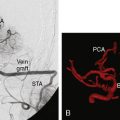
14 EC-IC Bypass Using ELANA Technique
Introduction
ELANA represents the results of the ideas and investigation of C. A. F. Tulleken and his group at the University Medical Centre in Utrecht, Holland, and spans a period from 1993 to the present. Their efforts arose partly in response to the EC-IC Bypass Study in the mid-1980s, which had disappointing results regarding the viability of STA-MCA bypasses in treating ischemic patients. Tulleken believed that the results of the study may have been different had the bypasses been more proximal in the circle of Willis with its resultant higher flows. However, making such high-flow bypasses would be problematic in the ischemic patient who was less likely to tolerate the temporary occlusion of the large proximal cerebral arteries while constructing the bypasses. In general, the incidence of perioperative stroke in creating high-flow bypasses is approximately 9.5%.1–3 Therefore, a nonocclusive method of augmenting flow would be of particular benefit to these patients.
The Utrecht group began to investigate the use of laser technology to develop a nonocclusive method of creating intracranial anastomoses. In the early 1990s, they developed a laser catheter and suction system designed to create a consistent attachment to the artery wall and a more consistent arteriotomy. The addition of a separate small platinum ring that allows the laser catheter to better interface with the recipient wall has led to an efficient system of creating nonocclusive cerebral bypasses. The stages of the development of ELANA have been reviewed in the literature4 with the first human cases performed in 1993. Minor modifications were made between 1993 and 1995 with the current technique and technology remaining quite stable over the past 15 years with minimal substantive change. This technique has similarly been reviewed previously in the literature.4 The ELANA system is currently being utilized in primarily three centers in Europe, one in Canada, and four in the United States. This chapter aims to review the ELANA system and technique, examine the data from clinical studies in human cases, and discuss its benefits along with its disadvantages over conventional high-flow bypass techniques while updating current thinking on its future with an eye toward future modifications that may make the technique even more desirable.
Technique
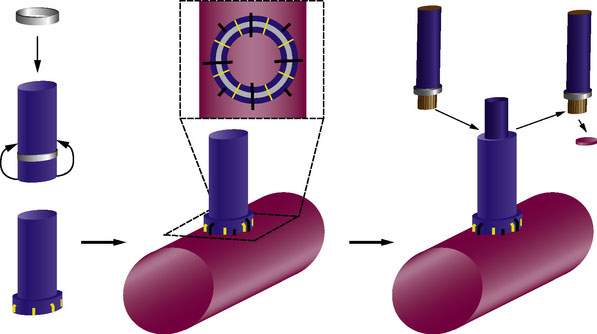
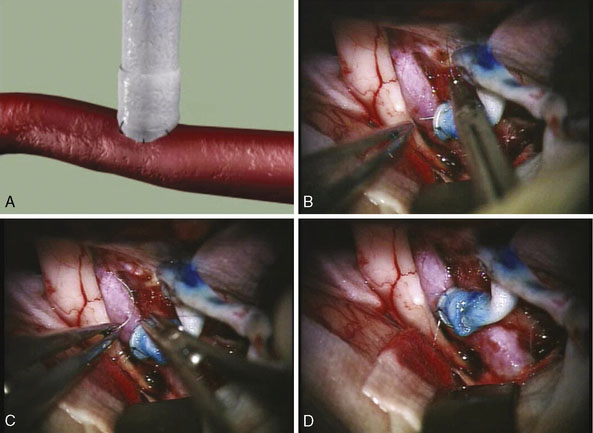
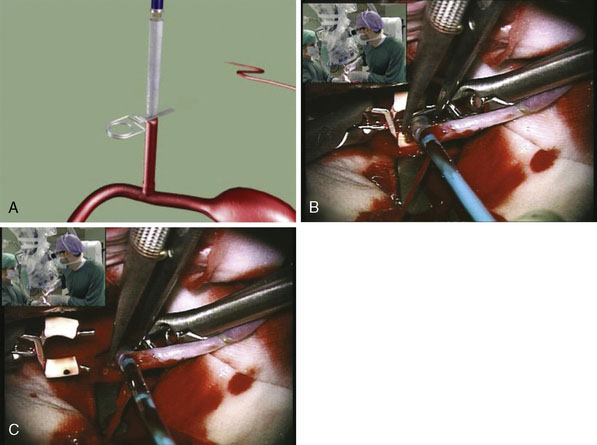
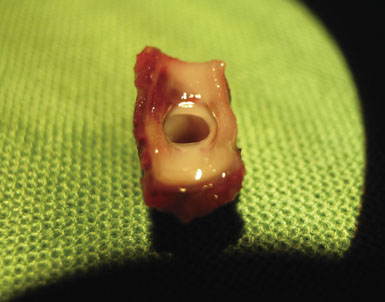
Two unique steps are necessary to create an ELANA anastomosis. First, a platinum ring of 2.6 to 2.8 mm in diameter is attached to the distal part of the donor vessel at a side table by flipping the distal end of the donor around the ring and microsurgically suturing using 4-8 😯 nylon sutures to secure it in place. Second, following microsurgically attaching the donor/ring construct to the recipient using 8-10 😯 prolene sutures and after the proximal anastomosis has been created, the ELANA catheter is used to create the arteriotomy using an excimer laser energy source with removal of the arterial “flap” using vacuum suction within the catheter (Figures 14–1 and 14–2). The laser catheter is composed of an outer array of laser fibers representing the “cutting” element surrounding an inner core of central suction (see Figure 14–2). The ELANA catheter is passed through the open end of the donor vessel or through a side slit of the donor to the recipient outer wall (Figure 14–3) and after 2 minutes of suction activation, the laser is activated for two to three 10-second bursts to create the arteriotomy. After the arteriotomy has been made, the catheter is withdrawn with the arterial flap attached to the central suction core (Figure 14–4).

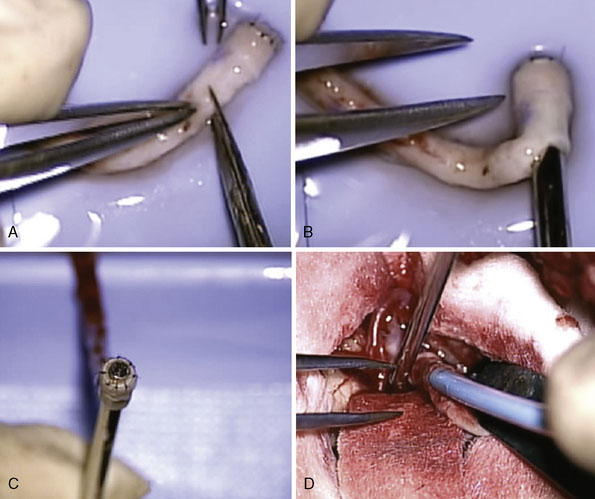
Figure 14–3 Photograph showing the ELANA catheter tip. Note inner suction surrounded by outer laser array.
(From Langer DJ, Van Der Zwan A, Vajkoczy P, et al., Excimer laser-assisted nonocclusive anastomosis. An emerging technology for use in the creation of intracranial-intracranial and extracranial-intracranial cerebral bypass, Neurosurg Focus 2008;24(2):E6.)
Once the laser step has been completed, a temporary clip is placed just proximal to the anastomosis on the donor side. This prevents back bleeding through the proximal open end of the donor graft or through the side slit (Figure 14–5). Bypasses can be created in a single-piece or two-piece fashion. A one-piece graft is the same as conventional except for the creation of a temporary side slit in the donor to allow catheter access to the anastomosis. A two-piece graft can also be performed with the distal portion of the donor sewn to the proximal portion, which is attached to an extracranial carotid source (Figures 14–6 and 14–7). ELANA can also be used to create novel IC-IC bypasses where the proximal donor source is an intracranial vessel such as A1, M1, or the internal carotid, and the distal target is to nearly any target vessel of sufficient caliber. The proximal arteriotomy is made using ELANA, while the distal arteriotomy is created using ELANA or conventional technique.
Neuroprotective Considerations
To create most high-flow bypasses, the donor or recipient vessels must be temporarily occluded to allow for the construction of the anastomoses. Creating high-flow bypasses in the presence of inadequate collateral blood flow has a high morbidity rate due to the need for prolonged temporary clipping during the creation of the anastomosis. These patients are at high risk for ischemia and subsequent cerebral infarction due to the high occlusion times necessary for this complex procedure.5,6 Ogilvy et al.7 demonstrated that the risk for cerebral infarction increases when temporary occlusion exceeds 20 minutes. In high-flow bypass surgery, occlusion times can be up to 45 to 60 minutes and require neuroprotective protocols not often used for most neurosurgical procedures such as moderate to deep hypothermia, pentobarbital administration, and induction of arterial hypertension to prevent ischemia.5,8–15 These modalities help to protect the brain during temporary occlusion by suppressing metabolic demand and improving nutrient delivery to the brain. However, these protective strategies themselves have been associated with side effects that can contribute to poor outcomes in patients who are already at high risk due to the complexities of cerebral bypass surgery.
ELANA allows for the creation of complex end-to-side, high-flow bypasses without the need for temporary clipping. It minimizes the risk of cerebral ischemia and eliminates time constraints for the construction of the bypass since the vessel stays open throughout and blood flow is uninterrupted. The complex brain-protective strategies that would otherwise be necessary can be avoided. This was confirmed by Muench et al.5 who were able to successfully treat 29 patients who required high-flow bypasses for treatment of giant aneurysms or tumors. Rather than using brain-protective strategies as noted above, they were able to achieve good results by using standard induction protocols typical of most other intracranial cerebrovascular procedures. They also noted that ELANA allows for less retraction of the brain and less manipulation as compared to conventional bypass techniques, helping to reduce incidence of brain swelling and edema.1,5
Large/Giant ICA Aneurysms
The natural history for aneurysms above 1 cm in diameter is grave,16,17 and they are typically treated aggressively. Direct coil embolization can be unsafe due to the large size and broad neck of the aneurysm. Due to their complex angiographic anatomy, giant aneurysms are not very amenable to endovascular treatment, which results in a complete occlusion rate of 64%, a mortality rate of 9%, and a major morbidity rate of 12.7%.18 It is thought that cerebral bypass using high-flow anastomoses can assist with the direct surgical treatment of these lesions in order to achieve a higher treatment rate with fewer complications. Van Doormaal et al.19 reported treating 34 patients with aneurysms of the ICA proximal to the bifurcation. They reported a mean age of 53 years and aneurysm sizes ranging from 10 to 30 mm. Patients presented with symptoms of cranial nerve compression, a history of SAH, or were asymptomatic. All were thought to be at risk of ICA occlusion without the bypass due to failed BTOs or poorly developed collateral vessels on angiography. In all patients, a bypass was constructed between the ECA using a conventional technique, and the intracranial ICA, M1, or A1 segment using the ELANA technique.
Strokes and ICA Occlusions
Ischemic strokes associated with ICA occlusions result in a compromised hemodynamic state and have a risk of recurrent stroke as high as 9% to 18% per year. In patients for whom there are no other treatment options, EC-IC bypass is a consideration. The STA-MCA bypass was not shown to be effective in preventing stroke in patients with symptomatic ICA bypass.20 The drawback to the EC-IC Bypass Study may be due to lack of control for patients who were not hemodynamically compromised, and that STA-MCA flow may be inadequate. On average, STA-MCA bypass provides 15 to 25 ml/min. More proximal, high-flow bypasses may be able to better protect these patients from future ischemic events. In patients who have undergone cerebral revascularization for treatment of ischemic events in the setting of ICA occlusion, ELANA has been used to establish a high-flow bypass from the ECA to the intracranial ICA, proximal MCA, or ACA. In a smaller subset of patients treated for ICA occlusion with ELANA bypass, there were favorable results, with mean flow rates of 130 ml/min and no symptoms of hyperperfusion.16 Currently studies are underway to investigate the efficacy of high-flow ELANA bypasses in patients with symptomatic carotid artery occlusive disease. Indeed, ELANA was originally designed to treat such patients and prevent further ischemia resulting from surgical treatment.
Blood Flow in ELANA Bypasses
In general, the mean flow in the ICA is approximately 250 ml/min. Distal branches of the MCA may have flows ranging from 40 to 140 ml/min.1,3,21 Van der Zwan et al.1 quantified ELANA bypass flow in 36 patients who underwent high-flow ELANA EC-IC bypass for treatment of giant aneurysms or symptomatic occlusive disease of the ICA. They found that mean bypass flow was 140 ml/min, ranging from 60 to 220 ml/min. This far exceeds common flows through conventional STA-MCA bypasses described in the literature, which range from 15 to 20 ml/min.3 This is most likely because of the higher caliber of the recipient and graft vessels. The larger the recipient artery is, the larger its peripheral vascular tree; thus, the bypass graft encounters less peripheral resistance. By bringing in higher flow to the proximal circle of Willis, the bypass is able to make a greater contribution to distal cerebral circulation.
Patency of ELANA Bypasses
As the goal of bypass surgery is to provide long-term revascularization, examining the patency of ELANA bypasses as compared to conventional bypasses is necessary. Regli et al.22 found that EC-IC bypasses using conventional saphenous vein grafts were more likely to occlude when flow was below 50 ml/min, and that the overall patency rate of such conventional bypasses was approximately 86%. Bremmer et al.23 examined the patency rate of 159 consecutive ELANA bypasses in a prospective study. They found that intraoperative ELANA patency was 95%, and although intraoperative patency predicted long-term patency, the postoperative patency rate of ELANA bypasses was 77%. This may have been due to delayed aneurysm deconstruction, as opposed to immediate intraoperative vessel sacrifice. In addition, a number of the aneurysms bypassed required distal anastomoses at the A2 or M2 segments with somewhat lower flows obtained.24
In addition, Bremmer et al.23 noted that ELANA patency rates were highest in older patients, perhaps because collateral pathways can be more effectively recruited in younger patients. There was an increased risk of occlusion of the bypass in female patients, as female patients tended to have lower bypass flows. The administration of heparin to the patients during the bypass procedure increased intraoperative patency rates, but did not have a significant impact on postoperative patency rates, even though intraoperative patency predicted long-term patency. In addition, intraoperative trapping of the aneurysm improved bypass flows and improved patency rates. ELANA bypass patency rates are best when there is careful patient selection via test occlusion, immediate intraoperative entrapment of the aneurysm, and a patent intraoperative bypass. Intraoperative angiograms and infrared indocyanine green video angiography25 may assist in checking the patency of the bypass.
Advantages and disadvantages
The biggest single advantage of ELANA over conventional bypass is clearly the lack of temporary occlusion. This permits the operating surgeon to not only perform a high-flow bypass without ischemia time but also allows the creation of bypasses that could not be safely performed with conventional techniques such as bypasses to the ICA, P1, and the basilar artery. Grafting to larger, more proximal recipient vessels may be superior to more peripheral bypass and may provide more physiological inflow.1 In addition grafting into more proximal vessels such as the ICA or P1 provides more direct high-pressure flow to small perforating vessels as compared to bypasses to M2/3 or P3 in which perforators are more distal from the inflow zone putting them at risk. IC-IC operations such as M1-M2 or M3 as well as ICA to P1 can be more safely performed.26 By keeping the grafts entirely intracranial, the graft length is reduced with the elimination of a neck incision thus reducing morbidity related to neck dissection, graft compression, and thrombosis.
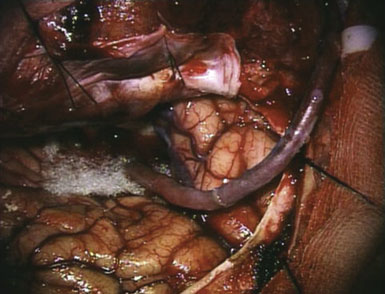
There are, however, distinct disadvantages that may affect the adoption of the ELANA technique throughout neurosurgery. First, the ELANA technique adds time to the surgical procedure because of its extra steps. In addition, flap retrieval is not 100%, and at this point hovers around 85% to 90%. Flap retrieval remains a clear technical hurdle that needs to be overcome. The surgical technique can be modified to retrieve a residual flap by incising the donor vessel close to the anastomosis under a short period of temporary occlusion (Figure 14–8). This allows the flap retrieval rate to be close to 100% but increases the technical factors that work against its adoption.
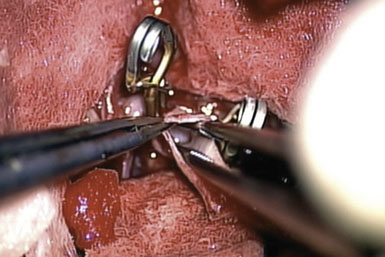
Figure 14–8 Microforceps within the graft to retrieve retained flap. Note temporary clips on the recipient vessel.
The biggest factors that need to be overcome when considering using ELANA are cost and training versus the perceived benefit to the patient. The ELANA catheter kit costs over $10,000 per use with the laser costs somewhat higher every year. The technical skill required to perform ELANA is no different than that for conventional bypass, but the technique is not straightforward27 and requires a commitment to laboratory training and mentoring that is time-consuming. These factors would probably play less of a role if high-flow EC-IC and IC-IC were commonly done. Unfortunately, they are rare. The total number of high-flow bypasses performed nationwide probably does not exceed 1000 per year, and in all likelihood this number will continue to erode with the advent of flow-diverter technology. When these factors are balanced against the perceived benefit of a nonocclusive technique, they weigh heavily and clearly work against its adoption. Most bypass surgeons believe that the risk of stroke related to temporary occlusion time is decidedly low or simply is not high enough to warrant the cost and effort of learning a new technique that adds operative time and requires an investment in training. ELANA is clearly the only safe option for patients with aneurysms and tumors on or around vessels that cannot be occluded at all, but these remain a tiny subset of an already small patient group.
The future of elana
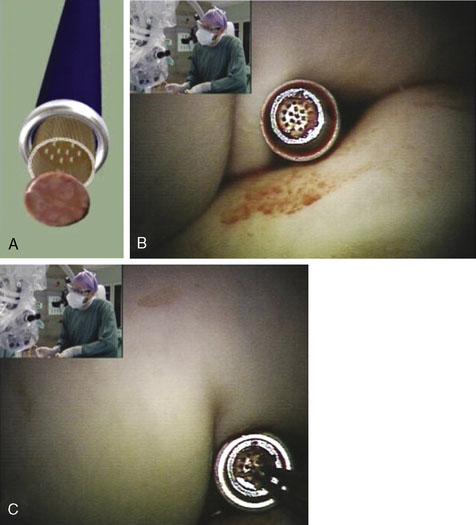
Currently the disadvantages of ELANA weigh heavily against its broad adoption. However, improvements in design of the ELANA ring may impact its clinical efficacy and eliminate some of these disadvantages. A newly designed ring (Figures 14–9 and 14–10) will potentially permit the creation of a nonocclusive sutureless bypass—sutureless ELANA or SELANA. This improvement will permit the surgeon to perform the distal intracranial and, with modification, the proximal extracranial anastomosis without microsuturing, vastly shortening operative time and making the operation much easier to perform. Studies evaluating the safety and efficacy of SELANA are ongoing in Holland with its clinical introduction expected in 2010.
1 van der Zwan A., Tulleken C.A., Hillen B. Flow quantification of the non-occlusive excimer laser-assisted EC-IC bypass. Acta Neurochir (Wien). 2001;143(7):647-654.
2 Iwai Y., Sekhar L.N., Goel A., et al. Vein graft replacement of the distal vertebral artery. Acta Neurochir (Wien). 1993;120(1–2):81-87.
3 Sekhar L., Kalavakonda C. Saphenous vein and radial artery grafts in the management of skill base tumors and aneurysms. In: Spetzler R.F., Schmiedeck P., editors. Operative Techniques in Neurosurgery, vol. 2. Philadelphia: WB Saunders; 1999:129-141. Extra-Intracranial Bypass Surgery
4 Langer D.J., Vajkoczy P. ELANA: Excimer laser assisted nonocclusive anastomosis for extracranial-to-intracranial and intracranial-to-intracranial bypass: a review. Skull Base. 2005;15:191-204.
5 Muench E., Meinhardt J., Schaeffer M., et al. The use of the excimer laser-assisted anastomosis technique alleviates neuroanesthesia during cerebral high-flow revascularization. J Neurosurg Anesthesiol. 2007;19(4):273-279.
6 Samson D., Batjer H.H., Bowman G., et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34(1):22-28. discussion 28–29
7 Ogilvy C.S., Carter B.S., Kaplan S., et al. Temporary vessel occlusion for aneurysm surgery: risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996;84(5):785-791.
8 Lavine S.D., Masri L.S., Levy M.L., et al. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg. 1997;87(6):817-824.
9 Spetzler R.F., Hadley M.N., Rigamonti D., et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868-879.
10 McDermott M.W., Durity F.A., Borozny M., et al. Temporary vessel occlusion and barbiturate protection in cerebral aneurysm surgery. Neurosurgery. 1989;25:54-61. discussion 61–62
11 Michenfelder J.D., Milde J.H. Influence of anesthetics on metabolic, functional and pathological responses to regional cerebral ischemia. Stroke. 1975;6:405-410.
12 Michenfelder J.D., Milde J.H. Cerebral protection by anaesthetics during ischaemia (a review). Resuscitation. 1975;4:219-233.
13 Michenfelder J.D., Milde J.H., Sundt T.M.Jr. Cerebral protection by barbiturate anesthesia. Use after middle cerebral artery occlusion in Java monkeys. Arch Neurol. 1976;33:345-350.
14 Shapiro H.M. Barbiturates in brain ischaemia. Br J Anaesth. 1985;57:82-95.
15 Yatsu F.M., Diamond I., Graziano C., et al. Experimental brain ischemia: protection from irreversible damage with a rapid-acting barbiturate (methohexital). Stroke. 1972;3:726-732.
16 Langer D.J., Van Der Zwan A., Vajkoczy P., et al. Excimer laser-assisted nonocclusive anastomosis. An emerging technology for use in the creation of intracranial-intracranial and extracranial-intracranial cerebral bypass. Neurosurg Focus. 2008;24(2):E6.
17 Tulleken C.A., van der Zwan A., van Rooij W.J., et al. High-flow bypass using nonocclusive excimer laser-assisted end-to-side anastomosis of the external carotid artery to the P1 segment of the posterior cerebral artery via the sylvian route. Technical note. J Neurosurg. 1998;88(5):925-927.
18 Parkinson R.J., Eddelman C.S., Batjer H.H., et al. Giant aneurysms: endovascular challenges. Neurosurgery. 2006;59(Suppl 5):S103-S112.
19 van Doormaal T.P., van der Zwan A., Verweij B.D., et al. Treatment of giant and large internal carotid artery aneurysms with a high-flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2006;59(Suppl 4):ONS328-ONS335.
20 Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313(19):1191-1200.
21 Charbel F.T., Misra M., Clarke M.E., et al. Computer simulation of cerebral blood flow in moyamoya and the results of surgical therapies. Clin Neurol Neurosurg. 1997;99(Suppl 2):S68-S73.
22 Regli L., Piepgras D.G., Hansen K.K. Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg. 1995;83(5):806-811.
23 Bremmer J.P., Verweij B.H., Klijn C.J., et al. Predictors of patency of excimer laser-assisted nonocclusive extracranial-to-intracranial bypasses. J Neurosurg. 2009;110(5):887-895.
24 Sundt T.M.Jr, Piepgras D.G., Marsh W.R., et al. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65(4):439-450.
25 Woitzik J., Horn P., Vajkoczy P., et al. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005;102(4):692-698.
26 Tulleken C.A., Streefkerk H.J., van der Zwan A. Construction of a new posterior communicating artery in a patient with poor posterior fossa circulation: technical case report. Neurosurgery. 2002;50(2):415-419. discussion 419–420
27 Heros R.C. Excimer laser-assisted nonocclusive anastomosis. Neurosurg Focus. 2008;24(2):E6a. discussion E6a