording the first dorsal interosseous muscle), musculocutaneous nerve (recording the biceps muscle), or axillary nerve (recording the deltoid muscle) may be indicated.5
Sensory Conduction Studies
Sensory NCSs are performed with the two-pronged stimulator and pair of recording electrodes over the nerve. These studies may performed antidromically (stimulating proximally and recording adjacent to the sensory receptor) or orthodromically (stimulating distally and recording proximally). These responses are relatively small and are measured in microvolts (μV, 1/1000 of the unit used to measure a CMAP). As with motor NCSs, current is applied over the nerve to induce an action potential and is gradually increased with subsequent stimulations. At supramaximal stimulation, the combined action potentials of the sensory fibers are recorded as a sensory nerve action potential (SNAP). SNAP latency, duration, and amplitude are measured. Latency is measured to onset (representing the conduction of the fastest fibers) or, more commonly, to the peak of the negative deflection (see Fig. 178-3).8
Particular advantages of NCSs compared with the NEEs include the ability to perform these studies with minimal patient cooperation (e.g., patients under anesthesia), provide key localizing information with regard to the sensory nerve fibers, and superior potential for identifying demyelinating lesions. It is, however, important to appreciate that NCSs only assess the large myelinated nerve fibers of the PNS, and thus disorders affecting only the smallest nerve fibers (e.g., small sensory fiber peripheral polyneuropathies) will not be demonstrated. NCSs are also relatively insensitive to motor axon loss compared with NEEs, underscoring the complementary nature of NCSs and NEEs.5
Needle Electrode Examination
Next, the examiner assesses for spontaneous electrical activity with the needle electrode at rest within the muscle. Normal muscle should be electrically silent during this phase. Fibrillation potentials are an important electrophysiologic marker of denervation. This form of spontaneous activity is characterized by regularly firing potentials, most commonly of a brief sharp spike configuration, derived from a single denervated muscle fiber. Larger-amplitude potentials are observed in acute disease9 and are present in greater density in more severe disorders. Fibrillation potentials are sensitive to denervation because disruption of a single motor axon results in fibrillation of all muscle fibers within that motor unit. They can thus be seen when motor axon loss is insufficient to cause clinical weakness. Although these potentials are most commonly associated with injury to motor axons, they may also be found in inflammatory myopathies and rarely in other neuromuscular disorders. Importantly, in the setting of denervation, fibrillation potentials require an average of 21 days to appear after injury and persist until reinnervation occurs or the muscle fibers degenerate at around 18 to 24 months after axon loss.10
Other forms of spontaneous activity can be observed during the resting phase. Fasciculation potentials, which are irregularly firing spontaneous discharges of an entire motor unit, are a marker of nerve fiber irritation as opposed to denervation. Complex repetitive discharges, which are recurrent, cyclic discharges of a series of muscle fibers, are a marker of chronicity, typically seen in neurogenic or myopathic disorders of at least 6 months’ duration.3
During the activation phase of the NEE, the muscle is contracted in order to analyze the firing pattern and morphology of voluntarily generated motor unit action potentials (MUAPs). A MUAP represents the summed electrical activity produced by depolarization of the muscle fibers of a single motor unit within the range of the recording electrode. The force generated by a muscle is a function of the number of different MUAPs firing and the rate at which they fire. At minimal contraction, a single MUAP may be observed firing in a semirhythmic pattern at a basal rate of approximately 5 to10 Hz. As the patient is asked to gradually contract the muscle with greater force, a second MUAP is “recruited,” and the firing rates of both MUAPs are increased until a third MUAP is recruited, and so on. A normal ratio of firing frequency to the number of different MUAPs firing is approximately 5:1 (e.g., when the MUAP firing rate reaches 20 Hz, four different MUAPs should be identifiable).8 At maximal contraction, MUAPs overlap and create an interference pattern, in which no single motor unit can be distinguished.
Finally, the morphology of the MUAPs is analyzed. In partial or gradual axon loss disorders, chronic neurogenic changes in the MUAP configuration can be appreciated after a period of 4 to 6 months. These changes occur due to collateral sprouting of the surviving motor axons, resulting in a greater number of muscle fibers belonging to each remaining motor unit. As more adjacent muscle fibers join the motor unit, the MUAP increases in duration and amplitude and appears more complex (polyphasic). Once present, these changes persist indefinitely and may be the sole evidence of a remote neurogenic injury.3
Late Responses
H Reflex
The H response amplitude and latency can be compared contralaterally and to values in normal controls adjusted for age and height, providing evidence of nerve fiber injury within the S1 reflex arc. Although H reflex abnormalities are considered sensitive for root impingement caused by S1 radiculopathy, the finding lacks specificity, also being found in patients with polyneuropathy, proximal tibial or sciatic mononeuropathies, and lumbosacral plexopathy. Absent H reflexes are also commonly observed in patients older than age 65 and in patients with a history of lumbar laminectomy and are thus of unclear clinical significance in this context.11 Overall, the usefulness of an absent H reflex is limited when the remainder of the EDX examination is within normal limits.
F waves
F waves12 are produced after a distal motor nerve stimulation. They can be measured during routine distal motor nerve conduction after reversing the stimulator orientation (the cathode is positioned proximal and the anode distal) and adjusting recording equipment settings to account for the low amplitude and long latency of these responses. F waves are different from H reflexes because they are conducted solely along motor axons, do not synapse within the spinal cord, and are thus not a measure of a true reflex arc. They are produced when some of the antidromic impulses cause a subpopulation of anterior horn cells to “backfire.” The resulting impulses travel back down the motor axons, and the latency of these late responses can be measured. These can be compared contralaterally and with height-adjusted normal values. A prolonged F wave latency is consistent with demyelination of the motor axon between the stimulus site and the recording muscle, as might be observed in acquired demyelinating polyradiculoneuropathy or demyelination in other causes or radiculopathy.5
Electrodiagnostic Localization of Spinal Disorders
Radiculopathy
Injury to a single spinal nerve root within the intraspinal canal usually results in normal sensory and motor NCSs. Since the lesion occurs proximal to the dorsal root ganglion (DRG), intact distal sensory fibers yield normal sensory conductions within the distribution of the suspected nerve root injury (Table 178-1). One important exception to this rule can occur at the L5 root, whose DRG can be found within the intraspinal canal in between 10% and 40% of patients.13 In these cases, an intraspinal canal lesion affecting the L5 level root (and DRG residing within the canal) causes loss of the superficial peroneal SNAP, complicating localization. Using the NEE to identify denervation changes within nonperoneal-innervated L5 muscles (e.g., gluteus medius, tensor fascia lata, semitendinosus, flexor digitorum longus, and tibialis posterior muscles) is required for proper localization in these cases. Motor NCSs are usually also normal, unless damage to a nerve root supplying the recording muscle results in sufficient axon loss to reduce the CMAP amplitude. Thus, the most important role of NCSs in radiculopathy evaluation is often to exclude mimicking causes, including plexopathy and mononeuropathy.
| Root | Sensory Response | Recording Site |
|---|---|---|
| C5 and C6 | Lateral antebrachial cutaneous | Lateral forearm |
| C6 | Radial | Thumb |
| C6 | Median | Thumb |
| C6 and C7 | Radial | Anatomic snuffbox |
| C6 and C7 | Median | Index finger |
| C7 | Median | Middle finger |
| C7 and C8 | Median | Ring finger |
| C7 and C8 | Ulnar | Ring finger |
| C8 | Ulnar | Fifth digit |
| C8 | Dorsal ulnar cutaneous | Dorsal forearm |
| T1 | Medial antebrachial cutaneous | Medial forearm |
| L4 | Saphenous | Medial ankle |
| L5 | Superficial peroneal | Lateral ankle |
| S1 | Sural | Posterior ankle |
A lesion affecting the nerve root within the intraspinal canal will not affect the postganglionic sensory nerve action potential.
Abnormalities on NEE are identified in muscles innervated by the same spinal root, or myotome. Finding evidence of neurogenic injury in more than one muscle (ideally both proximal and distal muscles) supplied by different nerves sharing the same spinal root is central to NEE localization. However, only a subset of motor fibers within the nerve root undergoes axon degeneration in all but the most severe radiculopathies, and thus findings on NEE may be limited to a few muscles within the affected myotome. A myotomal map based on systematic studies of the various NEE patterns occurring with single cervical root lesions, as defined by surgical localization for the upper (Table 178-2)14 and lower (Table 178-3)15 extremity, is provided, arranged by root. Note that due to overlap, particularly between the C5 and C6, C6 and C7, and L3 and L4 myotomes, the ability to differentiate root lesions at these levels from one another is limited.
TABLE 178-2 Typically Affected Muscles in Cervical Radioculopathy (by Root)
| Root | Abnormal Muscle | Nerve |
|---|---|---|
| C5 (or C6) | Supraspinatus | Suprascapular |
| Infraspinatus | Suprascapular | |
| Deltoid | Axillary | |
| Biceps brachii | Musculocutaneous | |
| Brachioradialis | Radial | |
| C7 (or C6) | Triceps | Radial |
| Anconeus | Radial | |
| Pronator teres | Median | |
| Flexor carpi radialis | Median | |
| C8 | Extensor indicis proprius | Radial |
| Flexor pollicis longus | Median | |
| First dorsal interosseous | Ulnar | |
| Abductor digiti minimi | Ulnar | |
| T1 (or C8) | Abductor pollicis brevis | Median |
TABLE 178-3 Typically Affected Muscles in Lumbosacral Radiculopathy (by Root)
| Root | Abnormal Muscle | Nerve |
|---|---|---|
| L3-4 | Iliopsoas | Femoral |
| Adductor longus | Obturator | |
| Rectus femoris | Femoral | |
| Vastus lateralis | Femoral | |
| Vastus medialis | Femoral | |
| L5 | Gluteus medius | Superior gluteal nerve |
| Tensor fascia lata | Superior gluteal nerve | |
| Semitendinosus | Sciatic (tibial division) | |
| Flexor digitorum longus | Tibial | |
| Tibialis posterior | Tibial | |
| Tibialis anterior | Peroneal (deep branch) | |
| Peroneus longus | Peroneal (superficial branch) | |
| Extensor hallucis longus | Peroneal (deep branch) | |
| Extensor digitorum brevis | Peroneal (deep branch) | |
| S1 | Gluteus maximus | Inferior gluteal nerve |
| Biceps femoris, long head | Sciatic (tibial division) | |
| Biceps femoris, short head | Sciatic (peroneal division) | |
| Medial gastrocnemius | Tibial | |
| Lateral gastrocnemius | Tibial | |
| Abductor hallucis | Tibial | |
| Abductor digiti quinti | Tibial |
NEE of the paraspinal muscles is also a routine component of the radiculopathy EDX assessment. Fibrillation potentials in the paraspinal muscles indicate axon loss affecting the posterior primary ramus of the spinal root, consistent with a lesion within or adjacent to the intraspinal canal (i.e., proximal to the brachial or lumbosacral plexus with regard to the arm or leg, respectively).10 However, due to the overlapping innervation of the paraspinal muscles, findings are not reliably segmental, often occurring caudal to the affected root. They are also not specific for radiculopathy, occurring in patients who have undergone prior spinal surgery via a posterior approach, motor neuron disease, or myopathy and in a portion of normal patients older than age 40. Thus, paraspinal fibrillation potentials cannot be interpreted in isolation and must be considered within the context of the rest of the EDX.11
Overall, the presence of fibrillation potentials in proximal and distal muscles of a myotome in the absence of chronic neurogenic MUAP changes is most consistent with a recent nerve root lesion. When chronic neurogenic MUAP changes and fibrillation potentials are both prominent within a myotome, either chronic progressive radiculopathy or an acute form superimposed on a more remote root lesion is likely. In contrast, chronic neurogenic motor unit potential changes limited to distal muscles of the myotome are supportive of a static, remote lesion.11
Case Examples
Case 1
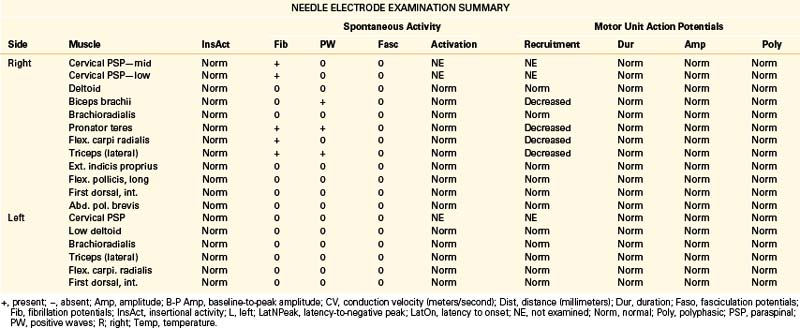
A 49-year-old, right-handed woman presented with dysesthesias and weakness in the right upper extremity, starting immediately after a fall onto her outstretched arm 8 weeks prior. No fractures were discerned at the time of the injury. She had pain in the right side of her neck and shoulder, particularly when trying to raise the arm up over her head, and numbness in the right index finger and thumb. Her examination revealed mild weakness of shoulder abduction, elbow extension, and forearm pronation. Triceps reflex was lower on the right. The patient was referred for EDX testing to evaluate for cervical root injury versus brachial plexopathy. See Table 178-4 for EDX results.
Notes
The NCSs are within normal limits. Additional sensory NCSs were performed for exclusion of a brachial plexus lesion, including the lateral and medial antebrachial cutaneous SNAPs. Changes in the sensory NCS are the most sensitive indicator of axon loss affecting the brachial plexus (Fig. 178-5) and are useful for differentiating a brachial plexopathy from a radiculopathy. The NEE revealed fibrillation potentials and decreased recruitment throughout the proximal and distal C6 and C7 myotome, consistent with an acute lesion. There is no evidence of early compensatory reinnervation (i.e., increased duration MUAPs), consistent with a lesion of less than 3 months in duration.
Case 2
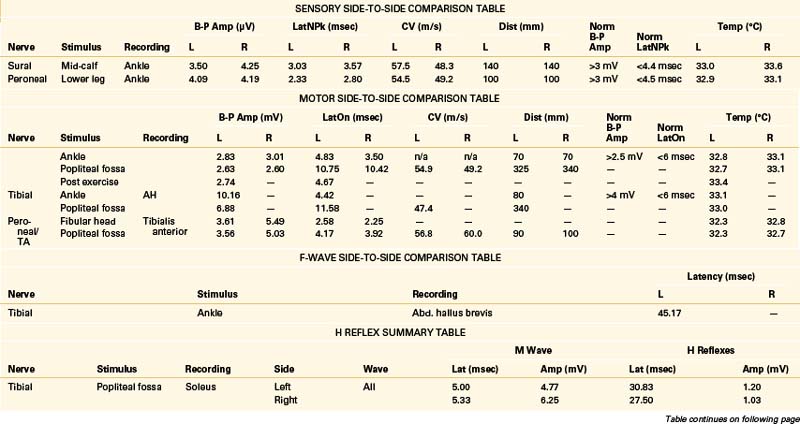
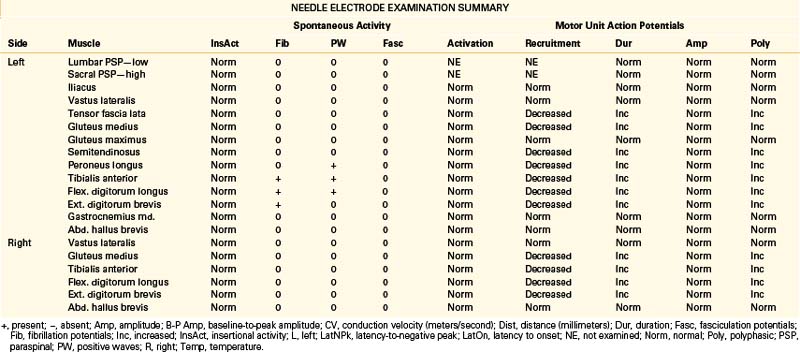
A 68-year-old, right-handed man presented with long-standing low back pain and more recent numbness of the top of the left foot of 4 weeks’ duration. Examination revealed mild weakness of the left ankle dorsiflexion, eversion, and toe extension and sensory loss over the dorsum of the left foot. The gait appeared normal, but the patient had some difficulty walking on his heels. The patient was referred for EDX testing to evaluate for lumbar root injury versus peroneal mononeuropathy. See Table 178-5 for the EDX results.
Lumbar Canal Stenosis
There is no characteristic EDX finding indicative of stenosis of the lumbar canal. Abnormalities depend entirely on the severity and duration of axon loss present. Findings can range from evidence of severe bilateral (most often asymmetrical) lumbosacral radiculopathies to a relatively normal study. Due to potential involvement of the cauda equina, H reflex recordings and lower-extremity NEE should be performed bilaterally in cases of suspected lumbar canal stenosis.11
Postoperative Electrodignostic Examination
In the remote postoperative period, active motor axon loss changes and their distribution in a given myotome can provide some helpful information. In particular, abundant fibrillation potentials within proximal and distal muscles of a myotome suggest the presence of ongoing or recurrent radiculopathy. As a general principle, the longer the time that has passed since surgery, the more likely that fibrillation potentials are caused by a progressive or postsurgical lesion. In contrast, chronic neurogenic motor unit potential changes may be permanent, particularly within the distal muscles, and may be the manifestation of a root injury 6 months to years prior.11
Recommendations for the Referring Physician
1. Refer with a specific question in mind. A patient should be referred after a complete history and neurologic examination. The EDX examination can then be focused to facilitate localization and attempt to answer the clinical question of interest. Referring for a general survey of a limb may result in more extensive testing than is required and is less likely to yield useful results.
2. Keep the limitations of the test in mind. In particular, with regard to radiculopathies, the EDX examination cannot detect all compressive radiculopathies and therefore cannot be used to exclude the diagnosis. In this context, the examination is more useful for establishing the presence of a radiculopathy, estimating its acuity/severity, and excluding other potential localizations as described earlier.
Other important limitations apply to patients with extensive peripheral edema and morbid obesity. In these cases, an NCS may be unelicitable due to increased distance between the recording electrode and the target muscle or nerve, and ability to access certain muscles may not be possible even with the longest-needle electrode. In these cases, the diagnostic utility of the EDX examination is limited.
Finally, EDX localization can be more difficult in elderly patients. In particular, a portion of normal individuals older than age 60 have bilaterally unelicitable sural and superficial peroneal SNAPs.3 In these cases, differentiation between an intraspinal and extraspinal canal localization can be challenging, particularly in the absence of localizing active motor axon loss changes (e.g., fibrillation potentials) throughout a particular myotome. Thus, in older patients, normal aging, a mild chronic peripheral polyneuropathy, and mild bilateral chronic L5 and S1 radiculopathies may appear electrodiagnostically similar, even with contralateral comparison and evaluation of an upper extremity to assess for evidence of a gradient (i.e., suggestive of a length-dependent polyneuropathy).
3. Consider the onset of symptoms and timing of the examination. A 3-week waiting period after symptom onset is recommended prior to EDX examination in order to allow fibrillation potentials to develop, maximizing the localizing value of the test. Studying the patient as soon as feasible after the initial waiting period is also advisable, due to the tendency for the EDX examination to normalize with time.
4. Prepare patients for the test. Although testing is generally well tolerated by most patients, some degree of discomfort is inherent to both NCSs and NEEs. Patients should be made aware of this prior to their arrival at the EDX testing site. Also, the EDX laboratory should be contacted in advance to determine their procedures for managing special situations such as coagulation abnormalities, use of the medication warfarin, and patients with implantable electronic devices (e.g., spinal cord stimulators), as protocols vary.
Levin K.H. Electrodiagnostic approach to the patient with suspected radiculopathy. Neurol Clin. 2002;20:397-421. vi, 2002
Levin K.H., Lüders H. Comprehensive clinical neurophysiology. Philadelphia: WB Saunders; 2000.
Levin K.H., Maggiano H.J., Wilbourn A.J. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46:1022-1025.
Preston D.C., Shapiro B.E. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations, ed 2. Philadelphia: Butterworth-Heinemann; 2005.
Wilbourn A.J. AAEE case report #12: Common peroneal mononeuropathy at the fibular head. Muscle Nerve. 1986;9:825-836.
Wilbourn A.J. Nerve conduction studies. Types, components, abnormalities, and value in localization. Neurol Clin. 2002;20(305):38.
Wilbourn A.J., Aminoff M.J. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1998;21:1612-1631.
1. Cohen M.S., Wall E.J., Brown R.A., et al. 1990 AcroMed Award in basic science. Cauda equina anatomy. II: Extrathecal nerve roots and dorsal root ganglia. Spine (Phila Pa 1976). 1990;15:1248-1251.
2. Drake R.L., Drake R.L., Gray H. Gray’s atlas of anatomy. Philadelphia: Churchill Livingstone; 2008.
3. Levin K.H., Lüders H. Comprehensive clinical neurophysiology. Philadelphia: WB Saunders; 2000.
4. Wilbourn A.J. AAEE case report #12: Common peroneal mononeuropathy at the fibular head. Muscle Nerve. 1986;9:825-836.
5. Wilbourn A.J. Nerve conduction studies. Types, components, abnormalities, and value in localization. Neurol Clin. 2002;20(305):38.
6. Seddon H.J. Surgical disorders of the peripheral nerves, ed 2. Edinburgh: Churchill Livingstone; 1975.
7. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516.
8. Preston D.C., Shapiro B.E. Electromyography and neuromuscular disorders: clinical-electrophysiologic correlations, ed 2. Philadelphia: Butterworth-Heinemann; 2005.
9. Kraft G.H. Fibrillation potential amplitude and muscle atrophy following peripheral nerve injury. Muscle Nerve. 1990;13:814-821.
10. Dumitru D., Amato A.A., Zwarts M.J. Electrodiagnostic medicine, ed 2. Philadelphia: Hanley & Belfus; 2002.
11. Wilbourn A.J., Aminoff M.J. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1998;21:1612-1631.
12. Fisher M.A., Shivde A.J., Teixera C., Grainer L.S. The F. response—a clinically useful physiological parameter for the evaluation of radicular injury. Electromyogr Clin Neurophysiol. 1979;19:65-75.
13. Levin K.H. L5 radiculopathy with reduced superficial peroneal sensory responses: intraspinal and extraspinal causes. Muscle Nerve. 1998;21:3-7.
14. Levin K.H., Maggiano H.J., Wilbourn A.J. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46:1022-1025.
15. Levin K.H. Electrodiagnostic approach to the patient with suspected radiculopathy. Neurol Clin. 2002;20:397-421.