CHAPTER 8 Electrodiagnostic Approach to Patients with Suspected Radiculopathy
SPINE AND NERVE ROOT ANATOMY: DEVIATIONS FROM THE EXPECTED
Spinal anatomy is discussed in detail in Chapters 46 and 80 by Russell Gilchrist and will not be emphasized here. From an electrodiagnostic perspective, however, there are several specific anatomical issues that merit further discussion.
Regarding the cervical nerve roots and the brachial plexus, there are many anatomic variations. Perneczky1 described an anatomic study of 40 cadavers. In all cases, there were deviations from accepted cervical root and brachial plexus anatomy. Levin, Maggiano, and Wilbourn2 examined the pattern of abnormalities on electromyography (EMG) in 50 cases of surgically proven cervical root lesions. A range of needle EMG patterns was found with EMG demonstrating less specificity for the C6 root level, but more specificity and consistent patterns for C8, C7, and C5 radiculopathies. In subjects with C6 radiculopathies, half the patients showed findings similar to those with C5 radiculopathies and the other half demonstrated C7 patterns. This surgical group was more severely affected than patients who do not require surgical interventions, and this pattern may not hold for less symptomatic patients.
In a prospective study of 100 patients with lumbosacral radiculopathy who underwent lumbar laminectomy, EMG precisely identified the involved root level 84% of the time.3 Needle EMG failed to accurately identify the compressed root in 16%. However, at least half of the failures were attributable to anomalies of innervation. Another component to this study involved stimulating the nerve roots intraoperatively with simultaneous recording of muscle activity in the lower limb using surface electrodes. These investigators demonstrated variations in root innervation, such as the L5 root innervating the soleus and medial gastrocnemius, in 16% of a sample of 50 patients. Most subjects demonstrated dual innervation for most muscles.3
PHYSICAL EXAMINATION
The electrodiagnostic examination is an extension of the standard clinical examination. The history and physical examination are vital initial steps in determining what conditions may be causing the presenting symptoms. Most radiculopathies present with symptoms in one limb. Multiple radiculopathies such as are seen in cervical spinal stenosis or lumbar stenosis may cause symptoms in more than one limb. A focused neuromuscular examination that assesses strength, reflexes, and sensation in the affected limb and the contralateral limb is important, providing a framework for electrodiagnostic assessment.
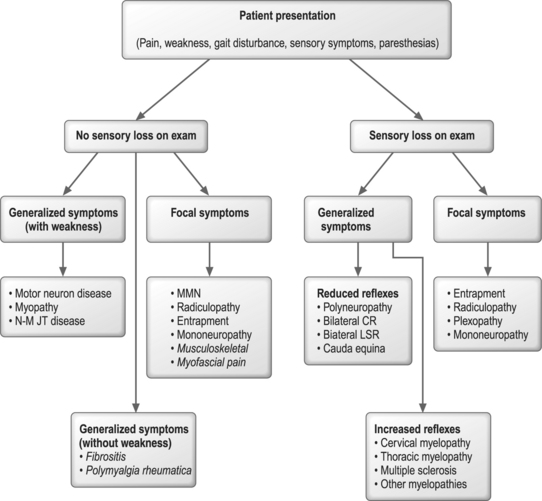
An algorithmic approach to utilizing physical examination and symptom information to tailor the electrodiagnostic evaluation is shown in Figure 8.1. In this approach, symptoms and physical examination signs create a conceptual framework for approaching these sometimes daunting problems. Admittedly, there are many exceptions to this approach with considerable overlap in medical disorders which might fall within multiple categories. Radiculopathies and entrapment neuropathies are examples of such conditions with a variety of clinical presentations and physical examination findings, such that they are included in both focal symptom categories with and without sensory loss. In the case of a person with lumbosacral radiculopathy, a positive straight leg raise test may be noted in the absence of motor, reflex, or sensory changes. Conditions such as myopathies and polyneuropathies better fit this algorithmic approach, given that symptoms and physical examination signs are more specific. Figure 8.1 also contains musculoskeletal disorders and denotes how they fall into this conceptual framework. The electrodiagnostician must be willing to modify the electrodiagnostic examination in response to nerve conduction and EMG findings and adjust the focus of the examination in light of new information.
The implications of symptoms and signs on electrodiagnostic findings were investigated by Lauder and colleagues for suspected cervical and lumbosacral radiculopathies.4,5 Even though physical examination findings were better at predicting who would have a radiculopathy, many patients with normal examinations had abnormal electrodiagnostic studies, indicating that clinicians should not curtail electrodiagnostic testing simply because the physical examination is normal. For lower limb symptoms, loss of a reflex or weakness dramatically increased the likelihood of having a radiculopathy by EMG. Losing the Achilles reflex, for instance, resulted in an odds ratio of 8.4 (p<0.01), in other words eight times the likelihood of having a radiculopathy by EMG with this physical examination finding compared to someone without loss of this reflex.4 Similar findings were noted for upper limb symptoms; if a reflex was lost or weakness was noted the likelihood of having a cervical radiculopathy confirmed by EMG was many times greater.5 Combinations of findings, particularly weakness plus sensory loss or plus reflex changes, resulted in a ninefold greater likelihood of cervical radiculopathy and two to three times greater likelihood of lumbosacral radiculopathy.4,5
The American Association of Neuromuscular and Electrodiagnostic Medicine Guidelines for Radiculopathy Evaluation
The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) guidelines recommend that for an optimal evaluation of a patient with suspected radiculopathy, a needle EMG screen of a sufficient number of muscles and at least one motor and one sensory nerve conduction study should be performed in the involved limb.6 The nerve conduction studies are necessary to exclude polyneuropathy. The sufficiency of the EMG screen and a recommended number of muscles is discussed in detail below. An EMG study is considered diagnostic for a radiculopathy if EMG abnormalities are found in two or more muscles innervated by the same nerve root, and different peripheral nerves, yet muscles innervated by adjacent nerve roots are normal.7 This assumes, of course, that other generalized conditions such as polyneuropathy are not present.
H-REFLEXES, F-WAVES, AND NERVE CONDUCTION
H-reflexes
H-reflexes have commonly been used to determine whether a radiculopathy demonstrates S1 involvement.7 It is a monosynaptic reflex that is an S1-mediated response and can differentiate to some extent L5 from S1 radiculopathy. Many researchers have evaluated their sensitivity and specificity with respect to lumbosacral radiculopathies and generally found a range of sensitivities from 32% to 88%.7–12 However, many of these studies suffered from lack of a control group, imprecise inclusion criteria, or small sample sizes.
Marin et al.12 prospectively examined the H-reflex and the extensor digitorum brevis reflex in 53 normals, 17 patients with L5, and 18 patients with S1 radiculopathy. Patients included in the study had all of the following: (1) radiating low back pain into the leg, (2) reduced sensation or weakness or positive straight leg raise test, and (3) either EMG evidence of radiculopathy or structural causes of radiculopathy on magnetic resonance imaging (MRI) or computed tomography (CT) imaging. The maximal (2 SD) value for the H-reflex side-to-side latency difference was 1.8 ms as derived from the normal group. They analyzed the sensitivity of the H-reflex for side-to-side differences greater than 1.8 ms or a unilaterally absent H-reflex on the affected side. The H-reflex only demonstrated a 50% sensitivity for S1 radiculopathy, 6% for L5 radiculopathy, but had a 91% specificity. Amplitudes were not assessed in this study. These results suggest that the H-reflex has a low sensitivity for S1 root level involvement.
H-reflexes may be useful to identify subtle S1 radiculopathy, yet there are a number of shortcomings related to these responses. They can be normal with radiculopathies12 and, because they are mediated over such a long physiological pathway, they can be abnormal due to polyneuropathy, sciatic neuropathy, or plexopathy.7 They are most useful in the assessment for polyneuropathy.
In order to interpret a latency or amplitude value and render a judgment as to the probability that it is abnormal, precise population-based normative values encompassing a large age range of normal subjects must be available for comparison of these nerve conduction findings. Falco et al.13 demonstrated in a group of healthy elderly subjects (60–88 years old) that the tibial H-reflex was present and recorded bilaterally in 92%. Most elderly are expected to have normal H-reflex studies and, when abnormalities are found in these persons, the electrodiagnostician should critically evaluate these findings and the clinical scenario before attributing H-reflex abnormalities to the aging process.
In patients with upper limb symptoms suggestive of cervical radiculopathy, H-reflexes and F-waves are not useful in diagnosis but rather help exclude polyneuropathy as an underlying cause of symptoms. One study by Miller and colleagues14 examined the H-reflexes in the upper limb in a set of patients defined by a combination of clinical criteria (no imaging or EMG studies) as having definite or probable cervical radiculopathy. They tested the H-reflex for the FCR, the ECR, the APB, and the biceps heteronymous reflex. The later reflex is derived by stimulating the median nerve in the cubital fossa and recording over the biceps brachii muscle, averaging 40–100 trials. These reflex studies had a 72% sensitivity overall for the group with 100% for the subset of patients with definite cervical radiculopathy. In contrast, needle EMG demonstrated 90% sensitivity for the definite group. Although these findings suggest a possible role for these upper limb H-reflexes, they are highly specialized, time consuming, and difficult to consistently elicit. They may have a role in sensory radiculopathies where needle EMG will not be positive and imaging findings are equivocal. Further studies are necessary to clarify whether the findings of Miller et al.14 can be duplicated at other centers.
F-waves
F-waves are late responses involving the motor axons and axonal pool at the spinal cord level. They can be assessed and classified by using the minimal latency, mean latency, and chronodispersion or scatter.7 As in the case of H-reflexes, they demonstrate low sensitivities and are not specific for radiculopathy; rather, they are a better screen for polyneuropathy. Published sensitivities range from 13% to 69%; however, these studies suffer from many of the same shortcomings that are found in the H-reflex studies.8,15,16
London and England17 reported two cases of persons with neurogenic claudication from lumbosacral spinal stenosis. They demonstrated that the F-wave responses could be reversibly changed after 15 minutes of ambulation, which provoked symptoms. This suggested an ischemia-induced conduction block in proximal motor neurons. A larger-scale study of this type might find a use for F-waves in the identification of lumbosacral spinal stenosis and assist with the delineation of neurogenic from vascular claudication.
Motor and sensory nerve conduction studies
Plexopathies often pose a diagnostic challenge as they are similar to radiculopathies in symptoms and signs. In order to distinguish plexopathy from radiculopathy, sensory responses which are accessible in a limb should be tested. In plexopathy, they are likely to be reduced in amplitude, whereas in radiculopathy they are generally normal. If substantial axonal loss has occurred at the root level, the compound muscle action potential recorded in muscles innervated by that root may be reduced in both plexopathies and radiculopathies. This is usually when severe axonal loss has occurred such as with cauda equina lesions or penetrating trauma that severely injures a nerve root. The distal motor latencies and conduction velocities are usually preserved as they reflect the fastest conducting nerve fibers.7
SOMATOSENSORY EVOKED POTENTIALS, DERMATOMAL SOMATOSENSORY EVOKED POTENTIALS, AND MAGNETIC EVOKED POTENTIALS
The AANEM guidelines recently examined the literature and concluded that somatosensory evoked potentials (SEPs) may be useful for cervical spondylosis with cord compression. Likewise, in lumbosacral spinal stenosis, dermatomal somatosensory evoked potentials (DSEPs) may be useful in defining levels of deficits.6 These tests are not necessary for electrodiagnostic testing for persons with suspected radiculopathies and their usefulness is limited to special circumstances. These tests are not recommended for the routine evaluation of persons with suspected radiculopathy.
DSEPs can document physiological evidence of multiple or single root involvement in lumbosacral spinal stenosis and may be useful in the case where spinal canal narrowing is minimal and the patient has symptoms. This testing also complements standard needle EMG. Snowden et al.18 found that for single and multilevel lumbosacral spinal stenosis, DSEPs revealed 78% sensitivity relative to spinal imaging. In this well-designed prospective study, DSEP criteria as well as inclusion criteria were precisely defined. The predictive value for a positive test was 93%.
Yiannikas, Shahani, and Young19 demonstrated that SEPs may be useful for cervical myelopathy. In this study of 10 patients with clinical signs of myelopathy, all 10 had abnormal peroneal SEPs and seven had abnormal median SEPs.
Maertens de Noordhout et al.20 examined motor and SEPs in 55 persons with unequivocal signs and symptoms of cervical spinal myelopathy. In this group 87% showed gait disturbances, and 82% showed hyperreflexia. MRI was not the diagnostic standard as these authors felt that MRI was prone to overdiagnosis; rather, metrizamide myelography showed unequivocal signs of cervical cord compression for all of these patients. Magnetic stimulation of the cortex was performed and the responses measured with surface electrodes. In these subjects 89% demonstrated abnormalities in magnetic evoked potential (MEP) to the first dorsal interosseus muscle and 93% had one MEP abnormality. At least one SEP abnormality was noted in 73%.
Tavy et al.21 examined whether MEPs or SEPs assisted in identifying persons with radiological evidence of cervical cord compression but who were without clinical markers for myelopathy. All patients had clinical symptoms of cervical radiculopathy, but not myelopathy. In this group, MEPs were normal in 92% and SEPs were normal in 96%. These investigators concluded that MEPs and SEPs are normal in most cases of persons with asymptomatic cervical stenosis. This indicates that abnormal MEPs and SEPs are likely to be true-positive findings and not false positives related to mild asymptomatic cord compression. It is important to remember that cervical spondylosis is a process that causes a continuum of problems including both radiculopathy and myelopathy.
The inherent variability and difficulty in determinations of what constitutes normal SEPs prompted investigation. Dumitru and colleagues22 examined the variations in latencies with SEPs. In 29 normal subjects, they examined the ipsilateral intertrial variations, arithmetic mean side-to-side differences, and maximum potential side-to-side differences with stimulation of the superficial peroneal sensory nerve, sural nerve, and L5 and S1 dermatomes with respect to P1 and N1 latencies and peak-to-peak amplitudes. Considerable ipsilateral intertrial variation was observed and side-to-side comparisons revealed a further increase in this inherent variation regarding the above measured parameters. They suggested an additional parameter with which to evaluate SEPs: the maximum side-to-side latency difference.
Dumitru and colleagues,23 in a study involving persons with unilateral and unilevel L5 and S1 radiculopathies, evaluated DSEPs and segmental SEPs. History, physical examination, imaging studies, and electrodiagnostic medicine evaluations clearly defined patients with unilateral/unilevel L5 or S1 nerve root compromise. Regression equation analysis for cortical P1 latencies evaluating age and height based on comparable patient and control reference populations revealed segmental and dermatomal sensitivities for L5 radiculopathies to be 70% and 50%, respectively, at 90% confidence intervals. Similar sensitivities were obtained for 2 standard deviation mean cortical P1 latencies. Side-to-side cortical P1 latency difference data revealed segmental and dermatomal sensitivities for S1 radiculopathies to be 50% and 10%, respectively, at 2 standard deviations. These investigators questioned the clinical utility of both segmental and dermatomal SEPs in the evaluation of patients with suspected unilateral/unilevel L5 and S1 nerve root compromise, finding little utility for these tests in persons with single-level lumbosacral radiculopathy.
PURPOSE OF ELECTRODIAGNOSTIC TESTING
ELECTROMYOGRAPHY AND DIAGNOSTIC SENSITIVITIES
The need for EMG, particularly in relationship to imaging of the spine, has been recently highlighted.25 Needle EMG is particularly helpful in view of the fact that the false-positive rates for MRI of the lumbar spine are high, with 27% of normals having a disc protrusion.26 For the cervical spine, the false-positive rate for MRI is much lower, with 19% of subjects demonstrating an abnormality, but only 10% showing a herniated or bulging disc.27 Radiculopathies can occur without structural findings on MRI, and likewise without EMG findings. The EMG only evaluates motor axonal loss or motor axon conduction block and for these reasons a radiculopathy effecting the sensory root will not yield abnormalities by EMG. If the rate of denervation is balanced by reinnervation in the muscle, then spontaneous activity is less likely to occur.
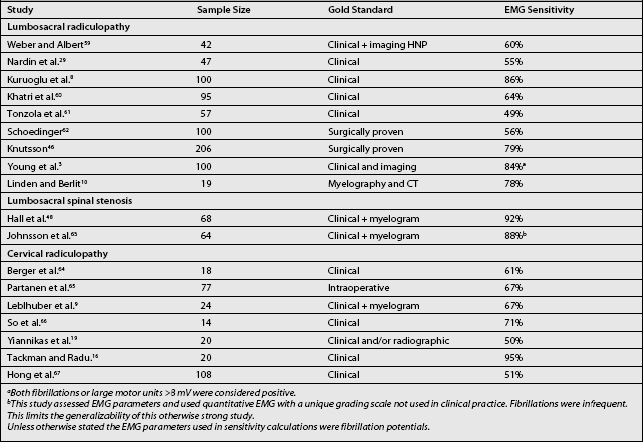
The sensitivity of EMG for cervical and lumbosacral radiculopathies has been examined in a number of studies. The results of some of these studies are displayed in Table 8.1, which lists the ‘gold standards’ against which these EMG findings were compared. Studies using a clinical standard may reflect a less severe group, whereas those using a surgical confirmation may indicate a more severely involved group. The sensitivity for EMG is unimpressive, ranging from 49% to 92% in these studies. Electromyography is not a sensitive test, yet likely has higher specificity. The issue of specificity and its value in electrodiagnosis was underscored by Robinson.25 It is apparent that EMG is not a very good screening test. In terms of screening tests, MRI is better for identifying subtle structural abnormalities, with EMG to assess their clinical relevance and to exclude other disorders.
PARASPINAL MUSCLE EXAMINATION
Several reports suggested high rates of false-positive fibrillations in lumbar paraspinal muscles.28,29 Dumitru, Diaz, and King30 conducted a well-designed study to examine whether or not fibrillations are found in the lumbar paraspinal muscles of normal asymptomatic volunteers. These investigators examined lumbosacral paraspinal muscles and intrinsic foot muscles with monopolar EMG and recorded potentials for analysis. Regular firing rate was required in order for classification as fibrillation potentials. They found many irregularly firing potentials with similar waveform characteristics as fibrillations and positive sharp waves (PSW). By excluding these irregularly firing potentials (atypical endplate spikes) they found much lower false-positive paraspinal fibrillation prevalences than other investigators. Only 4% of these normal subjects had lumbar fibrillations or PSW potentials by EMG testing. The investigators felt that the higher prevalences of spontaneous activity reported by other investigators28,29 were due to not fully appreciating the similarity between innervated and denervated spontaneous single muscle fiber discharges. This well-designed quantitative study underscores the need to assess both firing rate and rhythm as well as discharge morphology when evaluating for fibrillations and positive waves in the lumbar paraspinal muscles. Electrodiagnosticians should take care not to overcall fibrillations in lumbosacral paraspinal muscles by mistaking irregularly firing endplate spikes for fibrillations.
Paraspinal muscles may be abnormal in patients with spinal cancers31–33 or amyotrophic lateral sclerosis,34 and following spinal surgery35 or lumbar puncture.36 In fact, fibrillations can be found years after lumbar laminectomy.35 The absence of paraspinal muscle fibrillations in such patients is helpful, but finding fibrillations in someone after laminectomy is of uncertain relevance as these fibrillations may be residual from the previous muscle damage or relatively new denervation.
Investigations over the last decade have provided insights into better quantification and examination of lumbosacral paraspinal muscles. The lumbar paraspinal muscle examination has been refined through investigations that used a grading scale for the findings.37–40 The ‘mini PM’ score provides a quantitative means of deriving the degree of paraspinal muscle denervation.40 It distinguishes normal findings from persons with radiculopathy. This novel and quantitative technique may prove useful to identify subtle radiculopathies or spinal stenosis with greater precision.
HOW MANY AND WHICH MUSCLES TO STUDY
The cervical radiculopathy screen
Dillingham et al.41 conducted a prospective multicenter study evaluating patients referred to participating electrodiagnostic laboratories with suspected cervical radiculopathy. A standard set of muscles were examined by needle EMG for all patients. Those with electrodiagnostically confirmed cervical radiculopathies, based upon EMG findings, were selected for analysis. The EMG findings in this prospective study also encompassed other neuropathic findings: (1) positive sharp waves, (2) fibrillation potentials, (3)complex repetitive discharges (CRD), (4) high-amplitude, long-duration motor unit action potentials, (5) increased polyphasic motor unit action potentials, or (6) reduced recruitment. There were 101 patients with electrodiagnostically confirmed cervical radiculopathies representing all cervical root levels. When paraspinal muscles were one of the screening muscles, five-muscle screens identified 90–98% of radiculopathies, six-muscle screens identified 94–99%, and seven-muscle screens identified 96–100% (Tables 8.2 and 8.3). When paraspinal muscles were not part of the screen, eight distal limb muscles recognized 92–95% of radiculopathies. Six-muscle screens, including paraspinal muscles, yielded consistently high identification rates, and studying additional muscles lead to only marginal increases in identification. Individual screens useful to the electromyographer are listed in Tables 8.2 and 8.3. In some instances a particular muscle cannot be studied due to wounds, skin grafts, dressings, or infections. In such cases the electromyographer can use an alternative screen with equally high identification. These findings were consistent with those derived from a large retrospective study.42
Table 8.2 Five-muscle screen identifications of patients with cervical radiculopathies
| Muscle screen | Neuropathic | Spontaneous activity |
|---|---|---|
| Without paraspinals | ||
| Deltoid, APB, FCU | 92% | 65% |
| Triceps, PT | ||
| Biceps, triceps | 85% | 54% |
| EDC, FCR, FDI | ||
| Deltoid, triceps | 84% | 58% |
| EDC, FDI, FCR | ||
| Biceps, triceps | 91% | 60% |
| PT, APB, FCU | ||
| With paraspinals | ||
| Deltoid, triceps, PT | 98% | 80% |
| APB, PSM | ||
| Biceps, triceps, EDC | 95% | 73% |
| FDI, PSM | ||
| Deltoid, EDC, FDI | 90% | 73% |
| PSM, FCU | ||
| Biceps, FCR, APB | 95% | 77% |
| PT, PSM | ||
The screen detected the patient with cervical radiculopathy if any muscle in the screen was one of the muscles which were abnormal for that patient.
Neuropathic findings for nonparaspinal muscles included positive waves, fibrillations, increased polyphasic potentials, neuropathic recruitment, increased insertional activity, CRDs, or large amplitude/long duration motor unit action potentials.
For paraspinal muscles the neuropathic category included fibrillations, increased insertional activity, positive waves, or CRDs. Spontaneous activity referred only to fibrillations or positive sharp waves.
APB, abductor pollicis brevis; FCU, flexor carpi ulnaris; FCR, flexor carpi radialis; PSM, cervical paraspinal muscles; FDI, first dorsal interosseous; PT, pronator teres, supra-supraspinatus, infra-infraspinatus; EDC, extensor digitorum communis.
Adapted with permission, Dillingham et al.41
Table 8.3 Six-muscle screen identifications of the patients with cervical radiculopathies
| Muscle Screen | Neuropathic | Spontaneous Activity |
|---|---|---|
| Without paraspinals | ||
| Deltoid, APB, FCU | 93% | 66% |
| Triceps, PT, FCR | ||
| Biceps, triceps, FCU | 87% | 55% |
| EDC, FCR, FDI | ||
| Deltoid, triceps | 89% | 64% |
| EDC, FDI, FCR, PT | ||
| Biceps, triceps, EDC | 94% | 64% |
| PT, APB, FCU | ||
| With paraspinals | ||
| Deltoid, triceps, PT | 99% | 83% |
| APB, EDC, PSM | ||
| Biceps, triceps, EDC | 96% | 75% |
| FDI, FCU, PSM | ||
| Deltoid, EDC, FDI | 94% | 77% |
| PSM, FCU, triceps | ||
| Biceps, FCR, APB | 98% | 79% |
| PT, PSM, triceps | ||
Muscle abbreviations, identification criteria, and definitions are described in Table 8.2.
The lumbosacral radiculopathy screen
A similar prospective multicenter study was conducted at five institutions by Dillingham et al.43 Patients referred to participating electrodiagnostic laboratories with suspected lumbosacral radiculopathy were recruited and a standard set of muscles examined by needle EMG. Patients with electrodiagnostically confirmed lumbosacral radiculopathies, based upon EMG findings, were selected for analysis. As described above for the prospective cervical study, neuropathic findings were analyzed along with spontaneous activity. There were 102 patients with lumbosacral radiculopathies representing all lumbosacral root levels. When paraspinal muscles were one of the screening muscles, four-muscle screens identified 88–97%, five-muscle screens identified 94–98%, and six-muscle screens 98–100% (Tables 8.4–8.6). When paraspinal muscles were not part of the screen, identification rates were lower for all screens and eight distal muscles were necessary to identify 90%. If only four muscles can be tested due to limited patient tolerance, as seen in Table 8.4, and if one of these muscles are the paraspinals, few electrodiagnostically confirmable radiculopathies will be missed. A large retrospective study noted consistent findings, concluding that five muscles identified most electrodiagnostically confirmable radiculopathies.44
Table 8.4 Four-muscle screen identifications of patients with lumbosacral radiculopathies
| Screen | Neuropathic | Spontaneous Activity |
|---|---|---|
| Four muscles without paraspinals | ||
| ATIB, PTIB, MGAS, RFEM | 85% | 75% |
| VMED, TFL, LGAS, PTIB | 75% | 58% |
| VLAT, SHBF, LGAS, ADD | 52% | 35% |
| ADD, TFL, MGAS, PTIB | 80% | 67% |
| Four muscles with paraspinals | ||
| ATIB, PTIB, MGAS, PSM | 97% | 90% |
| VMED, LGAS, PTIB, PSM | 91% | 81% |
| VLAT, TFL, LGAS, PSM | 88% | 77% |
| ADD, MGAS, PTIB, PSM | 94% | 86% |
The screen identified the patient if any muscle in the screen was abnormal for that patient. The muscle either demonstrated neuropathic findings or spontaneous activity.
Neuropathic findings for nonparaspinal muscles included positive waves, fibrillations, increased polyphasic potentials, neuropathic recruitment, increased insertional activity, CRDs, or large amplitude long duration motor unit action potentials. Spontaneous activity referred only to fibrillations or positive sharp waves.
For paraspinal muscles the neuropathic category included fibrillations, increased insertional activity, positive waves, or CRDs.
PSM, lumbosacral paraspinal muscles; PTIB, posterior tibialis; ATIB, anterior tibialis; MGAS, medial gastrocnemius, LGAS, lateral gastrocnemius, TFL, tensor fascia lata, SHBF, short head biceps femoris; VMED, vastus medialis; VLAT, vastus lateralis; RFEM, rectus femoris; ADD, adductor longus.
Adapted from Dillingham, et al.43, with permission.
Table 8.5 Five-muscle screen identifications of patients with lumbosacral radiculopathies
| Screen | Neuropathic | Spontaneous activity |
|---|---|---|
| Five muscles without paraspinals | ||
| ATIB, PTIB, MGAS, RFEM, SHBF | 88% | 77% |
| VMED, TFL, LGAS, PTIB, ADD | 76% | 59% |
| VLAT, SHBF, LGAS, ADD, TFL | 68% | 50% |
| ADD, TFL, MGAS, PTIB, ATIB | 86% | 78% |
| Five muscles with paraspinals | ||
| ATIB, PTIB, MGAS, PSM, VMED | 98% | 91% |
| VMED, LGAS, PTIB, PSM, SHBF | 97% | 84% |
| VLAT, TFL, LGAS, PSM, ATIB | 97% | 86% |
| ADD, MGAS, PTIB, PSM, VLAT | 94% | 86% |
Abbreviations and definitions of muscle abnormalities are the same as in Table 8.4.
Table 8.6 Six-muscle screen identifications of patients with lumbosacral radiculopathies
| Screen | Neuropathic | Spontaneous Activity |
|---|---|---|
| Six muscles without paraspinals | ||
| ATIB, PTIB, MGAS, RFEM, SHBF, LGAS | 89% | 78% |
| VMED, TFL, LGAS, PTIB, ADD, MGAS | 83% | 70% |
| VLAT, SHBF, LGAS, ADD, TFL, PTIB | 79% | 62% |
| ADD, TFL, MGAS, PTIB, ATIB, LGAS | 88% | 79% |
| Six muscles with paraspinals | ||
| ATIB, PTIB, MGAS, PSM, VMED, TFL | 99% | 93% |
| VMED, LGAS, PTIB, PSM, SHBF, MGAS | 99% | 87% |
| VLAT, TFL, LGAS, PSM, ATIB, SHBF | 98% | 87% |
| ADD, MGAS, PTIB, PSM, VLAT, SHBF | 99% | 89% |
| VMED, ATIB, PTIB, PSM, SHBF, MGAS | 100% | 92% |
| VMED, TFL, LGAS, PSM, ATIB, PTIB | 99% | 91% |
| ADD, MGAS, PTIB, PSM, ATIB, SHBF | ||
Abbreviations and definitions of muscle abnormalities are the same as in Table 8.4.
Dillingham and Dasher45 re-analyzed data from a study published by Knutsson almost 40 years earlier.46 In this detailed study, 206 patients with sciatica underwent lumbar surgical exploration. All subjects underwent standard EMG by the author (Knutsson) with a standard set of 14 muscles using concentric needles. The examiner was blinded to other test results and physical examination findings. In addition to the EMG and surgical information, myelogram and physical examination data were derived. In this contemporary re-analysis, screens of four muscles with one being the PSM yielded an identification rate of 100%, a 92% sensitivity with respect to the intraoperative anatomical nerve root compressions, and an 89% sensitivity with respect to the clinical inclusion criteria.45 This study, using data from four decades ago, confirmed that four-muscle screening examinations provide high identification. These findings are consistent with contemporary work showing that screens with relatively few muscles (six) are optimal.
As described above, these research efforts were undertaken to refine and streamline the EMG examination. The strongest studies, contemporary prospective multicenter investigations, provide the best estimates for a sufficient number of muscles.41,43 In summary, for both cervical and lumbosacral radiculopathy screens the optimal number of muscles appears to be six muscles, including the paraspinal muscles and muscles that represent all root level innervations. When paraspinal muscles are not reliable, then eight nonparaspinal muscles must be examined. Another way to think of this:
LUMBAR SPINAL STENOSIS
With the aging population in the United States and the increasing prevalence of lumbar spinal stenosis that occurs in the elderly, this condition takes on greater public health significance. In fact, an entire edition of the Physical Medicine and Rehabilitation Clinics of North America was recently dedicated to this complex topic.47 There are few studies involving spinal stenosis and electromyography. For lumbosacral spinal stenosis, Hall and colleagues48 showed that 92% of persons with imaging-confirmed stenosis had a positive EMG. They also underscored the fact that 46% of persons with a positive EMG study did not demonstrate paraspinal muscle abnormalities, only distal muscle findings. In 76%, the EMG showed bilateral myotomal involvement.48 These results suggest that in such patients, distal limb findings may be the most prominent and electromyographers should not expect fibrillations in lumbosacral paraspinal muscles.
In the United States, diabetes is on the increase, with increasing prevalence and incidence.49 Diabetes often confounds accurate diagnosis of radiculopathy and spinal stenosis.50,51 Inaccurate recognition of sensory polyneuropathy, diabetic amyotrophy, or mononeuropathy can lead to unnecessary surgical interventions. In a recent prospective study by Adamova and colleagues,50 the value of electrodiagnostic testing was assessed. There were three groups; one group composed of 29 persons with imaging confirmed clinically mild lumbar spinal stenosis, 24 subjects with both diabetes and polyneuropathy, and 25 healthy age-matched volunteers served as control subjects. In this well-designed study, sural sensory amplitudes distinguished the diabetic polyneuropathy group (an amplitude of 4.2 microvolts or less was found in 47% of diabetic patients and only 17% of stenosis patients). The ulnar F-wave was prolonged in polyneuropathy patients and not lumbar stenosis patients and the radial SNAP was similarly reduced in the group with polyneuropathy.50 These findings underscore the value of performing sensory testing in the involved extremity as well as an upper limb to fully recognize diabetic polyneuropathy when present and differentiate this condition from lumbar spinal stenosis or radiculopathy.
LIMITATIONS OF THE EMG SCREEN
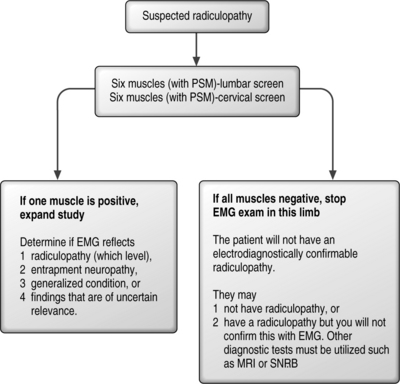
If one of the six muscles studied in the screen is positive, there is the possibility of confirming electrodiagnostically that a radiculopathy is present. In this case, the examiner must study additional muscles to determine the radiculopathy level and to exclude a mononeuropathy. If the findings are found in only a single muscle, they remain inconclusive and of uncertain clinical relevance. If none of the six muscles are abnormal, the examiner can be confident of not missing the opportunity to confirm by EMG that a radiculopathy is present, and can curtail the painful needle examination. The patient may still have a radiculopathy, but other tests such as MRI will be necessary to confirm this clinical suspicion. This logic is illustrated in Figure 8.2.
SYMPTOM DURATION AND THE PROBABILITY OF FIBRILLATIONS
In the past, a well-defined temporal course of events was thought to occur with radiculopathies despite the absence of studies supporting such a relationship. It was a commonly held notion that in acute lumbosacral radiculopathies, the paraspinal muscles denervated first, followed by distal muscles, and that reinnervation started with paraspinal muscles and then the distal muscles. This paradigm was recently addressed with a series of investigations.52–55 For both lumbosacral and cervical radiculopathies, symptom duration had no significant relationship to the probability of finding spontaneous activity in paraspinal or limb muscles.
IMPLICATIONS OF AN ELECTRODIAGNOSTICALLY CONFIRMED RADICULOPATHY
There are few studies that examine outcomes and the usefulness of electrodiagnosis in predicting treatment success, the exception being surgical outcomes for lumbar discectomy in patients with herniated nucleus pulposus. Tullberg et al.56 evaluated 20 patients with lumbosacral radicular syndromes who underwent unilevel surgery for disc herniations. They evaluated these patients before surgery and 1 year later with lower limb EMG, nerve conduction studies, F-waves, and SEPs. They showed that the electrodiagnostic findings did not correlate with the level defined by CT for 15 patients. However, those patients in whom electrodiagnostic testing preoperatively was normal were significantly more likely to have a poor surgical outcome (p<0.01). In spite of the fact that the sample size in this study was small, the significant correlation of a normal electrodiagnostic study with poor surgical outcome suggests that this may be a true relationship.
Spengler and Freeman57 described an objective approach to the assessment of patients preoperatively for laminectomy and discectomy for lumbosacral radiculopathy. Spengler et al.58 confirmed and underscored these previous findings regarding objective methods to assess the probability of surgical success preoperatively. In this preoperative screening evaluation, the EMG findings were combined with imaging, clinical, and psychological assessments. The EMG findings figured prominently (one-quarter of the scale): those patients with positive EMGs were more likely to have a better surgical outcome. This was particularly true when the EMG findings correlated with the spinal imaging findings, in a person without psychological or dysfunctional personality issues.
1 Perneczky A, Sunder-Plassmann M. Intradural variant of cervical nerve root fibres. Potential cause of misinterpreting the segmental location of cervical disc prolpases from clinical evidence. Acta Neurochir (Wien). 1980;52(1–2):79-83.
2 Levin KH, Maggiano HJ, Wilbourn AJ. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46(4):1022-1025.
3 Young A, Getty J, Jackson A, et al. Variations in the pattern of muscle innervation by the L5 and S1 nerve roots. Spine. 1983;8(6):616-624.
4 Lauder TD, Dillingham TR, Andary M, et al. Predicting electrodiagnostic outcome in patients with upper limb symptoms: are the history and physical examination helpful? Arch Phys Med Rehabil. 2000;81(4):436-441.
5 Lauder TD, Dillingham TR, Andary M, et al. Effect of history and exam in predicting electrodiagnostic outcome among patients with suspected lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79(1):60-68.
6 Guidelines in electrodiagnostic medicine. American Association of Electrodiagnostic Medicine. Muscle Nerve. 8(supplement), 1999.
7 Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612-1631.
8 Kuruoglu R, Oh SJ, Thompson B. Clinical and electromyographic correlations of lumbosacral radiculopathy. Muscle Nerve. 1994;17(2):250-251.
9 Leblhuber F, Reisecker F, Boehm-Jurkovic H, et al. Diagnostic value of different electrophysiologic tests in cervical disk prolapse. Neurology. 1988;38(12):1879-1881.
10 Linden D, Berlit P. Comparison of late responses, EMG studies, and motor evoked potentials (MEP’s) in acute lumbosacral radiculopathies. Muscle Nerve. 1995;18:1205-1207.
11 Sabbahi MA, Khalil M. Segmental H-reflex studies in upper and lower limbs of patients with radiculopathy. Arch Phys Med Rehabil. 1990;71(3):223-227.
12 Marin R, Dillingham TR, Chang A, et al. Extensor digitorum brevis reflex in normals and patients with radiculopathies. Muscle Nerve. 1995;18(1):52-59.
13 Falco F, Hennessey WJ, Goldberg G, et al. H reflex latency in the healthy elderly. Muscle Nerve. 1994;17:161-167.
14 Miller TA, Pardo R, Yaworski R. Clinical utility of reflex studies in assessing cervical radiculopathy. Muscle Nerve. 1999;22:1075-1079.
15 Scelsa SN, Herskovitz S, Berger AR. The diagnostic utility of F waves in L5/S1 radiculopathy. Muscle Nerve. 1995;18(12):1496-1497.
16 Tackmann W, Radu EW. Observations of the application of electrophysiological methods in the diagnosis of cervical root compressions. Eur Neurol. 1983;22:397-404.
17 London SF, England JD. Dynamic F waves in neurogenic claudication. Muscle Nerve. 1991;14:457-461.
18 Snowden ML, Haselkorn JK, Kraft GH, et al. Dermatomal somatosensory evoked potentials in the diagnosis of lumbosacral spinal stenosis: comparison with imaging studies. Muscle Nerve. 1992;15(9):1036-1044.
19 Yiannikas C, Shahani BT, Young RR. Short-latency somatosensory-evoked potentials from radial, median, ulnar, and peroneal nerve stimulation in the assessment of cervical spondylosis. Arch Neurol. 1986;43:1264-1271.
20 Maertens de Noordhout A, Remacle JM, Pepin JL, et al. Magnetic stimulation of the motor cortex in cervical spondylosis. Neurology. 1991;41(1):75-80.
21 Tavy DL, Franssen H, Keunen RW, et al. Motor and somatosensory evoked potentials in asymptomatic spondylotic cord compression. Muscle Nerve. 1999;22(5):628-634.
22 Dumitru D, Newton BY, Dreyfuss P. Segmental v dermatomal somatosensory-evoked potentials. Normal intertrial variation and side-to-side comparison. Am J Phys Med Rehabil. 1993;72(2):75-83.
23 Dumitru D, Dreyfuss P. Dermatomal/segmental somatosensory evoked potential evaluation of L5/S1 unilateral/unilevel radiculopathies. Muscle Nerve. 1996;19(4):442-449.
24 Haig AJ, Tzeng HM, LeBreck DB. The value of electrodiagnostic consultation for patients with upper extremity nerve complaints: a prospective comparison with the history and physical examination. Arch Phys Med Rehabil. 1999;80(10):1273-1281.
25 Robinson LR. Electromyography, magnetic resonance imaging, and radiculopathy: it’s time to focus on specificity. Muscle Nerve. 1999;22(2):149-150.
26 Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69-73.
27 Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178-1184.
28 Date ES, Mar EY, Bugola MR, et al. The prevalence of lumbar paraspinal spontaneous activity in asymptomatic subjects. Muscle Nerve. 1996;19(3):350-354.
29 Nardin RA, Patel MR, Gudas TF, et al. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22(2):151-155.
30 Dumitru D, Diaz CAJ, King JC. Prevalence of denervation in paraspinal and foot intrinsic musculature. Am J Phys Med Rehabil. 2001;80(7):482-490.
31 Boruta PM, LaBan MM. Electromyographic findings in patients with low back pain due to unsuspected primary and metastatic spinal or paraspinal muscle disease. Clin Orthop. 1981;161:235-241.
32 LaBan MM, Meerschaert JR, Perez L, et al. Metastatic disease of the paraspinal muscles: electromyographic and histopathologic correlation in early detection. Arch Phys Med Rehabil. 1978;59(1):34-36.
33 LaBan MM, Tamler MS, Wang AM, et al. Electromyographic detection of paraspinal muscle metastasis. Correlation with magnetic resonance imaging. Spine. 1992;17(10):1144-1147.
34 Kuncl RW, Cornblath DR, Griffin JW. Assessment of thoracic paraspinal muscles in the diagnosis of ALS. Muscle Nerve. 1988;11(5):484-492.
35 See DH, Kraft GH. Electromyography in paraspinal muscles following surgery for root compression. Arch Phys Med Rehabil. 1975;56(2):80-83.
36 Danner R. Occurrence of transient positive sharp wave like activity in the paraspinal muscles following lumbar puncture. Electromyogr Clin Neurophysiol. 1982;22(1–2):149-154.
37 Haig AJ, Moffroid M, Henry S, et al. A technique for needle localization in paraspinal muscles with cadaveric confirmation. Muscle Nerve. 1991;14(6):521-526.
38 Haig AJ, Talley C, Grobler LJ, et al. Paraspinal mapping: quantified needle electromyography in lumbar radiculopathy. Muscle Nerve. 1993;16(5):477-484.
39 Haig AJ, LeBreck DB, Powley SG. Paraspinal mapping. Quantified needle electromyography of the paraspinal muscles in persons without low back pain. Spine. 1995;20(6):715-721.
40 Haig AJ. Clinical experience with paraspinal mapping. II: A simplified technique that eliminates three-fourths of needle insertions. Arch Phys Med Rehabil. 1997;78(11):1185-1190.
41 Dillingham TR, Lauder TD, Andary M, et al. Identification of cervical radiculopathies: optimizing the electromyographic screen. Am J Phys Med Rehabil. 2001;80(2):84-91.
42 Lauder TD, Dillingham TR. The cervical radiculopathy screen: optimizing the number of muscles studied. Muscle Nerve. 1996;19(5):662-665.
43 Dillingham TR, Lauder TD, Andary M, et al. Identifying lumbosacral radiculopathies: an optimal electromyographic screen. Am J Phys Med Rehabil. 2000;79(6):496-503.
44 Lauder TD, Dillingham TR, Huston CW, et al. Lumbosacral radiculopathy screen. Optimizing the number of muscles studies. Am J Phys Med Rehabil. 1994;73(6):394-402.
45 Dillingham TR, Dasher KJ. The lumbosacral electromyographic screen: revisiting a classic paper. Clin Neurophysiol. 2000;111(12):2219-2222.
46 Knutsson B. Comparative value of electromyographic, myelographic, and clinical-neurological examinations in diagnosis of lumbar root compression syndrome. Acta Orthop Scand. 1961;Suppl 49:1-123.
47 Rittenberg JD, editor. Lumbosacral spinal stenosis. Physical Medicine and Rehabilitation Clinics of North America. Philadelphia: WB Saunders, 2003.
48 Hall S, Bartleson JD, Onofrio BM, et al. Lumbar spinal stenosis. Clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103(2):271-275.
49 Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11-4. C11-14
50 Adamova B, Vohanka S, Dusek L. Differential diagnostics in patients with mild lumbar spinal stenosis: the contributions and limits of various tests. Eur Spine J. 2003;12:190-196.
51 Cinotti G, Postacchini F, Weinstein JN. Lumbar spinal stenosis and diabetes. Outcome of surgical decompression. J Bone Joint Surg Br. 1994;76(2):215-219.
52 Dillingham TR, Pezzin LE, Lauder TD. Cervical paraspinal muscle abnormalities and symptom duration: a multivariate analysis. Muscle Nerve. 1998;21(5):640-642.
53 Dillingham TR, Pezzin LE, Lauder TD. Relationship between muscle abnormalities and symptom duration in lumbosacral radiculopathies. Am J Phys Med Rehabil. 1998;77(2):103-107.
54 Dillingham TR, Pezzin LE, Lauder TD, et al. Symptom duration and spontaneous activity in lumbosacral radiculopathy. Am J Phys Med Rehabil. 2000;79(2):124-132.
55 Pezzin LE, Dillingham TR, Lauder TD, et al. Cervical radiculopathies: relationship between symptom duration and spontaneous EMG activity. Muscle Nerve. 1999;22(10):1412-1418.
56 Tullberg T, Svanborg E, Isacsson J, et al. A preoperative and postoperative study of the accuracy and value of electrodiagnosis in patients with lumbosacral disc herniation. Spine. 1993;18(7):837-842.
57 Spengler DM, Freeman CW. Patient selection for lumbar discectomy: an objective approach. Spine. 1979;4(2):129-134.
58 Spengler DM, Ouellette EA, Battie M, et al. Elective discectomy for herniation of a lumbar disc: additional experience with an objective method. J Bone Joint Surg Am. 72(A(2)), 1990.
59 Weber F, Albert U. Electrodiagnostic examination of lumbosacral radiculopathies. Electromyogr Clin Neurophysiol. 2000;40(4):231-236.
60 Khatri BO, Baruah J, McQuillen MP. Correlation of electromyography with computed tomography in evaluation of lower back pain. Arch Neurol. 1984;41(6):594-597.
61 Tonzola RF, Ackil AA, Shahani BT, et al. Usefulness of elctrophysiological studies in the diagnosis of lumbosacral root disease. Ann Neurol. 1981;9(3):305-308.
62 Schoedinger GR. Correlation of standard diagnostic studies with surgically proven lumbar disk rupture. South Med J. 1987;80:44-46.
63 Johnsson KE, Rosen I, Uden A. Neurophysiologic investigation of patients with spinal stenosis. Spine. 1987;12(5):483-487.
64 Berger AR, Busis NA, Logigian EL, et al. Cervical root stimulation in the diagnosis of radiculopathy. Neurology. 1987;37(2):329-332.
65 Partanen J, Partanen K, Oikarinen H, et al. Preoperative electroneuromyography and myelography in cervical root compression. Electromyogr Clin Neurophysiol. 1991;31(1):21-26.
66 So YT, Olney RK, Aminoff MJ. A comparison of thermography and electromyography in the diagnosis of cervical radiculopathy. Muscle Nerve. 1990;13(11):1032-1036.
67 Hong CZ, Lee S, Lum P. Cervical radiculopathy. Clinical, radiographic and EMG findings. Orthop Rev. 1986;15(7):433-439.