40 Electrical Safety and Iatrogenic Complications of Electrodiagnostic Studies
Electrical Issues
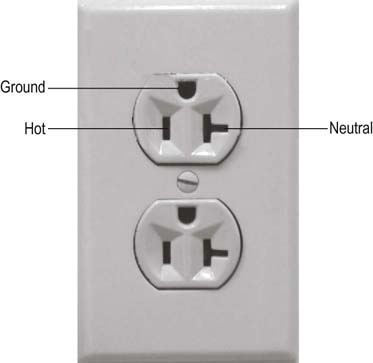
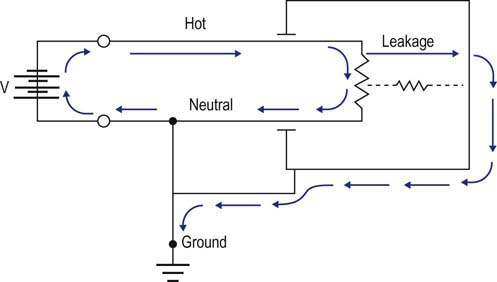
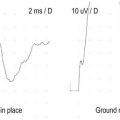
All electrical devices, including EMG machines, require current to operate. Current is delivered from an electrical cord plugged into a wall receptacle (Figure 40–1). A typical electrical receptacle in the United States contains three inputs: a black “hot” lead that carries 120 volts (V) of 60 Hz alternating current, a white “neutral” lead near 0 V, and a green ground lead that is used to dissipate leakage currents. When a circuit is created, current flows from the hot lead to the EMG machine and then returns via the neutral lead, based on the amount of resistance between the two leads as determined by Ohm’s law (see Chapter 39). Every wire, including power cords, has some small resistance; thus, a small voltage develops on the neutral lead, which equals the current flowing multiplied by the resistance in the power cord (Figure 40–2). The voltage increases with the length of the power cord and increases further if extension cords are added to the power cord. In addition, small voltage leaks often are present on the machine chassis, caused by stray capacitance and inductance from internal electronics (Figure 40–3). Thus, leakage currents may be transmitted onto the patient either from stray voltages on the machine chassis or on the neutral (reference) lead. As the ground electrode is close to true electrical neutral, the ground lead allows a pathway for stray current leaks to harmlessly dissipate.
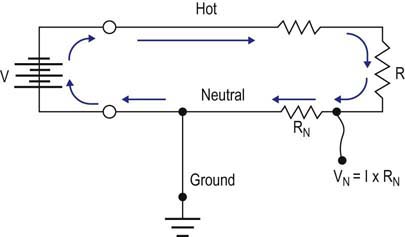
FIGURE 40–2 Stray leakage current: reference lead.
(Adapted from Kimura, J., 1983. Electrodiagnosis in diseases of muscle and nerve. FA Davis, Philadelphia, pp. 615–619, with permission.)
The risk of electrical injury from leakage current increases in the following situations
To prevent the possibility of an electrical injury during EDX studies, it is essential for equipment to be regularly maintained, to always use a ground electrode, and to follow simple guidelines when using electrical devices attached to the patient (Box 40–1). A wooden examining table is preferable to a metal table, as it does not conduct electricity. Machines should be turned on before attaching electrodes to the patient and turned off after disconnecting the patient, to minimize the risk of power surges. Equipment should be periodically inspected by a biomedical engineer to measure leakage current and verify proper grounding. In general, the maximum amount of acceptable leakage current is 100 µA or less, measured from chassis to ground, and 50 µA or less from any input lead to ground. Extension cords should be avoided to reduce the risk of voltages developing on the reference electrodes. Ground electrodes should always be used to avoid current flows from reaching the patient. The ground needs to be placed on the same limb as the active electrodes so that leakage currents cannot flow in a path through the heart (Figure 40–5A).
Box 40–1
Measures to Ensure Proper Grounding
• Always use a three-hole power receptacle with properly grounded outlet.
• Unnecessary electrical equipment should be kept outside the EMG examining room.
• Suspect improper grounding if
• Use a wooden examining table if possible (metal conducts electricity) (Figure 40–4).
• Avoid patient contact with any metal objects or any part of the EMG machine.
From Al-Shekhlee, A., Shapiro, B.E., Preston, D.C., 2003. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve 27, 517–526, with permission.
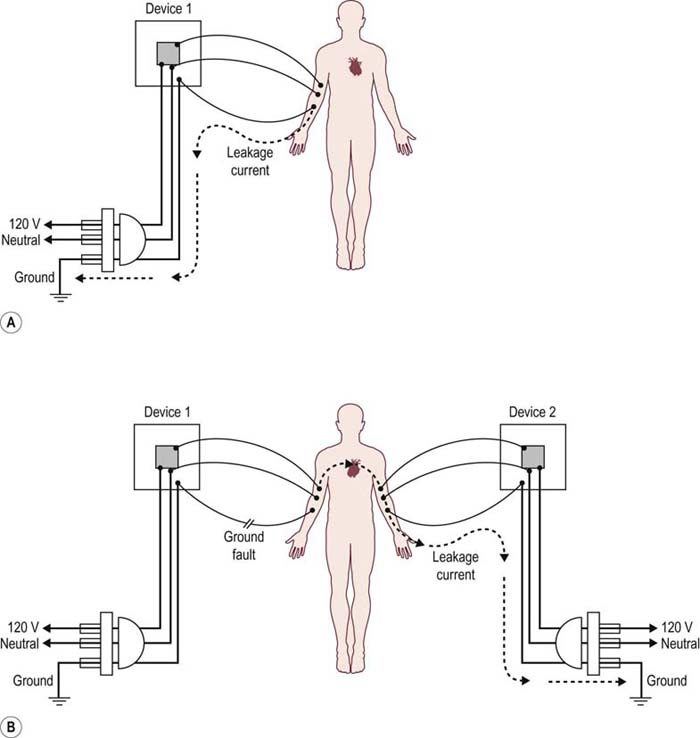
FIGURE 40–5 Leakage current and the risk of electrical injury.
(Adapted from Starmer, C.F., McIntosh, H.D., Whalen, R.E., 1971. Electrical hazards and cardiovascular function. N Engl J Med 284, 181–186, with permission.)
The issue of an intact ground electrode and proper ground placement is most important when a patient is connected to other electrical devices. If the ground from the EMG machine is not functioning (i.e., ground fault), stray current from the EMG machine could flow to a ground electrode from a different electrical device. If the pathway included the heart and the amount of current was large enough, a cardiac arrhythmia could theoretically occur (Figure 40–5B).
Risk of Electrical Injury
Central Lines and Electrical Wires
One of the more common ways a patient can become electrically sensitive is when the normal protective function of the skin is breached by intravenous lines and wires. This danger increases if the lines are actually in contact with or in close proximity to the heart, as occurs in central intravenous catheters (Figure 40–6). Most dangerous is the presence of an external wire near or in the heart, such as occurs with placement of a temporary external pacemaker and during the use of a guidewire while placing or changing a central line. Skin resistance typically is several million Ohms (MΩ). A central catheter traversing the skin reduces this resistance to 300,000 Ohms (kΩ). Any fluid spill where a catheter enters the body decreases the resistance even further. If a catheter has an internal guidewire, the resistance drops to 70 Ohms (Ω). An external pacemaker wire essentially has no resistance. In situations where the resistance is so low, small leakage voltages may result in small leakage currents, known as microcurrents. Whereas microcurrents are completely harmless in a patient with intact skin, they are potentially very dangerous in an electrically sensitive patient (i.e., a patient with a central line, external pacemaker wires, etc.).
Implanted Pacemakers and Cardioverter-Defibrillators
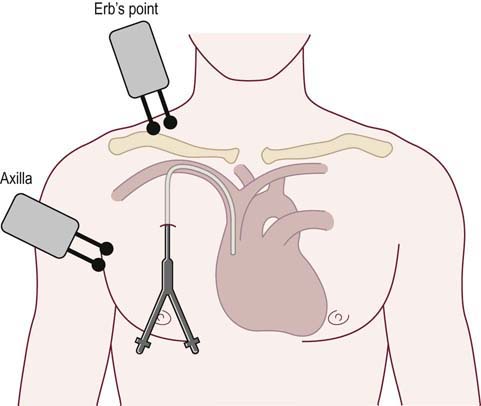
If NCSs are performed in patients with implantable pacemakers or IACDs, several simple procedures are recommended to be followed in order to preserve safety (Box 40–2). Stimulation should not be performed near the actual implanted device. There should always be a minimum of 6 inches between the implanted device and the stimulator. Just as with NCSs performed in a patient with a central line, it is preferable to use the contralateral arm if possible. High stimulus intensities should be avoided and stimulus pulse duration should be 0.2 ms or less so that the stimulation is not misinterpreted as a QRS complex. Stimulation rates should be no greater than 1 Hz so as to prevent the theoretical risk that the stimulation is misinterpreted as a cardiac rhythm. Thus, the typical repetitive stimulation done during neuromuscular junction testing is best avoided.
Box 40–2
Guidelines for Pacemakers and Implantable Cardioverter-Defibrillators
• Do not perform studies on patients with external pacer wires.
• Ensure all ground electrodes are functional.
• Limit all electrodes, including the ground, to the extremity of interest, and keep all electrodes as far away from the heart as possible, without crossing cardiac devices or their wires.
• Do not stimulate near the device (allow a minimum of 6 inches) and avoid ipsilateral proximal stimulation sites (i.e., axilla, Erb’s point, root stimulation).
• Use a stimulus duration of 0.2 ms or shorter and a stimulus rate of 1 Hz or slower. Thus, the typical repetitive stimulation done during neuromuscular junction testing is best avoided.
• Consult a cardiologist regarding performing studies in patients with an implantable automatic cardioverter-defibrillator.
• Laboratory emergency drugs should be available, including crash carts.
From Al-Shekhlee, A., Shapiro, B.E., Preston, D.C., 2003. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve 27, 517–526, with permission.
Pneumothorax
Pneumothorax is the most potentially serious iatrogenic complication of needle EMG. At any time during or just after the EMG examination, unexpected chest pain, shortness of breath, or cyanosis in a patient should alert the electromyographer to the possibility of a pneumothorax. If such symptoms develop, a prompt chest X-ray film is indicated to confirm the diagnosis, followed by urgent consultation with a thoracic surgeon as to whether a chest tube or observation is required. Although rare, this complication has been reported when sampling the following muscles (Figure 40–7):
• Diaphragm. Needle EMG of the diaphragm is sometimes used to help determine whether respiratory insufficiency has a neuromuscular basis. However, because the pleural fold is in close proximity to the diaphragm, a relatively small error in needle position may increase the risk of inadvertent pleural puncture and possible pneumothorax. The decision to sample the diaphragm must depend on the experience of the electromyographer and the potential benefit to the patient versus the risk of pneumothorax in that particular patient. Because patients for whom this study is ordered often have respiratory problems that prompt the study to be ordered, they may be the least able to handle an additional respiratory complication. In this text, we have purposely not included needle EMG of the diaphragm in Chapter 13. In our opinion, the risk-to-benefit ratio of sampling this muscle is too high to justify its use as a routine muscle to be sampled.
• Serratus anterior. The serratus anterior muscle lies between the scapula and the chest wall and inserts laterally on the ribs. An inadvertent puncture through the muscle between the ribs may allow the needle to enter the pleural space. To reduce the possibility of pneumothorax, the muscle can be sampled with the electromyographer’s fingers placed in two adjacent inter-rib spaces while the needle is inserted into the muscle directly over the rib.
• Supraspinatus. The supraspinatus muscle lies within the supraspinous fossa of the scapula. The middle of the fossa may be very shallow in some individuals. Thus, if the muscle is sampled too deeply at this point, the needle may puncture the pleura (Figure 40–8). Complications can be prevented by either avoiding the muscle altogether or sampling it more medially in the supraspinous fossa. This is performed by first palpating the acromion, the spine of the scapula, and the vertebral border of the scapula. The needle is then inserted just above the spine of the scapula at a point three quarters of the distance from the acromion to the vertebral border of the scapula. Often the supraspinatus can be avoided by sampling the infraspinatus instead. The infraspinatus muscle and infraspinous fossa are much larger than the supraspinatus and supraspinous fossa above. When screening for a suprascapular neuropathy, the infraspinatus muscle is the preferred muscle to study. Only if the infraspinatus muscle is abnormal is it then necessary to sample the supraspinatus to differentiate a lesion at the spinoglenoid notch from one at the suprascapular notch or above (see Chapter 31).
• Rhomboids. The rhomboids are infrequently sampled. However, they are useful to study in two situations: (1) to differentiate a C5 from a C6 radiculopathy (the rhomboids are derived from the C4–C5 roots), and (2) to differentiate an upper trunk brachial plexopathy from a more proximal radiculopathy (the rhomboids are innervated by the dorsal scapular nerve, which arises directly off the nerve roots proximal to the brachial plexus). Because the rhomboids originate on the dorsal spine and insert onto the medial border of the scapula, a needle placed too deeply may pass through the rhomboids and thoracic paraspinal muscles resulting in a pleural puncture.
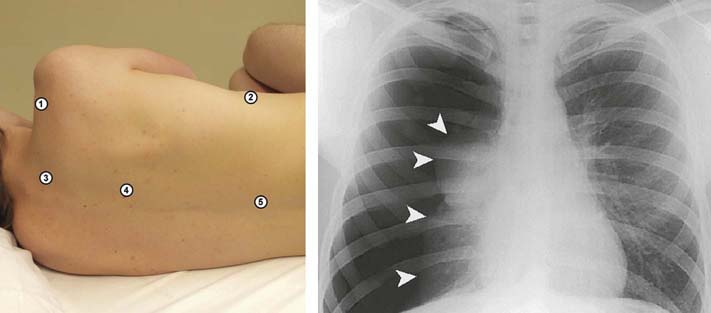
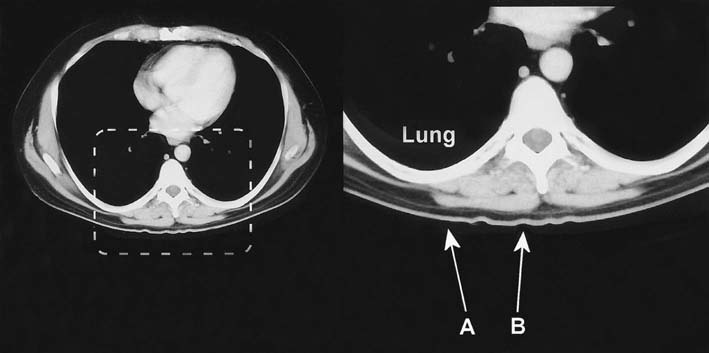
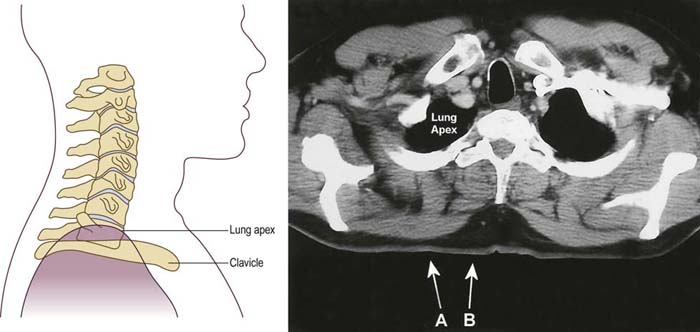
• Cervical and thoracic paraspinal muscles. The cervical paraspinal muscles are commonly sampled in the evaluation of cervical radiculopathy. Thoracic paraspinal muscles are one of the key sites to study in the evaluation of suspected motor neuron disease. These muscles can be safely studied, provided the needle placement is neither too lateral nor too deep. Considering the proximity of the thoracic paraspinal muscles to the lungs in the thorax, it is not unexpected that pneumothorax is a potential complication of thoracic paraspinal muscle sampling (Figure 40–9). However, pneumothorax can also occur during EMG examination of the lower cervical paraspinal muscles or when an EMG needle is used for cervical nerve root stimulation. Some patients, especially those who are thin with longer necks, may have lung tissue that reaches above the clavicle (Figure 40–10). In one study of 23 patients, 22% had lung tissue above the level of the clavicle. The average distance between skin and lung in these individuals was 3.3 cm, a distance clearly within the reach of a conventional 37 or 50 mm EMG needle. This complication is easily prevented by ensuring that the needle remains close to the midline, within the bulk of the paraspinal muscles.
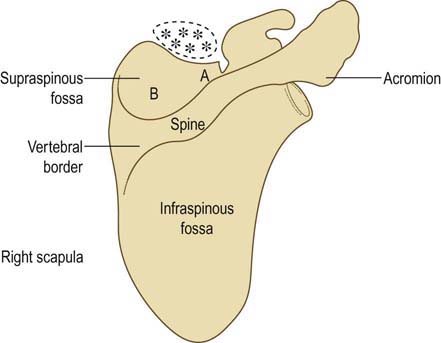
FIGURE 40–8 Supraspinatus muscle and risk of pneumothorax.
(Adapted from Reinstein, L., Twardzik, F.G., Mech, K.F. Jr., 1987. Pneumothorax: complication of needle electromyography of supraspinatus muscle. Arch Phys Med Rehabil 68, 561–562, with permission.)
Bleeding
Risk of Bleeding
Recommendations
Needle Electromyography and Patients at Risk of Bleeding
• Use the smallest gauge EMG needle available (e.g., 30 gauge).
• Limit the study to a few superficial muscles where prolonged compression over a puncture site can be performed if necessary.
• Avoid deep muscles that cannot be manually compressed and theoretically could result in a compartment syndrome if a hematoma developed. Most important among these are the antecubital fossa muscles (i.e., pronator teres and flexor carpi radialis), tibialis posterior, and flexor digitorum longus.
• Avoid muscles where hematomas theoretically could compress adjacent neurologic structures. Most important among these are the gluteal muscles near the sciatic nerve and the paraspinal muscles near the exiting spinal nerves.
• Avoid muscles with large arteries or veins located nearby so that inadvertent puncture of the vessel does not occur. Most important among these are the flexor pollicis longus near the radial artery, the iliacus near the femoral artery/vein, and the antecubital fossa muscles near the brachial artery.
Infection
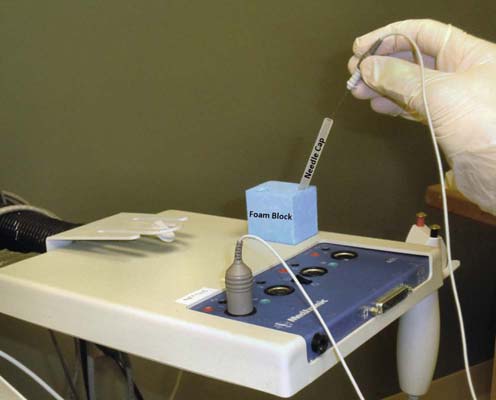
Inadvertent needle sticks are a risk during needle EMG. Transmissible diseases include HIV as well as other infections, especially viral hepatitis, underscoring the importance of hepatitis B vaccinations for all electromyographers. Similar to precautions used with any needle, the EMG needle should not be recapped using the contralateral hand. The risk of a needle stick is markedly reduced if the needle is placed safely out of the way when it is not being used (e.g., in between sampling muscles or when explaining the next muscle movement to the patient). In our laboratory, we successfully use a foam rubber block attached to the preamp arm of the EMG machine (Figure 40–11). The block holds the needle cap so that the needle can be recapped safely with one hand.
Local Injury
AAEM guidelines in electrodiagnostic medicine. Risks in electrodiagnostic medicine. Muscle Nerve. 1999;22:S53–S58.
Al-Shekhlee A., Shapiro B.E., Preston D.C. Iatrogenic complications and risks of nerve conduction studies and needle electromyography. Muscle Nerve. 2003;27:517–526.
Bolton C.F. Electromyographic studies in special settings. In: Brown W.F., Bolton C.F. Clinical electromyography. second ed. Stoneham, MA: Butterworth-Heinemann; 1993:770–774.
Boon A.J., Gertken J.T., Watson J.C., et al. Hematoma risk after needle electromyography. Muscle Nerve. 2012;45:9–12.
Butler M.L., Dewan R.W. Subcutaneous hemorrhage in a patient receiving anticoagulation therapy: an unusual EMG complication. Arch Phys Med Rehabil. 1984;65:733–734.
Caress J.B., Rutkove S.B., Carlin M., et al. Paraspinal muscle hematoma after electromyography. Neurology. 1997;47:269–272.
Cheema P., El-Mefty O., Jazieh A.R. Intraoperative haemorrhage associated with the use of extract of Saw Palmetto herb: a case report and review of literature. J Intern Med. 2001;250:167–169.
Davison B.L., Kosmatka P.K., Ferlic R.J. Acute radial nerve compression following routine venipuncture in an anticoagulated patient. Am J Orthop. 1996;25:712–713.
Farrell C.M., Rubin D.I., Haidukewych G.J. Acute compartment syndrome of the leg following diagnostic electromyography. Muscle Nerve. 2003;27:374–377.
Gertken J.T., Hunt C.H., Montes Chinea N.I., et al. Risk of hematoma following needle electromyography of the paraspinal muscles. Muscle Nerve. 2011;44:439–440.
Gruis K.L., Little A.A., Zebarah V.A., et al. Survey of electrodiagnostic laboratories regarding hemorrhagic complications from needle electromyography. Muscle Nerve. 2006;34(3):356–358.
Hawley R.J. Preventing complications of electromyography. Electromyogr Clin Neurophysiol. 2000;40:323–325.
Honet J.E., Honet J.C., Cascade P. Pneumothorax after electromyographic electrode insertion in the paracervical muscles: case report and radiological analysis. Arch Phys Med Rehabil. 1986;67:601–603.
Horowitz S.H. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology. 1994;44:962–964.
LaBan M.M., Petty D., Hauser A.M., et al. Peripheral nerve conduction stimulation: its effect on cardiac pacemakers. Arch Phys Med Rehabil. 1988;69:358–362.
Lynch S.L., Boon A.J., Smith J., et al. Complications of needle electromyography: hematoma risk and correlation with anticoagulation and antiplatelet therapy. Muscle Nerve. 2008;38(4):1225–1230.
Mellion M.L., Buxton A.E., Iyer V., et al. Safety of nerve conduction studies in patients with peripheral intravenous lines. Muscle Nerve. 2010;42(2):189–191.
Miller J. Pneumothorax. Complication of needle EMG of thoracic wall. N J Med. 1990;87:653.
Nora L.M. American Association of Electrodiagnostic Medicine guidelines in electrodiagnostic medicine: implanted cardioverters and defibrillators. Muscle Nerve. 1996;19:1359–1360.
O’Flaherty D., Adams A.P. Pacemaker failure and peripheral nerve stimulation. Anaesthesia. 1994;49:181.
O’Flaherty D., Wardill M., Adams A.P. Inadvertent suppression of a fixed rate ventricular pacemaker using a peripheral nerve stimulator. Anaesthesia. 1993;48:687–689.
Parziale J.R., Marino A.R., Herndon J.H. Diagnostic peripheral nerve block resulting in compartment syndrome. Am J Phys Med Rehabil. 1988;67:82–84.
Preston D., Logigian E. Iatrogenic needle-induced peroneal neuropathy in the foot. Ann Intern Med. 1988;109:921–922.
Raj G., Kumar R., McKinney W. Safety of intramuscular influenza immunization among patients receiving long-term warfarin anticoagulation therapy. Arch Intern Med. 1995;155:1529–1531.
Reinstein L., Twardzik F.G., Mech K.F., Jr. Pneumothorax: complication of needle electromyography of supraspinatus muscle. Arch Phys Med Rehabil. 1987;68:561–562.
Roberge R.J., McLane M. Compartment syndrome after simple venipuncture in an anticoagulated patient. J Emerg Med. 1999;17:647–649.
Rosioreanu A., Dickson A., Lypen S., et al. Pseudoaneurysm of the calf after electromyography: sonographic and CT angiographic diagnosis. Am J Roentgenol. 2005;185:282–283.
Sander H.W., Quinto C.M., Murali R., et al. Needle cervical root stimulation may be complicated by pneumothorax. Neurology. 1997;48:288–289.
Schoeck A.P., Mellion M.L., Gilchrist J.M., et al. Safety of nerve conduction studies in patients with implanted cardiac devices. Muscle Nerve. 2007;35(4):521–524.
Starmer C.F., McIntosh H.D., Whalen R.E. Electrical hazards and cardiovascular function. N Engl J Med. 1971;284:181–186.
Vaienti L., Vourtsis S., Urzola V. Compartment syndrome of the forearm following an electromyographic assessment. J Hand Surg Br. 2005;30(6):656–657.