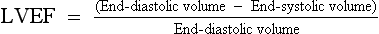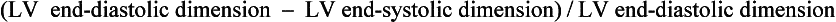Chapter 5
Echocardiography
1. How does echocardiography work?
Appropriateness criteria for obtaining an echocardiogram are given in Box 5-1.
2. What is the difference between echocardiography and Doppler?
 Pulsed Doppler (Fig. 5-1, A), which can localize the site of flow acceleration but is prone to aliasing
Pulsed Doppler (Fig. 5-1, A), which can localize the site of flow acceleration but is prone to aliasing
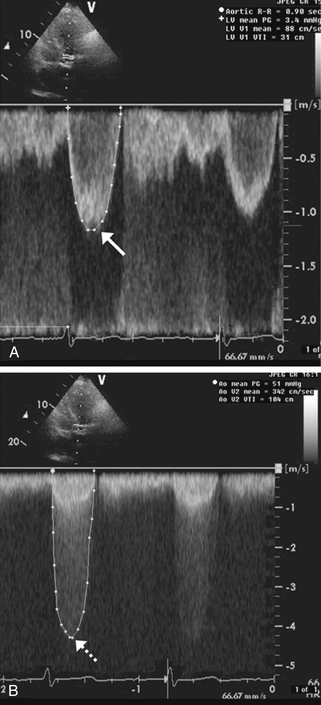
Figure 5-1 Doppler assessment used in patients with aortic stenosis. A shows pulsed Doppler in the left ventricular outflow tract in a patient with aortic stenosis. The peak velocity of the spectral tracing (arrow) is 1.2 msec, indicating normal flow velocity proximal to the aortic valve. B shows continuous Doppler across the aortic valve revealing a peak velocity of 4.5 msec (dashed arrow). Therefore, the blood-flow velocity nearly quadrupled across the stenotic aortic valve, consistent with severe aortic stenosis.
 Continuous-wave Doppler (Fig. 5-1, B), which cannot localize the level of flow acceleration but can identify very high velocities without aliasing
Continuous-wave Doppler (Fig. 5-1, B), which cannot localize the level of flow acceleration but can identify very high velocities without aliasing
 Color Doppler (Fig. 5-2), which uses different colors (usually red and blue) to identify flow toward and away from the transducer, respectively, and identify flow acceleration qualitatively by showing a mix of color to represent high velocity or aliased flow
Color Doppler (Fig. 5-2), which uses different colors (usually red and blue) to identify flow toward and away from the transducer, respectively, and identify flow acceleration qualitatively by showing a mix of color to represent high velocity or aliased flow
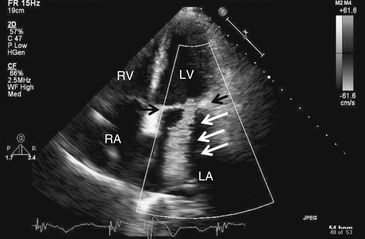
Figure 5-2 Mitral regurgitation. Apical four-chamber view with color Doppler revealing severe mitral regurgitation (white arrows). Black arrows point to the mitral valve. Note that in actuality, the regurgitant jet is displayed in color, corresponding to the flow of blood. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Doppler is particularly useful for assessing the hemodynamic significance of cardiac structural disease, such as the severity of aortic stenosis (see Fig. 5-1), degree of mitral regurgitation (see Fig. 5-2), flow velocity across a ventricular septal defect, or severity of pulmonary hypertension. The great majority of echocardiograms are ordered as echocardiography with Doppler to answer cardiac morphologic and hemodynamic questions in one study (e.g., a mitral stenosis murmur); 2-D echo to identify the restricted, thickened, and calcified mitral valve (Fig. 5-3); and Doppler to analyze its severity based on transvalvular flow velocities and gradients.
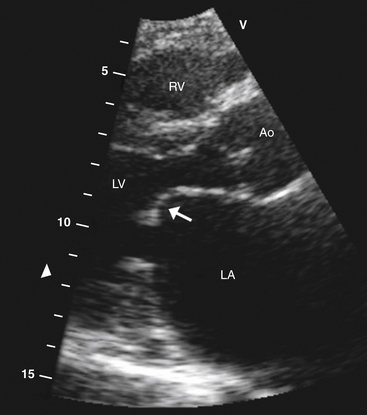
Figure 5-3 Parasternal long-axis view showing typical hockey stick appearance of the mitral valve (arrow) in rheumatic mitral stenosis. Ao, Aortic valve; LA, left atrium; LV, left ventricle; RV, right ventricle.
3. How is systolic function assessed using echocardiography?
 The Simpson method (method of discs) in which the LV endocardial border of multiple “slices” of the left ventricle is traced in systole and diastole, and the end-diastolic and end-systolic volumes are computed from these tracings, is one of the most common methods of calculating LVEF.
The Simpson method (method of discs) in which the LV endocardial border of multiple “slices” of the left ventricle is traced in systole and diastole, and the end-diastolic and end-systolic volumes are computed from these tracings, is one of the most common methods of calculating LVEF.
 The Teicholz method, in which the shortening fraction:
The Teicholz method, in which the shortening fraction:
is multiplied by 1.7, can also be used to estimate LVEF (although this method is inaccurate in patients with regional wall motion abnormalities).
 Visual estimation of LVEF by expert echocardiography readers is also commonly used.
Visual estimation of LVEF by expert echocardiography readers is also commonly used.
 Increasingly, state-of-the-art full volume acquisition using 3-dimensional (3-D) echocardiography can be used to provide accurate LVEF.
Increasingly, state-of-the-art full volume acquisition using 3-dimensional (3-D) echocardiography can be used to provide accurate LVEF.
 Systolic dysfunction in the presence of preserved LVEF (more than 50%-55%)—such as is found in patients with hypertrophic hearts, ischemic heart disease, or infiltrative cardiomyopathies—can be identified by depressed systolic tissue Doppler velocities.
Systolic dysfunction in the presence of preserved LVEF (more than 50%-55%)—such as is found in patients with hypertrophic hearts, ischemic heart disease, or infiltrative cardiomyopathies—can be identified by depressed systolic tissue Doppler velocities.
4. What is an echocardiographic diastolic assessment? What information can it provide?
 LV relaxation is usually best determined using tissue Doppler imaging, which assesses early diastolic filling velocity (Ea) of the LV myocardium. Normal hearts have Ea of 10 cm/sec or greater; impaired relaxation is present when Ea is less than 10 cm/sec.
LV relaxation is usually best determined using tissue Doppler imaging, which assesses early diastolic filling velocity (Ea) of the LV myocardium. Normal hearts have Ea of 10 cm/sec or greater; impaired relaxation is present when Ea is less than 10 cm/sec.
 An indicator of LV preload is peak transmitral early diastolic filling velocity (E), which measures the velocity of blood flow across the mitral valve. An estimate of the LV filling pressure can be made using the ratio of blood flow velocity across the mitral valve (E) to the velocity of myocardial tissue during early diastole (Ea). A high ratio (e.g., E/Ea ≥ 15) indicates elevated LV filling pressure (LA pressure ≥ 15 mm Hg); a lower ratio (e.g., E/Ea ≤ 10) indicates normal LV filling pressure (LA pressure < 15 mm Hg).
An indicator of LV preload is peak transmitral early diastolic filling velocity (E), which measures the velocity of blood flow across the mitral valve. An estimate of the LV filling pressure can be made using the ratio of blood flow velocity across the mitral valve (E) to the velocity of myocardial tissue during early diastole (Ea). A high ratio (e.g., E/Ea ≥ 15) indicates elevated LV filling pressure (LA pressure ≥ 15 mm Hg); a lower ratio (e.g., E/Ea ≤ 10) indicates normal LV filling pressure (LA pressure < 15 mm Hg).
5. How can echocardiography with Doppler be used to answer cardiac hemodynamic questions?
 Stroke volume and cardiac output can be obtained with measurements of the LV outflow tract and time-velocity integral (TVI) of blood through the LV outflow tract.
Stroke volume and cardiac output can be obtained with measurements of the LV outflow tract and time-velocity integral (TVI) of blood through the LV outflow tract.
 Doppler evaluation of right ventricular outflow tract diameter and TVI similarly allow measurement of right ventricular output.
Doppler evaluation of right ventricular outflow tract diameter and TVI similarly allow measurement of right ventricular output.
 Tricuspid regurgitation peak gradient can be added to estimate of right atrial pressure to in turn estimate pulmonary artery systolic pressure.
Tricuspid regurgitation peak gradient can be added to estimate of right atrial pressure to in turn estimate pulmonary artery systolic pressure.
 Mitral inflow velocities, deceleration time, pulmonary venous parameters, and tissue Doppler imaging of the mitral annulus can give accurate assessment of LV diastolic function, including LV filling pressures.
Mitral inflow velocities, deceleration time, pulmonary venous parameters, and tissue Doppler imaging of the mitral annulus can give accurate assessment of LV diastolic function, including LV filling pressures.
 Measurement of TVI and valve annular diameters can be used to assess intracardiac shunts (Qp/Qs) and regurgitant flow volumes, where Qp is flow out the right ventricular outflow tract and Qs is flow out the left ventricular outflow tract.
Measurement of TVI and valve annular diameters can be used to assess intracardiac shunts (Qp/Qs) and regurgitant flow volumes, where Qp is flow out the right ventricular outflow tract and Qs is flow out the left ventricular outflow tract.
 Pressure gradients across native and prosthetic valves and across cardiac shunts can be used to assess hemodynamic severity of valve stenosis, regurgitation, or shunt severity, respectively.
Pressure gradients across native and prosthetic valves and across cardiac shunts can be used to assess hemodynamic severity of valve stenosis, regurgitation, or shunt severity, respectively.
 Respiratory variation in valvular flow can aid in the diagnosis of cardiac tamponade or constrictive pericarditis.
Respiratory variation in valvular flow can aid in the diagnosis of cardiac tamponade or constrictive pericarditis.
6. How is echocardiography used to evaluate valvular disease?
 Two-dimensional echocardiography can provide accurate visualization of valve structure to assess morphologic abnormalities (calcification, prolapse, flail, rheumatic disease, endocarditis). Figure 5-3 demonstrates the restricted movement of the mitral valve in a patient with mitral stenosis.
Two-dimensional echocardiography can provide accurate visualization of valve structure to assess morphologic abnormalities (calcification, prolapse, flail, rheumatic disease, endocarditis). Figure 5-3 demonstrates the restricted movement of the mitral valve in a patient with mitral stenosis.
 Color Doppler can provide semiquantitative assessment of the degree of valve regurgitation (mild, moderate, severe) in any position (aortic, mitral, pulmonic, tricuspid).
Color Doppler can provide semiquantitative assessment of the degree of valve regurgitation (mild, moderate, severe) in any position (aortic, mitral, pulmonic, tricuspid).
 Pulsed Doppler can help pinpoint the location of a valvular abnormality (e.g., subaortic vs aortic vs supraaortic stenosis). Pulsed Doppler can also be used to quantitate regurgitant volumes and fractions using the continuity equation.
Pulsed Doppler can help pinpoint the location of a valvular abnormality (e.g., subaortic vs aortic vs supraaortic stenosis). Pulsed Doppler can also be used to quantitate regurgitant volumes and fractions using the continuity equation.
 Continuous-wave Doppler is useful for determining the hemodynamic severity of stenotic lesions, such as aortic or mitral stenosis.
Continuous-wave Doppler is useful for determining the hemodynamic severity of stenotic lesions, such as aortic or mitral stenosis.
7. How can echocardiography help diagnose and manage patients with suspected pericardial disease?
 Echocardiography can diagnose pericardial effusions (Fig. 5-4) because fluid in the pericardial space readily transmits ultrasound (appears black on echo).
Echocardiography can diagnose pericardial effusions (Fig. 5-4) because fluid in the pericardial space readily transmits ultrasound (appears black on echo).
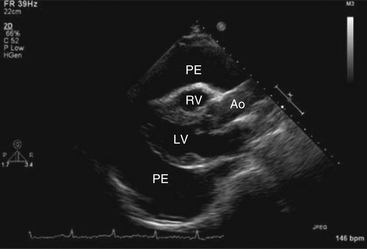
Figure 5-4 Parasternal long-axis view showing a large pericardial effusion (PE) surrounding the heart. Ao, Aorta; LV, left ventricle; RV, right ventricle. (From Kabbani SS, LeWinter M: Cardiac constriction and restriction. In Crawford MH, DiMarco JP, editors: Cardiology, St Louis, 2001, Mosby.)
 Two-dimensional echocardiography and Doppler are pivotal in determining the hemodynamic impact of pericardial fluid; that is, whether the patient has elevated intrapericardial pressure or frank cardiac tamponade.
Two-dimensional echocardiography and Doppler are pivotal in determining the hemodynamic impact of pericardial fluid; that is, whether the patient has elevated intrapericardial pressure or frank cardiac tamponade.
 The following are indicators of elevated intrapericardial pressure in the setting of pericardial effusion:
The following are indicators of elevated intrapericardial pressure in the setting of pericardial effusion:
 Diastolic indentation or collapse of the right ventricle (RV)
Diastolic indentation or collapse of the right ventricle (RV)
 Compression of the right atrium (RA) for more than one-third of the cardiac cycle
Compression of the right atrium (RA) for more than one-third of the cardiac cycle
 Lack of inferior vena cava (IVC) collapsibility with deep inspiration
Lack of inferior vena cava (IVC) collapsibility with deep inspiration
 25% or greater variation in mitral or aortic Doppler flows
25% or greater variation in mitral or aortic Doppler flows
 50% or greater variation of tricuspid or pulmonic valves flows with inspiration
50% or greater variation of tricuspid or pulmonic valves flows with inspiration
 Echocardiographic signs of constrictive pericarditis include thickened or calcified pericardium, diastolic bounce of the interventricular septum, restrictive mitral filling pattern with 25% or greater respiratory variation in peak velocities, and lack of inspiratory collapsibility of the inferior vena cava.
Echocardiographic signs of constrictive pericarditis include thickened or calcified pericardium, diastolic bounce of the interventricular septum, restrictive mitral filling pattern with 25% or greater respiratory variation in peak velocities, and lack of inspiratory collapsibility of the inferior vena cava.
 Echocardiography is additionally useful for guiding percutaneous needle pericardiocentesis by identifying the transthoracic or subcostal window with the largest fluid cushion, monitoring decrease of fluid during pericardiocentesis, and in follow-up studies, assessing for reaccumulation of fluid.
Echocardiography is additionally useful for guiding percutaneous needle pericardiocentesis by identifying the transthoracic or subcostal window with the largest fluid cushion, monitoring decrease of fluid during pericardiocentesis, and in follow-up studies, assessing for reaccumulation of fluid.
8. What is the role of echocardiography in patients with ischemic stroke?
 Depressed LV ejection fraction, generally less than 40%
Depressed LV ejection fraction, generally less than 40%
 Left ventricular or left atrial clot (Fig. 5-5)
Left ventricular or left atrial clot (Fig. 5-5)
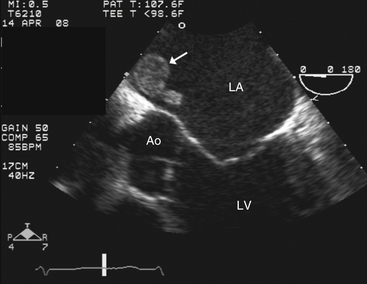
Figure 5-5 Transesophageal echocardiography showing a left atrial thrombus (arrow). Ao, Aortic valve; LA, left atrium; LV, left ventricle.
 Intracardiac mass such as tumor or endocarditis
Intracardiac mass such as tumor or endocarditis
 Mitral stenosis (especially with a history of atrial fibrillation)
Mitral stenosis (especially with a history of atrial fibrillation)
 Prosthetic valve in the mitral or aortic position
Prosthetic valve in the mitral or aortic position
 Significant atherosclerotic disease in the aortic root, ascending aorta, or aortic arch
Significant atherosclerotic disease in the aortic root, ascending aorta, or aortic arch
 Saline contrast study indicating a significant right-to-left intracardiac shunt, such as atrial septal defect
Saline contrast study indicating a significant right-to-left intracardiac shunt, such as atrial septal defect
Note: A normal transthoracic echocardiogram in a patient without atrial fibrillation generally excludes a cardiac embolic source of clot and generally obviates the need for transesophageal echocardiography (TEE).
9. What are the echocardiographic findings in hypertrophic cardiomyopathy (HCM)?
 Septal, concentric, or apical hypertrophy (walls greater than 1.5 cm in diameter)
Septal, concentric, or apical hypertrophy (walls greater than 1.5 cm in diameter)
 The presence of systolic anterior motion (SAM) of the mitral valve in some cases of obstructive hypertrophic cardiomyopathy (Fig. 5-6)
The presence of systolic anterior motion (SAM) of the mitral valve in some cases of obstructive hypertrophic cardiomyopathy (Fig. 5-6)
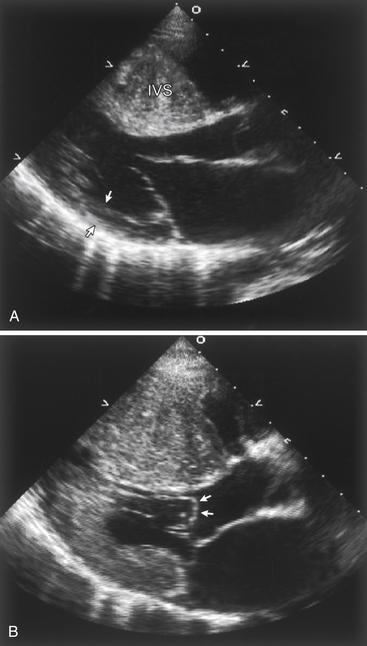
Figure 5-6 Echocardiographic findings in hypertrophic cardiomyopathy. A, Parasternal long axis image during diastole demonstrating massive thickening of the interventricular septum (IVS) when compared to the thickness of the posterior wall (arrows). B, During systole, echocardiography demonstrates systolic anterior motion (SAM) of the mitral valve, with the leaflets actually bowing in to the left ventricular outflow tract (arrows).
 Dynamic left ventricular outflow tract (LVOT) gradients caused by SAM, midcavitary obliteration, or apical obliteration
Dynamic left ventricular outflow tract (LVOT) gradients caused by SAM, midcavitary obliteration, or apical obliteration
10. What are the common indications for transesophageal echocardiography?
 Significant clinical suspicion of endocarditis in patients with suboptimal transthoracic windows
Significant clinical suspicion of endocarditis in patients with suboptimal transthoracic windows
 Significant clinical suspicion of endocarditis in patients with prosthetic heart valve
Significant clinical suspicion of endocarditis in patients with prosthetic heart valve
 Suspected aortic dissection (Fig. 5-7)
Suspected aortic dissection (Fig. 5-7)
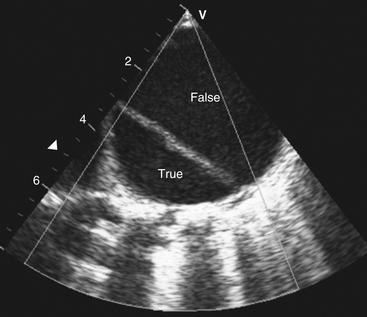
Figure 5-7 Transesophageal echocardiography revealing dissection of the descending thoracic aorta. The true aortic lumen (True) is seen separated from the false lumen (False) by the dissection.
 Suspected atrial septal defect (ASD) or patent foramen ovale in patients with cryptogenic embolic stroke
Suspected atrial septal defect (ASD) or patent foramen ovale in patients with cryptogenic embolic stroke
 Embolic stroke with nondiagnostic transthoracic echo
Embolic stroke with nondiagnostic transthoracic echo
 Endocarditis with suspected valvular complications (abscess, fistula, pseudoaneurysm)
Endocarditis with suspected valvular complications (abscess, fistula, pseudoaneurysm)
 Evaluation of the mitral valve in cases of possible surgical mitral valve
Evaluation of the mitral valve in cases of possible surgical mitral valve
 Intracardiac shunt in which the location is not well seen on transthoracic echocardiography
Intracardiac shunt in which the location is not well seen on transthoracic echocardiography
 Assessment of the left atria and left atrial appendage for the presence of thrombus (clot) (see Fig. 5-5) prior to planned cardioversion
Assessment of the left atria and left atrial appendage for the presence of thrombus (clot) (see Fig. 5-5) prior to planned cardioversion
11. What is contrast echocardiography?
Because synthetic microbubbles are smaller than saline bubbles, they cross the pulmonary capillaries and are used to image left heart structures. Most commonly, synthetic microbubbles are used to achieve better endocardial border definition in patients with suboptimal echocardiographic windows. Contrast echocardiography is also used to better visualize structures such as possible LV clots or other masses.
Both synthetic and saline contrast agents can be used to augment Doppler signals, for example, in patients with pulmonary hypertension in whom a tricuspid regurgitation jet is needed to estimate pulmonary artery pressure.
12. What is stress echocardiography?
Other uses of stress echocardiography include:
 Assessment of mitral or aortic valve disease in patients who have moderate disease at rest but significant symptoms with exercise
Assessment of mitral or aortic valve disease in patients who have moderate disease at rest but significant symptoms with exercise
 Assessment of patients with suspected exercise-induced diastolic dysfunction
Assessment of patients with suspected exercise-induced diastolic dysfunction
 Assessment of viability in patients with depressed ejection fractions. Improvement in left ventricular function with infusion of low-dose dobutamine (less than 10 μg/kg/min) suggests viable myocardium.
Assessment of viability in patients with depressed ejection fractions. Improvement in left ventricular function with infusion of low-dose dobutamine (less than 10 μg/kg/min) suggests viable myocardium.
 Distinguishing between true aortic stenosis and pseudo aortic stenosis in patients with mild-to-moderate aortic stenosis at rest and depressed ejection fraction with low cardiac output
Distinguishing between true aortic stenosis and pseudo aortic stenosis in patients with mild-to-moderate aortic stenosis at rest and depressed ejection fraction with low cardiac output
Bibliography, Suggested Readings, and Websites
1. Abraham, T.P., Dimaano, V.L., Liang, H.Y. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609.
2. Armstrong, W.F., Zoghbi, W.A. Stress echocardiography: current methodology and clinical applications. J Am Coll Cardiol. 2005;45:1739–1747.
3. Douglas, P.S., Khandheria, B., Stainback, R.F., et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography. J Am Coll Cardiol. 2007;50:187–204.
4. Evangelista, A., Gonzalez-Alujas, M.T. Echocardiography in infective endocarditis. Heart. 2004;90:614–617.
5. Grayburn, P.A. How to measure severity of mitral regurgitation: valvular heart disease. Heart. 2008;94:376–383.
6. Kirkpatrick, J.N., Vannan, M.A., Narula, J., et al. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50:381–396.
7. Lang, R.M., Mor-Avi, V., Sugeng, L., et al. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol. 2006;48:2053–2069.
8. Lester, S.J., Tajik, A.J., Nishimura, R.A., et al. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689.
9. Otto, C.M. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151.
10. Peterson, G.E., Brickner, M.E., Reimold, S.C. Transesophageal echocardiography: clinical indications and applications. Circulation. 2003;107:2398–2402.
11. Stewart, M.J. Contrast echocardiography. Heart. 2003;89:342–348.