12 Electrodiagnostic examination
Nerve Conduction Studies
Nerve conduction in the upper limb
Motor nerve conduction
Stimulation
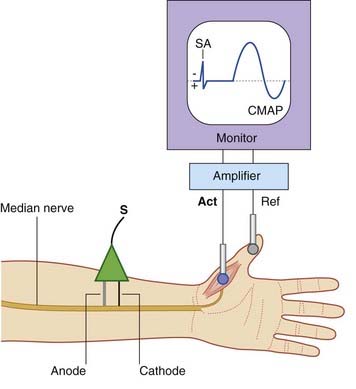
A typical stimulating electrode is one with an anode and a cathode in the form of two blunt prongs which are applied to the skin surface overlying the nerve. In Figure 12.1 it has been placed over the median nerve at the wrist (just lateral to the cordlike palmaris longus tendon). The cathode is placed nearer to the recording site than the anode in order to prevent any conduction block by the anode. When sufficient current is passed from cathode to anode, transmembrane ionic movements initiate impulse propagation in both directions along the nerve. Large myelinated nerve fibers lying nearest the cathode are the first to become depolarized; these include the Aα diameter axons of anterior horn motor neurons. A pulse of 20–40 mA with a duration of 0.1 ms is usually sufficient to activate all motor units in abductor pollicis brevis.
Recording
Increasing the applied voltage activates additional motor units until all are activated by each pulse. The required stimulus is called maximal. For good measure, the final stimulus is often supramaximal at 5–10% above maximal. The final waveform observed constitutes the compound motor action potential, or CMAP. It is produced by summation of the individual muscle fiber potentials (Figure 12.2).
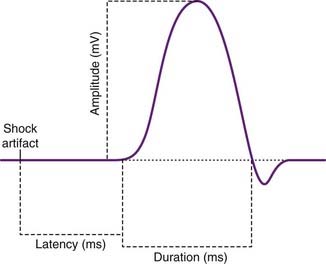
Routine measurements of the final CMAP are shown in Figure 12.3. They include the latency (time interval) between stimulus and depolarization onset, and the amplitude and duration of the negative phase of the waveform. (The final, positive phase is produced by inward ion movement during collective repolarization of the muscle fibers.)
Motor nerve conduction velocity (MNCV)
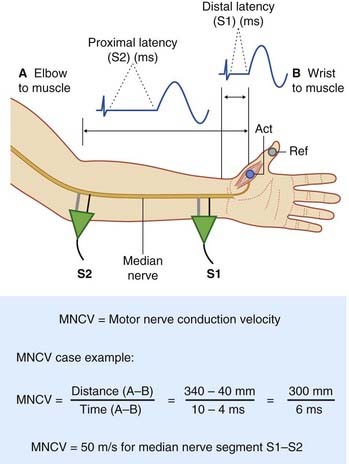
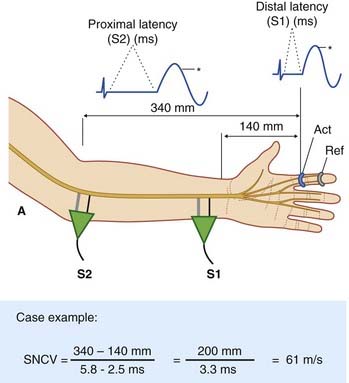
The setup required to determine motor nerve conduction velocity for the median nerve is straightforward, as shown in Figure 12.4. Here the nerve has first been activated at the wrist (S1) to generate and store a ‘wrist to muscle’ velocity record. The stimulator has then been placed over the median nerve at the elbow (S2) to provide an ‘elbow to muscle’ record. Speed being the product of distance over time, the elbow-to-wrist conduction velocity is given by subtracting one value from the other, as illustrated by the case example.
Sensory nerve conduction
For studies of sensory nerve conduction velocity (SNCV), the median is again the nerve of choice (Figure 12.5). Again it is large myelinated nerve fibers that will be stimulated, and the site and manner of stimulation at elbow and wrist will be the same. On this occasion, however, we are selectively recording antidromic stimulation of cutaneous sensory fibers – specifically, of the digital branches of the median nerve to the skin of the index finger, which is wearing an active recorder in the form of a ring.
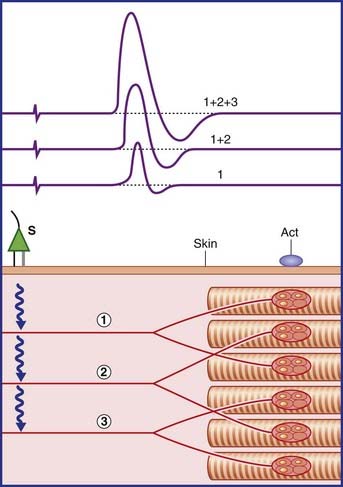
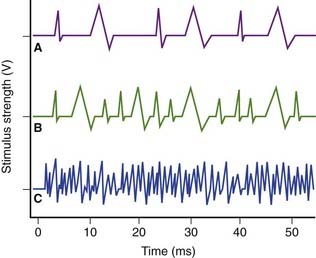
The prime function of the myelinated nerve fibers to be sampled by the ring recorder are those supplying the highly sensitive and discriminatory skin of the finger pad, described in Chapter 11. The largest, serving Meisssner and Pacinian corpuscles and Merkel cell–neurite complexes, are known to normally conduct at a speed of 60–100 m/s and the finest, serving mechanical nociceptors, at 10–30 m/s. This tenfold variation is in marked contrast to that of the relatively uniform fiber size of the stem axons supplying the small motor units of the abductor muscle and conducting at 45–55 m/s. One consequence is that, when stimulating sensory nerves at increasing distances from the recording site, a change in the waveform shape is normally noted. In the figure, the asterisks are intended to highlight the difference in the shape of the waveforms of the two compound sensory nerve action potentials (CSNAPs). Two factors are involved:
Nerve conduction in the lower limb
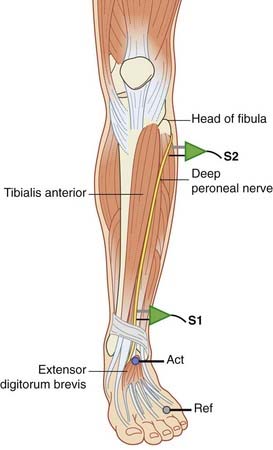
The lower limb muscle most frequently sampled for motor nerve conduction velocity is extensor digitorum brevis on the dorsum of the foot (Figure 12.6). The deep peroneal nerve is stimulated first in front of the ankle and then at the level of the neck of the fibula. At times, tibialis anterior is also sampled; in this case the nerve is stimulated first at the neck of the fibula and then at the lateral edge of the popliteal fossa next to the biceps femoris tendon.
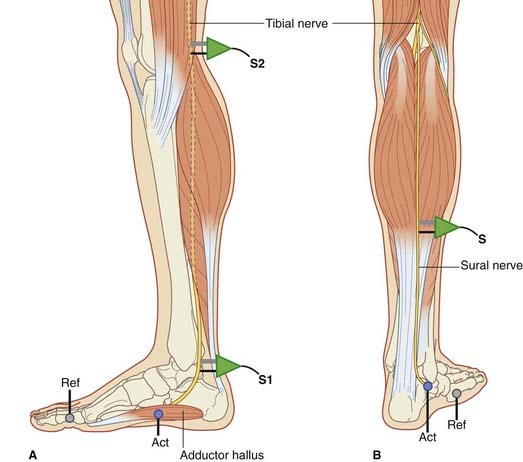
A second choice for MNCV is the tibial nerve recording from adductor hallucis, located on the medial side of the foot (Figure 12.7A).
For SNCV assessment, the sural is the nerve of choice. The sural arises from the tibial and receives a contribution from the peroneal; it supplies the skin along the lateral margin of the foot. The recorder is applied to the skin below the lateral malleolus, and the nerve is stimulated antidromically at the levels shown in Figure 12.7B.
Nerve root pathology
Nerve root pathology is known as radiculopathy (L. radix, ‘root’). Radiculopathies are encountered:
Clinical Panel 12.1 Peripheral neuropathies
Peripheral neuropathies are amenable to several classifications, each with its own relevance:
One kind of acute polyneuropathy and two kinds of chronic polyneuropathy will now be described.
Chronic polyneuropathy originating in myelin sheaths
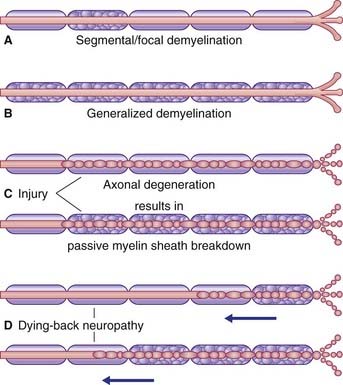
This type of neuropathy is associated with chronic vitamin deficiency, longstanding diabetes or chronic hypothyroidism. The myelin sheaths of the peripheral nerves degenerate while the axons remain relatively intact. Because potassium channels are largely obliterated when the sheaths are initially laid down, and because saltatory conduction is lost along with the sheaths, sensory conduction is progressively impaired. As might be anticipated, the longest nerves are the most affected, yielding ‘glove and stocking’ paresthesia (Figure CP 12.1.2). (The term paresthesia refers to sensations of numbness, pins-and-needles and/or tingling.)
Initially, demyelination may be segmental/focal as shown in Figure CP 12.1.1.
After several months, motor weakness and wasting may become evident in the muscles of the hands and feet. With further progression, joint sense and vibration sense may be lost there. (Joint sense refers to the ability to detect passive movement at a joint performed by a clinician; vibration sense refers to the ability to feel the buzz of a tuning fork applied to bone; see Ch. 16.)
Clinical Panel 12.2 Entrapment neuropathies
Upper limb
Cervical spondylosis, described in Chapter 14, is a potential source of confusion. This disorder is another example of ’nerve entrapment’, being caused by compression of one or more cervical spinal nerves by bony outgrowths next to apophyseal facet joints in the neck. Most commonly affected nerves are C6 (sensory to skin of lateral forearm, lateral hand and entire thumb) and C7 (sensory to skin of outer three fingers front and back). Arm and forearm tendon reflexes may be diminished and some motor weakness may be apparent in the distribution of the affected ventral roots. In addition to the more extensive cutaneous symptoms and signs, cervical spondylosis is unrelated to manual activities, seldom causes nocturnal symptoms and has a relatively advanced age profile.
Confirmatory of carpal tunnel syndrome is a prolonged distal latency in the motor nerve conduction test (Figure 12.4) and/or in the sensory nerve conduction test (Figure 12.5).
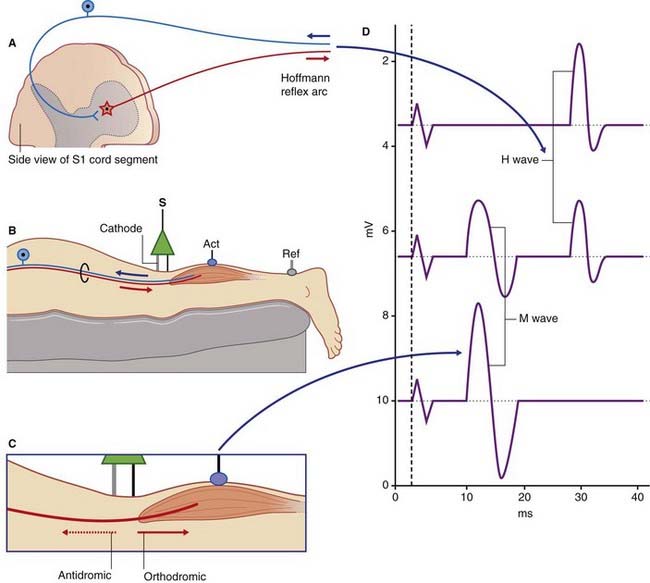
The H response (see Ch. 12 tutorial onsite)
Owing to their deep location, nerve conduction in spinal nerve roots can only be assessed indirectly, by activating sensorimotor reflex arcs at appropriate levels. The standard test, named after Hoffmann who first described it, is known as the H response or H wave test. This is frequently used to assess overall conduction velocity in the S1 reflex arc – the same neurons that are evaluated clinically by the Achilles reflex (Figure 12.8). The tibial nerve is stimulated using the minimum current sufficient to elicit a muscle twitch. The objective here is to excite the largest myelinated afferent fibers, namely those serving annulospiral nerve endings in neuromuscular spindles, thereby eliciting a monosynaptic, minimal latency twitch in the triceps surae (gastrocnemius/soleus). The minimal latency is in fact quite long – up to 35 ms depending on the patient’s overall height – because S1 segment of the spinal cord lies behind the body of vertebra L1, creating a 130–150 cm upandown (sic!) trip. Increasing the current reaches the point where the M wave appears (Figure 12.8D). The M wave is produced by direct orthodromic activation of the motor end plates. Antidromic conduction accounts for progressive canceling out of the action potentials descending in the efferent limb of the H reflex arc.
Electromyography
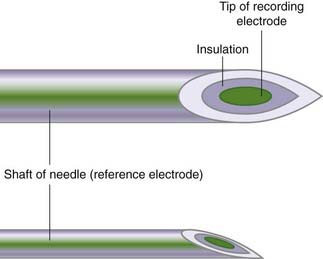
Needle electrode
The recording electrode occupies the lumen of a fine needle (Figure 12.9). An insulation sleeve isolates the recording electrode from the barrel of the needle, which functions as a reference electrode. As in the case of nerve conduction studies, the EMG record is based on the potential difference between the recording and reference electrodes. During muscle contractions, an extracellular record is taken of the low-voltage potentials that originate from the muscle membrane depolarizations.
The normal electromyogram
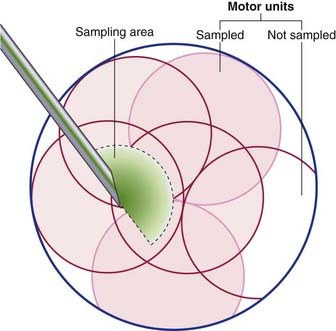
The sensitivity settings on the machine are adjusted in order to record voluntary muscle contraction. The patient is asked to slightly contract the muscle and, as they do, irregular waveforms appear representing motor unit action potentials (MUAPs). Each of these individual waveforms represents activation of the muscle fibers that belong to an individual motor unit. While the electrode is stationary, all MUAPs that are of similar shape originate from the same individual anterior horn cell and reflect depolarization of that cell. Their shape, in normal situations, is similar to the familiar QRS of an EKG (ECG) recording, and measurements are made of their amplitude, duration and morphology. Each individual MUAP is a sample of the summated depolarizations of the fibers of a single motor unit. We must bear in mind that the electrode can only record from the muscle fibers which are the closest – not all fibers of a unit contribute to the observed response. As indicated in Figure 12.10, overlap of the territories of individual anterior horn cells permits several motor units to be sampled simultaneously.
The recorded waveforms tell us about the form and function of the motor units, and about changes under various pathological conditions. Each depolarization of an anterior horn cell (AHC) results in a virtually synchronous depolarization of all of its target muscle fibers. The needle electrode records a summation of the individual action potentials closest to its exposed tip, to provide a MUAP. As long as the recording electrode remains stationary, the MUAP waveform will remain the same. On the monitor they ‘march across’ at a frequency that is the same as the firing rate of the neurons being sampled. The stronger the voluntary muscle contraction, the greater the number of motor neurons recruited by the corticospinal tract and the more frequent the firing rate (Figure 12.11).
Some clinical applications
Denervation of muscle
Skeletal muscle may become denervated as a result of:
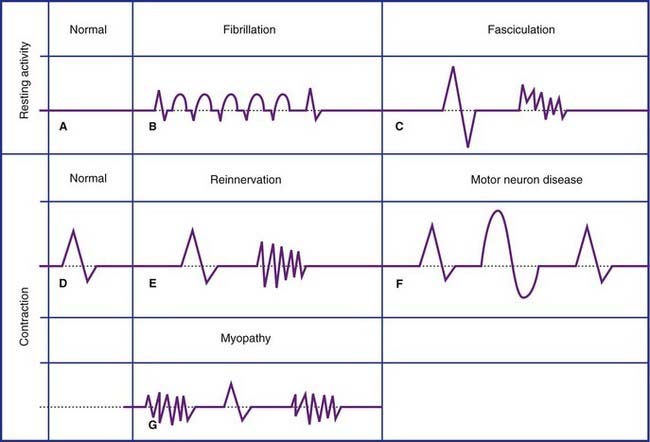
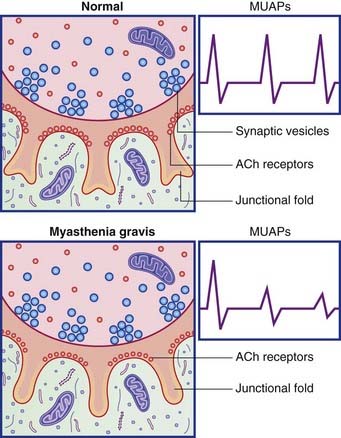
Some abnormal motor unit action potentials are illustrated and explained in Clinical Panel 12.3. Clinical Panel 12.4 is an account of the autoimmune disorder known as myasthenia gravis (‘grave muscle weakness’)
Clinical Panel 12.3 Abnormal motor unit action potentials
(Myasthenia gravis is described in Clinical Panel 12.4.)
Prolonged polyphasic and giant MUAPs
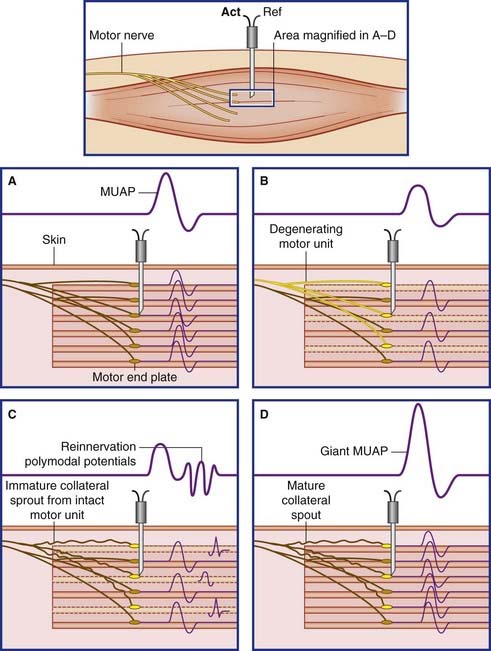
The term polyphasic signifies an abnormally large number of positive and negative phases. Polyphasic MUAPs signify reinnervation of motor end plates vacated by earlier degeneration of their nerve supply, followed by takeover by neighboring healthy axons. Figure 12.12 provides a basic explanation. In this figure two separate motor neurons are each represented by a single parent axon supplying three muscle fibers. Following interruption of one parent axon, its vacated nerve sheaths exert a chemotropic effect, inducing the surviving stem and/or branch axons to issue collateral sprouts which eventually reinnervate the vacated end plates. The outcome is the production of giant motor unit potentials by the enlarged motor unit.
Giant MUAPs are called ‘neuropathic’ because they signify motor neuron pathology. As mentioned in Chapter 10, they occur in the elderly as a result of ‘fall out’ of spinal motor neurons. Motor neuron disease (Ch. 16) is associated with progressive loss of spinal and cranial nerve motor neurons on a much greater scale; even the neurons that provide reinnervation are eventually lost. Radiculopathy (Ch. 14) resulting from from compression of nerve roots, and axonal polyneuropathy are other causes.