28 EC-IC and IC-IC Bypass for Giant Aneurysms Using the ELANA Technique
Natural history
Giant intracranial aneurysms, approximately 5% of all intracranial aneurysms, are one of the most challenging lesions in neurosurgery. By definition their fundus size is 2.5 cm or larger, and they are predominantly located in those proximal parts of the vascular tree with high blood flow, such as the internal carotid artery (cavernous and supraclinoid ICA), the proximal middle cerebral artery (MCA), the basilar artery (BA), and vertebral artery (VA). Two-thirds of these aneurysms are located in the anterior circulation and are slightly more common in the female population. Approximately 8% present in the pediatric age, but most commonly they become manifest during the fifth to seventh decades. Most of these aneurysms (20% to 70%) present with rupture and they have the same re-rupture rate as other smaller aneurysms.1,2 The second most common presentation is the mass effect of the aneurysm causing ocular movement disturbances due to cranial nerve palsies, vision or vision field loss and focal weakness/gait disorders, headaches and seizures. Third, approximately 8% of giant aneurysms present with ischemic syndromes most likely due to embolic phenomena originating from the aneurysm sac to more distally located vascular territories. The natural history of these aneurysms is very poor with high morbidity and mortality rates between 65% to 85% within 2 years after discovery.1,2
Therapeutic modalities
Endovascular Treatment
As primary clipping can be technically very difficult or impossible, the endovascular approach to these aneurysms has been increasingly popular. Although in smaller aneurysms endovascular treatment has proven to be effective in many cases, the results of endovascular treatment of giant aneurysms are still very disappointing.3,4 Endovascular Hunterian obstruction in the case of a giant ICA aneurysm of the parent vessel seems to be the most effective and simplest method of treatment. The functionality of the ICA and its intracranial collaterals as the circle of Willis and the leptomeningeals should be thoroughly evaluated before definitive occlusion. Therefore, at our center in patients harboring these aneurysms, first a balloon test occlusion (BTO) of the ICA is performed with venous outflow study to observe if the patient tolerates definitive ICA occlusion. If the patient clinically does not tolerate the occlusion, or if asymmetry in venous outflow is over 1 second, a replacement bypass procedure is planned before definitive ICA occlusion. In more distal giant aneurysms, however, BTO is less reliable due to the complexity of the aneurysm and its afferent arteries. In the explicit review of Parkinson et al., a total of 316 patients with giant aneurysms treated endovascularly using coils, parent vessel occlusion, Onyx®, stenting with or without coil or Onyx® were reviewed. In only 57% of patients was a complete occlusion or cure achieved, with 7.7% mortality and 17.2% major neurological morbidity.2 In addition, in distally located giant aneurysms the results of endovascular treatment are poor.4 Endosaccular coiling proves to be an ineffective long-term treatment of these aneurysms due to recanalization and further aneurysm growth. Parent vessel occlusion can be considered, but risks of late ischemia remain relatively large. Selective embolization of these distal giant aneurysms harbors high complication risks (20% mortality). In those cases, especially in more distal giant aneurysms of the MCA, ACA, and PCA where mass effects also play a role in symptomatology, endovascular treatment seems to be less effective.
Parent Vessel Replacement Bypass Surgery
No major reviews on results of flow replacement bypass surgery are available but smaller series are available.5,6 From these studies the results of parent artery bypass surgery using mostly VSM or radial arterial grafts with consequent trapping or proximal occluding with or without additional endovascular therapy seem to be more promising than the results of endovascular treatment alone.
Before planning bypass surgery it is mandatory to determine the amount of flow to be replaced by the bypass. As most giant aneurysms are located in the more proximal cerebropetal arteries conducting high flows like the ICA, MCA, ACA, BA, and VA, the flow to be replaced by any bypass is most highly dependent on the functional capacity of the Circle of Willis and collaterals as the leptomeningeal vessels, ophthalmic artery, and choroidal plexus. Exact determination of flow needed to replace is therefore hard to make. However, in case of BTO failure in a giant ICA aneurysm, it seems to be safe to choose for a bypass type with potential high flows. In cases of giant MCA, ACA, BA, or VA aneurysms the flows to be replaced in the parent arteries should be determined using single vessel flow MRA flowmetry or multivessel NOVA technique (VasSol®).7–9Consequently, partially based on these data a custom made plan for an EC-IC or IC-IC bypass has to be defined to rebuild vascular architecture. The anatomy must be studied in detail to determine which vessels are approachable for bypass grafting. Currently, conventional angiography, and CT perfusion and MRA are used to study this.
Before bypass attachment, intraoperative quantitative flow measurements are performed on different intracerebral arteries using a dedicated flow meter (Transonic®, Ithaca, NY), again to assess the flow, which has to be replaced by the bypass. If we use the superficial temporal artery (STA) as a donor, cut flow is measured before bypass completion to use in the cut flow index.10
It is important here to recall Poiseuille’s law, which says that flow varies proportionally to the pressure drop over the bypass, the length of the bypass, and the fourth power of the radius: Q = π r4 P/8μL (Q = flow rate; r = radius of pipe; P = pressure drop over the pipe; μ = viscosity of fluid; L = length of the pipe). For example, because cortical vessel and the STA have a relatively small diameter, the possible flow through the conventional STA-cortical MCA bypass is limited to 10 to 40 cc/min. Increased flows of 84 to 100 mL/min are possible when the STA is connected to the larger M3.11 High flows of 130 to 200 cc/min can be achieved when a venous or arterial interponate is used to connect the ECA with the intracranial ICA. So, in cases where higher flows are needed, a more proximal anastomosis should be made. However, anastomosing techniques on more proximally located arteries are technically demanding for surgeons and anesthesiologists. The conventional anastomosing technique with temporal occlusion of the major cerebral arteries theoretically provides an extra risk of ischemia during the classical procedure, and measures to prevent hypothermia and cardiac arrest and pentothal protection are described to minimize this risk. To circumvent this risk, the ELANA technique was developed by our senior author (Tulleken).
The elana technique
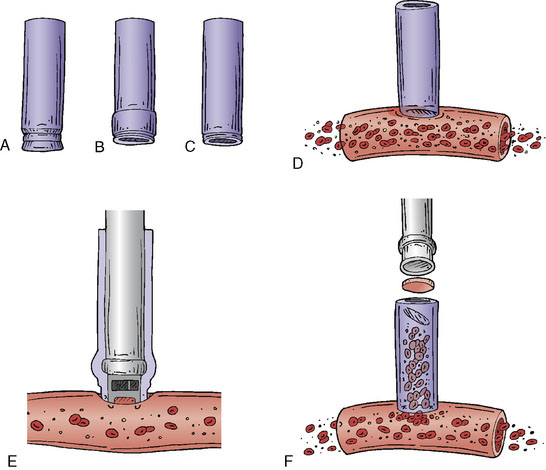
The ELANA technique (Figure 28–1) facilitates the construction of an end-to-side anastomosis without temporary occlusion of the recipient artery. It was primarily developed for cerebral augmentative revascularization (1992); however, it turned out that the technique was also quite feasible for creating protective or replacement bypasses.12–15 A vein (mostly the vena saphena magna, VSM) or artery (radial artery, RA) can be used as the donor vessel. The inner diameter should be minimally 2 mm to enable the laser catheter to pass through the donor vessel. First, depending on the size of the recipient vessel, a platinum ring of 2.6 mm or 2.8 mm is attached to the distal segment of the donor vessel using eight microsutures (see Figures 28–1A through 28–1C). The ring with the attached distal donor segment is subsequently stitched end-to-side to the recipient using again eight microsutures (see Figure 28–1D). This is followed by the passing of the ELANA 2.0® laser suction catheter down the lumen of the open donor vessel. The tip of the catheter is placed against the sidewall of the recipient vessel (see Figure 28–1E and Figure 28–2). After 2 minutes of active suctioning from the dedicated inside portion of the catheter, the laser fibers on the outside of the catheter are activated within 5 seconds. The laser broaches the recipient arterial wall and separates an arteriotomy flap from the recipient (see Figure 28–1F). The suction portion of the catheter maintains contact with the small arteriotomy flap, thus preventing its migration into the lumen of the recipient.
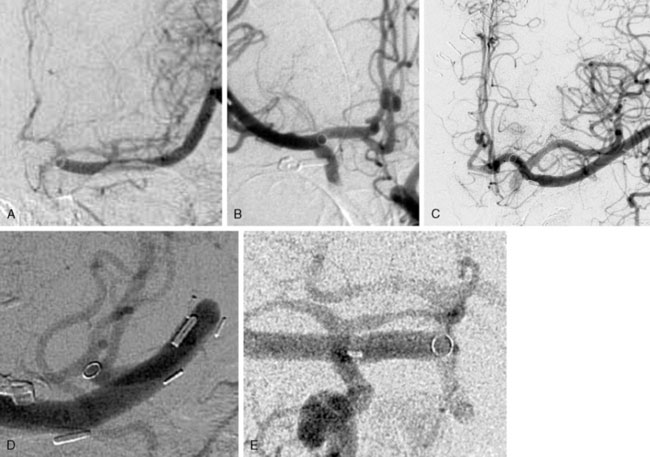
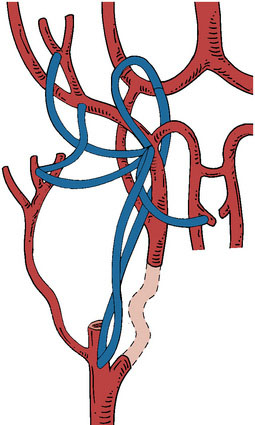
A major advantage of this procedure is the nonocclusive character of the anastomosis, and to our knowledge this is the first nonocclusive anastomosis technique in neurosurgery. Second, for creating this nonocclusive type of anastomosis less intracranial arterial exposure is needed as no temporal clips are used. Third, no measures as hypothermia are needed, as the complete procedure is nonischemic. Fourth, as there is no exposure to the ring and maximally four through-the-vessel-microsutures are needed, adequate re-endothelialization is achieved.16 Due to these characteristics safe anastomoses can be made on proximal recipients (ICA, proximal MCA, proximal ACA, P1, and BA) (Figure 28–3A through 28–3E) and various bypass types have been constructed (Figure 28–4). At our institute more than 300 patients and worldwide a large cohort of more than 400 patients were treated using the ELANA technique between 1992 and 2010 with good results and good long-term patency rates (up to 93%). After finalizing the ELANA technique in the form described, we treated 265 patients with large and giant aneurysms since 1999. These aneurysms were located in the cavernous ICA, supraclinoid ICA, ICA bifurcation, proximal MCA, proximal ACA, BA, and at the VA-BA junction. Although this technique introduces the major advantage of its nonocclusiveness and therefore the absence of intraoperative stress moments of temporal occlusion of major arteries, the technical challenges are mainly formed by the stitching of the donor vessel with the ring on the recipient vessel, most often deep intracranially. Mainly as the result of this part of the procedure, one ELANA anastomosis takes between 90 to 120 minutes, depending on its location and the neurosurgeon’s experience.
Perioperative preparation and monitoring
Preoperative Flow Measurements
For preoperative planning of surgery, bypass indication, type of bypass and location, and an estimation of the flow to be replaced can be made by using single-vessel flow MRA flowmetry or multivessel NOVA technique (VasSol®). At our institute, only single-vessel MRA flowmetry is available, and this technique is also used for postoperative evaluation of bypass flow.9
Intraoperative Flow Measurements
In all procedures since 1998 we intraoperatively measured flow through parent vessels, distal efferent vessels, and the bypass using a dedicated flow meter (Transonic®, Ithaca, NY).9 Especially in flow replacement bypass surgery it is important to measure the flow in the artery that is to be replaced. In addition, after bypass completion, it is important to monitor bypass flow during parent vessel occlusion and aneurysm trapment to measure the increase in the flow through the bypass during these maneuvers. Any complication, such as kinking of the bypass, compression of the bypass during closure, or thrombosis of the bypass, can be detected by flow monitoring during the rest of the procedure until the end of closure.
Intraoperative Near-Infrared Indocyanine Green Video Angiography (ICGA)
Before and after bypass construction as well as during aneurysm trapment or reconstruction, the use of indocyanine green video angiography (ICGA)17 can be very useful to monitor involvement of perforating arteries. Although this method is not replacing intraoperative DSA, which is not available in our hospital, it can be very helpful as long as it is taken into account that this method can only be used on those vessels in the field of surgery that are visible, and that deeper vessels are missed.
ELANA EC-IC bypass for giant aneurysms of the ICA
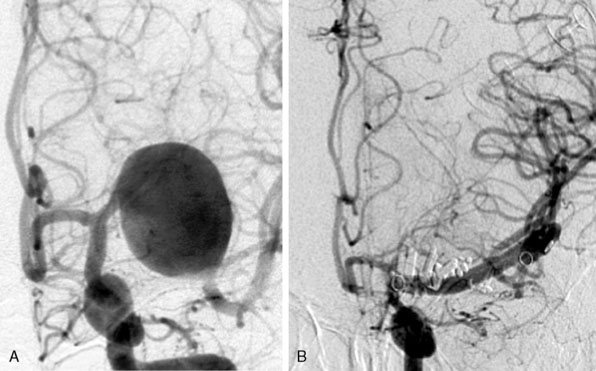
Between 1999 and 2009 we operated on 45 patients with giant aneurysms of the cavernous and supraclinoid ICA18 (Figure 28–5). Mean age was 53 years (standard deviation [SD] 15) and predominantly female (75%). Thirty-two patients (71%) failed occlusion tests, and the remaining patients lacked sufficient collaterals on imaging. Presentation of symptoms included cranial nerve compression signs exclusively in 27 patients (75%) and SAH, with or without cranial nerve compression signs, in 14 patients (31%). In the remaining four patients, no objective symptoms were registered. In all patients the VSM was used for bypass. The vein was cut in half and firstly the ELANA anastomosis was created on the intracranial ICA, ICA bifurcation, or proximal MCA. In a second phase the other half of the vein was used to make a conventional end-to-side anastomosis on the ECA and finally both vein pieces were connected in an end-to-end anastomosis. The mean surgical time for the bypass procedure of all patients was 443 minutes (range, 300 to 750 min). The mean time until catheter vacuum suction—meaning the anastomosis between recipient, platinum ring, and donor vessel was made, but the recipient artery wall was still intact—was 246 minutes (range, 150 to 330 min). During surgery, the recipient artery was never occluded as part of the bypass procedure. With an intact bypass, we proceeded with intraoperative ligation of the ICA if an acute danger of aneurysm bleeding existed or if the compression symptoms increased rapidly before the operation. In the first patients of our series, the ICA was left patent during surgery to prevent ischemic complications. In these patients, we occluded the ICA after surgery with detachable balloons after confirmation that the bypass remained patent for at least 24 hours as demonstrated on contrast imaging. However, at present, we choose for immediate intraoperative occlusion of the ICA, because in the early years five out of 23 patients (22%) had to be re-operated on. In these cases, the bypass occluded within a day due to low bypass flow because the ICA was left open postoperatively, waiting for endovascular occlusion.
ELANA bypass for giant aneurysms of the MCA
As MCA giant aneurysms are even more life-threatening lesions prone to rupture compared to giant aneurysms of the ICA, an aggressive therapeutic strategy is even more warranted1 (Figure 28–6). The preferred treatment is to take the aneurysm completely out of circulation and in some cases even debulking the mass of the aneurysm. The results of filling these MCA aneurysms with coils or glue are mostly disappointing by recanalization, impaction, or sustaining mass effect. Although literature provides many case reports of flow replacement bypasses using the STA as donor end-to-side connected to a small distal MCA branch for reversal of flow in these aneurysms, this technique still demands a temporal MCA occlusion with a theoretical risk of ischemia. In addition there is also a risk of ischemia due to insufficient flow replacement. According to the mathematical model of Hillen et al., there are hemodynamic arguments that, for replacing higher flows in the MCA (up to 120 ml/min), larger diameter bypasses (>2 mm) should be preferred.19 An intracranial IC-IC bypass should be able to replace the MCA in an orthograde, and therefore, more physiological direction; this approach also minimizes surgical exposure, thus avoiding neck carotid surgery for the donor part of a regular conventional EC-IC bypass.
Since 1999, we have treated 25 patients with giant aneurysms with a mean diameter of 38 mm (SD, ±12 mm) measured on imaging.20 The mean age was 45 years (SD ±16 years) and 12 patients were female (48%). All aneurysms were assessed as being not safely clippable or coilable. Preoperative flow measurements were performed as far as possible, as coils previously unsuccessfully delivered into these aneurysms (in six patients, 24%) disturb flow measurements.
Complications of ELANA Bypass for Giant Aneurysms of the MCA
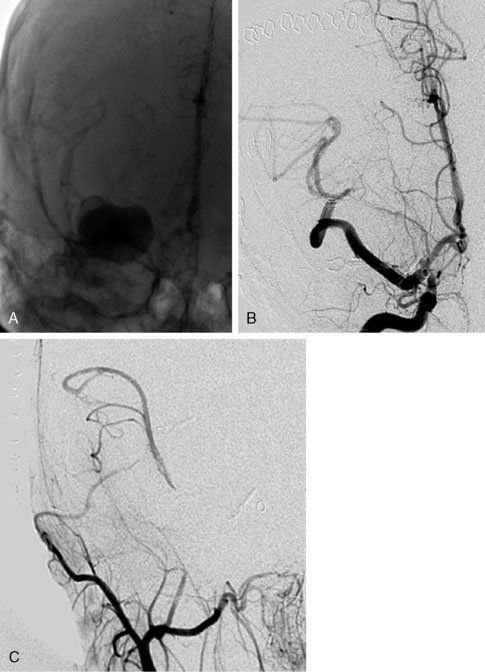
Finally, in one patient (4%) an intraoperative infarct occurred. She was a 22-year-old patient with a left-sided giant MCA aneurysm with only headache symptoms (see Figure 28–6). An IC-IC bypass (type I) was planned and during surgery, when both proximal and distal ELANA anastomoses were constructed and only the final stage of end-to-end anastomosing of the two venous bypass segments was to be finished, a spontaneous hemorrhage of the (until this part) asymptomatic aneurysm occurred at the neck of the aneurysm. The massive bleeding that followed urged us to temporally trap the MCA and the aneurysm to make further finishing of the bypass possible. This turned the procedure from a nonocclusive one into a temporal ischemic procedure and it took us 12 minutes to finish the bypass and remove the temporary clips on the bypass. During this time we checked collateral leptomeningeal capacity by removing the distal clip on the MCA, and there was no backflow indicating that in this patient collateral capacity was minimal. Although the occlusion time was only 12 minutes, which is much shorter than the occlusion time usually necessary for a conventional anastomosis on larger proximal arteries, the patient woke up with right-sided hemiparesis and mild dysphasia. Postoperative CT showed a left-sided putamen infarct, and 2 months were required for full recovery. This incident, together with the three ischemic incidents of the distal conventional anastomoses, clearly shows that conventional anastomosing techniques with temporal occlusion bear significant risk of infarction and that the nonocclusive character of the ELANA anastomosing technique does prevent infarcts in certain patient groups. As there are currently no reliable methods to test leptomeningeal capacity in the treatment of giant MCA aneurysms, we prefer to use the ELANA nonocclusive anastomosing technique in these patients as much as possible.
Discussion of ELANA Bypass for Giant Aneurysms of the MCA
The phenomenon of the long-term bypass occlusion in two patients with extensive leptomeningeal collaterals with previous successful and patent bypasses reveals that the human cerebral vasculature is dynamic and that it can adapt itself to changing circumstances. After bypass construction, a new equilibrium is slowly established in the MCA flow territory, which varies inter-individually but also intra-individually in time depending on preexisting collaterals and bypass location. The bypass could then function as a slowly fading, nonphysiologic bridge to reach a new physiologic situation with the aid of leptomeningeal and intracerebral collaterals. This hypothesis is supported by earlier reports on leptomeningeal function after MCA occlusion.21Although in literature numbers of bypass patencies are often used in the discussions of treatment modalities in giant aneurysms, this finding of long-term occlusion of bypasses without symptomatology, due to extensive collaterals, clearly demonstrates that follow-up in terms of functional outcome as the mRS is much more important.
ELANA bypass for giant aneurysms of the ACA
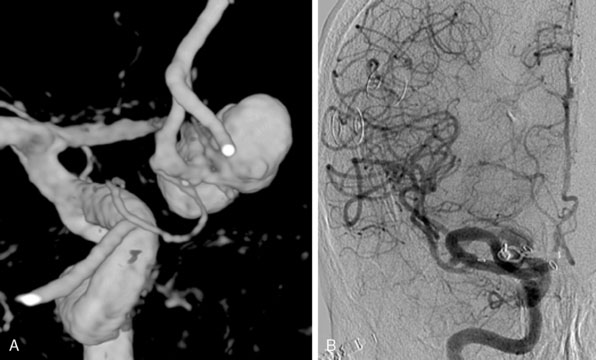
Although we frequently see patients with giant aneurysms of the ACA, it is rarely necessary to use ELANA technique for bypassing these lesions. An example of this is a 61-year-old patient with an SAH and a giant aneurysm of the ACA that was not coilable and because of multicalcifications not easily clippable (Figure 28–8). As there are no possibilities of measuring distal leptomeningeal anastomoses, we decided to perform a IC-IC bypass from the A1 to the proximal A2, and then clipping the aneurysm, including the distal A1 and ACA. Intraoperative flow measurements on the A2 showed a flow of 30 cc/min to be replaced. As the STA in this case was very small we decided to construct an IC-IC bypass and to trap the aneurysm. According to intraoperative flow measurements, initial flow in the bypass with the A1 still open was 15 cc/min but went up to 30 after trapping the aneurysm, including the A1-A2 segment, demonstrating that the bypass functioned as a 100% replacement bypass. The patient recovered very well and is at work (follow-up 40 months), and CTA control after 2 years showed the bypass to be fully patent.
ELANA bypass for giant aneurysms of the posterior circulation
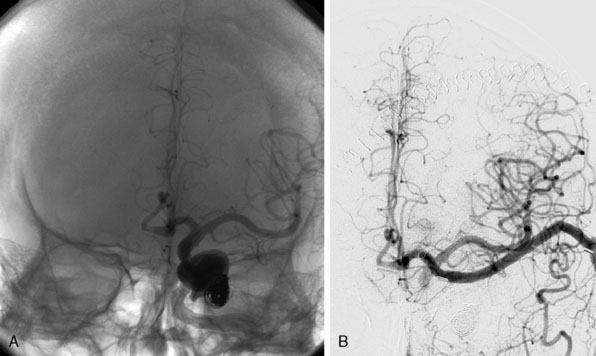
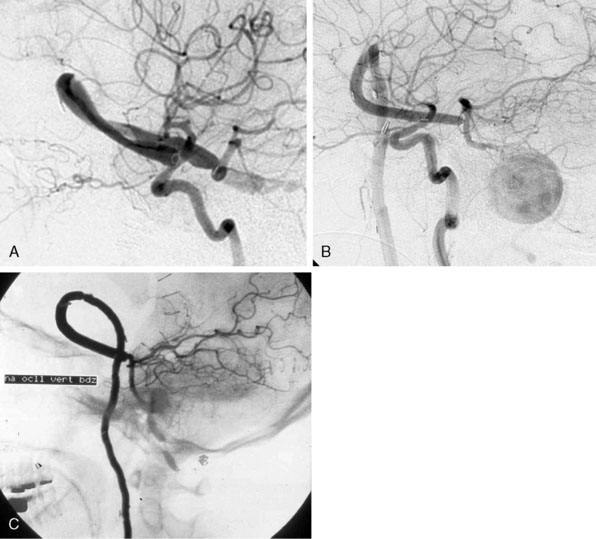
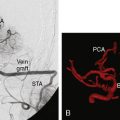
We treated 10 patients with a giant aneurysm of the posterior circulation with an ELANA bypass on the BA (Figures 28–9A and 28–9B), P1 (Figure 28–9C), P2, or the SCA.22–24 An example of progressive brainstem ischemia after reversal of flow is the case of a 55-year-old male who suffered a SAH from a giant fusiform BA aneurysm. We constructed an IC-IC bypass from the ICA to the BA, thus creating a large artificial posterior communicating artery (PCom) (Figure 28–9A). Both anastomoses were made using the ELANA technique. The next day both VAs were endovascularly occluded and the flow through the bypass, which first was directed from the P1 to the ICA, now reversed to the posterior circulation supplying the BA vascular territories. The aneurysm slowly thrombosed, but the next day a progressive brainstem ischemia developed with the bypass still patent and the patient died.
A clear example of the risk of aneurysm rupture after reversal of flow treatment and therefore not complete trapping of the aneurysm is a 44-year-old male patient with a giant BA aneurysm and progressive brainstem symptomatology (see Figure 28–9B). He was treated with an EC-IC bypass from the external carotid artery (ECA) to the BA using the ELANA technique for the latter anastomosis. Both VAs were occluded in the same session. Postoperative angiography showed partly thrombosis of the aneurysm due to reversal of flow. The patient was slowly recovering but died after 2 months as the result of a massive SAH from the aneurysm remnant.
Of the group of 10 patients, only two had a good functional outcome (20%). One of these two cases is a 31-year-old female with a history of SAH from a giant basilar aneurysm and unsuccessful coiling of the aneurysm 2 months before. An EC-IC ELANA bypass was constructed from the ECI to the P1 (ELANA anastomosis) (see Figure 28–9C). The intraoperative flow in this bypass was 70 cc/min with the VAs still open, and we planned an endovascular occlusion of the VAs and aneurysm the next day. The aneurysm occluded and the bypass completely took over posterior circulation. MR flowmetry after 2 weeks showed that the flow in the bypass increased to 120 cc/min, and on follow-up (12 years) she had maximum outcome scores and is fully active in daily life.
1 Barrow D.L., Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214-244.
2 Drake C.G. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
3 Parkinson R.J., Eddleman C.S., Hunt Batjer H., et al. Giant intracranial aneurysms: endovascular challenges. Neurosurgery. 2006;59(Suppl 3):S103-S113.
4 Ross I.B., Weil A., Piotin M., et al. Endovascular treatment of distally located giant aneurysms. Neurosurgery. 2000;47:1147-1153.
5 Jafar J.J., Russel S.M., Woo H.H. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: indications, operative technique, and results in 29 patients. Neurosurgery. 2002;51:138-144. discussion 144–146
6 Sekhar L.N., Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002;50:321-331.
7 Bakker C.J., Kouwenhoven M., Hartkamp C.J. Accuracy and precision of the time-averaged flow as measured by non-triggered 2D phase-contrast angiography. Magn Reson Imaging. 1995;13:959-965.
8 Charbel F.T., Hoffman W.E., Misra M., et al. Ultrasonic perivascular flow probe: technique and application in neurosurgery. Neurol Res. 1998;20:439-442.
9 van der Zwan A., Tulleken C.A., Hillen B. Flow quantification of the non-occlusive excimer laser-assisted EC-IC bypass. Acta Neurochir (Wien). 2001;143:647-654.
10 Amin-Hanjani S., Du X., Mlinarevich N., et al. The cut flow index: an intraoperative predictor of the success of extracranial-intracranial bypass for occlusive cerebrovascular disease. Neurosurgery. 2005;56(Suppl 1):75-85.
11 Alaraj A., Ashley W.W.Jr, Charbel F.T., et al. The superficial temporal artery trunk as a donor vessel in cerebral revascularization: benefits and pitfalls. Neurosurg Focus. 2008;24:E7.
12 Tulleken C.A., Hoogland P., Slooff J. A new technique for end-to-side anastomosis between small arteries. Acta Neurochir Suppl (Wien). 1979;28:236-240.
13 Tulleken C.A., Verdaasdonk R.M., Berendsen W., et al. Use of the excimer laser in high-flow bypass surgery of the brain. J Neurosurg. 1993;78:477-480.
14 Tulleken C.A., Verdaasdonk R.M. First clinical experience with Excimer assisted high flow bypass surgery of the brain. Acta Neurochir (Wien). 1995;134:66-70.
15 Tulleken C.A., Verdaasdonk R.M., Beck R.J., et al. The modified excimer laser-assisted high-flow bypass operation. Surg Neurol. 1996;46:424-429.
16 Streefkerk H.J., Kleinveld S., Koedam E.L., et al. Long-term reendothelialization of ecximer laser-assisted nonocclusive anastomoses compared with conventionally sutured anastomoses in pigs. J Neurosurg. 2005;103:328-336.
17 Raabe A., Beck J., Gerlach R., et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132-139. discussion 139
18 van Doormaal T.P., van der Zwan A., Verweij B.H., et al. Treatment of giant and large internal carotid artery aneurysms with a high-flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2008;62:1411-1418.
19 Hillen B., Hoogstraten H.W., Post L. A mathematical model of the flow in the circle of Willis. J Biomech. 1986;19:187-194.
20 van Doormaal T.P., van der Zwan A., Verweij B.H., et al. Treatment of giant middle cerebral artery aneurysms with a flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2008;63:12-20.
21 Harvey J., Rasmussen T. Occlusion of the middle cerebral artery: an experimental study. AMA Arch Neurol Psychiatry. 1951;66:20-29.
22 Tulleken C.A.F., Streefkerk H.J.N., van der Zwan A. Construction of a new posterior communicating artery in a patient with poor posterior fossa circulation: technical case report. Neurosurgery. 2002;50:415-419.
23 Tulleken C.A., van der Zwan A., van Rooij W.J., et al. High-flow bypass using nonocclusive excimer laser-assisted end-to-side anastomosis of the external carotid artery to the P1 segment of the posterior cerebral artery via the sylvian route. Technical note. J Neurosurg. 1998;88:925-927.
24 Streefkerk H.J., Wolfs J.F., Sorteberg W., et al. The ELANA technique: constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta Neurochir (Wien). 2004;146:1009-1019.