CHAPTER 232 Electrodiagnostic Evaluation of Peripheral Nerves
Electromyography and Nerve Conduction Studies
Electromyography (EMG) and nerve conduction studies (NCSs), also referred to as electrodiagnostic studies, are used to test the function of the nervous system.1,2 They are generally used to test the integrity of the peripheral nervous system (PNS) but can also be used to evaluate movement disorders, such as cervical dystonia (torticollis). EMG and NCSs are best used as an extension of the neurological examination to help localize and define a lesion. They can help determine whether weakness or numbness is due to a lesion in the central nervous system (CNS) or PNS. Once a lesion is determined to be in the PNS, EMG and NCSs can localize the lesion to the anterior horn cell, nerve root, dorsal root ganglion, plexus, nerve, neuromuscular junction, or muscle. In addition, the degree of involvement of the sensory and motor nerves can be determined. Lesions can also be localized to the cell body, axon, or myelin. The pattern of abnormalities on EMG and NCSs is used to provide a definitive diagnosis. These electrodiagnostic studies can also be used to determine the duration, severity, and prognosis of a lesion. Finally, they can provide an objective measure of improvement or worsening, which is often useful when determining response to treatment.
Fundamentals of Electrodiagnostic Testing
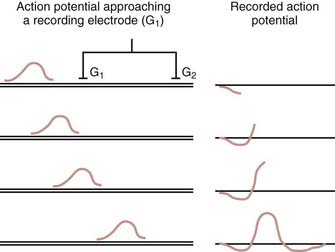
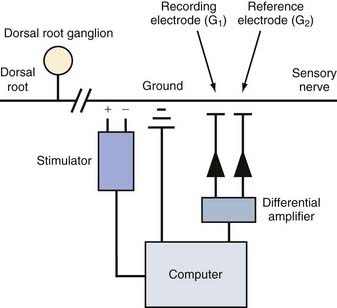
EMG and NCSs use different means of measuring action potentials of nerve axons or muscle fibers. The physiology of an action potential is discussed elsewhere.1 Measurement of action potentials involves placing two recording electrodes along a nerve axon or muscle fiber (Fig. 232-1). The difference in electrical potential between the two electrodes is amplified through a differential amplifier and plotted on a monitor for analysis. Because the recording electrodes are close to each other (usually within a few centimeters), in the absence of an action potential, there is no significant difference in electrical potential between them. As an action potential approaches one of the electrodes, this electrode measures an electrical potential that is not measured by the other electrode. A triphasic wave is recorded as the action potential passes under the first electrode. Most recordings during EMG and NCSs involve the summation of a number of action potentials from nerve or muscle fibers. For example, a sensory NCS involves recording the summation of individual action potentials from all the hundreds or thousands of sensory axons of a particular nerve.
Sensory Nerve Action Potential
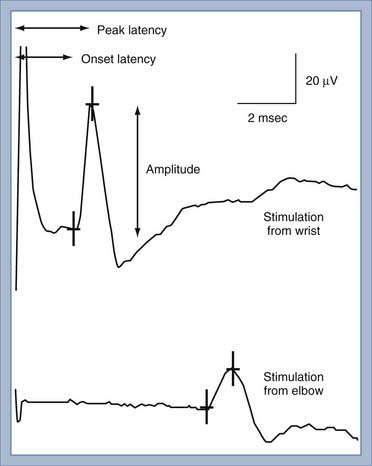
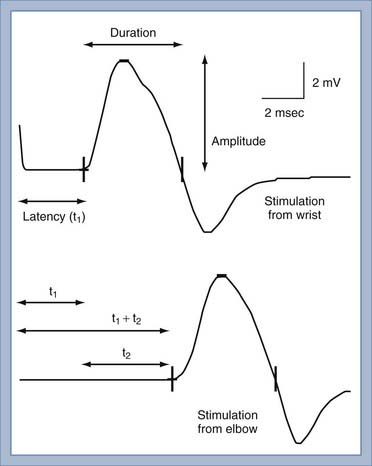
NCS of the sensory nerves generates a recording referred to as a sensory nerve action potential (SNAP). In this study, the sensory nerve is stimulated with sufficient electrical current that all the large-diameter sensory axons are simultaneously depolarized (Fig. 232-2). This stimulation is referred to as supramaximal stimulation because a higher electrical current than the minimum required for stimulation of all the axons is used to ensure that all the axons are depolarized. Action potentials of the depolarized axons immediately travel away from the site of stimulation at various velocities, depending on a number of factors. For instance, conduction velocity increases with larger axon diameter, increased myelination, and higher nerve temperature. The action potentials travel along the axons and are recorded by the recording electrodes over the nerve (Fig. 232-2). Each individual action potential generates a triphasic recording (see Fig. 232-1). A typical sensory nerve contains up to several hundred sensory axons, and an equal number of action potentials is recorded from the nerve. The SNAP is the sum of the individual action potentials recorded from each sensory axon. Under normal conditions, the action potentials of large-diameter sensory axons travel at similar velocities and thus pass under the recording electrode nearly simultaneously. The sum of these action potentials results in the SNAP (Fig. 232-3). The action potentials of small-diameter myelinated and unmyelinated axons travel at slower and more variable velocities. Thus, these action potentials pass under the recording electrode at variable times and do not summate sufficiently to generate enough amplitude to be a visible waveform. The amplitude of the SNAP is calculated from the baseline, or first positive peak, to the negative peak (while keeping in mind that negative is upward) and is a reflection of the number of normal large-diameter myelinated sensory axons. Conduction velocity is calculated by dividing the distance between the sites of stimulation and recording by the time that the first action potentials reach the recording electrodes, which is represented as the beginning of the upward slope from the baseline or first positive peak. Under normal circumstances, a longer distance between the stimulation and recording sites results in less synchronized action potentials from the variety of slow- and fast-conducting axons. This phenomenon is referred to as temporal dispersion and results in a decreased SNAP amplitude and increased duration when recording over large distances (Fig. 232-3).
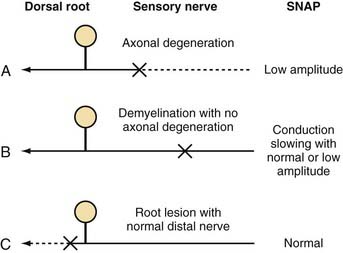
In the presence of nerve injury or disease, there is often a change in SNAP conduction velocity or amplitude. Changes in the SNAP depend on the site and mechanism of the lesion, but all such lesions cause numbness, paresthesias, and other sensory symptoms. Any lesion at or distal to the dorsal root ganglion that causes wallerian degeneration results in fewer sensory axons, fewer action potentials recorded by the electrodes, and a decreased SNAP amplitude (Fig. 232-4A). Conduction velocity is normal or nearly normal because the remaining axons are myelinated and function normally. With marked axonal loss, conduction velocity may be slightly decreased because of the loss of faster conducting axons. Conduction velocity can be decreased to 80% of the lower limit of normal for mild axonal loss and to 70% of normal when SNAP amplitude is less than 50% of the lower limit of normal.
Demyelination distally between the sites of nerve stimulation and recording leads to slowing of the individual action potentials and thus more markedly reduced conduction velocity or increased latency (time from stimulation to the initial waveform) of the SNAP (Fig. 232-4B). Typical clinical manifestations include carpal tunnel syndrome with demyelination of the median sensory nerve at the carpal tunnel or a demyelinating polyneuropathy such as Guillain-Barré syndrome. Because acquired demyelinating lesions frequently result in varying degrees of slowing of the individual axons, there is often increased temporal dispersion. If the demyelination is severe enough, the action potential may be unable to continue propagating down the axon across the site of demyelination to the recording electrode, thereby resulting in conduction block. Conduction block, which is almost always caused by demyelination, can result in a low-amplitude or absent SNAP. In most cases of conduction block there is concomitant conduction slowing because some axons are demyelinated to the point of conduction block but others are only partially demyelinated, which causes conduction slowing. Thus, a low-amplitude SNAP with conduction slowing suggests a demyelinating lesion, whereas a low-amplitude SNAP without conduction slowing suggests a lesion causing axonal degeneration without primary demyelination.
A demyelinating lesion proximal to the point of nerve stimulation and recording leaves the distal nerve intact. A lesion causing axonal loss proximal to the dorsal root ganglion, such as at the root, results in wallerian degeneration of the proximal axon. However, the sensory axons distal to the dorsal root ganglion maintain their continuity to the cell body and thus remain normal. The SNAP remains normal under these circumstances (Fig. 232-4C), even in the presence of anesthetic sensations. A normal SNAP in a patient with numbness suggests that the lesion is proximal to the dorsal root ganglion (root or CNS) or is a proximal demyelinating lesion.
Compound Muscle Action Potential
NCS of the motor nerves generates a recording referred to as the compound muscle action potential (CMAP). Unlike the situation with a sensory NCS, the CMAP is recorded from the muscle and not the motor nerve. Similar to a sensory NCS, the motor nerve is stimulated with supramaximal stimulation such that all the motor axons are simultaneously depolarized. Action potentials from the depolarized axons immediately travel away from the site of stimulation at nearly identical velocities. The action potentials travel along the axons to the neuromuscular junction. Each motor axon innervates up to several hundred muscle fibers. Because the typical motor nerve contains up to a few hundred motor axons, the amplitude of the CMAP—a summation of action potentials from muscle fibers—is 100 to 1000 times the magnitude of the SNAP. When compared with sensory nerves, the motor axons of motor nerves are much more similar in diameter and degree of myelination, thereby resulting in more similar individual axonal conduction velocities and very little temporal dispersion (Fig. 232-5). Moreover, the duration of motor unit action potentials (MUAPs) is long enough that the degree of overlap is affected relatively little by temporal dispersion.
The conduction velocity of the distal motor nerve segment cannot be calculated for the CMAP as it can for the SNAP because the time from nerve stimulation to recording of muscle fiber action potentials includes the release of acetylcholine. Acetylcholine must diffuse across the neuromuscular junction and bind to the acetylcholine receptor before the muscle fiber action potential can be recorded. This process takes about 0.5 to 1 msec. Instead of calculating conduction velocity from a point of stimulation to the muscle, the time from stimulation at a distal point of the nerve to the onset of the CMAP—referred to as the distal motor latency—is compared with that in normal controls to determine whether distal conduction slowing is present. The conduction velocity of the proximal portion of the motor nerve can be calculated as described in Figure 232-5. The three most important aspects of the CMAP are the amplitude, conduction velocity, and distal motor latency.
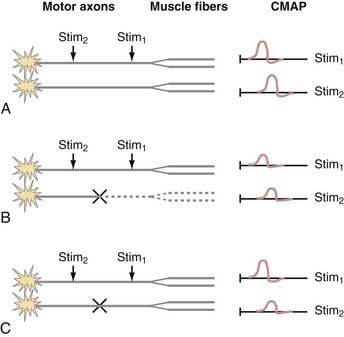
In the presence of injury or disease of the motor nerve or muscle, there is often a change in CMAP conduction velocity, amplitude, or distal motor latency. Changes in CMAP depend on the site and mechanism of the lesion, but all such lesions cause weakness and possibly muscle atrophy in the case of denervation. The more common scenarios are presented in Figure 232-6. For the sake of illustration, consider a motor nerve consisting of two motor axons, each of which innervates two muscle fibers. Under conditions of normal PNS functioning, supramaximal stimulation of the motor nerve distally and proximally leads to action potentials in both motor axons and all four muscle fibers. The CMAP recordings consist of the sum of the muscle action potentials from all four muscle fibers (Fig. 232-6A).
When there is motor axonal degeneration from distal dying-back axonopathy, wallerian degeneration from a proximal lesion, or loss of motoneurons, only one of the two motor axons is present and can transmit an action potential to its two innervated muscle fibers (Fig. 232-6B). The other two muscle fibers cannot be stimulated, which leads to decreased CMAP amplitudes from both the distal and proximal stimulation sites.
In this model, conduction block between the distal and proximal sites of stimulation results in a characteristic CMAP abnormality (Fig. 232-6C). A typical demyelinating lesion, such as from entrapment neuropathy, may result in conduction block of one motor axon and conduction slowing of the other. Distal to the site of demyelination, the motor axons and myelin are normal. Thus, stimulation of the motor nerve distally results in activation of both motor axons and all four muscle fibers, which yields a normal CMAP amplitude and distal motor latency. Stimulation of the motor nerve proximal to the site of demyelination leads to action potentials in both motor axons. However, because one of the action potentials is blocked at the site of demyelination, it is unable to proceed with activation of the corresponding muscle fibers. The other action potential is slowed but can still activate two of the muscle fibers. Hence, the CMAP from the proximal point of stimulation is the sum of two muscle fiber action potentials; its amplitude and area are smaller than normal, and it is delayed because of conduction slowing. Therefore a scenario in which CMAP amplitude and area at the proximal point of stimulation are decreased in comparison to the distal site is indicative of a conduction block between the two points of stimulation. If there is a decrease in CMAP amplitude but area is maintained, temporal dispersion is suggested. Unlike sensory nerves, there is normally very little temporal dispersion of motor nerves, and its presence indicates an area of demyelination.
Late Responses
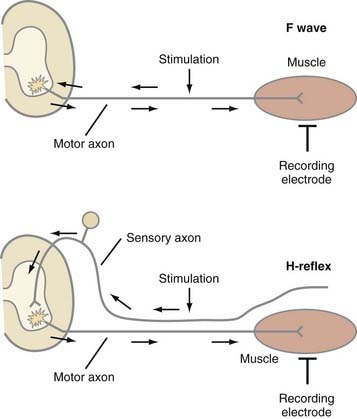
SNAP and CMAP studies are best at evaluating distal nerves. Stimulation and recording from nerves at proximal sites, such as the root or plexus (e.g., Erb’s point), are often unreliable. When stimulating proximally, it is often difficult to ensure supramaximal stimulation or to limit stimulation to one nerve. Alternatively, evaluation of late responses can provide useful data regarding the proximal portions of nerves. The two most commonly evaluated late responses are the F wave and H-reflex (Fig. 232-7).
Repetitive Stimulation
Repetitive stimulation is used to diagnose abnormalities of the neuromuscular junction, such as myasthenia gravis, botulism, Lambert-Eaton myasthenic syndrome, and congenital myasthenia. The technique involves rapid, repetitive CMAP recordings from 2 to 50 Hz. Defects in neuromuscular transmission, especially at the postsynaptic site, lead to successive decrements in CMAP amplitude with stimulation at low frequencies of 2 to 3 Hz. An increase in CMAP amplitude at high frequencies such as 50 Hz is indicative of a presynaptic neuromuscular transmission defect, such as Lambert-Eaton syndrome. The principles underlying these abnormalities can be found in other textbooks.1,2
Needle Electromyography
Spontaneous Activity
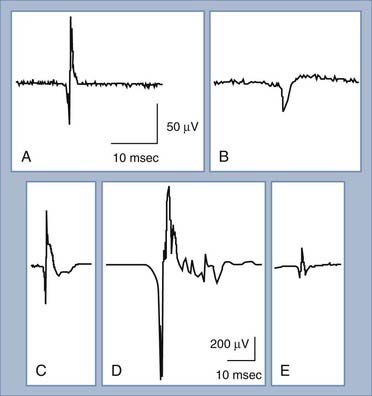
Under normal conditions, when a patient is relaxed, muscle fibers are electrically silent, with no significant spontaneous muscle fiber action potentials. Different types of abnormal spontaneous muscle fiber action potentials can be seen and indicate specific abnormalities of the PNS (Table 232-1). The most common and significant spontaneous activities consist of fibrillation potentials and positive sharp waves (Fig. 232-8A and B). These are spontaneous action potentials from individual muscle fibers in response to either acute denervation or acute muscle fiber injury. Muscle disorders associated with these discharges include muscle fiber necrosis from muscle trauma, muscular dystrophies or inflammatory myopathies, and other muscle diseases, such as acid maltase deficiency or hyperkalemic periodic paralysis. Because fibrillation potentials and positive sharp waves are caused by the same group of PNS abnormalities, they usually occur together. Denervation of muscle results in fibrillation potentials and positive sharp waves within 2 to 3 weeks. These findings persist until the muscle fiber is reinnervated, usually within 3 to 4 months in mild injuries, or until the denervated muscle fiber undergoes complete atrophy after up to a few years of persistent denervation without reinnervation.
TABLE 232-1 Abnormal Spontaneous Muscle Fiber Action Potentials and Associated Peripheral Nervous System Abnormalities
| MUSCLE FIBER ACTION POTENTIAL | ABNORMALITY |
|---|---|
| Fibrillation potential | Acute denervation, acute muscle fiber necrosis |
| Positive sharp wave | Acute denervation, acute muscle fiber necrosis |
| Complex repetitive discharge | Chronic denervation, chronic muscle fiber necrosis |
| Fasciculation potential | Normal finding, motoneuron disease, radiculopathy, neuropathy |
| Myotonic discharge | Myotonic dystrophy, myotonia congenita, paramyotonia |
| Myokymic discharge | Radiation plexopathy or myelopathy, multiple sclerosis, brainstem glioma with facial myokymia |
| Cramp discharge | Normal finding, motoneuron disease, radiculopathy, neuropathy |
| Neuromyotonic discharge | Isaac’s disease, neuropathy |
Complex repetitive discharges are generated from muscle fibers that have been denervated for more than 2 months or from injured muscle fibers, usually associated with muscle fiber necrosis. The neurological disorders that cause complex repetitive discharges are similar to those associated with fibrillation potentials and positive sharp waves, except that complex repetitive discharges occur under chronic conditions. The other spontaneous abnormalities listed in Table 232-1 are seldom encountered in patients with neurosurgical conditions and are not discussed.
Motor Unit Action Potential Size and Polyphasic Nature
The MUAP consists of the sum of action potentials from all the muscle fibers of a single motor unit (Fig. 232-8C). To evaluate MUAPs, an EMG recording needle is inserted into the muscle, and the patient contracts the muscle slightly so that one or a few motor units are activated and recorded. The size of the MUAP is related to the number of muscle fibers within the recording range of the EMG needle. If the MUAP is larger than normal (increased duration or amplitude), there must be an increased number of summated muscle fiber action potentials per motor unit (Fig. 232-8D). An increased number of muscle fibers per motor unit can occur only through reinnervation, thus suggesting that the denervation took place at least 2 months ago. If the MUAP is smaller than usual (decreased duration or amplitude), there are decreased numbers of muscle fibers per motor unit, which occurs in myopathies or neuromuscular junction disorders (Fig. 232-8E).
A normal MUAP is usually triphasic (Fig. 232-8C). An MUAP with five or more phases is polyphasic and results from increased temporal dispersion of the individual muscle fiber action potentials within a motor unit because of chronic denervation with reinnervation, myopathy, or a neuromuscular junction disorder (Fig. 232-8D).
Common Clinical Disorders
Carpal Tunnel Syndrome
Three conduction studies are most commonly performed to evaluate median sensory latency across the wrist.3,4
Commonly, one or more of these studies are performed to evaluate patients referred for possible carpal tunnel syndrome. One should be aware, however, that the more studies one performs, the greater the chance of false-positive results.5,6 Recent studies have shown that performing all three studies and simply adding together the differences in latency is more sensitive, specific, and reliable than performing a single study or performing multiple studies and considering them independently.7,8 When the three latency comparisons are added, a sum (referred to as the combined sensory index) of 1 msec or greater is considered abnormal and is suggestive of carpal tunnel syndrome.
It should be noted that some improvement in latencies is usually expected after surgical release of the median nerve at the wrist. However, in many cases, latencies do not return to normal despite a good postsurgical clinical outcome.3 Thus, in patients with persistent postoperative symptoms, it is important to compare the results with preoperative conduction studies. If preoperative results are not available, postoperative testing separated by several months should be performed to see whether the latencies are getting better or worse over time.
Ulnar Neuropathy at the Elbow
Motor NCSs are often the most useful technique for localizing the site of ulnar neuropathy at the elbow and determining the pathophysiology of the lesion. Recording from the abductor digiti minimi is the most common method. Some authors, however, have found that recording from the first dorsal interosseous muscle, the most distal muscle supplied by the ulnar nerve, is more sensitive.9–11 A two-channel technique may be used to record from both muscles simultaneously so that extra stimulation is not required.
Stimulation is usually performed at the wrist, below the elbow, above the elbow, and sometimes at the axilla. Study of the across-elbow segment requires much care in technique and interpretation. The position of the elbow greatly influences the measured conduction velocity. When the elbow is extended, the ulnar nerve may become redundant in the ulnar groove, and surface measurements may not reflect the true distance of the underlying nerve. Flexing the elbow stretches the nerve to its full length, and measurement of the distance over the ulnar groove more closely reflects the distance along the nerve. Because there is room for considerable error in measurement of across-elbow conduction velocity as a result of distance measurements and elbow position, many electromyographers allow up to an 11- to 15-m/sec difference between the across-elbow and forearm segments before calling the finding “abnormal.”12 Recent studies, however, have demonstrated that it is better to compare the velocity with established reference values than to compare it with forearm velocity because the latter also slows in ulnar neuropathy.13
Slowed conduction velocity is not the only finding that should be considered diagnostic of ulnar neuropathy at the elbow. Such patients may also have a drop in amplitude in the across-elbow segment. A reduction in amplitude of more than 10% in the across-elbow segment is probably abnormal.12
It is often found that studying very short segments yields higher sensitivity for focal lesions. With short-segment studies, the area of demyelination occupies a higher percentage of the distance studied than with studies of longer segments, in which normal nerve dilutes the measurement. Inching studies (or perhaps more appropriately called “centimetering” studies) can be performed by stimulating the nerve at 2-cm increments across the elbow.14,15 With this technique, a conduction delay of more than 0.7 msec across 2-cm segments is probably abnormal.14 More impressive are focal changes in amplitude or waveform morphology across a segment.
Sensory NCSs are often of less localizing value than motor studies. Nevertheless, sensory responses are frequently helpful for measuring the degree of sensory axon loss. A drop in amplitude of the ulnar SNAP is probably one of the more sensitive indicators of ulnar neuropathy at the elbow.16
Needle EMG of the ulnar-innervated muscle is critical, both to determine whether any axon loss has occurred and to help localize lesions that may be purely axonal in nature. Thus, even if NCS results are entirely normal, when ulnar neuropathy is clinically suspected, needle EMG should still be performed. The most helpful hand muscles to assess are the abductor digiti minimi and the first dorsal interosseus, two muscles commonly involved in ulnar neuropathy at the elbow.9 Study of the flexor carpi ulnaris and the ulnar half of the flexor digitorum profundus is marginally helpful. Although the branch to these muscles usually comes off distal to most entrapment sites at the elbow, the fascicles supplying these muscles are in a relatively protected position within the nerve, so these muscles are frequently spared.
Radiculopathies
The practitioner should keep in mind the relative sensitivity and specificity of various imaging and electrodiagnostic testing. Although magnetic resonance imaging (MRI) provides a very sensitive method for assessing nerve roots in the back and neck, it is a highly nonspecific technique. Many asymptomatic people have disk bulges and disk protrusions, and their frequency increases with age. In one study, 61% of asymptomatic 40- to 49-year-olds had a disk bulge on MRI and 33% had a disk protrusion; the incidence is considerably higher in older individuals.17 Other studies have shown a specificity of only about 50%.18,19 Hence, there is about an equal chance that a disk abnormality will or will not correspond with clinical symptoms at that site. Electrophysiologic studies are somewhat less sensitive than MRI in detecting mild root compression, but their specificity is considerably higher—probably greater than 85% to 90%. It is often useful to combine the highly sensitive but nonspecific imaging modalities with the more specific electrophysiologic testing when evaluating someone with possible radiculopathy.
Needle EMG is probably the best electrophysiologic test for detecting radiculopathy.20 After the onset of radiculopathy, evidence of denervation can be seen in proximal muscles such as the paraspinal muscles in as little as 10 to 14 days. More distal muscles in the limb become abnormal later, with up to 3 to 4 weeks needed to show evidence of denervation. For the diagnosis of radiculopathy, at least two muscles in the same myotome, but supplied by different peripheral nerves, should show evidence of denervation (fibrillations, positive sharp waves). In chronic root lesions, evidence of reinnervation (long-duration, polyphasic, large-amplitude MUAPs) may be seen in a myotomal distribution; however, this is a softer finding than evidence of recent denervation.
Somatosensory evoked potentials (SEPs) should theoretically be better at detecting root abnormalities affecting sensory fibers. However, data indicate that SEPs are not as good as needle EMG in detecting isolated radiculopathy, probably because of the overlap of dermatomes, such that multiple roots are stimulated simultaneously during SEPs; normal roots can produce a normal result. In contrast, SEPs are probably better than EMG at detecting spinal stenosis in which more than one root is involved.21
Assessment of Traumatic Peripheral Nerve Injury
Nerve Conduction Studies
Electrodiagnostically, complete axonotmesis and complete neurotmesis look the same; the difference between these lesions lies in the integrity of the supporting structures, which have no electrophysiologic function. Thus, these lesions can be grouped together as axonotmesis for purposes of this discussion. Immediately after axonotmesis and for a few days thereafter, motor conduction studies look the same as those seen in a neurapraxic lesion. Nerve segments distal to the lesion remain excitable and demonstrate normal conduction, whereas proximal stimulation results in an absent or small response from distal muscles. Early on, this picture looks the same as conduction block and can be confused with neurapraxia. Hence, neurapraxia and axonotmesis cannot be distinguished until sufficient time for the occurrence of wallerian degeneration in all motor fibers has passed, typically about 9 days after injury.22
After this time, the amplitude of the motor response elicited with distal stimulation falls. This decrease in amplitude starts at about day 3 and is complete by about day 9.22 Thus, in complete axonotmesis, by day 9 the picture is very different from that of neurapraxia. Responses are absent both above and below the lesion. Lesions with partial axon loss produce small-amplitude motor responses, with the amplitude being roughly proportional to the number of surviving axons.
Needle Electromyography
After a lesion causing axon loss (axonotmesis or neurotmesis), needle EMG demonstrates fibrillation potentials and positive sharp waves a number of days after injury. The time between injury and the onset of fibrillation potentials depends in part on the length of the distal nerve stump. When the distal stump is short, it takes only 10 to 14 days for fibrillations to develop. With a longer distal stump (e.g., ulnar-innervated hand muscles in a brachial plexopathy), 21 to 30 days is required for the full development of fibrillation potentials and positive sharp waves.23
Fibrillation and positive sharp wave density are usually graded on a scale of 1 to 4. This is an ordinal scale, which means that as numbers increase, the findings are worse. However, it is not an interval or ratio scale; that is, 4+ is not twice as bad as 2+ or four times as bad as 1+. Moreover, 4+ fibrillation potentials do not indicate complete axon loss and in fact may represent only a minority of axons lost.9,24 Evaluation of recruitment and particularly of distally elicited CMAP amplitude is necessary before one can decide whether complete axon loss has occurred.
When there are surviving axons after an incomplete axonal injury, the remaining MUAPs are initially normal in morphology but demonstrate reduced or discrete recruitment. Axonal sprouting is manifested by changes in the morphology of existing motor units. Amplitude increases, duration becomes prolonged, and the percentage of polyphasic MUAPs increases as motor unit territory increases.25,26
In complete lesions, the only possible mechanism of recovery is axonal regrowth. The earliest needle EMG finding in this case is the presence of small, polyphasic, often unstable motor unit potentials previously referred to as “nascent potentials.” (This term is now discouraged because it implies a cause; it is preferable to simply describe the size, duration, and phasic nature of the MUAP.) Observation of these potentials is dependent on establishing axon regeneration, as well as new neuromuscular junctions, and such observation represents the earliest evidence of reinnervation, usually preceding the onset of clinically evident voluntary movement.26 These potentials represent the earliest definitive evidence of axonal reinnervation in complete lesions.
Localization
Another indirect inference that can be made based on sensory NCSs is localization of the lesion at a preganglionic versus postganglionic site. Lesions proximal to the dorsal root ganglion—that is, at the preganglionic level (proximal root, cauda equina, spinal cord)—tend to have normal SNAP amplitudes, even if there is reduced or absent sensation.27,28 This is a particularly bad prognostic sign when seen in the setting of possible root avulsion. Conversely, lesions occurring distal to the dorsal root ganglion have small or absent sensory responses (when recorded in the appropriate distribution).
The other major electrodiagnostic method for determining the site of nerve injury is needle EMG. Conceptually, if one knows the branching order to various muscles under study, one can determine that the nerve injury is between the branches to the most distal normal muscle and the most proximal abnormal muscle. There are, however, a number of potential limitations with this approach. First, the branching and innervation for muscles are not necessarily consistent from one person to another. Sunderland demonstrated a great deal of variability in the branching order to muscles in the limbs, in the number of branches going to each muscle, and in which nerve or nerves supply each muscle.29 Thus, the typical branching scheme may not apply to the patient being studied, and the site of the lesion can be misconstrued.
Additionally, the existence of partial lesions can lead to misdiagnosis of more distal sites. In partial ulnar nerve lesions at the elbow, for example, the forearm ulnar-innervated muscles are often spared.24 This is thought to be due at least partially to sparing of the fascicles in the nerve that are preparing to branch to the flexor digitorum profundus and flexor carpi ulnaris (i.e., they are in a relatively protected position). This finding could lead one to inadvertently localize the lesion distal to the distal part of the forearm or wrist. Intraneural topography needs to be considered when making a diagnosis based on branching.30
Prognostication of Awakening From Coma
A number of electrophysiologic methods have been studied for their ability to predict outcomes in comatose patients. Of these methods, median nerve SEPs have the strongest evidence of utility in predicting outcome after coma.31 Specifically, bilateral absence of cortical responses to median nerve stimulation is usually associated with a very poor prognosis. Pathologic studies in small numbers of patients have shown that essentially complete cortical necrosis is required to obliterate the cortical response in patients with nontraumatic encephalopathy.32
It has been shown that for adult patients with coma caused by hypoxic-ischemic encephalopathy, bilateral absence of cortical responses to median nerve stimulation confidently predicts nonawakening. Of more than 200 patients with this finding, no one has awakened, with an upper 95% confidence interval (CI) of 1%.31 Although normal SEPs in this group do not necessarily predict a good outcome, they do suggest a better outcome than average.
SEPs are also useful but not quite as predictive in adults who are comatose from traumatic causes. Bilateral absence of cortical responses in this group predicts about a 5% chance of awakening (95% CI, 2% to 8%), but the great majority who have awakened had severe disability.33
Aminoff M. Electromyography in Clinical Practice: Clinical and Electrodiagnostic Aspects of Neuromuscular Disease, 3rd ed. Philadelphia: Churchill Livingstone; 1997.
Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic Medicine, 2nd ed. Philadelphia: Hanley & Belfus; 2002.
Jablecki CK, Andary MT, Floeter MK, et al. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2002;58:1589-1592.
Kincaid J. AAEE minimonograph #31: the electrodiagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 1988;11:1005-1015.
Lew H, Wang L, Robinson L. Test-retest reliability of combined sensory index: implications for diagnosing carpal tunnel syndrome. Muscle Nerve. 2000;23:1261-1264.
Nardin R, Patel M, Gudas T, et al. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151-155.
Preston D, Shapiro B. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. Oxford: Butterworth-Heinemann; 1997.
Robinson L. Electromyography, magnetic resonance imaging, and radiculopathy: it’s time to focus on specificity. Muscle Nerve. 1999;22:149-150.
Robinson L, Micklesen P, Wang L. Strategies for analyzing nerve conduction data: superiority of a summary index over single tests. Muscle Nerve. 1998;21:1166-1171.
Shakir A, Micklesen P, Robinson LR. Which motor nerve conduction study is best in ulnar neuropathy at the elbow? Muscle Nerve. 2004;29:585-590.
Snowden ML, Haselkorn JK, Kraft GH, et al. Dermatomal somatosensory evoked potentials in the diagnosis of lumbosacral spinal stenosis: comparison with imaging studies. Muscle Nerve. 1992;15:1036-1044.
Stevens J. AAEE minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1987;10:99-113.
Wilbourn A, Aminoff M. AAEE minimonograph #32: the electrophysiologic examination in patients with radiculopathies. Muscle Nerve. 1988;11:1099-1114.
1 Aminoff M. Electromyography in Clinical Practice: Clinical and Electrodiagnostic Aspects of Neuromuscular Disease, 3rd ed. Philadelphia: Churchill Livingstone; 1997.
2 Preston D, Shapiro B. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. Oxford: Butterworth-Heinemann; 1997.
3 Stevens J. AAEE minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1987;10:99-113.
4 Jablecki C, Andary M, So Y, et al. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve. 1993;16:1392-1414.
5 Rivner H. Statistical errors and their effect on electrodiagnostic medicine. Muscle Nerve. 1994;17:811-814.
6 Robinson L, Temkin N, Fujimoto W, et al. Effect of statistical methodology on normal limits in nerve conduction studies. Muscle Nerve. 1991;14:1084-1090.
7 Robinson L, Micklesen P, Wang L. Strategies for analyzing nerve conduction data: superiority of a summary index over single tests. Muscle Nerve. 1998;21:1166-1171.
8 Lew H, Wang L, Robinson L. Test-retest reliability of combined sensory index: implications for diagnosing carpal tunnel syndrome. Muscle Nerve. 2000;23:1261-1264.
9 Jabre J, Wilbourn A. The EMG findings in 100 consecutive ulnar neuropathies. Acta Neurol Scand. 1979;60(suppl 73):91.
10 Payan J. Electrophysiological localization of ulnar nerve lesions. J Neurol Neurosurg Psychiatry. 1969;32:208-220.
11 Stewart J. The variable clinical manifestations of ulnar neuropathies at the elbow. J Neurol Neurosurg Psychiatry. 1987;50:252258.
12 Kincaid J. AAEE minimonograph #31: the electrodiagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 1988;11:1005-1015.
13 Shakir A, Micklesen P, Robinson LR. Which motor nerve conduction study is best in ulnar neuropathy at the elbow? Muscle Nerve. 2004;29:585-590.
14 Kanakamamedala R, Simons D, Porter R, et al. Ulnar nerve entrapment at the elbow localized by short segment stimulation. Arch Phys Med Rehabil. 1988;69:959-963.
15 Miller R. The cubital tunnel syndrome: diagnosis and precise localization. Ann Neurol. 1979;6:56-59.
16 Eisen A. Early diagnosis of ulnar nerve palsy: an electrophysiologic study. Neurology. 1974;24:256-262.
17 Jensen M, Brant-Zawadzki M, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
18 Nardin R, Patel M, Gudas T, et al. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151-155.
19 Robinson L. Electromyography, magnetic resonance imaging, and radiculopathy: it’s time to focus on specificity. Muscle Nerve. 1999;22:149-150.
20 Wilbourn A, Aminoff M. AAEE minimonograph #32: the electrophysiologic examination in patients with radiculopathies. Muscle Nerve. 1988;11:1099-1114.
21 Snowden ML, Haselkorn JK, Kraft GH, et al. Dermatomal somatosensory evoked potentials in the diagnosis of lumbosacral spinal stenosis: comparison with imaging studies. Muscle Nerve. 1992;15:1036-1044.
22 Chaudry V, Cornblath D. Wallerian degeneration in human nerves: serial electrophysiological studies. Muscle Nerve. 1992;15:687-693.
23 Thesleff S. Physiological effects of denervation of muscle. Ann N Y Acad Sci. 1974;228:89-103.
24 Campbell W, Pridgeon R, Riaz G, et al. Sparing of the flexor carpi ulnaris in ulnar neuropathy at the elbow. Muscle Nerve. 1989;12:965-967.
25 Buchthal F. Fibrillations: clinical electrophysiology. In: Culp WJ, Ochoa J, editors. Abnormal Nerves and Muscle Generators. New York: Oxford University Press; 1982:632-662.
26 Dorfman L. Quantitative clinical electrophysiology in the evaluation of nerve injury and regeneration. Muscle Nerve. 1990;13:822-828.
27 Brandstater M, Fullerton M. Sensory nerve conduction studies in cervical root lesions. Can J Neurol Sci. 1983;10:152.
28 Tackman W, Radu E. Observations of the application of electrophysiological methods in the diagnosis of cervical root compressions. Eur Neurol. 1983;22:397-404.
29 Sunderland S. Nerves and Nerve Injuries, 2nd ed. New York: Churchill Livingstone; 1978.
30 Wertsch J, Oswald T, Roberts M. Role of intraneural topography in diagnosis and localization in electrodiagnostic medicine. Phys Med Rehabil Clin N Am. 1994;5:465-475.
31 Zandbergen E, deHaan R, Stoutenbeek C, et al. Systematic review of early prediction of poor outcome in anoxic ischemic coma. Lancet. 1998;352:1808-1812.
32 Rothstein T, Thomas E, Sumi S. Predicting outcome in hypoxicischemic coma: a prospective clinical and electrophysiologic study. Electroencephalogr Clin Neurophysiol. 1991;79:101-107.
33 Robinson LR, Micklesen PJ, Tirschwell DL, et al. Predictive value of somatosensory evoked potentials for awakening from coma. Crit Care Med. 2003;31:960-969.