Eikenella and Similar Organisms
1. Identify and explain the key morphologic and biochemical characteristics for Eikenella corrodens.
2. Describe the normal habitat for Eikenella spp. and situations that provide optimal conditions for the opportunistic bacteria to become a pathogen.
3. Define the acronym HACEK; what organisms does this acronym refer to, and what medical conditions are associated with these organisms?
4. Define the general characteristics for Eikenella corrodens, Methylobacterium spp., Weeksella virosa, and Bergeyella zoohelcum, and explain how the organisms are distinguished from one another.
5. Describe the Gram stain characteristics for each type of bacteria listed in objective 4.
6. Identify the normal habitat for Methylobacterium and explain why the organism is frequently isolated from water distribution systems.
7. Explain how the pink colonies produced in culture by Methylobacterium spp. are differentiated from other species of bacteria capable of producing pink colonies.
8. Describe the culture techniques used to isolate Eikenella corrodens and Methyolobacterium spp.
9. Correlate patient signs, symptoms, and laboratory results to identify the most probable etiologic agent associated with the data.
Epidemiology, Spectrum of Disease, and Antimicrobial Therapy
The organisms listed in Table 29-1 are not commonly associated with human infections, but they are occasionally encountered in clinical specimens. Eikenella corrodens is normal flora of the human oral cavity. The organism is a facultative anaerobe, nonmotile, gram-negative rod. Among the organisms considered in this chapter, it is the organism most frequently isolated and is usually found in mixed infections resulting from human bites or clenched-fist wounds. The organism can be isolated from dental plaque and has been implicated in periodontitis, osteomyelitis, bite wound infections, bacteremia, and endocarditis. It is an opportunistic pathogen predominantly in immunocompromised patients, causing abscesses and infections, and may lead to death. Patients with diabetes are often at risk for Eikenella infections as a result of the daily microtrauma to their skin via glucose monitoring, insulin injections, and the potential for introduction of the organism from oral secretions by licking or biting their skin. The organism is often the cause of soft tissue infections in intravenous drug abusers who lick the injection site.
TABLE 29-1
Epidemiology, Pathogenesis, and Spectrum of Disease
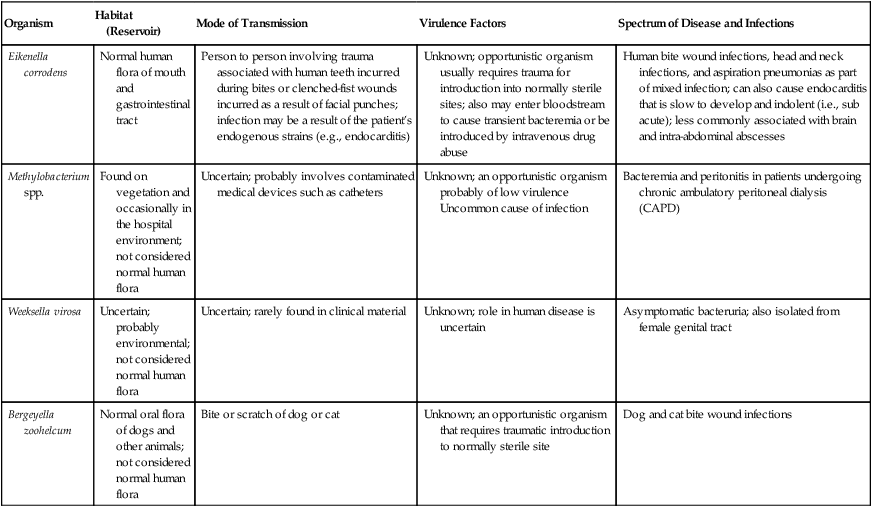
| Organism | Habitat (Reservoir) | Mode of Transmission | Virulence Factors | Spectrum of Disease and Infections |
| Eikenella corrodens | Normal human flora of mouth and gastrointestinal tract | Person to person involving trauma associated with human teeth incurred during bites or clenched-fist wounds incurred as a result of facial punches; infection may be a result of the patient’s endogenous strains (e.g., endocarditis) | Unknown; opportunistic organism usually requires trauma for introduction into normally sterile sites; also may enter bloodstream to cause transient bacteremia or be introduced by intravenous drug abuse | Human bite wound infections, head and neck infections, and aspiration pneumonias as part of mixed infection; can also cause endocarditis that is slow to develop and indolent (i.e., sub acute); less commonly associated with brain and intra-abdominal abscesses |
| Methylobacterium spp. | Found on vegetation and occasionally in the hospital environment; not considered normal human flora | Uncertain; probably involves contaminated medical devices such as catheters | Unknown; an opportunistic organism probably of low virulence Uncommon cause of infection |
Bacteremia and peritonitis in patients undergoing chronic ambulatory peritoneal dialysis (CAPD) |
| Weeksella virosa | Uncertain; probably environmental; not considered normal human flora | Uncertain; rarely found in clinical material | Unknown; role in human disease is uncertain | Asymptomatic bacteruria; also isolated from female genital tract |
| Bergeyella zoohelcum | Normal oral flora of dogs and other animals; not considered normal human flora | Bite or scratch of dog or cat | Unknown; an opportunistic organism that requires traumatic introduction to normally sterile site | Dog and cat bite wound infections |
This organism also is the “E,” for Eikenella, in the HACEK group of bacteria known to cause subacute bacterial endocarditis (see Chapter 68 for more information regarding endocarditis and bloodstream infections). HACEK is an acronym used to represent the slow-growing gram-negative bacilli associated with endocarditis. The additional members of the HACEK group of bacteria include Aggregatibacter aphrophilus, Actinobacillus actinomycetemcometans, Cardiobacterium hominis, and Kingella kingae.
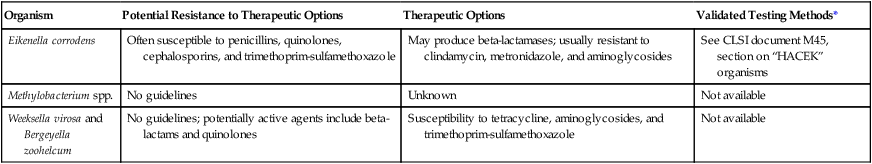
The rarity with which these organisms are encountered in the clinical laboratory and the lack of validated in vitro susceptibility testing methods do not provide enough data to recommend definitive treatment guidelines (Table 29-2). Although ß-lactamase production has been described in E. corrodens, this species is usually susceptible to penicillin and other ß-lactam antimicrobials. Penicillin-resistant strains of E. corrodens have been identified.
TABLE 29-2
Antimicrobial Therapy and Susceptibility Testing
| Organism | Potential Resistance to Therapeutic Options | Therapeutic Options | Validated Testing Methods* |
| Eikenella corrodens | Often susceptible to penicillins, quinolones, cephalosporins, and trimethoprim-sulfamethoxazole | May produce beta-lactamases; usually resistant to clindamycin, metronidazole, and aminoglycosides | See CLSI document M45, section on “HACEK” organisms |
| Methylobacterium spp. | No guidelines | Unknown | Not available |
| Weeksella virosa and Bergeyella zoohelcum | No guidelines; potentially active agents include beta-lactams and quinolones | Susceptibility to tetracycline, aminoglycosides, and trimethoprim-sulfamethoxazole | Not available |
*Validated testing methods include those standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and those commercial methods approved by the Food and Drug Administration (FDA).
Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport for the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Direct Detection Methods
Other than Gram stain and microscopic examination, there are no specific procedures for the direct detection of these organisms in clinical material. E. corrodens is a slender, medium-length gram-negative, straight rod with rounded ends. Methylobacterium is a vacuolated, pale-staining, short to medium-length gram-negative bacillus that may resist decolorization. Weeksella virosa and Bergeyella zoohelcum are medium to long gram-negative rods with parallel sides and rounded ends that may form “II-forms” (parallel sides) similar to the Sphingobacterium (see Chapter 24 for more information regarding this genus).
Cultivation
Media of Choice
Colonial Appearance
Table 29-3 describes the colonial appearance and other distinguishing characteristics (e.g., odor and pigment) of each genus on 5% sheep blood agar.
TABLE 29-3
Colonial Appearance and Characteristics
| Organism | Medium* | Appearance |
| Bergeyella zoohelcum | BA | Colonies may be sticky; tan to yellow in color |
| Eikenella corrodens | BA | Colonies are tiny at 24 hours; mature colonies have moist, clear centers surrounded by flat, spreading growth; colonies may pit or corrode the agar surface; slight yellow pigmentation in older cultures; sharp odor of bleach |
| Methylobacterium spp. | BA | Pink to coral pigment; does not grow well on blood agar |
| Weeksella virosa | BA | Small colonies at 24 hours; mature colonies mucoid and adherent with a tan to brown pigment |
BA, 5% sheep blood agar.
Approach to Identification
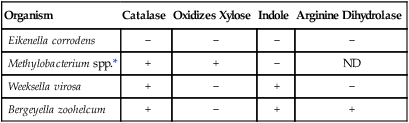
The ability of most commercial identification systems to accurately identify the organisms discussed in this chapter is limited or, at best, uncertain. Therefore, strategies for identification of these genera are based on the use of conventional biochemical tests. Table 29-4 outlines basic criteria useful for differentiating the genera discussed in this chapter.
TABLE 29-4
Key Biochemical and Physiologic Characteristics
| Organism | Catalase | Oxidizes Xylose | Indole | Arginine Dihydrolase |
| Eikenella corrodens | − | − | − | − |
| Methylobacterium spp.* | + | + | − | ND |
| Weeksella virosa | + | − | + | − |
| Bergeyella zoohelcum | + | − | + | + |
ND, No data; +, >90% of strains positive; −, >90% of strains negative.
*Colonies are pigmented pink and must be differentiated from Roseomonas spp.; Roseomonas spp. usually grow on MacConkey agar and will grow at 42° C.
Data compiled from Weyant RS, Moss CW, Weaver RE, et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1997, Williams & Wilkins.







