CHAPTER 73 Elbow Disarticulation Amputation
INTRODUCTION
Over the years, advances in upper extremity prosthetics have included improved surgical techniques, preoperative management, postoperative management, and advances in prosthetic technology. In the past decade, the greatest advances have occurred in prosthetic technologies, fabrication techniques, and components to more effectively replace the lost function of the extremity.7
DEMOGRAPHICS
Trauma is the leading cause of upper extremity limb loss, accounting for 80% of upper extremity amputations. Tumor is the most common cause of upper extremity amputation in children. From 1988 to 1996, the rate of trauma and cancer-related amputations declined. This decline was likely due to improved surgical reconstruction, advances in limb-sparing techniques, and prevention through improved occupational safety awareness.6
Following trauma, the decision to attempt limb salvage or proceed with amputation is complex. There is a bias toward limb salvage in upper extremity trauma surgery. The functional demands of the upper extremity are different from the lower extremity. Lack of weight bearing forces, the ability to function with partial sensation, and limited function of upper extremity prostheses are reasons sited for limb salvage and reimplantation.8
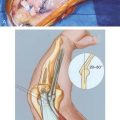
ELBOW DISARTICULATION AMPUTATION
In children with upper limb deficiency or amputation, growth and development, bony overgrowth and more rigorous use of a prosthetic device need to be considered. An elbow disarticulation amputation level for this population optimizes residual limb length and avoids bony overgrowth. The slowed humeral growth after elbow disarticulation results in a humeral length at maturity that allows the use of a prosthetic elbow while retaining the suspension and rotational control of an elbow disarticulation.1 In children, transhumeral amputation results in a high incidence of bony overgrowth. An elbow disarticulation preserves the epiphysis, prevents bony overgrowth, and maintains growth potential; therefore, elbow disarticulation is the level of choice.2
AMPUTATION SURGERY
NERVES AND BLOOD VESSELS
Meticulous hemostasis is mandatory to avoid postoperative hematoma formation. All wounds are drained for 48 hours postoperatively.
BONE
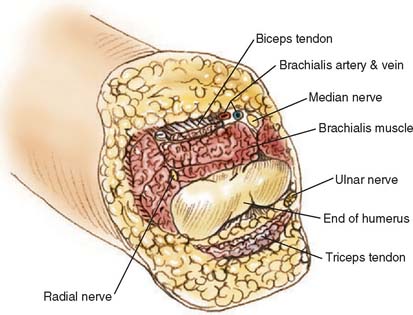
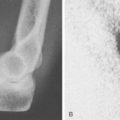
Through-elbow amputation is carried out as a true disarticulation (Fig. 73-1). Minor contouring of the margins of the distal humerus is usually required to eliminate sharp condylar prominences. For above-elbow amputations, the bone edges should be slightly beveled so that there are no sharp prominences or rough bone edges.
MUSCLE STABILIZATION
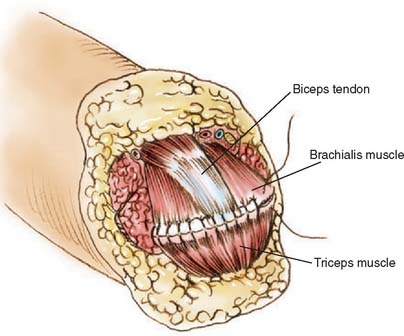
Whenever possible, the sectioned muscles and tendons are sewn to each other over the end of the humerus at the amputation site (Fig. 73-2). If possible, the triceps tendon and aponeurosis is retained and brought forward through and over the trochlea to be sewn under minimal tension to the brachialis muscles. The biceps tendon also can be interwoven into the brachialis muscle near the amputation site, giving excellent residual limb muscle control.
NERVE TRANSFERS
In an attempt to improve functional control of myoelectric prostheses, the possibility of transferring residual nerves to spare muscles in or near the residual limb has been studied. Using nerves to reinnervate targeted muscle, the surface myoelectric signals from these muscles have been used to simultaneously control multiple degrees of freedom in a prosthesis.13
Research into this concept has been promising. Studies have shown that when large nerves (such as the brachial plexus nerves) are transferred onto relatively small muscle areas, the recovery of the muscle is very good.12 With targeted reinnervation, control of a prosthesis will be easier and more natural because the myoelectric signals are physiologically correlated to the movements of the lost arm. Although targeted reinnervation shows exciting potential, ongoing research is needed.13
ACUTE POST-AMPUTATION MANAGEMENT
Following surgery, the goals of the preprosthetic period are to
Immediate and early postsurgical prosthetic fitting provides edema control, pain reduction, and protects the surgical incision.14 Successful prosthetic use is higher when fitting is completed within a “golden period” of 30 days after surgery.14 If prosthetic fitting and training are delayed, the patient can become adept at one-handed techniques, making it difficult to incorporate a prosthesis in their daily living activities. A second study has shown that even delayed fitting can be successful.22 Common reasons for rejecting a prosthetic limb include the perception of limited usefulness, excessive weight, and residual limb pain.
PROSTHETIC MANAGEMENT
Careful surgery, evaluation, prosthetic fitting, and training are critical to successful outcome and optimal prosthetic function. The long-term rate of prosthetic wear for transhumeral and elbow disarticulation amputations is less than 50%.16 Although a prosthesis is not necessary for an amputee to function, recent studies suggest that overuse in the remaining hand and arm occurs in up to 50% of persons with upper limb amputations.10 Active use of a prosthesis is believed to reduce this risk.
Because it is difficult to predict long-term acceptance of an upper limb prosthesis or an individual’s preferred type of prosthetic system (body or external powered), the initial definitive prosthesis should be used to explore prosthetic options. Individuals with a recent upper extremity amputation should be allowed time to explore advantages and disadvantages using a prosthesis and different terminal devices in a variety of home, work, and social settings.6
The difference between body-powered and electric components should be reviewed. Advantages of a body-powered prosthesis with a hook terminal device include lighter weight, better durability, increased sensory feedback, less expense, and greater ease in seeing the manipulated object. Advantages of a myoelectric prosthesis include better appearance, moderate or no harnessing, less body movement to operate the prosthesis, the ability to reach overhead, better grip strength, and the ability to grasp larger objects.3 If the patient’s workplace has magnetic fields or large electrical currents, a myoelectric prostheses may not function unless special shielding materials are used during fabrication to prevent interference.
TERMINAL DEVICE
The human hand is a very complex anatomic and physiologic structure whose function cannot be replaced by the current level of prosthetic technology. A variety of prosthetic terminal devices are available including passive, body powered, and externally powered hooks and hands. The most commonly prescribed passive terminal device is the passive hand. Many passive hands have bendable or spring loaded fingers that can provide a static grasp for objects. Passive hands do not require cables or batteries for operation; they are light in weight and have a socially acceptable appearance. Prosthetic hands provide a three-jaw chuck pinch. Prosthetic hooks provide the equivalent of lateral or tip pinch.
Body-powered terminal devices transmit grasping forces and proprioception (hand positioning) from the harness and cables used to operate the hand.17 Externally powered devices can have a digital or proportional (stronger signal/faster action) control system.
Traditional myoelectric prosthetic hands do not provide feedback regarding the force exerted on a grasped object. The degree of control is imprecise; often more force is applied than necessary.17 Although the kinematics of currently available hand systems have not changed a great deal, significant progress has been made in control options to allow greater precision and ease of operation. A new feedback system with a miniature vibration motor, a piezoresistive force sensor, and control electronics has improved the ability to regulate grasping without the help of vision. The results suggest that more precise control and grasping force are possible with a feedback system.17
Multiple control options are now available, allowing better customization of prosthetic systems to the demands of individual users. Using a computer, a prosthetist can modify the parameters of every component integrated into the prosthesis. State-of-the-art hands have integrated sensors that reduce the user’s need to concentrate on controlling the grasping action.4 Onboard sensors for hand control can signal when to adjust grasping force (magnitude and direction), opening width, and speed of movement.
PROSTHETIC SOCKET
Advances in prosthetic materials such as acrylic laminates, carbon graphite, and flexible thermoplastics allow prostheses to be more comfortable, lighter, and more durable. An elbow disarticulation socket is broad and flat distally to conform to the anatomic configuration of the distal humerus (Fig. 73-3). A total contact interface should be attempted at the elbow disarticulation level to allow efficient energy transfer from the residual limb to the prosthetic device. This design provides some self-suspension and allows active rotation of the prosthesis (internal and external rotation of the humerus).
Recent use of silicone materials for the fabrication of prosthetic sockets has expanded the fitting options for upper limb amputees. Silicone suction technology allows suction suspension. Material thickness, stiffness, and color can be precisely controlled.20
SUSPENSION SYSTEM
The goal of suspension systems is to secure a prosthesis to the body. The elbow disarticulation prosthesis can be suspended by a harness, suction, anatomic, or a silicone sleeve suspension. The traditional figure-of-eight chest strap and shoulder saddle harnesses provide suspension and control of body-powered prostheses (Fig. 73-4). A figure-of-eight harness provides the greatest available excursion, but it can create pressure problems in the contralateral axilla. A shoulder saddle and chest strap suspension/control system reduces axillary forces and provides better lifting capability but does not harness contralateral shoulder excursion. Typically, a split Pelite insert, a windowed socket, or a flexible wall socket is used to provide entry and anatomic suspension at the elbow disarticulation level.
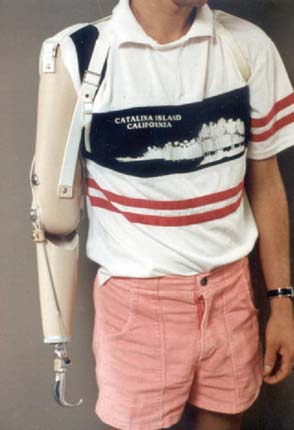
FIGURE 73-4 Body-powered elbow disarticulation prostheses with a figure-of-eight suspension and control system.
(Courtesy of Mike Gozola, C.P., Prosthetic Laboratories, Rochester, MN.)
Suction suspension is often used in conjunction with externally powered components to eliminate or reduce the amount of harnessing necessary. If the patient has a great deal of scarring or decreased skin integrity, a thicker liner can be used to enhance soft tissue supplementation. Liner thicknesses range from 3 to 9 mm. These suspensions also provide excellent skin protection and suspension for patients who are very active users.
FUNCTIONAL OUTCOMES
Prosthetic-specific, adult functional status measures need to be developed and validated. Building a core set of outcome measures will provide a comprehensive picture of different aspects of hand function and prosthetic use.21
SUMMARY
The rehabilitation process for a person with an elbow disarticulation is best accomplished with a comprehensive, multidisciplinary, specialized treatment team. A well-fitting prosthesis with appropriate components, supervised training, and ongoing follow-up optimizes prosthetic use and function. Targeted reinnervation and other research on the control of upper limb prostheses hold promise to allow more agile, intuitive control systems to increase prosthetic function, use, and quality of life for persons with limb loss.13
1 Abraham E., Pellicor J.R., Hamilton R.C., Hallman D.W., Ghosh L. Stump overgrowth in juvenile amputees. J. Pediatr. Orthop. 1986;6:66.
2 Atkin G.T. Surgical amputation in children. J. Bone Joint Surg. Am. 1963;45:1735.
3 Atkins D. Managing self-care in adults with upper extremity amputations. In: Christian C., editor. Ways of Living: Self-Care Strategies for Special Needs. 2nd ed. Bethesda, MD: American Occupational Therapy Association; 2000:221.
4 Chappell P.H., Kyberd P.J. Prehensile control of a hand prosthesis by microcontroller. J. Biomed. Eng. 1991;13:363.
5 Daly W. Clinical applications of roll-on sleeves for myoelectrically-controlled transradial and transhumeral prostheses. J. Prosthet. Orthot. 2000;12:88.
6 DeLisa J.A. Physical Medicine and Rehabilitation Principles and Practices, 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2005.
7 Dillingham T.R., Pezzin L.E., MacKenzie E.J. Limb amputations and limb deficiency—epidemiology and most recent trends in the United States. South. Med. J. 2002;95:75.
8 Graham B., Adtkins P., Tsai T.M. Major reimplantation versus revision amputation and prosthetic fitting in the upper extremity—a late functional outcome study. J. Hand Surg. 1998;23:783.
9 Hoffer J.A., Loeb G.E. Implantable electrical and mechanical interfaces with nerve and muscle. Ann. Biomed. Eng. 1980;8:351.
10 Jones L.E., Davidson J.H. Save that arm: a study of problems in the remaining arm of unilateral upper limb amputees. Prosthet. Orthot. Int. 1999;23:55.
11 Kuiken T.A. Use of nerve-muscle grafts to improve the control of artificial limbs. J. Technol. Disabil. 2003;15:105.
12 Kuiken T.A., Rymer W.Z., Childress D.S. The hyper-reinnervation of rat skeletal muscles. Brain Res. 1995;1676:113.
13 Lipschutz R.D., Kuiken T.A., Miller L.A., Dumanian G.A., Stubblefield K.A. Shoulder disarticulation externally powered prosthetic fitting following targeted muscle reinnervation for improved myoelectric control. J. Prosthet. Orthot. 2006;18:28.
14 Malone J.M., Fleming L.L., Roberson J., Whitesides T.E.Jr, Leal J.M., Poole J.U., Grodin R.S. Immediate, early, and late post-surgical management of upper limb amputation. J. Rehabil. Res. Dev. 1984;21:33.
15 Michael J.W., Bowker J.H. Atlas of Amputations and Limb Deficiencies – Surgical, Prosthetic, and Rehabilitation Principles, 2nd ed. St. Louis: Mosby-Year Book, Inc, 1992.
16 Pinzur M.S., Angeltas J., Light T.R., Izuierdor R., Pluthat T. Functional outcome following traumatic upper limb amputation and prosthetic limb fitting. J. Hand Surg. 1994;19:836.
17 Pylatiuk C., Kargov A., Schulz S. Design and evaluation of a low-cost force feedback system for myoelectric prosthetic hands. J. Prosthet. Orthot. 2006;18:57.
18 Salam Y. The use of silicone suspension sleeves with myoelectric fitting. J. Prosthet. Orthop. 1994;6:119.
19 Smith D.G., Michael J.W., Bowker J.H., editors. Atlas of Amputations and Limb Deficiencies—Surgical, Prosthetic, and Rehabilitation Principles, 3rd ed., Rosemont, IL: American Academy of Orthopedic Surgeons, 2004.
20 Uellendahl J.E., Mandacina S., Ramdial S. Custom silicone sockets for myoelectric prostheses. J. Prosthet. Orthot. 2006;18:35.
21 Wright F.V. Measurement of functional outcome with individuals who use upper extremity prosthetic devices: current and future directions. J. Prosthet. Orthot. 2006;18:46.
22 Wright T.W., Hagen A.D., Wood M.B. Prosthetic usage in major upper extremity amputations. J. Hand Surg. 1995;28:619.