Chapter 38 Electrical Therapy for Bradycardia
Future Directions
Since its introduction more than a half century ago, progressive improvements in technology have led to the dramatic evolution of cardiac pacing therapy. The changing demographics of the United States have led to an increase in the pacemaker implantation rate from 37 per 100,000 person years 30 years ago to 99 per 100,000 patient years during the first decade of the twenty-first century.1 The technology implemented in cardiac resynchronization devices and implantable cardiac defibrillators has received more widespread attention, but it should not be forgotten that technological improvements for cardiac pacemakers continue to evolve at a rapid pace. Even more importantly, the maturity and relative constancy of the basic indications for cardiac pacemaker therapy have helped clarify the shortcomings of current devices and identify areas for future basic and clinical research. For example, more widespread use of magnetic resonance imaging (MRI) for diagnostic purposes has provided the incentive for manufacturers to develop pacemakers using materials that will not be affected by the magnetic and electrical fields generated by scanners.
Remote Follow-up of Cardiac Pacing Devices
Currently, all commercially available cardiac pacemakers have some type of remote monitoring system.2 Information stored in the memory of the pacemaker can be downloaded wirelessly to a receiver located in the patient’s home. Both patient-activated downloads and automatic downloads are possible, depending on the manufacturer. Information is transmitted via cell phone or over landlines to a central server that is maintained by the pacemaker manufacturer. This downloaded information can then be accessed via the Internet in a “two-way” manner, initiated by the physician as well as automatically, with automatic alerts sent from the server to the physician. Initial studies have suggested that remote monitoring can identify potential problems more quickly than can in-office or clinic visits.2,3 In one study of patients with implantable defibrillators, remote monitoring identified lead failure (conductor wire break or insulation breach) 2 months earlier than can standard monitoring; consequently, lead failure–related inappropriate shocks were experienced by only 27% of patients with remote monitoring compared with 53% of patients with traditional follow-up.4
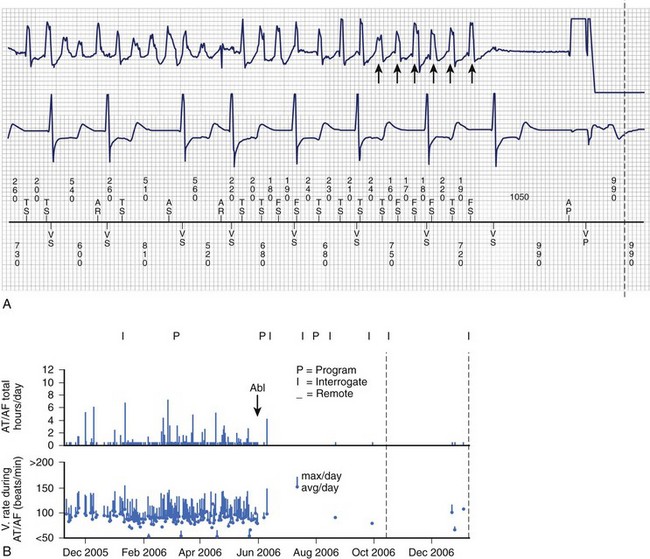
Identification of atrial arrhythmias in patients with bradycardia has been shown to provide important prognostic information. In a nested cohort of 312 patients from the Mode Selection Trial (MOST), the presence of a 5-minute or longer episode of atrial tachycardia was associated with an increased risk of death (heart rate [HR], 2.48; confidence interval [CI], 1.25 to 4.91) and development of atrial fibrillation (HR, 5.93; CI, 2.88 to 12.2).5 The usefulness of early identification of atrial arrhythmias by remote monitoring in improving clinical outcomes is the subject of several large multicenter trials. Preliminary results from the TRENDS study suggest that atrial fibrillation “burden” (>5.5 arrhythmia hours per day) is associated with a higher thromboembolic rate (2.4% per year) compared with that in patients without atrial arrhythmias identified by the device (1.1%).6 Another multicenter study, Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT), has completed its enrollment and will also evaluate whether atrial arrhythmias identified by and stored in the device can identify a group of patients at higher risk for stroke. Coupled with remote follow-up, early documentation of atrial arrhythmias may be beneficial in reducing the incidence of stroke by identifying patients who would benefit from anticoagulant therapy. Figure 38-1 shows an example of a patient with atrial fibrillation and an implanted pacemaker. Interrogation of the pacemaker after ablation of the atrial fibrillation confirms successful elimination of detected episodes of this arrhythmia.
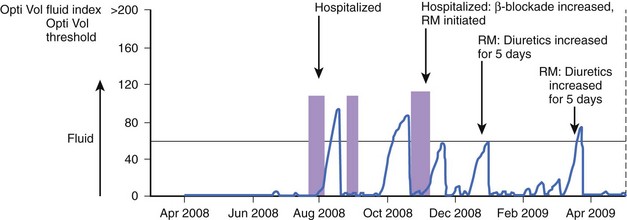
Early studies have provided interesting data indicating that device-based hemodynamic monitoring may be beneficial in patients with heart failure. Devices currently in use can estimate patient activity via motion sensors and specific respiratory parameters and can estimate pulmonary venous congestion via intrathoracic impedance measurements.7 Fluid accumulation within pulmonary spaces decreases intrathoracic impedance, since fluid offers less resistance to electrical current than does air. In a small clinical study of 33 patients, a decrease in thoracic impedance was observed approximately 2 weeks before hospitalizations for heart failure. An example of the clinical use of intrathoracic impedance is shown in Figure 38-2. In the study, after remote monitoring was initiated, decreased impedance was used along with clinical variables to increase the diuretic dose of a patient with severe systolic dysfunction, which may have prevented rehospitalization.
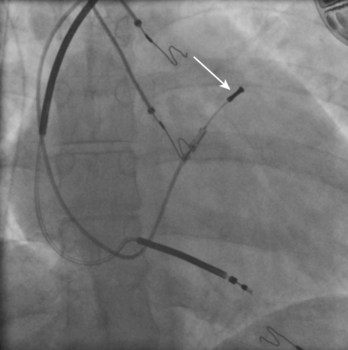
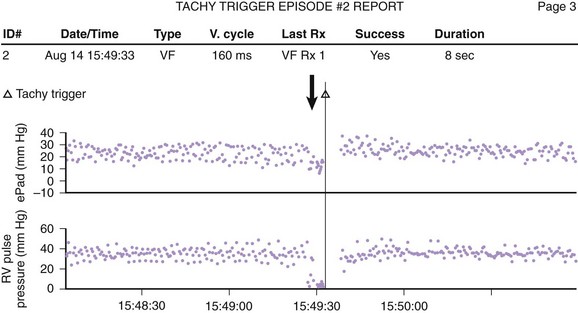
Indirect estimates of volume status using technologies such as intrathoracic impedance can be incorporated into any lead-device system, but direct pressure measurements using specialized investigational leads may provide theoretical or real advantages. One lead system uses a specially designed pressure transducer that is placed in the right ventricular outflow tract; the transducer directly measures right ventricular pressure and uses an algorithm to estimate pulmonary artery diastolic pressure (Figure 38-3). Pulmonary artery diastolic pressure, as a correlate of pulmonary capillary wedge pressure, can be used as an estimate of left ventricular preload to guide heart failure management. In addition, the pressure monitor may also be useful in confirming the presence of ventricular arrhythmias by identifying accompanying hemodynamic compromise (Figure 38-4). In the largest study to date, the Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial, 274 patients with class III or IV heart failure were randomized to two groups: one in which information was available to clinicians and the other in which clinicians were blinded to hemodynamic data. After a 6-month follow-up, clinical use of a hemodynamic monitor resulted in a nonsignificant 21% reduction in heart failure–related events.8 Another specialized lead system under clinical investigation uses a pressure tip placed at the inter-atrial septum to directly measure left atrial pressure.
In addition to intracardiac pressures, new technologies that monitor other non-heart rate–related parameters such as ST-segment deviation and systemic blood pressure are being developed.8–11 ST-segment changes can be monitored by analyzing the intracardiac electrogram from a tip electrode located in the right ventricle and the device “can.” The device continuously monitors the relationship between the ST segment and the P-Q segment. If a significant shift between the ST segment and the P-Q segment persists for a programmed number of beats, the device can either vibrate or provide an audio alert. In an animal study, changes in the ST segment recorded by implanted leads occurred earlier than in those recorded from surface electrocardiogram (ECG) leads; these changes therefore confer an ability to quickly detect myocardial ischemia in any of the three major coronary arteries. Initial data from experimental studies suggest that systemic blood pressure changes can be estimated by measuring bioimpedance between two electrodes placed in the subcutaneous tissue. Incorporation of this technology into implanted devices may provide a method for monitoring antihypertensive therapy.
It is hoped that freer and more widespread access to medical information by medical providers will improve healthcare outcomes and reduce costs. Implantation of devices will likely be the first clinical field where this information revolution will take place. Current devices provide simple audible alerts when the implanted device detects a potential problem, but it is an easy leap to imagine providing patients with access to information in a simplified and understandable format. In this way, implanted devices coupled with remote follow-up can initiate a major paradigm shift, with patients taking a more active role in their medical care, based on actual data rather than symptoms alone.12
Newer Cardiac Pacing System Algorithms
The development of specialized and complex algorithms for cardiac pacing systems has had a significant impact on pacemaker programming and thus on the delivery of more appropriate pacing therapy. Large randomized studies performed in the last decade of the twentieth century identified the deleterious effects of right ventricular pacing, especially if performed from the right ventricular apex.13,14 Importantly, this deleterious effect occurs even when only moderate ventricular pacing is present. In a post hoc analysis of MOST, cumulative ventricular pacing greater than 40% of the time was associated with a 2.6-fold increased risk of hospitalization for heart failure.15
Several studies have shown that right ventricular pacing is also associated with an increased likelihood of developing persistent atrial fibrillation. For example, in MOST, a linear dose-response relationship was noted between ventricular pacing and the likelihood of developing atrial fibrillation, with a 20% increase in the likelihood of developing atrial fibrillation with each 10% increase in cumulative ventricular pacing.15 More recently, it has become apparent that specific algorithms that minimize right ventricular pacing can reduce the likelihood of atrial fibrillation in patients with sinus node dysfunction. In the Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) trial, 1065 patients with sinus node dysfunction and normal AV conduction were randomized either to an algorithm specifically designed to minimize ventricular pacing or to conventional dual-chamber pacing without this function.16 The algorithm resulted in a significant reduction in ventricular pacing (minimizing ventricular pacing algorithm: 9%; conventional programming: 99%) and after an almost 2-year follow-up, minimizing right ventricular pacing was associated with a 40% reduction in the relative risk for persistent atrial fibrillation (conventional DDD pacing: 12.7% versus minimizing pacing algorithm: 7.9%). The MINimizE Right Ventricular Pacing to Prevent Atrial Fibrillation and Heart Failure (MINERVA) trial is currently under way to evaluate whether algorithms that minimize ventricular pacing will reduce the burden of atrial fibrillation in those patients who already have an established diagnosis of atrial arrhythmias.17
The clinical benefits of other complex algorithms appear to have limited benefit in the aggregate; it is quite certain that these complex algorithms can be useful in selected individual patients.18 In the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT), 872 patients who had a blunted heart rate response to exercise (chronotropic incompetence) were randomized to standard dual-chamber pacing or to dual-chamber pacing that modulated heart rate on the basis of input from an accelerometer and a minute ventilation sensor (rate adaptation).18 Although the use of rate-adaptation algorithms was associated with an increased heart rate in response to exercise, this did not translate into significant differences in quality-of-life and activity indices between the two groups. Initial unblinded trials had suggested that specialized pacing algorithms that provided higher pacing rates in response to sudden changes in heart rate could be useful for patients with severely symptomatic vasovagal syncope.19 However, a subsequent double-blind trial did not show any clinical benefit of pacing using specialized algorithms in patients with vasovagal syncope.20
It is expected that in the next 5 years, pacemakers will be characterized by increased automatic functions. Automated algorithms for adjusting pacing output to minimize energy requirements and extend battery life have already been developed for atrial and ventricular pacing (e.g., the “autocapture” feature, St. Jude, Sylmar, CA).21,22 In a multicenter randomized trial of 910 patients who underwent implantation of a dual-chamber pacing system, automatic threshold evaluation of ventricular capture was found to be reliable and comparable with manual threshold evaluation and led to a projected 16% increase in longevity from 8.9 to 10.3 years.23 Large registry studies (ULTRA and Automaticity, both sponsored by Boston Scientific, St. Paul, MN) are currently under way to evaluate whether automatic algorithms will increase pacemaker longevity and reduce the use of health care resources.
Site Selection
It has been known for more than 40 years that pacing from the right ventricular apex alters the ventricular contraction pattern by producing functional left bundle branch block; this, in turn, can be associated with adverse acute hemodynamic effects and long-term structural, metabolic, and functional effects.24 The realization that techniques to “resynchronize” ventricular activation patterns could improve outcomes in patients with systolic left ventricular dysfunction and increased QRS duration has brought this issue to the forefront. It has been proposed that pacing from the right ventricular septum could allow for more physiological ventricular activation in patients with atrioventricular block. Small acute hemodynamic studies and chronic studies with short-term follow-up have yielded mixed results with regard to the benefits of septal pacing.25,26 Three large studies—Optimize RV Selective Site Pacing Clinical Trial (Optimize RV), Right Ventricular Apical and High Septal Pacing to Preserve Left Ventricular Function (Protect Pace) Trial, and Right Ventricular Apical versus Septal Pacing (RASP) Trial—with left ventricular ejection fraction as the primary endpoint and planned long-term follow-up (24 to 36 months)—are currently under way. Short-term hemodynamic data suggest that biventricular pacing may have beneficial effects in patients with reduced left ventricular function and a normal QRS duration (<0.12 seconds) who will require a significant percent of ventricular pacing.27 Similarly, in the Multicenter Automatic Defibrillator Implant Trial–Cardiac Resynchronization Therapy (MADIT-CRT) trial, biventricular pacing was associated with a 41% reduction in heart failure events in patients with reduced left ventricular function (ejection fraction [EF] <30%) and mild heart failure symptoms (NYHA classes I and II).28
Certain atrial pacing sites may have some beneficial effect with regard to the development of atrial arrhythmias. Early small prospective studies have suggested that pacing from sites along the inter-atrial septum, including the coronary sinus os and Bachmann’s bundle, may be associated with a decreased incidence of atrial fibrillation and other atrial arrhythmias.29,30 However, subsequent moderately sized trials have suggested that the benefits of atrial pacing from selected sites such as the atrial septum are not uniform, with either no benefit or only modest benefit (30% reduction in atrial fibrillation burden).31,32 The Septal Pacing for Atrial Fibrillation Suppression Evaluation (SAFE) trial has been designed as a large multicenter trial to evaluate the association of septal pacing with a decrease in the development of persistent (>7 days) atrial fibrillation. Early studies have suggested that multiple-site atrial pacing (usually from a lateral right atrial site and a second atrial site at the coronary sinus os or within the coronary sinus), in addition to using specific atrial sites, results in a more normal atrial activation sequence with an accompanying improvement in the contribution of atrial contraction.33,34 Finally, multiple-site atrial pacing may reduce atrial vulnerability to induction of atrial fibrillation.33
Power Sources
Lithium iodine has been the traditional source of battery power for the past three decades because of its stable and predictable characteristics. Currently available batteries can have a lifetime of 8 to 12 years, depending on use and programmed output. Pulse generator replacement is associated with a twofold increased risk of pocket infection compared with initial pacemaker implants. Therefore, the development of reliable leads, and thus devices with longer battery life, would be extremely beneficial and result in a significant reduction in the use of health care resources.2 Lithium carbon monofluoride batteries that may offer higher energy density, thus allowing for longer service life, are being developed. It should be noted that the longest-lasting pacemaker reported in the medical literature is a nuclear-powered pacemaker that was implanted in 1973 and was still operating in 2007.35 Although they are long-lasting, nuclear pacemakers are no longer available because of high initial costs and the rapid evolution of lithium iodine power sources in the 1970s. However, engineers are evaluating the feasibility of using newer-generation nuclear batteries in implantable medical devices. Another avenue for improving cardiac longevity has been the development of energy-efficient circuit components (e.g., capacitors) that use unusual materials such as wet tantalum. Finally, theories on the possibility of “harvesting and storing” the kinetic energy of heart motion and using it to power a pacemaker have been proposed.
Lead Design, Lead Reliability, and “Leadless” Pacing
Significant improvements in lead design have taken place in the past 10 years. Manufacturers have developed high-impedance electrodes that reduce the amount of current required for myocardial stimulation. In one small clinical study of 40 patients, high-impedance electrodes were shown to reduce by 50% the current required for pacing, but unfortunately this did not lead to a longer time to pulse generator replacement, perhaps partly because of the small number of patients (24 of 40) who had complete follow-up.36
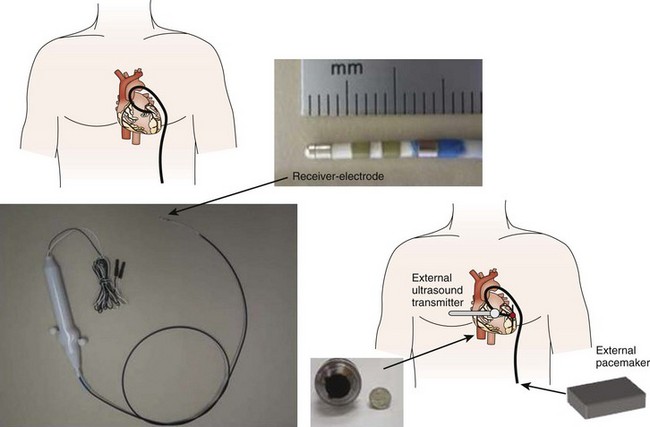
The focus of modern lead design has shifted to increasing reliability, since the pacing lead remains the most common cause of device failure. Recent studies have suggested that the lead failure rate is approximately 20% to 30% in the first 10 years and that attrition of lead integrity continues thereafter.37,38 Manufacturers are investigating new lead designs that employ cables rather than coils and multiple layers of insulation to improve lead longevity. Several novel technologies incorporating a remote “receiver” that is placed within the heart and is coupled wirelessly to a “transmitter” located in the shoulder area are being developed.39–41 Ultrasound bursts from a transmitter on the chest have resulted in successful ventricular pacing in animal models and during acute hemodynamic studies in humans (Figure 38-5).39,40 More recently, pacing has been achieved in an experimental model by using magnetic fields to generate the power required for cardiac stimulation.41
Biologic Pacing
Over the past decade, interest has been growing in developing a biologic pacemaker. Several approaches to developing such a device exist.42–45 The first method is to change the function of existing cardiac myocytes by increasing β-adrenergic receptor expression or by encouraging the development of diastolic depolarization; the latter can be achieved by blocking the background potassium (K+) currents responsible for the resting membrane potential or by enhancing diastolic currents (If).42,43 The second general strategy is to use cell therapy approaches to differentiate stem cells into cardiac myocytes with pacing characteristics.44 In canine models, a “tandem system” that uses both a biologic pacing system and an electronic system has been implanted.45 The biologic system functions most of the time and responds appropriately to catecholamine challenge. When the biologic system slows, the electronic system takes over. As this technology matures, it is likely that pacemakers using hybrid biology hardware architecture or perhaps even a stand-alone biologic design will become commercially available for clinical use.
Key References
Borek PP, Wilkoff BL. Pacemaker and ICD leads: Strategies for long-term management. J Interv Card Electrophysiol. 2008;23(1):59-72.
Bourge RC, Abraham WT, Adamson PB, et al. COMPASS-HF Study Group. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J Am Coll Cardiol. 2008;51(11):1073-1079.
Cleland JG, Coletta AP, Yassin A, et al. Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta-analysis of Cox-2 studies, HAT, ON-TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON-HF and REVERSE. Eur J Heart Fail. 2008;10(6):614-620.
Echt DS, Cowan MW, Riley RE, Brisken AF. Feasibility and safety of a novel technology for pacing without leads. Heart Rhythm. 2006;10:1202-1206.
Glotzer TV, Hellkamp AS, Zimmerman J, et al. MOST Investigators: Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614-1619.
Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: An 8-year prospective multicenter study. Heart Rhythm. 2007;4(2):154-160.
Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity—evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007;4(9):1125-1132.
Matchett M, Sears SF, Hazelton G, Kirian K, Wilson E, Nekkanti R. The implantable cardioverter defibrillator: Its history, current psychological impact and future. Expert Rev Med Devices. 2009;6(1):43-50.
Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132-133.
Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace. 2008;10(5):556-557.
Parsonnet V, Cheema A. The nature and frequency of postimplant surgical interventions: A realistic appraisal. Pacing Clin Electrophysiol. 2003;26(12):2308-2312.
Sweeney MO, Bank AJ, Nsah E, et al. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial: Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000-1008.
Wilkoff BL, Auricchio A, Brugada J, et al. Heart Rhythm Society; European Heart Rhythm Association; American College of Cardiology; American Heart Association; European Society of Cardiology; Heart Failure Association of ESC; Heart Failure Society of America: HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5(6):907-925.
Winters SL, Kusumoto FM, Miller JM, Slotwiner DJ. The role of the Heart Rhythm Society in integrating the healthcare enterprise. Heart Rhythm. 2007;4(1):122-124.
Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841-848.
1 Uslan DZ, Tleyjeh IM, Baddour LM, et al. Temporal trends in permanent pacemaker implantation: A population-based study. Am Heart J. 2008;155(5):896-903.
2 Wilkoff BL, Auricchio A, Brugada J, et al. Heart Rhythm Society; European Heart Rhythm Association; American College of Cardiology; American Heart Association; European Society of Cardiology; Heart Failure Association of ESC; Heart Failure Society of America: HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5(6):907-925.
3 Sweesy MW, Erickson SL, Crago JA, et al. Analysis of the effectiveness of in-office and transtelephonic follow-up in terms of pacemaker system complications. Pacing Clin Electrophysiol. 1994;17(11 Pt 2):2001-2003.
4 Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace. 2008;10(5):556-557.
5 Glotzer TV, Hellkamp AS, Zimmerman J, et al. MOST Investigators: Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614-1619.
6 Cleland JG, Coletta AP, Yassin A, et al. Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta-analysis of Cox-2 studies, HAT, ON-TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON-HF and REVERSE. Eur J Heart Fail. 2008;10(6):614-620.
7 Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841-848.
8 Bourge RC, Abraham WT, Adamson PB, et al. COMPASS-HF Study Group: Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J Am Coll Cardiol. 2008;51(11):1073-1079. 18
9 Fischell TA, Fischell DR, Fischell RE, et al. Real-time detection and alerting for acute ST-segment elevation myocardial ischemia using an implantable, high-fidelity, intracardiac electrogram monitoring system with long-range telemetry in an ambulatory porcine model. J Am Coll Cardiol. 2006;48(11):2306-2314.
10 Asbach S, Weiss I, Wenzel B, et al. Intrathoracic far-field electrocardiogram allows continuous monitoring of ischemia after total coronary occlusion. Pacing Clin Electrophysiol. 2006;29(12):1334-1340.
11 Venugopal D, Patterson R, Jhanjee R, et al. Subcutaneous bioimpedance recording: Assessment of a method for hemodynamic monitoring by implanted devices. J Cardiovasc Electrophysiol. 2009;20(1):76-81.
12 Winters SL, Kusumoto FM, Miller JM, Slotwiner DJ. The role of the Heart Rhythm Society in integrating the healthcare enterprise. Heart Rhythm. 2007;4(1):122-124.
13 Wilkoff BL, Cook JR, Epstein AE, et al. Dual Chamber and VVI Implantable Defibrillator Trial Investigators: Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115-3123.
14 Goldenberg I, Moss AJ, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Investigators: Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113(24):2810-2817. 20
15 Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. MOde Selection Trial Investigators: Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107(23):2932-2937. 17
16 Sweeney MO, Bank AJ, Nsah E, et al. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial: Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000-1008.
17 Funck RC, Boriani G, Manolis AS, et al. MINERVA Study Group: The MINERVA study design and rationale: A controlled randomized trial to assess the clinical benefit of minimizing ventricular pacing in pacemaker patients with atrial tachyarrhythmias. Am Heart J. 2008;156(3):445-451.
18 Lamas GA, Knight JD, Sweeney MO, et al. Impact of rate-modulated pacing on quality of life and exercise capacity—evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT). Heart Rhythm. 2007;4(9):1125-1132.
19 Ammirati F, Colivicchi F, M Santini, Syncope Diagnosis and Treatment Study Investigators. Permanent cardiac pacing versus medical treatment for the prevention of recurrent vasovagal syncope: A multicenter, randomized, controlled trial. Circulation. 2001;104(1):52-57.
20 Connolly SJ, Sheldon R, Thorpe KE, et al. VPS II Investigators: Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): A randomized trial. JAMA. 2003;289(17):2224-2229.
21 Sperzel J, Goetze S, Kennergren C, et al. Performance evaluation of a right atrial automatic capture verification algorithm using two different sensing configurations. Pacing Clin Electrophysiol. 2009;32(5):579-587.
22 Pecora D, Morandi F, Liccardo M, et al. Performance of a ventricular automatic-capture algorithm in a wide clinical setting. Pacing Clin Electrophysiol. 2008;31(12):1546-1553.
23 Koplan BA, Gilligan DM, Nguyen LS, et al. A randomized trial of the effect of automated ventricular capture on device longevity and threshold measurement in pacemaker patients. Pacing Clin Electrophysiol. 2008;31(11):1467-1474.
24 Lister JW, Klotz DH, Jomain SL, et al. Effect of pacemaker site on cardiac output and ventricular activation in dogs with complete heart block. Am J Cardiol. 1964;14:494-503.
25 Gold MR, Shorofsky SR, Metcalf MD, et al. The acute hemodynamic effects of right ventricular septal pacing in patients with congestive cardiac failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:679-681.
26 Blanc JJ, Etienne Y, Gilard M, et al. Evaluation of different ventricular pacing sites in patients with severe heart failure: Results of an acute hemodynamic study. Circulation. 1997;96:3273-3277.
27 Bildirici U, Vural A, Agacdiken A, et al. Comparison of the effects of left vs. right ventricular pacing on left ventricular remodelling. Europace. 2008;10(12):1387-1391.
28 Moss AJ, Hall WJ, Cannom DS, et al. MADIT-CRT Trial Investigators: Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338.
29 Delfaut P, Saksena S, Prakash A, Krol RB. Long-term outcome of patients with drug-refractory atrial flutter and fibrillation after single- and dual-site right atrial pacing for arrhythmia prevention. J Am Coll Cardiol. 1998;32(7):1900-1908.
30 Camm AJ, Sulke N, Edvardsson N, et al. AFTherapy Investigators: Conventional and dedicated atrial overdrive pacing for the prevention of paroxysmal atrial fibrillation: The AFTherapy study. Europace. 2007;9(12):1110-1118.
31 Hakacova N, Velimirovic D, Margitfalvi P, et al. Septal atrial pacing for the prevention of atrial fibrillation. Europace. 2007;9(12):1124-1128.
32 Bailin SJ, Adler S, Giudici M. Prevention of chronic atrial fibrillation by pacing in the region of Bachmann’s bundle: Results of a multicenter randomized trial. J Cardiovasc Electrophysiol. 2001;12(8):912-917.
33 Hansen JC, Latchamsetty R, Lavi N, et al. High-density biatrial pacing protects against atrial fibrillation by synchronizing left atrial tissue. Interv Card Electrophysiol. 2010;27(2):81-87.
34 Dabrowska-Kugacka A, Lewicka-Nowak E, et al. Atrial electromechanical sequence and contraction synchrony during single- and multisite atrial pacing in patients with brady-tachycardia syndrome. Pacing Clin Electrophysiol. 2009;32(5):591-603.
35 Parsonnet V. A lifetime pacemaker revisited. N Engl J Med. 2007;357(25):2638-2639.
36 Etsadashvili K, Hintringer F, Stühlinger M, et al. Long-term results of high vs. normal impedance ventricular leads on actual (Real-Life) pacemaker generator longevity. Europace. 2009;11(2):200-205.
37 Parsonnet V, Cheema A. The nature and frequency of postimplant surgical interventions: A realistic appraisal. Pacing Clin Electrophysiol. 2003;26(12):2308-2312.
38 Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: An 8-year prospective multicenter study. Heart Rhythm. 2007;4(2):154-160.
39 Echt DS, Cowan MW, Riley RE Brisken AF. Feasibility and safety of a novel technology for pacing without leads. Heart Rhythm. 2006;10:1202-1206.
40 Lee KL, Tse HF, Echt DS, Lau CP. Temporary leadless pacing in heart failure patients with ultrasound-mediated stimulation energy and effects on the acoustic window. Heart Rhythm. 2009;6(6):742-748.
41 Wieneke H, Konorza T, Erbel R, Kisker E. Leadless pacing of the heart using induction technology: A feasibility study. Pacing Clin Electrophysiol. 2009;32(2):177-183.
42 Edelberg JM, Aird WC, Rosenberg RD. Enhancement of murine cardiac chronotropy by the molecular transfer of the human beta2 adrenergic receptor cDNA. J Clin Invest. 1998;101:337-343.
43 Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002;419:132-133.
44 Qu J, Plotnikov AN, Danilo PJr, et al. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106-1109.
45 Rosen MR, Brink PR, Cohen IS, et al. Regenerative therapies in electrophysiology and pacing. J Interv Card Electrophysiol. 2008;22(2):87-98.