Echocardiography in cardiac arrest
Overview
The incidence of out-of-hospital cardiac arrest is estimated to be 50 to 55 per 100,000 in the United States.1 When a primary electrical cause of cardiac arrest is found, definitive treatment is directed toward reversing the rhythm disturbance (cardioversion, defibrillation, pacing). When a nonelectrical cause of cardiac arrest is diagnosed (pulseless electrical activity [PEA]/asystole), practitioners are urged to exclude and treat potentially reversible causes because otherwise the outlook is poor (Figure 31-1).2 Of the reversible causes, only hypoxia, hypokalemia, hyperkalemia, and hypothermia may be diagnosed definitively with existing standard point-of-care testing. Hypovolemia, tamponade, pulmonary embolism (PE), coronary thrombosis, and tension pneumothorax are diagnosed primarily clinically and toxins by laboratory investigations.
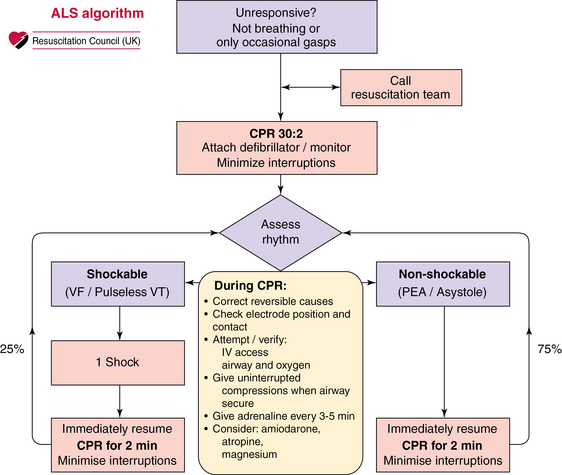
Figure 31-1 Advanced life support (ALS) algorithm. On the right side of the algorithm, in the nonshockable rhythm, the team is urged to seek and correct reversible causes of cardiac arrest. Such causes include the four H’s and four T’s: hypovolemia, hypocapnia/hypercapnia, hypoxemia, hypothermia, tamponade, thrombosis (coronary/pulmonary), toxins, and tension pneumothorax.
Appropriately applied and interpreted transthoracic echocardiography (TTE) may be used to diagnose or exclude many potentially reversible causes of cardiac arrest while guiding potentially lifesaving therapeutic interventions.3 Echocardiography has been used in several studies to determine the presence or absence of cardiac activity; indeed, in some cases it has been shown to predict outcome. Moreover, echocardiography facilitates the diagnosis of true electromechanical dissociation (EMD)—the absence of effective cardiac activity despite electrical activity, which in and of itself may also determine the probable outcome of resuscitation.4 Although the American Heart Association continues to recommend that the history and clinical examination be used to direct management of PEA/asystole (low sensitivity and specificity),5 the 2010 International Liaison Committee on Resuscitation (ILCOR) guidelines suggest that ultrasound imaging may help identify reversible causes of cardiac arrest and, when appropriately trained personnel are available, should be used to assist in the assessment and treatment of potentially reversible causes of cardiac arrest.6
Echocardiography for the diagnosis of cardiac arrest
Traditionally, detection of cardiac output is achieved by palpation of central pulses or by noninvasive blood pressure measurement, although both these methods have been shown to be imprecise,7,8 with up to 45% of health care professionals being unable to accurately assess central pulses during cardiac arrest, which can potentially result in prolonged periods with no chest compressions and premature cessation of resuscitation.9 Periresuscitation echocardiography has not only been shown to identify the presence or absence of cardiac motion during resuscitation but can also be performed without detracting from the advanced life support (ALS) protocol.10
Echocardiography and the underlying cause of cardiac arrest
The potential causes of cardiac arrest that can be diagnosed with TTE include tamponade, coronary artery disease, PE, and hypovolemia. Extension of ultrasound imaging beyond the heart can be used to diagnose or exclude pneumothorax with lung views.10,11
Hypovolemia
Left ventricular (LV) end-diastolic volume has been shown to correlate well with blood loss and can reportedly be used to detect small changes in intravascular volume.12 In the critically ill, a number of parameters have been found to indicate severe hypovolemia, including the presence of a small hyperkinetic left ventricle (Figure 31-2) (and a normal right ventricle) with obliteration of the cavity at end-systole,12 an LV end-diastolic area of less than 5.5 cm2/m2 body surface area (BSA), a small inferior vena cava (IVC) with inspiratory collapse in spontaneously breathing patients,13 or a small IVC at end-expiration14 with variable respiratory changes in mechanically ventilated patients.15 Hypovolemia leading to cardiac arrest is likely to be severe, and although there is debate in the literature regarding the sensitivity and specificity of echocardiographic features of hypovolemia in the arrested state, the finding of a small, underfilled left ventricle with collapsed caval veins suggests the need for aggressive volume resuscitation and a search for the cause of the hypovolemia.16
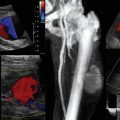
Tamponade and pericardiocentesis
The physiologic features of tamponade result from an increase in intrapericardial pressure causing impaired filling and an adverse effect on cardiac function. Echocardiographic features that indicate hemodynamic significance of a collection include the presence of a swinging heart, right ventricular (RV) diastolic collapse, right atrial diastolic collapse, pseudo-SAM (systolic anterior wall motion abnormality), an enlarged nonpulsatile vena cava (all parts of the respiratory cycle), reciprocal changes in the size of cardiac chambers, and transvalvular flow that varies with respiration.17 Demonstration of these echocardiographic features of tamponade has erroneously become synonymous with the diagnosis of tamponade, which is a clinical diagnosis. In cardiac arrest, demonstration of a pericardial collection (Figure 31-3) should lead to consideration of immediate drainage since the echocardiographic features of tamponade may not be present in certain circumstances (particularly following cardiac surgery).
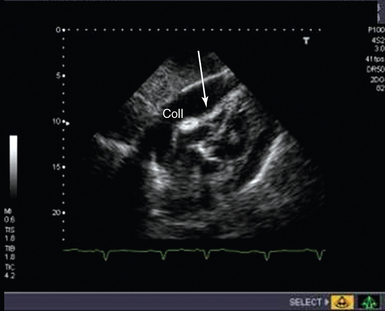
Figure 31-3 Subcostal view of a patient with tamponade. The image taken is shown in late diastole, where indentation and collapse of the right ventricular free wall are seen (arrows). coll, Collection.
It is recommended that pericardiocentesis be performed under echocardiographic guidance (class I), with success rates higher than 90% being achieved, depending on the volume and location of the collection and operator experience.18 Major complications include cardiac perforation, pneumothorax, coronary perforation, trauma to abdominal organs, and death, but the incidence of complications is significantly reduced with echocardiographic guidance.18 Indeed, TTE has been used routinely in some units for more than 30 years to guide pericardiocentesis with the aim of reducing complications.19
Coronary artery thrombosis
TTE is used routinely for the diagnosis of myocardial infarction (MI)/ischemia, evaluation of its complications, monitoring of therapeutic interventions, and risk stratification. In cardiac arrest with ongoing cardiac activity, echocardiography may reveal regional wall motion abnormalities (RWMAs) related to either previous MI or new ischemia. Although RWMA is not 100% sensitive or specific for coronary artery disease, in the context of cardiac arrest these findings may alter both the cardiovascular support and therapeutic interventions undertaken (e.g., avoidance of drugs that increase myocardial oxygen demand, insertion of an intraaortic balloon pump, and rapid/immediate revascularization). Free wall rupture is documented in 0.8% to 6.2% of patients after acute MI20 and frequently results rapidly in sudden death secondary to tamponade. When the findings are subacute, early diagnosis with TTE may be lifesaving. Other complications of coronary artery disease (ventricular septal defect, mitral regurgitation, intracardiac thrombus, RV infarction) resulting in cardiogenic shock are readily detectable with echocardiography, and when suspected, urgent TTE is indicated.18
Pulmonary embolism
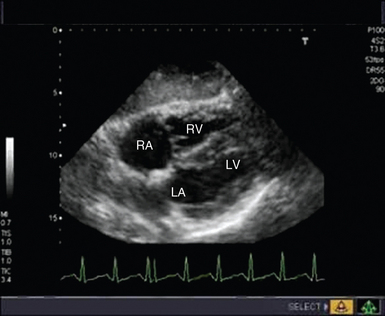
Cardiac arrest secondary to PE is likely to be massive and result from occlusion of more than two thirds of the pulmonary vascular bed. Echocardiography is recommended in the current guidelines for investigation of suspected PE only in patients too unstable to be transferred for computed tomography,21 the extreme of which is during cardiac arrest. TTE findings of RV dilatation (Figure 31-4) in the absence of significant left-sided valve or ventricular disease or known pulmonary disease are indicative of a high probability of PE, with the diagnosis confirmed by demonstration of thrombus in the right side of the heart, pulmonary arteries, or both.21
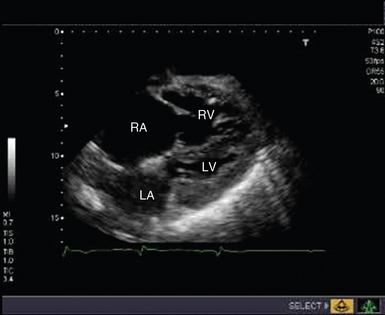
Figure 31-4 Subcostal view of a patient with massive pulmonary embolism. Note the hugely dilated right side of the heart and the small, squashed left ventricle. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The sensitivity and specificity of TTE for the diagnosis of PE are still being debated. In some studies the presence of RV hypokinesis has been found to identify patients with 30% or greater nonperfused lung, who may benefit from thrombolysis; others have not found any correlation between the extent of the perfusion abnormality and the degree of RV dilatation or dysfunction.22 The degree of RV dysfunction noted on TTE does correlate with mortality, however.23
Echocardiography in the post–cardiac arrest setting
TTE is routinely used in critical care to diagnose underlying cardiac pathology and to monitor the effects of therapeutic interventions. In the post–cardiac arrest setting, echocardiography may be used to determine the optimal volume status, identify further complications related to the initial cause of the cardiac arrest, and monitor cardiac output. In addition, echocardiography can be used to guide the clinician in maximizing cardiac output while minimizing any increase in myocardial oxygen demand—by optimizing filling status, heart rate, and atrioventricular delay.24
Performance of advanced life support–compliant periresuscitation echocardiography
In the peri-arrest setting, a fully comprehensive TTE study is neither necessary nor relevant. In this situation, performance of rapid, accurate, ALS-compliant focused echocardiography as an extension of the clinical examination with the aim of ruling in or ruling out potential causes of the cardiac arrest is required. Current American Society of Echocardiography guidelines recommend that limited, focused transesophageal echocardiography be performed when intraoperative cardiac arrest occurs,9 and the ILCOR guidelines support this recommendation for nontraumatic cardiac arrest when appropriately trained personnel are present. The 10-second pulse check provides an opportunity when TTE may be performed, and a practitioner experienced in echocardiography and trained in ALS compliance should be able to perform this study (Figure 31-5).25
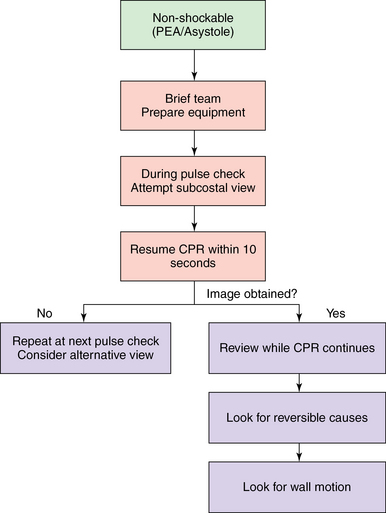
Figure 31-5 When to implement echocardiography in cardiac arrest. Here, in the nonshockable part of the algorithm, the subcostal view is attempted during the 10-second pulse check, and cardiopulmonary resuscitation is resumed at 10 seconds. The image obtained is then reviewed to look for reversible causes. If no image is obtained, at the next 10-second pulse check a further attempt is made with either the subcostal or another view. CPR, Cardiopulmonary resuscitation; PEA, pulseless electrical activity.
Because experienced echocardiographers are not a standard part of the cardiac arrest team, the question then arises whether it is possible to train members of the arrest team in performing focused echocardiography. There is increasing evidence that performance of focused echocardiography by nonexpert echocardiographic practitioners in the context of significant hemodynamic instability and cardiac arrest is feasible.25 Focused ultrasound is now considered a routine part of advanced trauma life support in patients with traumatic cardiac arrest, and in some hospitals in the intensive care setting, novice echocardiographers perform and interpret studies on the critically ill as part of their routine care.26 There is evidence that a focused, time-limited study is adequate and that noncardiologists/nonexperts can be successfully trained to perform such studies. However, although some studies suggest that focused echocardiography can be performed in an ALS-compliant manner and that clinicians can potentially predict the outcome depending on the echocardiographic findings,25,26 none have yet shown that the use of focused echocardiography improves outcomes per se.
To date, electrocardiography has been regarded as the “gold standard” for the diagnosis of asystole or ventricular fibrillation, but echocardiography may prove to be more accurate.27 The diagnosis of EMD is no longer used in ALS algorithms, but echocardiography is able to demonstrate its existence, as opposed to simply profound hypotension with an impalpable pulse. The outcomes of patients with true EMD (vs. profound hypotension) are significantly worse, and it may be that their management can be more tailored to the findings on echocardiography.27 Finally, routine use of TTE as a coordinated part of the ALS algorithm should be investigated for both in-hospital and out-of-hospital arrest and with physician and nonphysician (e.g., paramedic) operators, perhaps by using telemetry of images for expert review, to determine whether it improves outcomes. The introduction of lightweight, handheld echocardiographic machines has provided important opportunities to extend research beyond the hospital setting, such as for assessment of the prevalence of important echocardiographic findings in a critically ill patient some distance from the hospital and for evaluation of the role of interventions that could correct these findings before the patient reaches the hospital (or trigger transfer direct to a specialist center).
Pearls and highlights
• Paramount is not interrupting chest compressions/ALS algorithm. TTE images must be obtained during the 10-second pulse check.
• Potentially reversible causes are readily recognizable—even by novice echocardiographers, provided that they have received appropriate training.
References
1. Nichol, G, Thomas, E, Callaway, CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008; 300:1423–1431.
2. Neumar, RW, Otto, CW, Link, MS, et al, Part 8: adult advanced cardiovascular life support2010. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S729–S767.
3. Arntfield, RT, Millington, SJ. Point of care cardiac ultrasound applications in the emergency department and intensive care unit—a review. Curr Cardiol Rev. 2012; 8:98–108.
4. Blyth, L, Atkinson, P, Gadd, K, Lang, E, Bedside focused echocardiography as predictor of survival in cardiac arrest patients: a systematic review. Acad Emerg Me. 2012; 19:1119–1126.
5. Field, JM, Hazinski, MF, Sayre, RM, et al, Part 1: executive summary 2010. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science. Circulatio. 2010; 122:S640–S656.
6. Hazlinski, MR, Nolan, JP, Billi, JE, et al, Part 1: executive summary 2010. International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulatio. 2010; 122:S250–S581.
7. Tibballs, J, Russell, P. Reliability of pulse palpation by healthcare personnel to diagnose paediatric cardiac arrest. Resuscitation. 2009; 80:61–64.
8. Lapostolle, F, Le Toumelin, P, Agostinucci, JM, et al, Basic cardiac life support providers checking the carotid pulse: performance, degree of conviction and influencing factors. Acad Emerg Me. 2004; 11:878–880.
9. Travers, AH, Rea, TD, Bobrow, BJ, et al, Part 4: CPR overview2010. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science. Circulatio. 2010; 122:S676–S684.
10. Price, S, Uddin, S, Quinn, T. Echocardiography in cardiac arrest. Curr Opin Crit Care. 2010; 16:211–215.
11. Overland NP, Lossius HM, Wemmelund K, et al: Using transthoracic ultrasound to accurately assess pneumothorax progression during positive pressure ventilationa: a comparison with computed tomography, Chest (in press)
12. Renner, J, Gruenewald, M, Brand, P, et al. Global end-diastolic volume as a variable of fluid responsiveness during acute changing loading conditions,. J Cardiothorac Vasc Anesth. 2009; 21:650–654.
13. Leung, J, Levine, E. Left ventricular end-systolic cavity obliteration as an estimate of intraoperative hypovolaemia. Anesthesiology. 1994; 81:1102–1109.
14. Kircher, B, Himelman, R, Schiller, N. Noninvasive estimation for right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990; 66:493–496.
15. Lyon, M, Blaivas, M, Branman, M. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am J Emerg Med. 2005; 23:45–50.
16. Charron, C, Caille, V, Jardin, F, Viellard-Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006; 12:249–254.
17. Tsang, TS, Barnes, ME, Hayes, SN, et al, Clinical and echocardiographic characteristics of significant pericardial effusions following cardiothoracic surgery and outcomes of echo-guided pericardiocentesis for management: Mayo Clinic experience, 1979. 1998. Ches. 1999; 116:322–331.
18. Cheitlin, MD, Armstrong, WF, Aurigemma, GP, et al, ACC/AHA/ASE guideline update for the clinical application of echocardiography: summary articlea report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997. Guideline for the Clinical Application of Echocardiography). Circulatio. 2003; 108:1146–1162.
19. Callahan, JA, Seward, JB. Pericardiocentesis guided by two-dimensional echocardiography. Echocardiography. 1997; 14:497–504.
20. Leff, RA, Hoffman, I, Ventricular free wall rupture: ten year survival after surgical repair. Open Cardiovasc Med . 2008; 2:1–2.
21. European Society of Cardiology. Taskforce for the diagnosis and management of acute pulmonary embolism for the ESC. Eur Heart J. 2008; 29:2276–2315.
22. Otto, C. The practice of clinical echocardiography, ed 3. Philadelphia: Saunders; 2007.
23. Zhu, L, Yang, Y, Wu, Y, et al. Value of right ventricular dysfunction for prognosis in pulmonary embolism. Int J Cardiol. 2008; 127:40–45.
24. Lin, T, Chen, Y, Lu, C, Wang, M. Use of transoesophageal echocardiography during cardiac arrest in patients undergoing elective noncardiothoracic surgery. Br J Anaesth. 2006; 96:167–170.
25. Breitkreutz, R, Price, S, Steiger, HV, et al, Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitatio. 2010; 81:1527–1533.
26. Price, S, Via, G, Sloth, E, et al, World Interactive Network Focused on Critical Ultrasound ECHO-ICU Group. Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Care Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;10(6):49.
27. Salen, P, Meiniker, L, Chooljan, C, et al. Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients. Am J Emerg Med. 2005; 23:459–462.