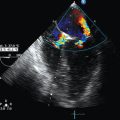
Echocardiography for intensivists
Overview
Since its early use in intensive care unit (ICU) settings by pioneers,1 echocardiography has been increasingly performed in critically ill patients because it provides unparalleled information on central hemodynamics.2 Initially, real-time morphologic and hemodynamic information, ease of use, portability, and safety constituted definite advantages of echocardiography over more invasive techniques, such as right heart catheterization. Subsequently, ultrasound systems have become smaller, higher quality, and less expensive. This facilitated the diffusion of echocardiography in the ICU environment.3
Rapidly, the use of echocardiography by intensivists appeared to be distinct from that of the cardiology community because of specific indications and requirements. Critical care echocardiography (CCE) refers to an examination performed and interpreted by an intensivist to establish diagnoses and guide therapeutic management of patients with cardiopulmonary compromise.4 The required training of intensivists to reach competence in CCE has been diversely implemented among countries, in Europe and worldwide.5 Scientific societies of critical care medicine have recently published international recommendations on the competence4 and the training6 required to perform CCE (see Chapter 61).
This chapter illustrates the current clinical use of echocardiography in the ICU settings and provides insights into future modalities of ultrasound-based clinical assessment of unstable patients with cardiopulmonary compromise.
Critical care echocardiography
Specific requirements of critical care echocardiography
Hemodynamic assessment of ventilated patients with circulatory or respiratory failure is the main indication for performing CCE.2,7,8 As opposed to conventional, state-of-the-art echocardiography performed in the cardiology laboratory, CCE has distinct requirements (Table 27-1). CCE must be available around-the-clock at the time of the clinical deterioration. Transesophageal echocardiography (TEE) is frequently required when the imaging quality of transthoracic echocardiography (TTE) is inadequate. Heart-lung interactions should be taken into account in the interpretation of CCE studies because critically ill patients are commonly mechanically ventilated. CCE may be focused on the hemodynamic assessment by using a qualitative rather than a quantitative approach.9 CCE may be repeated to assess the efficacy and tolerance of induced therapeutic changes as a monitoring tool, or if the patient’s condition changes over time.10 Additional indications of echocardiography in the ICU settings are closely related to the specific recruitment of institutions (Box 27-1) and require extensive training to reach competence.3
TABLE 27-1
Distinct Requirements of Critical Care and Conventional Echocardiography
| Critical Care Echocardiography | Conventional Echocardiography |
| Main indications: cardiopulmonary compromise | Main indications: cardiopathies |
| Performed at the bedside by the ICU physician | Performed in the cardiology laboratory by the sonographer |
| Online interpretation by the ICU physician | Off-line interpretation by the cardiologist |
| Interpretation in light of the critical care medicine background of the physician | Interpretation in light of the cardiology background of the physician |
| Guides diagnostic workup and invasive procedures | Expertise allows identification and interpretation of complex findings |
| Around-the-clock availability | Daytime schedule |
| Ventilated patients (heart-lung interactions) | Spontaneously breathing (out)patients |
| TEE frequently required and easy to perform | TTE is most commonly performed |
| Frequently goal-oriented examination | State-of-the-art exhaustive examination |
| Qualitative or quantitative evaluation using simple yet robust parameters | Quantitative assessment using all existing imaging tools |
| Immediate diagnostic/therapeutic impact | Delayed diagnostic/therapeutic impact |
| Monitoring tool/short-term follow-up | Diagnostic tool/long-term follow-up |
Transthoracic versus transesophageal echocardiography
TTE is the first-line approach because of its versatility, tolerance, and availability.7,11 It typically allows an optimal Doppler beam alignment with intracardiac flows and a broader field of examination of relatively superficial anatomic structures (Table 27-2).
TABLE 27-2
Respective Advantages of Transthoracic and Transesophageal Echocardiography in the ICU Settings
| Favors Transthoracic Echocardiography | Favors Transesophageal Echocardiography |
| Versatility, strictly noninvasive, availability, no contraindication (even in spontaneously breathing patients) Assessment of superficial anatomic structures (apical thrombus, pericardial space, inferior vena cava) Optimal alignment of Doppler beam with transvalvular blood flows (mitral, aortic, and tricuspid valves), and abnormal jets (valvulopathy, left ventricular outflow tract obstruction) Evaluation of pulmonary artery pressure (tricuspid and pulmonary regurgitant jets) |
Consistent high imaging quality, reproducibility, and stability of imaging planes (especially in ventilated patients) Assessment of deep anatomic structures (great vessels, base of heart, mediastinum, prosthetic valves, atria, and appendages) Precise identification of the mechanism of certain native or prosthetic valve dysfunctions (eccentric mitral regurgitation, prosthetic valve dysfunction) Identification of intracardiac shunts Identification of great vessel diseases (proximal pulmonary embolism, spontaneous or traumatic acute aortic conditions) |
TEE is usually used as an adjunct or subsequent test to TTE when surface examination is nondiagnostic. This may be related to poor imaging quality or to the inaccessibility of deep anatomic structures. Because of reduced interference with image acquisition, TEE has a greater diagnostic capability than TTE in ventilated ICU patients.12 TEE is first performed when examination of deep anatomic structures is required, especially after cardiac surgery.7,11 Finally, TEE provides more reproducible imaging planes than TTE when a hemodynamic monitoring is needed in unstable patients (see Table 27-2). Nevertheless, TEE is cumbersome to perform repeatedly and is contraindicated in the presence of any risk of esophageal injury (see Chapter 30). In ventilated ICU patients, TEE is safe, and unsuccessful probe insertion is rare when using laryngoscopic guidance under adequate sedation.8 In spontaneously breathing patients, the major risk of TEE is related to the development of acute respiratory failure precipitated by the esophageal intubation.13 Accordingly, TEE should be discouraged in unstable patients who are not on ventilatory support, especially when a tamponade or a massive pulmonary embolism is suspected.10
Impact of critical care echocardiography on patient management
CCE has a direct impact on management in a large proportion of ICU patients.8,10 Although TEE has been shown to prompt reoperation without further workup in patients with complicated open-heart surgery and has a greater therapeutic impact than TTE,12 surface echocardiography is also diagnostic in patients with shock.14 CCE frequently corrects initial diagnoses derived from invasive hemodynamic monitoring.10 When performed at the time of the acute insult, CCE best depicts the origin of the cardiopulmonary compromise before the effects of therapy, which may rapidly alter the hemodynamic profile. The anticipated therapeutic impact of CCE is maximal in the most unstable ICU patients at the time of examination.
Clinical use of critical care echocardiography
Circulatory failure
Septic shock
Hemodynamic disturbances associated with septic shock are complex and may variously associate with hypovolemia, vasoplegia, and right ventricular (RV), or left ventricular (LV) dysfunction.15 When performed during the initial phase of septic shock in fluid-resuscitated patients, who are usually under vasopressor support, CCE helps the intensivist in guiding acute therapy.16
CCE provides information on cardiac preload, reflected by end-diastolic ventricular volume or area (see Chapter 39), and allows prediction of fluid responsiveness in both mechanically ventilated and spontaneously breathing septic patients (see Chapter 40). CCE findings consistent with profound hypovolemia typically are associated with small cardiac cavities end-systolic obliteration of LV cavity in the presence of a hyperkinetic ventricle, small inferior vena cava size with full inspiratory collapse in spontaneously breathing patients, and large oscillations of interatrial septum that reflect low pressures of both atria (Figure 27-1). Nevertheless, most of these findings individually have a poor diagnostic capacity17,18 because “static” indices of preload fail to accurately predict the cardiac response to fluid loading.19 Of note, in fluid-resuscitated septic patients, overt hypovolemia is uncommon, and CCE is then useful in predicting fluid-responsiveness. Preload-dependent ventilated patients typically exhibit marked respiratory variations of aortic Doppler velocities and of the size of both the superior and inferior vena cavae.20 In these patients, fluid loading significantly increases the stroke distance of LV outflow (i.e., stroke volume) (Figure 27-2). In patients with spontaneous breathing activity or nonsinus rhythms, the effect of passive leg raising on LV outflow stroke distance helps in predicting responders to a fluid challenge from nonresponders.20
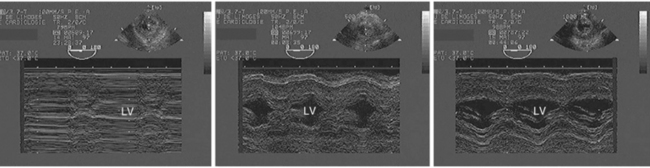
Figure 27-1 Profound hypovolemia detected by transesophageal echocardiography in a hypotensive ventilated patient after cardiac surgery. M-mode tracings obtained from the transgastric short-axis view of the left ventricle depict the presence of a virtual ventricular cavity with end-systolic obliteration (left panel), and the progressive increase of the ventricular size secondary to two consecutive fluid challenges which reflects increased preload (middle and right panels). Please note the improved thickening of left ventricular walls, which reflects the increase of stroke volume secondary to blood volume expansion in this fluid responder. LV, Left ventricle.
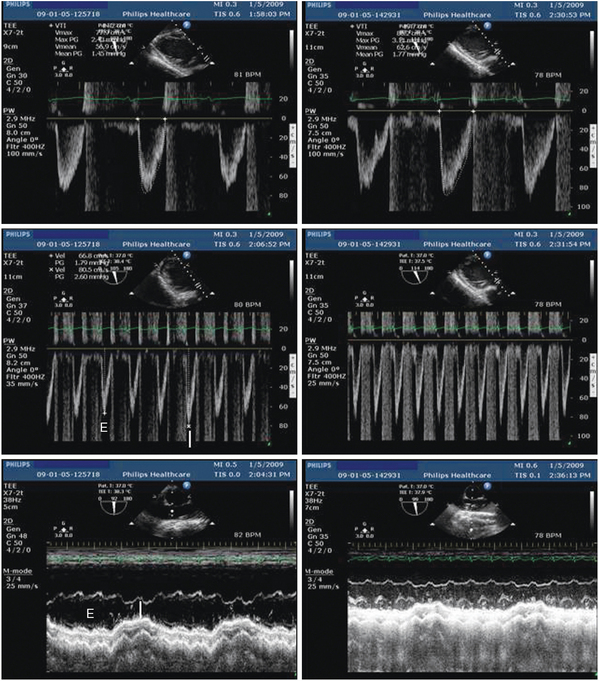
Figure 27-2 Assessment of fluid responsiveness by using transesophageal echocardiography in the early course of a ventilated patient with septic shock and sinus rhythm. At baseline, the stroke distance of left ventricular outflow was reduced (14.2 cm), reflecting decreased stroke volume (upper left), and associated with marked respiratory variations of maximal Doppler velocities (middle left) and superior vena cava diameter (lower left), which both suggested preload dependency. After 500-mL fluid loading, left ventricular outflow Doppler disclosed a 39% increase of stroke distance (19.7 cm), which confirmed fluid responsiveness (upper right), and both the respiratory variations of maximal Doppler velocities (middle right) and superior vena cava diameter (lower right) were attenuated. I, Insufflation; E, expiration.
![]() In approximately one third of patients examined at the early phase of septic shock, CCE clearly depicts a LV systolic dysfunction (Video 27-1
In approximately one third of patients examined at the early phase of septic shock, CCE clearly depicts a LV systolic dysfunction (Video 27-1![]() ). The myocardial depression related to septic shock positively responds to inotropic agents (Video 27-2), and fully recovers in survivors, with the treatment of sepsis, regardless of its severity.16,21,22 In contrast with congestive heart failure, LV filling pressures are typically not elevated.
). The myocardial depression related to septic shock positively responds to inotropic agents (Video 27-2), and fully recovers in survivors, with the treatment of sepsis, regardless of its severity.16,21,22 In contrast with congestive heart failure, LV filling pressures are typically not elevated.
RV failure is less frequently encountered in septic shock patients. When present, it is typically associated with an underlying acute respiratory distress syndrome (ARDS).16 Finally, sustained vasoplegia is suspected in the absence of preload dependence, cardiac dysfunction, or any other echocardiographic abnormality that could account for the circulatory failure (e.g., acute valvular regurgitation secondary to an infective endocarditis, tamponade related to a purulent pericarditis).
In 42 ventilated patients with septic shock, we compared the therapeutic changes derived from the Surviving Sepsis Campaign guidelines23 and TEE examination performed during the first hours of ICU admission.24 In 30% of patients who would have been fluid loaded based on a central venous pressure less than 12 mm Hg, CCE ruled out a preload dependance, and patients actually did not receive any blood volume expansion. In 14 patients (33%), CCE led to initiate an inotropic support that would have been decided for only 4 patients according to current guidelines.24
Complicated acute myocardial infarction
Circulatory failure secondary to acute myocardial infarction (AMI) may have various clinical presentations, including hypotension, low cardiac output, pulmonary edema, and cardiogenic shock, with frequent overlap. Cardiogenic shock is hemodynamically defined by a sustained systemic hypotension (systolic arterial pressure < 90 mm Hg) with adequate or elevated LV filling pressures (pulmonary artery wedge pressure > 15-18 mm Hg) and a reduced cardiac output (cardiac index < 2.2 L/min/m2).25
In patients with complicated AMI, cardiogenic shock typically results from an extensive infacted myocardial area or a mechanical complication (e.g., ruptured papillary muscle, ventricular septal, or free wall rupture). CCE clearly depicts the extension of AMI and allows the quantification of related LV systolic dysfunction.26 TTE is valuable for the identification of ventricular septal rupture and of potentially compressing hemopericardium secondary to LV free wall rupture (Figure 27-3). TEE is best suited to depict an eccentric mitral regurgitation resulting from a papillary muscle dysfunction or rupture, which may be frequently missed when using TTE (![]() Videos 27-3 and 27-4).
Videos 27-3 and 27-4).
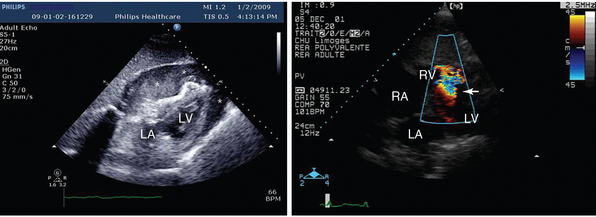
Figure 27-3 Mechanical complications of acute myocardial infarction diagnosed by using transthoracic echocardiography. In these two patients with severe shock, the subcostal view depicted a cardiac tamponade with compressed right cardiac cavities caused by a ruptured left ventricular free wall with hemopericardium (left panel, asterisks) and a ruptured ventricular septum with associated left-to-right shunt depicted by color Doppler mapping (right panel, arrow). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Acute aortic syndrome
Acute aortic syndrome (AAS) is characterized clinically by an “aortic pain” (i.e., severely intense, acute, searing or tearing, throbbing, and migratory chest pain) in a patient with a coexisting history of hypertension.27 AAS may be secondary to various acute aortic conditions, including the classic aortic dissection, intramural aortic hematoma, and penetrating atherosclerotic aortic ulcer. Associated circulatory failure is usually present when the ascending aorta is involved.28 In this setting, TTE should first be performed because it allows expeditous and accurate diagnosis of pericardial extravasation, which prompts surgical repair. TEE is then safely performed under general anesthesia during surgery, to precisely characterize the underlying aortic disease, its anatomic extension, and associated complications (Video 27-5![]() ). The ascending aorta is typically enlarged, and acute aortic regurgitation may be present. Associated findings depend on the causative aortic disease: intimal flap (aortic dissection), aortic wall thickening (intramural hematoma), or penetrating aortic ulcer.29
). The ascending aorta is typically enlarged, and acute aortic regurgitation may be present. Associated findings depend on the causative aortic disease: intimal flap (aortic dissection), aortic wall thickening (intramural hematoma), or penetrating aortic ulcer.29
Massive pulmonary embolism
Massive pulmonary embolism and ARDS are the main causes of acute cor pulmonale (ACP) in critically ill patients (see Chapter 33). ACP refers to any sudden increase in RV outflow impedance. Afterloaded RV typically exhibits a combined diastolic overload (RV enlargement) and systolic overload (paradoxic ventricular septal motion).30
TTE best depicts RV cavity dilatation in a longitudinal view of the heart (apical four-chamber view) and the end-systolic bulging of the ventricular septum toward the LV cavity in a transverse view of the heart (parasternal or subcostal short-axis view) (Figure 27-4). Associated findings include right atrial dilatation, various levels of pulmonary hypertension, and systemic venous congestion reflected by a dilatated inferior vena cava with reduced respiratory variations.30
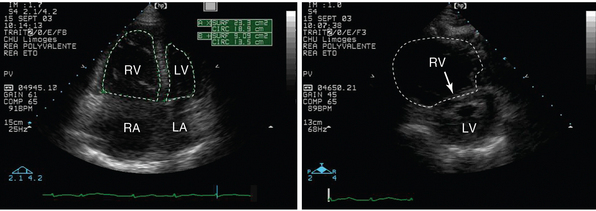
Figure 27-4 Transthoracic echocardiography performed in a patient presenting with hypotension and shortness of breath. Apical four-chamber view depicts a markedly enlarged right ventricle whose cavity size exceeds that of the left ventricle (left panel, dotted lines). Parasternal short-axis view confirms the right ventricular dilatation and depicts a flattened ventricular septum (right panel, arrow). These findings are consistent with an acute cor pulmonale, which is highly suggestive of a massive pulmonary embolism in the clinical context. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Cardiac tamponade
In critically ill patients, cardiac tamponade may result from a diffuse or loculated pericardial effusion, or from atrial or ventricular compression by extrapericardial collections.31 Classic clinical signs of cardiac tamponade are rarely encountered, especially in the presence of a localized tamponade resulting from the development of postoperative mediastinal hematomas in cardiac surgery patients. TTE rapidly depicts the presence of pericardial fluid around the heart, consistent with unloculated effusion (see Figure 27-3, left panel). Tamponade is present when any cardiac cavity is compressed (usually right cardiac cavities because of lower chamber pressures) and when interventricular dependence is increased, as reflected by augmented respiratory variations of Doppler ventricular inflow velocities in spontaneously breathing patients.32 TEE should first be performed in ventilated cardiac surgery or blunt chest trauma patients who present with shock and suspected localized cardiac tamponade. It typically depicts the presence of an inverted free wall curvature of the cardiac chamber that is compressed throughout the cardiac cycle by the mediastinal hematoma.31 Blood flow turbulences are frequently depicted by color Doppler mapping in the involved cardiac cavity (Video 27-7![]() ).
).
Cardiac arrest
CCE is valuable when performed during a cardiac arrest or after a successful resuscitation, to identify a potential cause (see Chapter 35). TTE can be performed during resuscitation using the subcostal approach. It may depict an ACP, a thrombus-in-transit consistent with massive pulmonary embolism, or a pericardial effusion with associated tamponade physiology.
Acute respiratory failure
Cardiogenic pulmonary edema
Cardiogenic pulmonary edema (CPE) is related to the rise of hydrostatic pressure in the pulmonary vascular bed.33 CCE allows the evaluation of LV filling pressures and the identification of a potential cardiopathy responsible for the CPE.34
In a patient presenting with suspected CPE, the first step is to confirm the presence of elevated filling pressures, which are frequently associated with (severe) LV diastolic dysfunction (see Chapter 32). Pulsed wave Doppler of the mitral inflow and pulmonary vein flow, combined with tissue Doppler imaging of the mitral annulus, can accurately identify elevated LV filling pressures. We have previously shown in mechanically ventilated patients that a ratio of maximal early diastolic velocities of Doppler mitral inflow and tissue Doppler of the mitral ring (E/E’ ratio) equal or less than 8 predicted a pulmonary artery occlusion pressure less than or equal to 18 mm Hg, with a sensitivity and a specificity of 83% and 88%, respectively.35 Other simple yet robust Doppler indices have been proposed to semiquantitatively assess LV filling pressures in ventilated critically ill patients.34 The presence of a dilatated left atrium or the consistent bulging of the septum toward the right atrium reflect elevated left atrial pressure.
The second step of CCE is to identify the origin of pulmonary vein congestion by using a systematic diagnostic algorithm.34 Congestive heart failure secondary to a cardiac ischemic disease or a dilatated cardiomyopathy is typically associated with a severe LV systolic dysfunction. Both the dilation of the LV cavity and thinning of LV walls are consistent with a chronic cardiomyopathy (Figure 27-5). Of interest, severe LV dysfunction with elevated filling pressures may be transient and secondary to noncardiac diseases, such as severe cerebral insults (![]() Video 27-8). Although congestive heart failure is the most common cause of CPE, a normal or increased LV systolic function fails to rule out the diagnosis.33 Elevated LV filling pressures with preserved ejection fraction should lead to search for severe valvulopathy, especially acute regurgitations.34 Eccentric and transient severe mitral regurgitation may easily be missed by using TTE. Apparently isolated LV diastolic dysfunction may result in CPE if severe and is potentially associated with a precipitating factor (inadequate fluid loading, dysrhythmia, severe systemic hypertension). CCE is pivotal in establishing diagnostic criteria of diastolic heart failure in depicting elevated LV filling pressures, preserved ejection fraction, absence of relevant valvulopathy, and altered LV diastolic properties at the time or in proximity to the acute CPE (see Chapter 32).36
Video 27-8). Although congestive heart failure is the most common cause of CPE, a normal or increased LV systolic function fails to rule out the diagnosis.33 Elevated LV filling pressures with preserved ejection fraction should lead to search for severe valvulopathy, especially acute regurgitations.34 Eccentric and transient severe mitral regurgitation may easily be missed by using TTE. Apparently isolated LV diastolic dysfunction may result in CPE if severe and is potentially associated with a precipitating factor (inadequate fluid loading, dysrhythmia, severe systemic hypertension). CCE is pivotal in establishing diagnostic criteria of diastolic heart failure in depicting elevated LV filling pressures, preserved ejection fraction, absence of relevant valvulopathy, and altered LV diastolic properties at the time or in proximity to the acute CPE (see Chapter 32).36
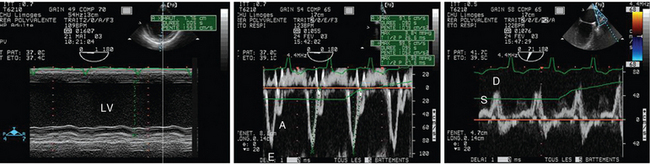
Figure 27-5 Transesophageal echocardiography performed in a ventilated patient with cardiogenic pulmonary edema. Two-dimensional imaging revealed a markedly dilatated and severely hypokinetic left ventricle with thin walls, as reflected by M-mode tracing (left panel). Pulsed wave Doppler depicted a restrictive mitral pattern with E/A greater than 2 and shortened E wave deceleration time (<100 msec) (middle panel). Pulmonary vein Doppler disclosed a blurred S wave in the absence of severe mitral regurgitation, which confirmed a marked increase of left atrial filling pressures (right panel). E/E′ was 13. LV, Left ventricle.
Acute respiratory distress syndrome
ARDS is the second most common cause of ACP in critically ill patients.30 CCE findings of ARDS-induced ACP are nonspecific. Nevertheless, CCE helps the front-line intensivist in setting the ventilator and adjusting therapeutic strategy (e.g., prone position, nitrous oxide [NO] inhalation) to treat or prevent RV afterloading (see Chapter 33). Because plateau pressure is significantly associated with the development of ACP,37 and RV failure appears as a prognostic factor in ARDS patients,38 CCE is valuable in monitoring RV function to guide therapy.39
Weaning failure from the ventilator
CCE allows the identification of patients at high risk of weaning failure and depicts in real time the effects of spontaneous breathing trials on central hemodynamics (see Chapter 34). We previously showed that patients who failed ventilator weaning had significantly lower LV ejection fraction and higher LV filling pressures when compared with patients who were successfully extubated. Spontaneous breathing trial significantly increased cardiac output and LV filling pressures (higher early to late maximal diastolic velocities of mitral Doppler inflow – E/A ratio – and shortened E wave deceleration time).40 CCE may also depict transient or worsened mitral regurgitation secondary to abrupt changes in cardiac loading conditions induced by the interruption of positive pressure ventilation. The combination of TTE and chest ultrasound promises to efficiently guide the diagnostic workup at bedside in patients exhibiting weaning failure from the ventilator (see Chapter 34).
Decompensated chronic respiratory failure
Severe long-standing respiratory failure may be associated with chronic cor pulmonale. Differential diagnosis with ACP mainly relies on the clinical context, the presence of RV free wall hypertrophy (close to 10 mm) and of marked pulmonary hypertension.30 A systolic pulmonary artery pressure derived from the maximal velocity of tricuspid regurgitant jet greater than 60 mm Hg is consistent with a chronically afterloaded and usually hypertrophied RV (see Chapter 33![]() ) (Video 27-9).
) (Video 27-9).
Unexplained hypoxemia
Anatomic shunts should be ruled out in patients with sustained hypoxemia but no relevant radiologic infiltrates or pulmonary ultrasound findings. Contrast study performed during a TEE examination is the reference method for the diagnosis of patent foramen ovale (PFO) with associated interatrial right-to-left shunt or for the identification of an anatomic shunt of the pulmonary vascular bed.41 Microcavitations obtained by agitated saline are injected intravenously to obtain a full opacification of the right atrium. Early visualization of microcavitations in the left atrium suggests the presence of an underlying PFO, when this occurs within three cardiac cycles after right atrial opacification, and this is consistent with an anatomic shunt of the pulmonary vasculature when the left atrial opacification is delayed.42 The volume of the anatomic shunt reflected by the amount of microcavitations observed in the left atrium and its consistency throughout the respiratory cycle determine its potential role in the sustained hypoxemia (Figure 27-6). In patients ventilated for ARDS, a PFO has recently been diagnosed by using TEE in 19% of the cases.43 These patients exhibited an associated ACP more frequently and had a blurred response to positive end-expiratory pressure titration when compared with ARDS patients without PFO.43
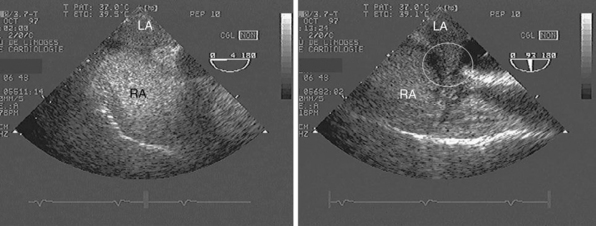
Figure 27-6 Positive contrast study performed during a transesophageal echocardiographic examination in a ventilated patient with unexplained hypoxemia. A large right-to-left interatrial shunt associated with a patent foramen ovale is seen both in the four-chamber view centered on the two atria (left panel) and in the 90-degree bicaval view (right panel, circle). The width of the anatomic shunt, the full opacification of left atrium, and sustained shunt throughout the respiratory cycle indicate a large shunted blood volume that could participate in severe and refractory hypoxemia. LA, Left atrium; RA, right atrium.
Specific indications
Cardiovascular injuries
Penetrating chest trauma should prompt focused TTE to rule out blood extravasation. In this specific setting, the identification of a pericardial effusion strongly suggests the presence of a hemopericardium secondary to a cardiac wound, which requires rapid surgery. Patients sustaining blunt chest trauma are best assessed by using TEE, for which diagnostic accuracy for the identification of aortic and cardiac injuries is greater than that of TTE (see Chapter 31).44 TEE findings associated with blunt aortic injuries are distinct from those encountered in patients with acute aortic dissections (Figure 27-7) and helps in guiding therapeutic management.45
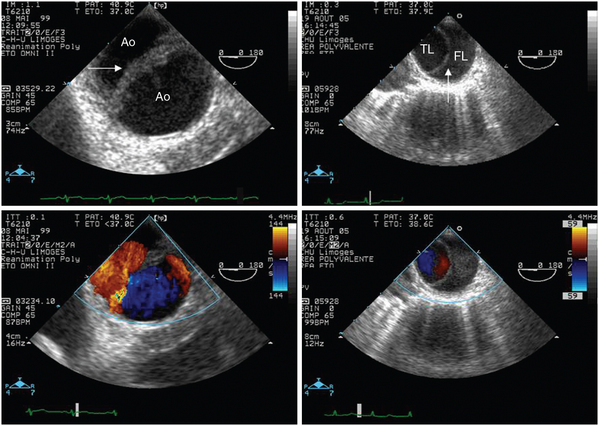
Figure 27-7 Differential diagnosis between aortic disruption and aortic dissection by using transesophageal echocardiography. In addition to the clinical context, echocardiographic findings allow distinguishing a spontaneous aortic dissection and a traumatic aortic disruption, which usually solely involves the aortic isthmus. In this transverse view of the descending aorta, the medial flap of the disrupted aorta appears thicker than the intimal flap associated with acute dissection (upper panels, arrows). Color Doppler mapping typically depicts similar blood flow velocities on both sides of the disrupted aortic wall because the medial flap fails to delimit two distinct channels, whereas lower velocities are observed in the false lumen of the aortic dissection (lower panels). Of importance, echocardiographic findings are usually confined within a few centimeters in the presence of an aortic disruption, whereas they are more extended according to the anatomic type of aortic dissection. Ao, Aortic isthmus; FL, false lumen; TL, true lumen.
Infective endocarditis
Modified Duke criteria for the definite diagnosis of infective endocarditis include TEE findings.46 In addition, TEE allows the clinician to accurately assess the severity of associated valvular regurgitation or prosthetic valve dysfunction and to evaluate the risk of systemic embolism of vegetations (Video 27-10![]() ). Because of a lower diagnostic accuracy, TTE should not be solely used to confidently exclude an infective endocarditis in a high-risk ICU patient.
). Because of a lower diagnostic accuracy, TTE should not be solely used to confidently exclude an infective endocarditis in a high-risk ICU patient.
Miscellaneous
Various additional clinical settings may lead to performance of CCE. A potential source of systemic embolism is best depicted by TEE (Video 27-11![]() ). Similarly, TEE allows guiding the initial positioning of intracardiac cannulae for extracorporeal life support (Figure 27-8). Subsequently, TEE allows monitoring the patient to identify potential cardiac complications and to determine the optimal time window to initiate weaning from circulatory assistance.47
). Similarly, TEE allows guiding the initial positioning of intracardiac cannulae for extracorporeal life support (Figure 27-8). Subsequently, TEE allows monitoring the patient to identify potential cardiac complications and to determine the optimal time window to initiate weaning from circulatory assistance.47
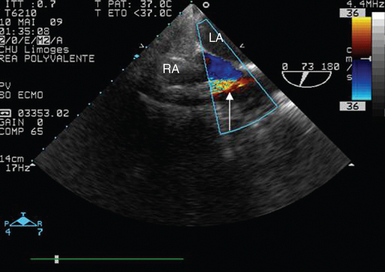
Figure 27-8 Transesophageal echocardiographic guidance of right atrial cannula insertion in a ventilated patient requiring extracorporeal life support for severe cardiogenic shock. The proper position of the cannula tip parallel to the atrial septum is confirmed and the adequate aspiration of venous blood is evidenced by color Doppler mapping (arrow). LA, Left atrium; RA, right atrium.
Current trends and future directions
The miniaturization of ultrasound systems led to the diffusion of pocket-size devices used as ultrasound stethoscopes for goal-directed assessment of ICU patients.48 Because goal-directed ultrasound assessment appears superior to standard physical examination both for diagnostic purposes and for procedural guidance, the concept of point-of-care ultrasonography emerged.3 It refers to an ultrasound examination performed and interpreted in real time by the provider at the patient’s bedside; it may be focused on limited clinical questions and can be repeated if the patient’s condition changes over time.49
Miniaturization of TEE probes promises to facilitate and safely prolonged esophageal insertion of the device for the purpose of hemodynamic monitoring. Recently, we showed that a miniaturized single-use monoplane TEE probe designed for a 72-hour esophageal insertion allowed serial qualitative assessment of central hemodynamics, which resulted in frequent therapeutic changes.50
Real-time three-dimensional TEE is feasible in ventilated ICU patients.51 In the near future, automated gain settings will presumably facilitate data acquisition and off-line multiplane analysis by less experienced operators. Accelerated reconstructions will provide accurate RV volume measurements, which promise to provide access to valuable information that are yet inaccessible to two-dimensional echocardiography.
Pearls and highlights
• CCE is a bedside examination performed and interpreted by the intensivist in charge of the patient on a 24-hour basis. This distinguishes CCE from conventional echocardiography.
• The diagnostic and monitoring power of echocardiography has led to its extensive use in ICUs and to a marked expansion of its indications in critically ill patients.
• When properly used after an adequate training, CCE increases the accuracy of clinical assessment, guides the diagnostic workup, saves time, and promises to reduce cost while improving the outcome of ICU patients.
References
1. Ozier, Y, Guéret, P, Jardin, F, et al. Two-dimensional echocardiographic demonstration of acute myocardial depression in septic shock. Crit Care Med. 1984; 12(7):596–599.
2. Vignon, P. Hemodynamic assessment of critically-ill patients using echocardiography Doppler. Curr Opin Crit Care. 2005; 11(3):227–234.
3. Vignon, P, PRO: physician-performed ultrasoundthe time has come for routine use in acute care medicine. Anesth Analg. 2012;115(5):999–1003.
4. Mayo, PH, Beaulieu, Y, Doelken, P, et al. American College of Chest Physicians/La Société Françoise de Réanimation de Langue Françoise Statement on competence in critical care ultrasonography. Chest. 2009; 135(4):1050–1060.
5. Vieillard-Baron, A, Slama, M, Cholley, B, et al, Echocardiography in the intensive care unit: from evolution to revolution?. Intensive Care Med. 2008;34(2):243–249.
6. Expert Round Table on Ultrasound in ICU. International expert statement on training standards for critical care ultrasonography. Intensive Care Med. 2011; 37(7):1077–1083.
7. Beaulieu, Y, Marik, PE. Bedside ultrasonography in the ICU (part 1). Chest. 2005; 128(2):881–895.
8. Huttemann, E, Schelenz, C, Kara, F, et al. The use and safety of transoesophageal echocardiography in the general ICU—a minireview. Acta Anaesthol Scand. 2004; 48(7):827–836.
9. Vieillard-Baron, A, Charron, C, Chergui, K, et al, Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative assessment sufficient. Intensive Care Med. 2006;32(10):1547–1552.
10. Vignon, P, Echocardiography in the critically ill: an overviewDe Backer D, Cholley B, Slama M, et al, eds.. Hemodynamic using echocardiography in the critically ill. Springer: Berlin, 2011; 1–9.
11. Douglas, PS, Khandheria, B, Stainback, RF, et al, ACCF/ASE/ACEP/ASNC/SCAL/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol. 2007;50(2):187–204.
12. Vignon, P, Mentec, H, Terré, S, et al. Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest. 1994; 106(6):1829–1834.
13. Gendreau, MA, Triner, WR, Bartfield, J. Complications of transesophageal echocardiography in the ED. Am J Emerg Med. 1999; 17(3):248–251.
14. Joseph, MX, Disney, PJS, Da Costa, R, et al. Transthoracic echocardiography to identify or exclude cardiac cause of shock. Chest. 2004; 126(5):1592–1597.
15. Parrillo, J. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993; 328(20):1471–1477.
16. Vieillard-Baron, A, Prin, S, Chergui, K, et al. Hemodynamic instability in sepsis. Bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med. 2003; 168(11):1270–1276.
17. Toussignant, CP, Walsh, F, Mazer, CD. The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg. 2000; 90(2):351–355.
18. Leung, JM, Levine, EH, Left ventricular end-systolic cavity obliteration as an estimate of intraoperative hypovolemia. Anesthesiology. 1994;81(5):1102–1109.
19. Michard, F, Teboul, JL, Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008.
20. Slama, M, Maizel, J, Assessment of fluid requirements: fluid responsivenessDe Backer D, Cholley B, Slama M, et al, eds.. Hemodynamic monitoring using echocardiography in the critically ill. Springer: Berlin, 2011; 61–69.
21. Etchecopar-Chevreuil C, Française B, Clavel Met al. Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med. 2008;34(2):43–49.
22. Vieillard-Baron, A, Caille, V, Charron, C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008; 36(6):1701–1706.
23. Dellinger, PR, Levy, MM, Carlet, JM, et al, Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2008;34(1):17–60.
24. Bouferrache, K, Amiel, JB, Chimot, L, et al, Initial resuscitation guided by the Surviving Sepsis Campaign recommendations and early echocardiographic assessment of hemodynamics in ICU septic patients: a pilot study. Crit Care Med. 2012;40(10):2821–2827.
25. Hollenberg, SM, Kavinsky, CJ, Parillo, JE. Cardiogenic shock. Ann Intern Med. 1999; 131(1):47–59.
26. Lang, RM, Bierig, M, Devereux, RB, et al. Recommendations for chamber quantification. 2006. Eur J Echocardiogr. 2006; 7(2):79–108.
27. Vilacosta, I, San Roman, JA. Acute aortic syndrome. Heart. 2001; 85(4):365–368.
28. Hagan, PG, Nienaber, CA, Isselbacher, EM, et al. The international registry of acute aortic dissection (IRAD). New insights into an old disease. JAMA. 2000; 283(7):897–903.
29. Vignon, P, Aboyans, V, Pascaud, JL. Pathologie aigu de l’aorte. In: Maury E, Mercat A, eds. Imagerie en reanimation. Paris: Elsevier; 2007:521–562.
30. Vieillard-Baron, A, Prin, S, Chergui, K, et al. Echo-doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002; 166(10):1310–1319.
31. Grumann, A, Baretto, L, Dugard, A, et al. Localized cardiac tamponade after open-heart surgery. Ann Thorac Cardiovasc Surg. 2012; 18(6):524–529.
32. Mayo, PH. Pericardial effusion and cardiac tamponade. In: De Backer D, Cholley B, Slama M, et al, eds. Hemodynamic monitoring using echocardiography in the critically ill,. Berlin: Springer; 2011:151–161.
33. Dickstein, K, Cohen-Solal, A, Filippatos, G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008; 29(19):2388–2442.
34. Vignon, P, Colreavy, F, Slama, M, Pulmonary edema: which role for echocardiography in the diagnostic work-upDe Backer D, Cholley B, Slama M, et al, eds.. Hemodynamic monitoring using echocardiography in the critically ill. Springer: Berlin, 2011; 177–194.
35. Vignon, P, Ait Hssain, A, Françoise, B, et al, Noninvasive assessment of pulmonary artery occlusion pressure in ventilated patients: a transesophageal study. Crit Care. 2008;12(1):R18.
36. Vasan, RS, Levy, D. Defining diastolic heart failure. A call for standardized diagnostic criteria. Circulation. 2000; 101(17):2118–2121.
37. Jardin, F, Vieillard-Baron, A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007; 33(3):444–447.
38. Monchi, M, Bellenfant, F, Cariou, A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med. 1998; 158(4):1076–1081.
39. Vieillard-Baron, A, Jardin, F. Why and how to use echocardiography in acute respiratory distress syndrome. In: De Backer D, Cholley B, Slama M, et al, eds. Hemodynamic monitoring using echocardiography in the critically ill. Berlin: Springer; 2011:195–202.
40. Caille, V, Amiel, JB, Charron, C, et al, Echocardiography: a help in the weaning process. Crit Care. 2010;14(3):R120.
41. Duperret, S, Vignon, P. Hypoxémies inexpliquées. In: Vignon P, Cholley B, Slama M, et al, eds. Echocardiographie Doppler chez le patient en état critique. Un outil de diagnostic et de monitorage. Echo-in-ICU-Group, Paris: Elsevier; 2008:353–375.
42. Gin, KG, Fenwick, JC, Pollick, C, et al. The diagnostic utility of contrast echocardiography in patients with refractory hypoxemia. Am Heart J. 1993; 125(4):1136–1141.
43. Mekontso Dessap, A, Boissier, F, Leon, R, et al. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med. 2010; 38(9):786–792.
44. Chirillo, F. Thoracic trauma. In: De Backer D, Cholley B, Slama M, et al, eds. Hemodynamic monitoring using echocardiography in the critically ill. Berlin: Springer; 2011:205–235.
45. Vignon, P, Guéret, P, Vedrinne, JM, et al. Role of transesophageal echocardiography in the diagnosis and management of traumatic aortic disruption. Circulation. 1995; 92(10):2959–2968.
46. Li, JS, Sexton, DJ, Mick, N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000; 30(4):633–638.
47. Aissaoui, N, Luyt, CE, Leprince, P, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011; 37(11):1738–1745.
48. Amiel, JB, Grümann, A, Lhéritier, G, et al. Assessment of left ventricular ejection fraction using an ultrasonic stethoscope in critically ill patients. Crit Care. 2012; 16(1):R29.
49. Moore, CL, Copel, JA. Point-of-care ultrasonography. N Engl J Med. 2001; 364(8):749–757.
50. Vieillard-Baron, A, Slama, M, Mayo, P, et al. A pilot study concerning the safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med. 2013; 39(4):629–635.
51. Vignon, P, Amiel, JB, Lhéritier, G, et al. Measurement of left ventricular volumes using real-time three-dimensional transesophageal echocardiography in ICU patients. Preliminary results. Intensive Care Med. 2011; 37(Suppl 1):S183.