Chapter 13 Ebstein’s Anomaly of the Tricuspid Valve
In June 1864, a 19-year-old laborer was admitted to the All-Saints Hospital in Breslau (now Wroclaw), Poland, where he died 8 days later. Wilhelm Ebstein, then assistant physician at All-Saints, performed a postmortem examination the following day and subsequently wrote a scholarly account of the clinical and necropsy findings entitled “On a Very Rare Case of Insufficiency of the Tricuspid Valve Caused by a Severe Congenital Malformation of the Same.”1 Ebstein’s meticulous correlation of pathology with clinical notes and hypotheses of pathophysiology resulted in a landmark publication.2 English translations are available and repay study.3–5
Ebstein’s report was virtually overlooked until 1937 when Yater and Shapiro6 published an account of radiologic and electrocardiographic observations of the anomaly.
In 1949, Tourniaire, Tourniaire, and Tartulier7 diagnosed the malformation in a living subject; and in 1950, Engle and colleagues8 analyzed data from three patients who died of Ebstein’s anomaly and asserted that clinical recognition was possible. In the same year, Reynolds9 published the first English-language report of cases recognized during life. Shortly thereafter, Soloff, Stauffer, and Zatuchni10,11 made the clinical diagnosis in a 34-year-old patient and confirmed the diagnosis at necropsy 6 years later. The anomaly or malformation that Ebstein described occurs in approximately 1 in 20,000 live births,5,12,13 accounts for 0.3% to 0.7% of all cases of congenital heart disease, and represents about 40% of congenital malformations of the tricuspid valve.14
Normal tricuspid leaflets consist of basal attachments to the annulus (right atrioventricular sulcus), peripheral zones into which chordae tendineae insert, and clear zones that lie between the basal attachments and the peripheral zones.15 The semicircular or quadrangular anterior leaflet is the largest of the three.15 The posterior leaflet is scalloped. The septal leaflet attaches chiefly to the ventricular septum as the name indicates, but part of its basal attachment is to the posterior wall of the right ventricle.15 The septal leaflet normally exhibits a slight but distinct apical displacement of its basal attachment compared with the mitral valve: 15 mm in children, and 20 mm in adults.16
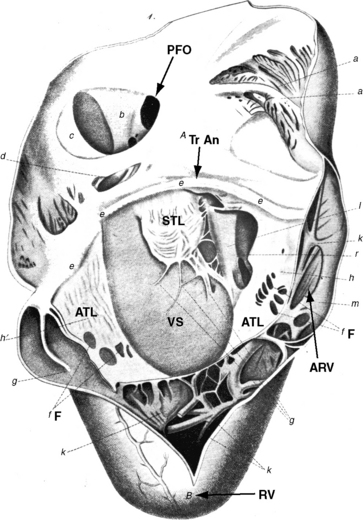
Ebstein wrote, “When we turn our attention to the description of the right ventricle, we see at once an extremely abnormal appearance of the tricuspid valve”1–4 (Figure 13-1). The septal and posterior leaflets do not attach normally to the tricuspid annulus, so the valve orifice is displaced downward into the right ventricular cavity at the junction of the inlet and trabecular components of the right ventricle.16,17
The right side of the heart therefore consists of three morphologic components: the right atrium proper; the inlet portion of the right ventricle, which is thin-walled and functionally integrated with the right atrium; and the trabecular and outlet portion, which constitute the functional right ventricle. The greater the apical displacement of the posterior and septal leaflets, the larger the atrialized right ventricle and the smaller the functional right ventricle (Figure 13-2). Downward displacement of the septal tricuspid leaflet is associated with discontinuity of the central fibrous body and the septal atrioventricular ring, which creates a potential substrate for accessory pathways and preexcitation. The leaflets and tensile apparatus of the tricuspid valve are thought to be formed chiefly by delamination of the inner layers of the inlet zone of the right ventricle. When the tricuspid leaflets are displaced downward, delamination fails to occur.
Morbid anatomy encompasses a broad range of severity,17,18 but certain features are relatively constant.19,20 The anterior leaflet is almost always attached to the atrioventricular junction. A salient anatomic feature is the level of the hinge points of the septal and posterior leaflets, which are characterized by apical displacement of their basal attachments,16 adherence to the underlying myocardium,17 and impaired movement because of short chordae tendineae and nodular fibrotic thickening (see Figure 13-1). When the septal leaflet is absent, the arrangement of the posterior leaflet distinguishes Ebstein’s anomaly from a congenitally unguarded tricuspid orifice (see subsequent). The anterior leaflet differs appreciably from the septal and posterior leaflets. The basal attachment is at the level of the atrioventricular sulcus (annulus). The large and potentially mobile anterior leaflet contains muscular strands instead of consisting entirely of a fibrous membrane as in the normal tricuspid valve. Mobility is impaired by thickening, modularity, fibrosis, and multiple short chordae.
The displaced insertions of the malformed septal and posterior leaflets allow free communication between the proximal (atrialized) and distal (functional) right ventricle. Occasionally, communication between the atrialized right ventricle and the functional right ventricle is confined to slits or perforations in the anterior tricuspid leaflet, as Ebstein originally described, or the two segments are separated by a muscular partition or shelf (see Figure 13-32) that restricts flow to the commissure between the anterior leaflet and the displaced septal leaflet.21 When the anteromedial commissure is fused and the anterior leaflet is intact, the tricuspid orifice is imperforate.21
The thin-walled atrialized right ventricle is relatively devoid of muscular tissue and is typically dilated, often aneurysmally.22 It expands paradoxically during ventricular systole, thus acting as a passive reservoir that decreases the volume of ejected blood.17 Morphometric analyses of the trabecular and infundibular portions of the functional right ventricle disclose an absolute decrease in the number of myocytes and an increase in fibrosis that are held responsible for infundibular dilation.19
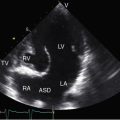
An ostium secundum atrial septal defect is present in over a third of hearts with Ebstein’s malformation, and most of the rest have a patent foramen ovale (see Figures 13-1 and 13-2).23 Ebstein believed that “[r]egurgitation of blood into the right atrium caused its dilatation and prevented complete closure of the valve of the foramen ovale.”1,17 Ebstein’s anomaly of an inverted tricuspid valve in congenitally corrected transposition of the great arteries is dealt with in Chapter 6.
Tricuspid regurgitation from a congenitally unguarded tricuspid orifice24 or tricuspid valve dysplasia differs fundamentally from Ebstein’s anomaly.25 A congenitally unguarded tricuspid orifice is characterized by absence of all three leaflets24 or by a muscular partition or shelf that leaves the atrioventricular orifice unguarded (see Figure 13-32). Congenital dysplasia of the tricuspid valve refers to nodular thickening and rolling of the edges of the leaflets without downward displacement.25
Idiopathic dilation of the right atrium has been reported in children without Ebstein’s anomaly.26,27 Transient tricuspid regurgitation of the newborn has no definable anatomic basis and resolves within a few weeks.28 Uhl’s anomaly is discussed at the end of this chapter.
Abnormalities of the left side of the heart have been reported in 39% of patients with Ebstein’s anomaly17,29 and consist of derangements in left ventricular geometry, impairment of systolic and diastolic function,29,30 and noncompaction.17,31 Superior systolic displacement of the mitral valve (prolapse) occurs because mitral leaflets with normal areas and chordal lengths are housed in a left ventricular cavity that is geometrically altered and reduced in size (see Figure 13-31).19,29,32,33 Depressed systolic function is the result of a combination of abnormal shape, impaired diastolic filling, and increased fibrous content of the free wall and the ventricular septum.19
The physiologic consequences of Ebstein’s anomaly are determined by the morphologic derangement of the tricuspid leaflets, by the hemodynamic burden imposed on an inherently flawed the right ventricle, by left ventricular function, and by atrial rhythm. The tricuspid orifice is typically incompetent, occasionally stenotic, and rarely imperforate. Functional impairment of the right ventricle depends on the severity of tricuspid regurgitation and on the size of the right atrium and atrialized right ventricle relative to the size of the functional right ventricle. The thin-walled atrialized right ventricle is either passive during the cardiac cycle or functions as an aneurysm that expands paradoxically during systole and therefore acts a physiologic impediment (see previous discussion). Exercise intolerance has been ascribed to an inadequate increment in pulmonary blood flow and a fall in systemic arterial oxygen saturation.34,35 Atrial tachyarrhythmias have serious physiologic implications, especially the rapid heart rates associated with accessory pathways (see sections The Electrocardiogram and The History).
The functionally inadequate right ventricle is especially vulnerable in utero and at birth. High neonatal pulmonary vascular resistance increases right ventricular afterload and augments tricuspid regurgitation. Right atrial pressure increases, and a right-to-left shunt is established across a patent foramen ovale or an atrial septal defect. The process is reversed as neonatal pulmonary vascular resistance falls and right ventricular afterload normalizes. The enlarging right atrium becomes sufficiently commodious to accommodate a large volume of regurgitant flow with little or no increase in pressure (Figure 13-5). In older patients, right ventricular filling pressure may increase, provoking a rise in right atrial pressure with reestablishment of the right-to-left interatrial shunt.
Ebstein’s original case was an example of obstruction at the tricuspid orifice (see Figure 13-1).1 He wrote, “The membrane divided the right ventricle into two halves. These halves communicated with each other in two ways: one through the oval opening which leads into the right conus arteriosus, and two through the already described multiple openings in the fenestrated membrane.”1 When the tricuspid orifice is stenotic or imperforate, the elevation of right atrial pressure reflects the degree of obstruction. The A wave is often giant, and a right-to-left interatrial shunt persists after the fall in neonatal pulmonary vascular resistance.
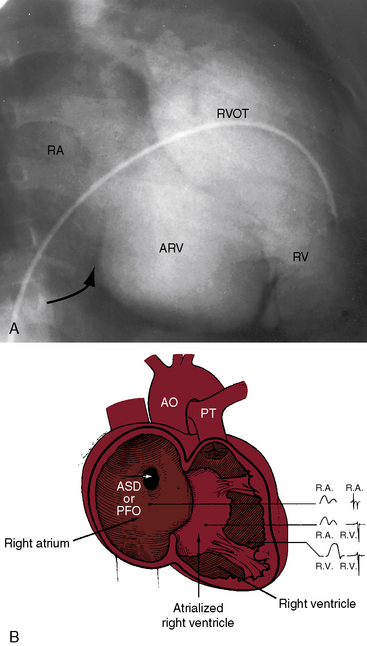
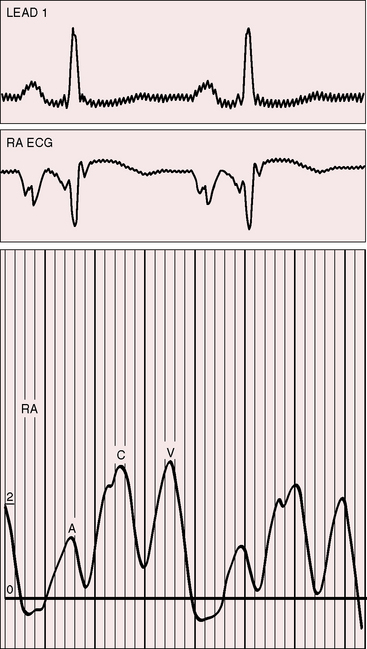
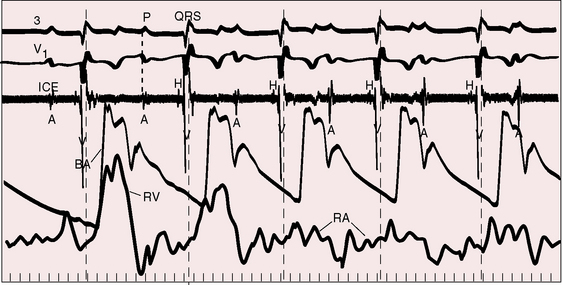
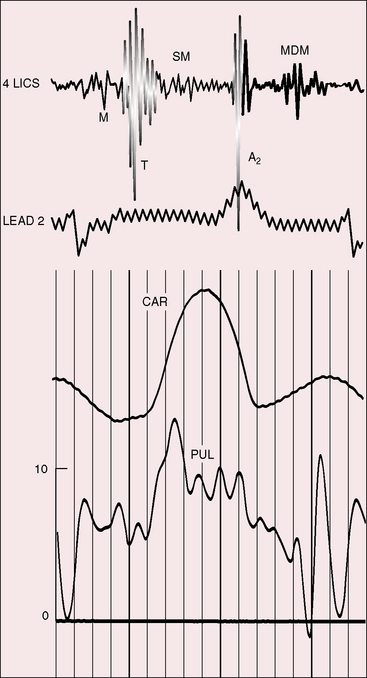
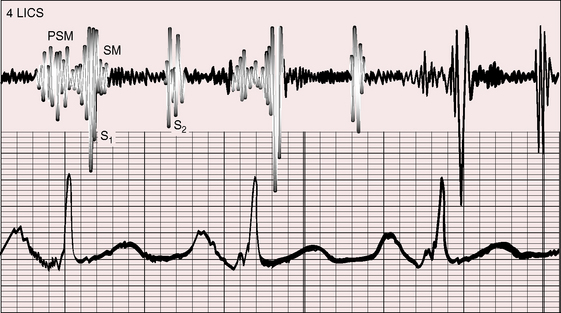
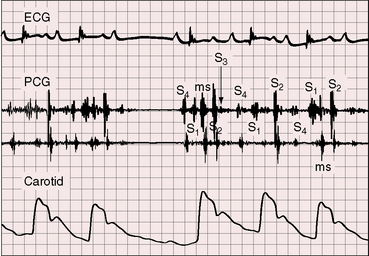
The electromechanical properties of the right atrium, atrialized right ventricle, and functional right ventricle provided the first secure basis for the clinical diagnosis of Ebstein’s anomaly (Figures 13-2 and 13-3).36,37 The right atrium proper generates a right atrial pressure pulse and an intracavitary atrial electrogram. The functional right ventricle generates a right ventricular pressure pulse and a right ventricular intracavitary electrogram. The atrialized right ventricle generates a right ventricular intracavitary electrogram but an atrial pressure pulse (see Figures 13-2 and 13-3). Mechanical stimulation of the atrialized right ventricle provokes a right ventricular electrogram and incurs the risk of triggering ventricular tachycardia.38,39 Clusters of ventricular cardiomyocytes are isolated within a fibrous matrix that prevents spiral/scroll reentrant waves from anchoring.40 When spiral/scroll waves do not anchor, they meander erratically as polymorphic ventricular tachycardia. Accordingly, mechanical stimulation of the atrialized right ventricle does not provoke monomorphic ventricular tachycardia that depends on slow conduction, unidirectional block, and a substrate that permits reentry but instead results in polymorphic ventricular tachycardia.38,40
The geometric configuration and function of the right and left ventricles are closely coupled in Ebstein’s anomaly.29 Leftward displacement of the ventricular septum (see Figure 13-31) reduces the volume of left ventricular diastolic filling and, accordingly, reduces the ejection fraction. Exercise provokes an increase in left ventricular ejection fraction because end-systolic volume decreases with little or no change in end-diastolic volume. The right ventricular free wall contributes feebly if at all to forward flow, which is materially assisted by paradoxical motion of the ventricular septum that functions as part of the right ventricle as in Uhl’s anomaly (see subsequent). In brief, left ventricular function is adversely affected by the diastolic position of the ventricular septum, geometric distortion of the ventricle (see Figure 13-31), reduced end-diastolic volume, paradoxical motion of the ventricular septum, an increase in fibrous tissue, and a decrease in cardiomyocytes in the free wall and septum.19
History
Males and females are equally affected.41–44 Familial Ebstein’s anomaly has been reported,41,43,45–51 and a patient with Ebstein’s anomaly of a right-sided tricuspid valve had a cousin with congenitally corrected transposition of the great arteries and Ebstein’s anomaly of an inverted left–sided atrioventricular valve.41 A novel mutation is held responsible for the genetic syndrome of ventricular preexcitation and conduction system disease of childhood onset,52 but these observations cannot be extrapolated to the general population of patients with preexication or to patients with Ebstein’s anomaly in the absence of preexcitation.
The Danish Registry estimates that the relative risk of Ebstein’s anomaly is increased by 500-fold in offspring exposed to in utero lithium carbonate, which is used for treatment of bipolar disorders.12,53,54
The probability of occurrence of Ebstein’s anomaly in the general population is 1:20,000, so the likelihood of the malformation occurring spontaneously in a pregnant woman taking lithium is 1:20 million.54 Estimates now show that the increased risk incurred with lithium does not exceed 28-fold, which is considerably less than estimates from the Danish Registry.12,53
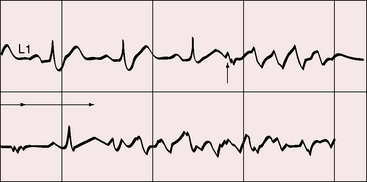
The clinical course of Ebstein’s anomaly ranges from intrauterine death to asymptomatic survival to late adulthood.43,55–58 The most common presentations are: 1, detection of the anomaly in a routine fetal echocardiogram; 2, neonatal cyanosis; 3, heart failure in infancy; 4, murmur in childhood; and 5, arrhythmias in adolescents and adults.41,57 The outlook for fetal Ebstein’s anomaly has aptly been characterized as appalling.57 Of neonates with Ebstein’s anomaly, 20% to 40% do not survive 1 month, and less than 50% survive to 5 years.17 Fetal hydrops is almost invariably fatal with rare exception.59,60 Neonates not only have high mortality rates but also a significant ongoing risk of morbidity and death.43,57,58 Even if symptoms resolve in the first month of life, the infant may then die suddenly. Transient neonatal cyanosis that recurs a decade or more later is an uncommon but distinctive and usually benign feature of Ebstein’s anomaly.57 A neonatal right-to-left interatrial shunt disappears as pulmonary vascular resistance normalizes; the shunt subsequently reappears as filling pressure rises in the functionally abnormal right ventricle. Tachyarrhythmic sudden death looms as a threat regardless of severity of the anomaly56,61 and is responsible for the decline in survival rate in the fifth decade.56 Wolff-Parkinson-White syndrome in otherwise healthy individuals carries an estimated sudden cardiac death risk of 0.02%,62 but in Ebstein’s anomaly, atrial flutter or fibrillation with accelerated conduction is accompanied by a major increase in the risk of sudden death. Stimulation of the arrhythmogenic atrialized right ventricle initiates polymorphic ventricular tachycardia that promptly degenerates into ventricular fibrillation (Figure 13-4; see previous discussion), and spontaneous ventricular tachycardia/fibrillation looms as a threat. The degree of cyanosis does not necessarily correspond with symptoms, but once cyanosis and symptoms develop, disability tends to be progressive, even in patients who were relatively asymptomatic before adulthood. The onset of chronic atrial fibrillation prefigures death within 5 years.56
Despite qualifications, legendary accounts are found of astonishing longevity in Ebstein’s anomaly, with survivals into the eighth and ninth decades.13,41,43,63,64 Ebstein’s anomaly was discovered at necropsy in a 75-year-old man who as a youth had been a lumberjack working on log booms.65 He was reportedly asymptomatic until his fifties when he was obliged to outrun an irate female bear.65 At necropsy 25 years later, his right atrium was thin-walled and greatly dilated, and the tricuspid valve was characteristically malformed.65 The oldest recorded patient with Ebstein’s anomaly lived to age 85 years and was devoid of cardiac symptoms until age 79 years.66
The chest pain that occasionally occurs with Ebstein’s anomaly is an enigma.41 The pain is retrosternal, epigastric or in the right or left anterior chest, and is sharp, stabbing, or shooting, features that suggest serous surface origin. A fibrinous pericardium has been found at necropsy over the atrialized right ventricle (see section on Auscultation).
Important although less frequent manifestations result from paradoxical emboli or brain abscess. Infective endocarditis is uncommon because regurgitant flow across the malformed tricuspid valve is low velocity with low turbulence. Prophylaxis for the low risk of infective endocarditis is open to question.17 Pregnancy incurs the risks inherent in a functionally inadequate volume-overloaded right ventricle that copes poorly with the additional hemodynamic burden of gestation.67 Paroxysmal atrial tachyarrhythmias are potential hazards during pregnancy, especially the rapid rates associated with accessory pathways. Cyanosis may first become manifest during pregnancy because of a rise in filling pressure in the volume-overloaded right ventricle. Hypoxemia increases the risk of fetal wastage,68 and a right-to-left interatrial shunt incurs a puerperal risk of paradoxical embolization.
Physical appearance
Growth and development are normal in patients who were asymptomatic as neonates and infants. Persistent cyanosis or intermittent exercise-induced cyanosis occurs in more than 50% of cases.41
Jugular venous pulse
The jugular pulse is normal except for a prominent C wave that coincides with mobility of the anterior tricuspid leaflet (Figures 13-5 and 13-6, first panel). The interval between the jugular A wave and the carotid pulse is often prolonged, reflecting prolongation of the PR interval (see section on The Electrocardiogram). Prominent A waves are seldom seen in the jugular pulse, but atrial contraction sometimes generates presystolic waves in the pulmonary arterial pressure pulse. A stenotic or imperforate tricuspid orifice is accompanied by A waves that may be giant. An attenuated X descent and a systolic venous V wave of tricuspid regurgitation seldom appear in the jugular pulse despite severe regurgitant flow because of the damping effect of the commodious right atrium and the thin-walled toneless atrialized right ventricle and because tricuspid regurgitation is low-pressure and hypokinetic (see Figures 13-5 and 13-6). Right ventricular failure induces a rise in mean jugular venous pressure and a rise in A and V wave crests, but systolic pulsations of the liver are inconspicuous because the right ventricle is hypokinetic.
Precordial movement and palpation
A right ventricular impulse and a tricuspid systolic thrill are reserved for neonates before normalization of pulmonary vascular resistance.41 With this exception, absence of a systolic impulse over the inflow portion of the right ventricle is an important negative sign in the clinical diagnosis of Ebstein’s anomaly.41,69 An undulating rippling motion over the atrialized right ventricle is sometimes seen in older patients. Enlargement of the infundibular portion of the right ventricle is accompanied by a systolic impulse in the third left intercostal space.70 Ebstein described a gentle left ventricular impulse, stating, “… the cardiac apex was visible under the sixth rib somewhat outside the mammary line.”1 Ebstein also percussed the enlarged right atrium: “… the cardiac dullness extended for two centimeters beyond the right border of the sternum at the level of the fourth rib and for three centimeters at the level of the sixth rib, where it merged with the liver dullness which was normal in extent.”1
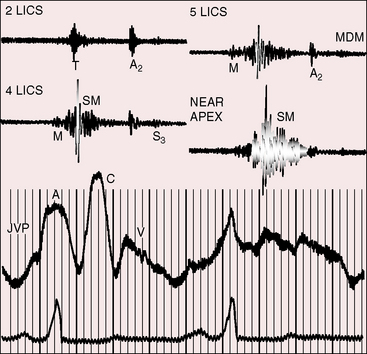
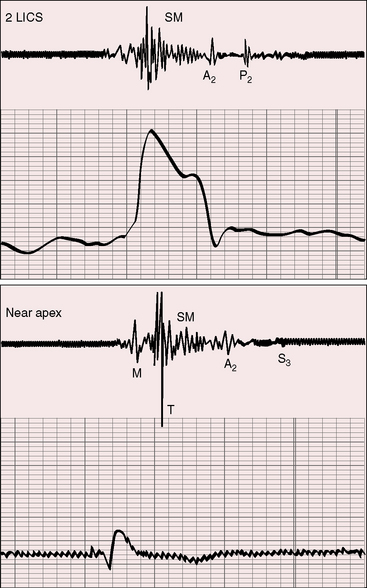
The initial component of a widely split first heart sound coincides with mitral valve closure, and the second component, which is delayed, coincides with closure of the large anterior tricuspid leaflet (Figures 13-6 through 13-9).69–72 The delay in tricuspid valve closure is not simply the result of complete right bundle branch block and a hypokinetic right ventricle but instead is the result of the large size and increased excursion of the anterior leaflet, which requires longer to reach its fully tensed closed position.72 The increased tension developed by the large anterior leaflet as it reaches the limits of its systolic excursion accounts for the loudness of the tricuspid component of the first heart sound—the sail sound—which is an important auscultatory sign of anterior leaflet mobility (see Figure 13-30).68,71 When a long PR interval softens the mitral component, the first heart sound is then represented by a loud single tricuspid component. Preexcitation of the right ventricle buries the mitral component of the first sound in an early loud tricuspid component (Figure 13-10).73
The systolic murmur of tricuspid regurgitation is typically grade 2/6 or 3/6, maximal over the displaced tricuspid valve, and therefore most prominent in a relatively leftward location toward the apex (see Figures 13-6 and 13-9). Intensity of the murmur does not increase during inspiration because the functionally inadequate right ventricle cannot increase its stroke volume and regurgitant flow. An early systole decrescendo murmur is a recognized feature of low-pressure tricuspid regurgitation because flow diminishes in latter systole as the large right atrial V wave reaches the height of normal right ventricular systolic pressure.74 The systolic murmur in Ebstein’s anomaly is medium-frequency and decrescendo because regurgitant flow is low-velocity from a hypokinetic low-pressure right ventricle (see Figures 13-7 through 13-10).69 However, in neonates with Ebstein’s anomaly, the tricuspid regurgitant murmur is holosystolic because right ventricular systolic pressure is elevated.
Wide splitting of the second heart sound is the result of delay in the pulmonary component caused by complete right bundle branch block (see Figure 13-9).69 However, the second sound is often single because pulmonary closure is inaudible from low pressure in the pulmonary trunk (see Figures 13-6 and 13-7).41,69 The split changes little if at all with respiration69 but is paradoxical when an accessory pathway prematurely activates the right side of the ventricular septum.70
Third and fourth heart sounds produce a distinctive triple or quadruple rhythm (see Figures 13-6, 13-7, and 13-9),69 often summate because of PR interval prolongation, and may increase during inspiration because of origin in the right side of the heart. Third and fourth heart sounds are sometimes sufficiently prolonged to produce short diastolic murmurs (Figures 13-8, 13-10, and 13-11), especially when the sounds occur in close proximity or when they summate (see Figure 13-7). Early diastolic sounds with the timing of opening snaps have been described in Ebstein’s anomaly and attributed to opening movements of the large mobile anterior tricuspid leaflet.69 The timing and quality of systolic and diastolic murmurs occasionally create the impression of a pericardial friction rub (Figure 13-12). A fibrinous pericardium has been found at necropsy over the atrialized right ventricle.
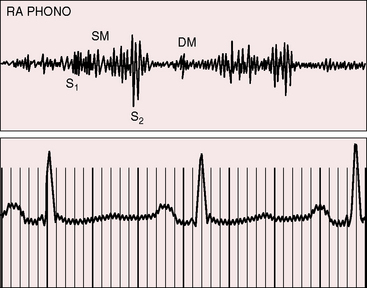
Figure 13-11 Right atrial intracardiac phonocardiogram (RA PHONO) from the 17-year-old boy with Ebstein’s anomaly. The tricuspid murmur (SM) was holosystolic when recorded with an intracardiac microphone but decrescendo when recorded on the thoracic wall (see Figure 13-6). DM is a short tricuspid mid-diastolic murmur.
A rublike systolic murmur, a rublike mid-diastolic murmur, and a rublike presystolic murmur create a distinctive cadence that resembles the rhythmic chugging of a locomotive engine as it gathers speed (see Figure 13-12).
Electrocardiogram
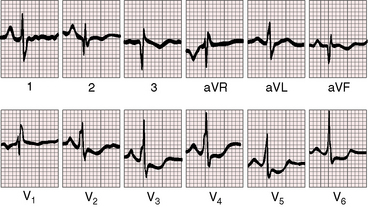
A confident diagnosis of Ebstein’s anomaly can often be made based on the electrocardiogram per se.36,75 The tracing is seldom normal even when the anomaly is mild (Figure 13-13), but rarely and oddly, the electrocardiogram can be normal even when the anomaly is severe (Figure 13-14).36 The major electrophysiologic features of Ebstein’s anomaly are summarized in Table 13-1.
Table 13-1 Major Electrophysiologic Abnormalities in Ebstein’s Anomaly
P waves are abnormal in height, duration, and configuration (Figures 13-15 through 13-17).41,75,76 Taussig aptly characterized the tall peaked P waves as Himalayan,41,77 patterns that have been ascribed to prolonged aberrant conduction in the enlarged right atrium.75,76 Permanent atrial standstill, which has been reported in familial Ebstein’s anomaly, is a rare and distinctive electrophysiologic abnormality in which atrial myocardium is unresponsive to electrical or mechanical stimuli, and P waves are absent in scalar and transesophageal electrocardiograms.49
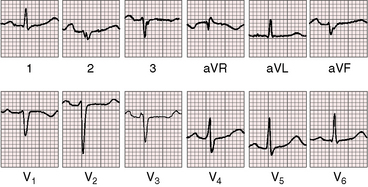
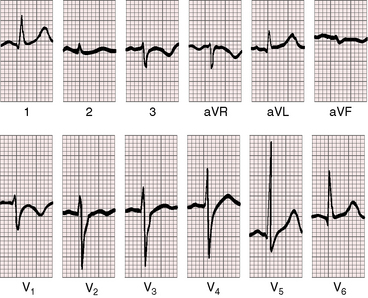
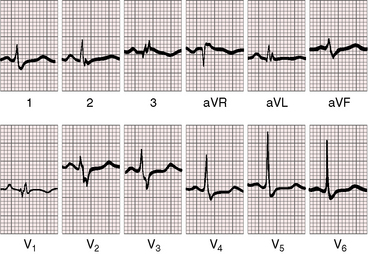
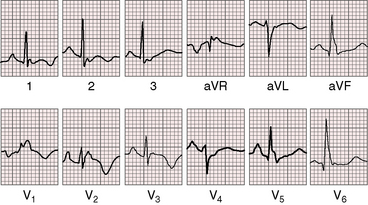
Figure 13-16 Electrocardiogram from a 39-year-old woman with a mild form of Ebstein’s anomaly and a patent foramen ovale confirmed with transesophageal echocardiography. She came to attention because of a cerebral ischemic event caused by a paradoxical embolus. Her x-ray is shown in Figure 13-21. The P waves are normal, the PR interval is 200 msec, and there is left axis deviation but without delta waves. The terminal R wave in aVR is slightly prolonged.
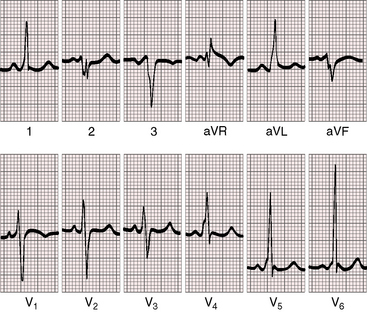
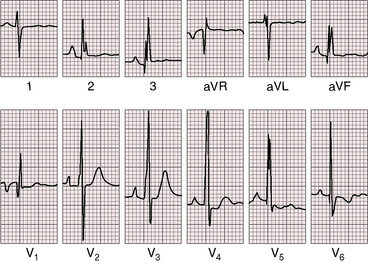
The PR interval is prolonged (Figures 13-13, 13-15, and 13-18), sometimes markedly so (see Figure 13-18).75 The duration of the PR interval and the width of the P wave correlate with prolonged conduction in the large right atrium.64,76 In the presence of preexcitation, the PR interval is usually (Figure 13-19) but not invariably short (see Figure 13-18) because of early inscription of the delta wave. However, a short PR interval occasionally occurs without a delta wave and without a history of paroxysmal rapid heart action (see Figure 13-14).
Electrophysiologic studies have identified intra-His and infra-His delay in Ebstein’s anomaly.76 Prolonged HV intervals have been ascribed to lengthened and impaired conduction within the atrialized right ventricle,76,78 conclusions that are in accord with the observation that complete heart block is rare despite prolongation of the PR interval and HV interval (see Figure 13-17).79
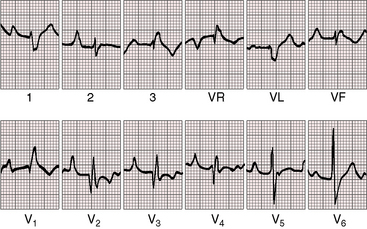
In 75% to 95% of cases, the QRS is characterized by a right ventricular conduction defect of the right bundle branch type (see Figures 13-16 and 13-17).76,80 QRS prolongation is largely if not exclusively the result of prolonged activation of the atrialized right ventricle and is less fully manifest in infants (see Figure 13-15). The conduction defect is therefore distal to the right bundle branch76 and is sometimes present despite a septal accessory pathway (see Figure 13-18). A distinctive second QRS complex attached to the preceding normal QRS complex originates in the atrialized right ventricle according to intracardiac mapping (Figure 13-20).76
The QRS axis is inferior, although a splintered polyphasic QRS makes the axis difficult to determine. Left axis deviation represents type B preexcitation (see Figure13-19), but a horizontal QRS axis occasionally occurs in the absence of accessory pathways (see Figure 13-13).81 Initial force abnormalities apart from delta waves are important features of the electrocardiogram. Deep Q waves appear in leads 2, 3, and aVF (see Figure 13-17),80,81 but most important are right precordial Q waves in lead V1 (see Figure 13-15) or in leads V1-4 (see Figures 13-16 and 13-17).80 This distinctive Q wave pattern occurs because the precordial surface leads record right ventricular intracavitary potentials unusually far leftward as a result of the large size of the right atrium.80 For the same reason, QRS patterns are similar, if not identical, in lead V1 and lead aVR (see Figures 13-16 and 13-17). Prominent Q waves in right precordial leads can be misleading when adults with Ebstein’s anomaly present with chest pain (see section The History).
Supraventricular tachycardia, atrial fibrillation, and atrial flutter occur in 25% to 30% of cases.36,41,61,75,76,82 Ebstein1 reported that his patient “has always been troubled with palpitations.” Wide QRS tachycardia via bypass tracts must be distinguished from ventricular tachycardia, which is uncommon if not as rare as a spontaneous tachyarrhythmia (see Figure 13-4).39,82 Right bundle branch block is a feature of Brugada’s syndrome, which occurs with ST segment elevation in leads V1-2 and ventricular fibrillation, but without structural heart disease.83–85
Wolff-Parkinson-White preexcitation, which was described in 193086 and characterized electrophysiologically in 1967,87 occurs in 5% to 25% of the electrocardiograms in Ebstein’s anomaly (see Figures 13-18 and 13-19).41,43,76,80 Downward displacement of the septal tricuspid leaflet is accompanied by discontinuity between the central fibrous body and the septal atrioventricular ring, which creates a substrate for preexcitation (see previous discussion). Recurrent tachyarrhythmias have been known to occur in Ebstein’s anomaly for more than six decades. Ebstein’s anomaly is the only cyanotic congenital cardiac malformation consistently associated with preexcitation, which is uniformly via a right bypass tract (i.e., type B Wolff-Parkinson-White; see Figures 13-18 and 13-19). Patients with preexcitation may not experience atrial tachyarrhythmias, and atrial tachyarrhythmias are not confined to patients with accessory atrioventricular conduction. Nevertheless, the combination of type B preexcitation and cyanosis constitutes presumptive evidence of Ebstein’s anomaly. Accessory pathway conduction can be permanent or intermittent and can occur without delta waves; and delta waves can occur with a short or normal PR interval (see Figure 13-14).81 The type B bypass conduction of Ebstein’s anomaly is associated with a left superior QRS axis (see Figures 13-18 and 13-19), but a left superior axis is not always associated with delta waves (see Figure 13-16). The QRS configuration and the direction of the delta wave vector assign the accessory atrioventricular pathway to the right posterolateral free wall or to the right posterior septum (see Figures 13-18 and 13-19).82 When accessory pathways are multiple, as they are in more than a third of patients with Ebstein’s anomaly, conduction tends to be via the septal pathway.82 Mahaim nodoventricular fibers are likely to be present when a left bundle branch block pattern occurs during sinus rhythm or during an episode of tachycardia.82
X-ray
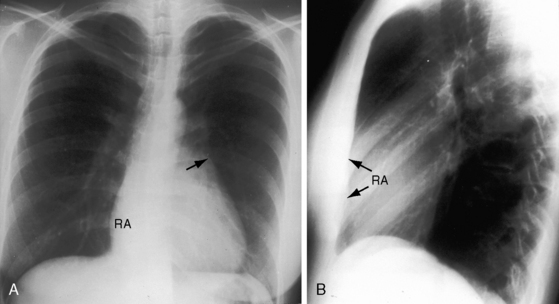
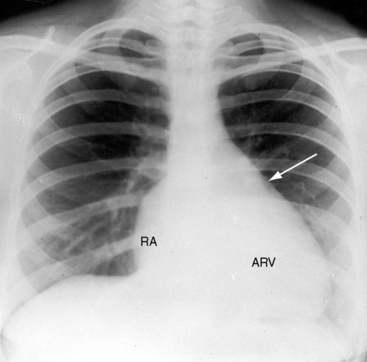
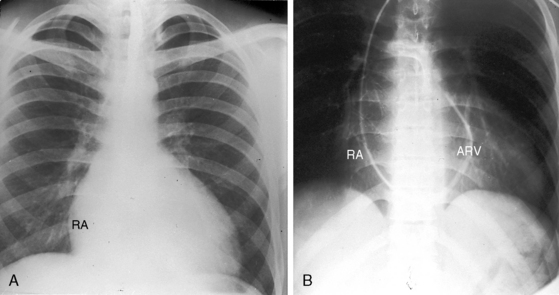
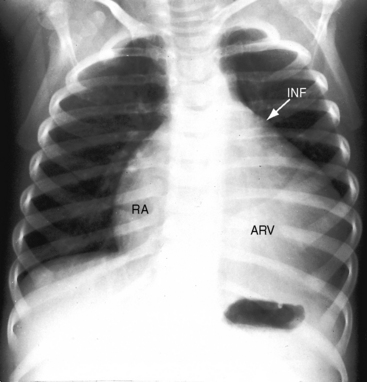
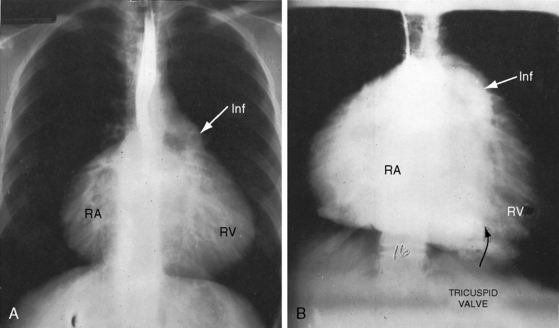
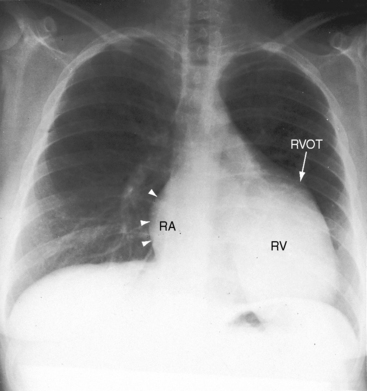
The cardiac silhouette varies from near normal (Figures 13-21 and 13-22) to diagnostic (Figures 13-23 through 13-27).41,88 Heart size in symptomatic infants is immense (Figures 13-28 and 13-29). Pulmonary vascularity is normal in mild acyanotic Ebstein’s anomaly (see Figures 13-21 and 13-22) and is reduced when the anomaly is severe and cyanotic (see Figures 13-25, 13-26, and 13-27).88 Cyanosis with normal or reduced pulmonary blood flow and a dominant left ventricle is a useful combination in the clinical diagnosis of Ebstein’s anomaly. The infundibulum either straightens the left cardiac border (see Figures 13-22, 13-23, and 13-24) or forms a conspicuous convex shoulder (see Figures 13-25, 13-26, and 13-27). The most consistent and dramatic radiologic feature is the right atrial silhouette, which is almost always enlarged (see Figures 13-23 through 13-25), sometimes dramatically so (see Figures 13-26 through 13-29), and is seldom normal even when the cardiac silhouette is otherwise normal (see Figures 13-21 and 13-22A). Marked rightward convexity of the enlarged right atrium together with marked leftward convexity of the enlarged infundibulum account for a boxlike configuration (see Figures 13-26 and 13-27).41 The vascular pedicle is narrow because the pulmonary trunk is not border forming and the ascending aortic shadow is inconspicuous or absent (see Figures 13-22 through 13-27), with rare exception (see Figure 13-21).
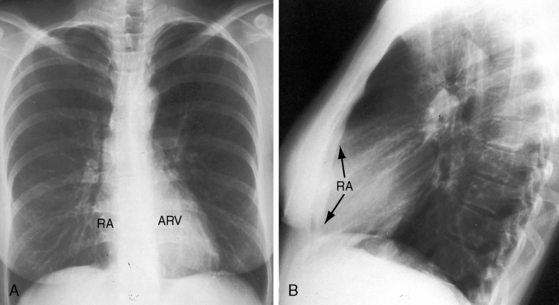
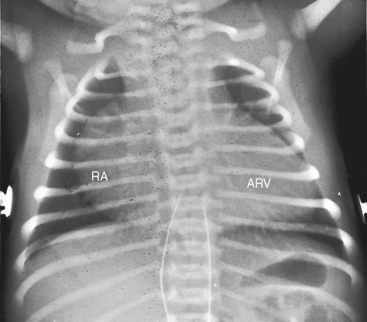
Figure 13-21 X-rays from the 39-year-old woman with mild Ebstein’s anomaly whose electrocardiogram is shown in Figure 13-16. A, The size and configuration of the heart are normal, but the ascending and transverse aortas are relatively prominent for age and gender. B, In the lateral projection, the retrosternal right atrium (RA) reveals itself. (RA = right atrium; ARV = atrialized right ventricle.)
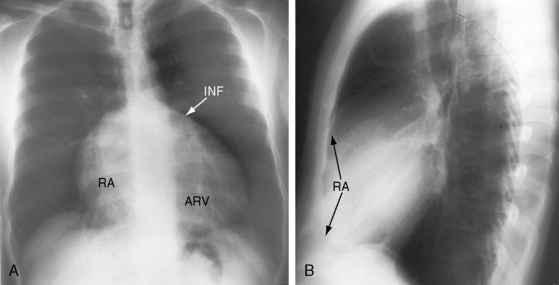
Figure 13-26 X-rays from an acyanotic 41-year-old man with Ebstein’s anomaly and exercise-induced hypoxemia. A, The lung fields are oligemic, although the film is overpenetrated. The infundibulum (INF) is prominent, but the vascular pedicle is narrow because neither the ascending aorta nor the pulmonary trunk is border-forming. Cardiac enlargement is the result of a huge right atrium (RA) and an atrialized right ventricle (ARV). The x-ray bears a striking resemblance to Figure 13-25 from the 19-month-old acyanotic female with Ebstein’s anomaly. B, The retrosternal space is occupied by the enlarged right atrium (RA, arrows).
Echocardiogram
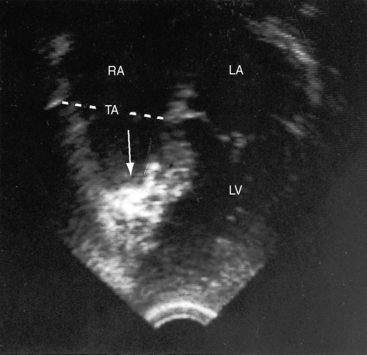
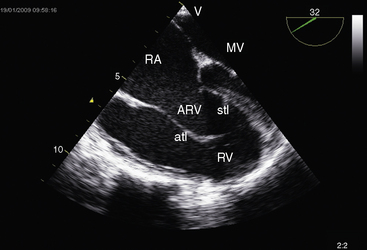
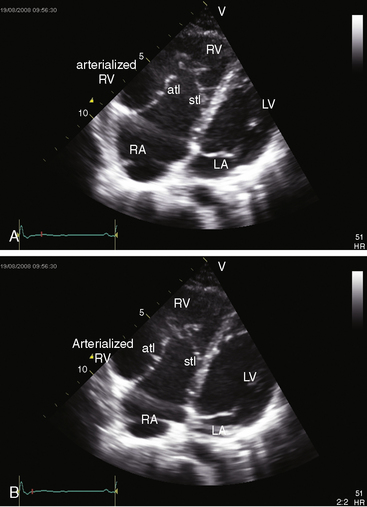
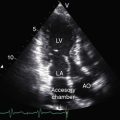
Echocardiography with color flow imaging and Doppler interrogation is the diagnostic test of choice and is used to establish the diagnosis and severity of Ebstein’s anomaly.17,25,89,90 The diagnosis can be made in utero.51,59,60 Apical displacement of the septal tricuspid leaflet in the anomaly exceeds 15 mm in children and 20 mm in adults (Figure 13-30).89 The right atrium proper lies proximal to the anatomic tricuspid annulus, and the atrialized right ventricle occupies the interval between the anatomic tricuspid annulus and the distally displaced septal tricuspid leaflet (see Figure 13-30). The displaced tethered septal tricuspid leaflet moves little if at all in contrast to the large elongated anterior leaflet, which may exhibit brisk sail-like movements in real-time imaging (Figures 13-30 and 13-31). Rarely, a broad echogenic band separates the atrialized right ventricle from the distal functional right ventricle, and the proximal annulus is unguarded by tricuspid leaflet tissue (Figure 13-32).
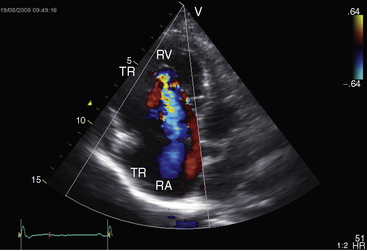
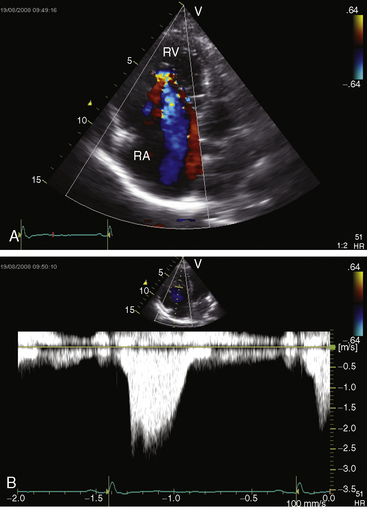
Echocardiography defines left ventricular geometry and cavity size, and real-time imaging identifies displacement of the ventricular septum into the left ventricular cavity with paradoxical septal motion (see Figure 13-31).29 Superior systolic displacement (mitral prolapse) occurs because normal-sized leaflets and chordae tendinae are housed in a left ventricular cavity that is reduced in size and altered in shape (see Figure 13-31).29 The right ventricular outflow tract in the short axis is dilated in contrast to the adjacent normal or small aortic root. Color flow imaging and Doppler interrogation quantify tricuspid regurgitation and establish the relatively low velocity of regurgitant flow, which begins at the level of the displaced septal and posterior leaflets and courses through the atrialized right ventricle into the right atrium proper (Figure 13-33, Video 13-1, and Figure 13-34).
Three-dimensional echocardiography58 and magnetic resonance imaging allow precise anatomic and functional assessment of the tricuspid valve and right ventricle.58
Uhl’s anomaly
The parchment heart was so designated by Osler91 in 1905 and was redescribed in 1950.92 Two years later, Uhl93 reported “almost total absence of myocardium of the right ventricle.” Aplasia or hypoplasia of most if not all of the myocardium in the trabecular portions of the right ventricle occurs with a structurally normal and functionally competent tricuspid valve.94,95 The parchment right ventricle of Uhl’s anomaly has been characterized as inexcitable in contrast to the arrhythmogenic atrialized right ventricle of Ebstein’s anomaly.96 Uhl’s anomaly has been reported in identical twins and has been diagnosed in utero.97
The essential hemodynamic fault in Uhl’s anomaly is lack of right ventricular contraction. The chamber functions as a passive conduit through which right atrial blood is channeled on its way into the pulmonary trunk. The thin-walled right ventricle balloons aneurysmally with each systole. The morphologically uninvolved ventricular septum exhibits vigorous paradoxical systolic motion and functions as part of the right ventricle, contributing materially to forward flow. Survival into adulthood is expected despite the functionally inadequate right ventricle. The jugular venous pulse is normal because the tricuspid valve is competent and because the force of right atrial contraction is not increased despite the parchment right ventricle. An elevation in right ventricular end-diastolic pressure and mean right atrial pressure rarely causes a right-to-left shunt interatrial shunt. A left ventricular impulse occupies the apex. A right ventricular impulse is absent by definition. A right ventricular third heart sound is generated by passive flow into the poorly compliant parchment right ventricle. The second heart sound is widely split even though the right ventricular conduction defect is thought to be peripheral (Figure 13-35). The electrocardiogram exhibits right atrial P waves (see Figure 13-35) because the right atrium tends to be larger than normal despite a competent tricuspid valve (Figure 13-36). The PR interval is normal, and preexcitation is unknown. The x-ray shows normal pulmonary vascularity and an increase in heart size chiefly from the dilated parchment ventricle (see Figure 13-36). The right atrial silhouette is rounded but unimpressive compared with Ebstein’s anomaly (see Figure 13-36). The morphologically spared infundibulum presents as a hump-shaped convexity at the upper the left cardiac border (see Figure 13-36). Echocardiography identifies a large right ventricle with an akinetic free wall, brisk paradoxical motion of the ventricular septum, and normal tricuspid leaflets (Figure 13-37). Doppler echocardiography with color flow imaging confirms that the tricuspid valve is competent.
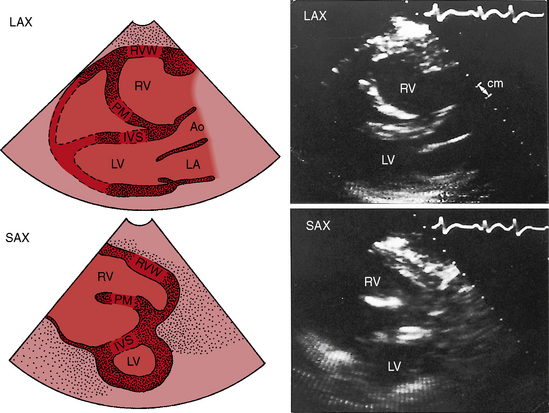
Figure 13-37 Echocardiograms (parasternal long-axis [LAX] and short-axis [SAX]) from the 25-year-old woman with Uhl’s anomaly whose x-ray is shown in Figure 13-36. The right ventricle (RV) is dilated, and its cavity contains a large refractile papillary muscle (PM) shown in the short axis sketch (SAX). The interventricular septum (IVS labeled in the sketches) exhibited brisk paradoxical systolic motion in real time, whereas the right ventricular wall (RVW labeled in the sketch) was akinetic. (Ao = aorta; LA = left atrium.)
(From Child JS, Perloff JK, et al. Uhl’s anomaly (parchment right ventricle). American Journal of Cardiology 53:635, 1984.)
Arrhythomegenic right ventricular dysplasia varies from mild focal lesions detected histologically to widespread segmental transmural involvement of the free wall of the right ventricle and infundibulum.95,98–101 Biventricular involvement is infrequent.102,103 Prevalence rate has been estimated at 1/5000.84 Dysplasia is represented by focal replacement of myocardium with fibrous and adipose tissue, not by focal congenital absence of the myocardium.99,100 The lesions can be detected with magnetic resonance imaging and with low-amplitude endocardial electrograms that identify replaced myocardium.104 Familial recurrence of right ventricular dysplasia was confirmed in a study of nine families.99,100 The echocardiogram discloses segmental and generalized abnormalities of right ventricular wall motion, abnormal trabecular architecture, and areas of saccular dilation.100,105 Isolated noncompaction of left ventricular myocardium, or Barth’s syndrome, is arrhythmogenic and is characterized morphologically by numerous prominent trabeculations and conspicuous intertrabecular recesses that penetrate deeply into the left ventricular myocardium.106,107
1 Ebstein W. On a very rare case of insufficiency of the tricuspid valve caused by a severe congenital malformation of the same. Am J Cardiol. 1968;22:867.
2 Konstantinov I.E. History of cardiology: Wilhelm Ebstein, MD. Interview by Mark Nicholls. Circulation. 2007;116:f101-f102.
3 Mann R.J., Lie J.T. The life story of Wilhelm Ebstein (1836-1912) and his almost overlooked description of a congenital heart disease. Mayo Clin Proc. 1979;54:197-204.
4 Sekelj P., Benfey B.G. Fundamentals of clinical cardiology: historical landmarks: Ebstein’s anomaly of the tricuspid valve. Am Heart J. 1974;88:108-114.
5 Van Son J.A., Konstantinov I.E., Zimmermann V. Wilhelm Ebstein and Ebstein’s malformation. Eur J Cardiothorac Surg. 2001;20:1082-1085.
6 Yater W.M., Shapiro M.J. Congenital displacement of the tricuspid valve (Ebstein’s disease): review and report of a case with electrocardiographic abnormalities and detailed histologic study of the conduction system. Ann Intern Med. 1937;11:1043.
7 Tourniaire M., Tourniaire F., Tartulier M. Maladie d’Epstein. Arch Mal Cœur Vaiss. 1949;42:1211.
8 Engle M.A., Payne T.P., Bruins C., Taussig H.B. Ebstein’s anomaly of the tricuspid valve; report of three cases and analysis of clinical syndrome. Circulation. 1950;1:1246-1260.
9 Reynolds G. Ebstein’s disease a case diagnosed clinically. Guys Hospital Reports. 1950;99:275-283.
10 Soloff L.A., Stauffer H.M., Zatuchni J. Ebstein’s disease: report of the first case diagnosed during life. Am J Med Sci. 1951;222:554-561.
11 Soloff L.A., Stauffer H.M., Zatuchni J. Ebstein’s disease: description of the heart of the first case diagnosed during life. Am J Med Sci. 1957;233:23-27.
12 Nora J.J., Nora A.H., Toews W.H. Letter: Lithium, Ebstein’s anomaly, and other congenital heart defects. Lancet. 1974;2:594-595.
13 Patane S., Marte F., Di Bella G., Chiribiri A. Ebstein’s anomaly in adult. Int J Cardiol. 2009;136:e6-e7.
14 Hauck A.J., Freeman D.P., Ackermann D.M., Danielson G.K., Edwards W.D. Surgical pathology of the tricuspid valve: a study of 363 cases spanning 25 years. Mayo Clin Proc. 1988;63:851-863.
15 Silver M.D., Lam J.H., Ranganathan N., Wigle E.D. Morphology of the human tricuspid valve. Circulation. 1971;43:333-348.
16 Gussenhoven E.J., Stewart P.A., Becker A.E., Essed C.E., Ligtvoet K.M., De Villeneuve V.H. “Offsetting” of the septal tricuspid leaflet in normal hearts and in hearts with Ebstein’s anomaly. Anatomic and echographic correlation. Am J Cardiol. 1984;54:172-176.
17 Attenhofer Jost C.H., Connolly H.M., Dearani J.A., Edwards W.D., Danielson G.K. Ebstein’s anomaly. Circulation. 2007;115:277-285.
18 Dearani J.A., Danielson G.K. Congenital Heart Surgery Nomenclature and Database Project: Ebstein’s anomaly and tricuspid valve disease. Ann Thorac Surg. 2000;69:S106-S117.
19 Celermajer D.S., Dodd S.M., Greenwald S.E., Wyse R.K., Deanfield J.E. Morbid anatomy in neonates with Ebstein’s anomaly of the tricuspid valve: pathophysiologic and clinical implications. J Am Coll Cardiol. 1992;19:1049-1053.
20 Zuberbuhler J.R., Becker A.E., Anderson R.H., Lenox C.C. Ebstein’s malformation and the embryological development of the tricuspid valve. With a note on the nature of “clefts” in the atrioventricular valves. Pediatr Cardiol. 1984;5:289-295.
21 Tabatabaei N., Katanyuwong P., Breen J.F., et al. Images in cardiovascular medicine. Uncommon variant of Ebstein anomaly with tricuspid stenosis. Circulation. 2009;120:e1-e2.
22 Anderson K.R., Lie J.T. The right ventricular myocardium in Ebstein’s anomaly: a morphometric histopathologic study. Mayo Clin Proc. 1979;54:181-184.
23 Frescura C., Angelini A., Daliento L., Thiene G. Morphological aspects of Ebstein’s anomaly in adults. Thorac Cardiovasc Surg. 2000;48:203-208.
24 Anderson R.H., Silverman N.H., Zuberbuhler J.R. Congenitally unguarded tricuspid orifice: its differentiation from Ebstein’s malformation in association with pulmonary atresia and intact ventricular septum. Pediatr Cardiol. 1990;11:86-90.
25 Lang D., Oberhoffer R., Cook A., et al. Pathologic spectrum of malformations of the tricuspid valve in prenatal and neonatal life. J Am Coll Cardiol. 1991;17:1161-1167.
26 Beder S.D., Nihill M.R., Mcnamara D.G. Idiopathic dilation of the right atrium in a child. Am Heart J. 1982;103:134-137.
27 Sheldon W.C., Johnson C.D., Favaloro R.G. Idiopathic enlargement of the right atrium. Report of four cases. Am J Cardiol. 1969;23:278-284.
28 Gewillig M., Dumoulin M., Van Der Hauwaert L.G. Transient neonatal tricuspid regurgitation: a Doppler echocardiographic study of three cases. Br Heart J. 1988;60:446-451.
29 Benson L.N., Child J.S., Schwaiger M., Perloff J.K., Schelbert H.R. Left ventricular geometry and function in adults with Ebstein’s anomaly of the tricuspid valve. Circulation. 1987;75:353-359.
30 Inai K., Nakanishi T., Mori Y., Tomimatsu H., Nakazawa M. Left ventricular diastolic dysfunction in Ebstein’s anomaly. Am J Cardiol. 2004;93:255-258.
31 Stollberger C., Kopsa W., Finsterer J. Non-compaction of the right atrium and left ventricle in Ebstein’s malformation. J Heart Valve Dis. 2006;15:719-720.
32 Cabin H.S., Roberts W.C. Ebstein’s anomaly of the tricuspid valve and prolapse of the mitral valve. Am Heart J. 1981;101:177-180.
33 Roberts W.C., Glancy D.L., Seningen R.P., Maron B.J., Epstein S.E. Prolapse of the mitral valve is described in two patients with the Ebstein’s anomaly of the tricuspid. Am J Cardiol. 1976;38:377-382.
34 Barber G., Danielson G.K., Heise C.T., Driscoll D.J. Cardiorespiratory response to exercise in Ebstein’s anomaly. Am J Cardiol. 1985;56:509-514.
35 Driscoll D.J., Mottram C.D., Danielson G.K. Spectrum of exercise intolerance in 45 patients with Ebstein’s anomaly and observations on exercise tolerance in 11 patients after surgical repair. J Am Coll Cardiol. 1988;11:831-836.
36 Lowe K.G., Emslie-Smith D., Robertson P.G., Watson H. Scalar, vector, and intracardiac electrocardiograms in Ebstein’s anomaly. Br Heart J. 1968;30:617-629.
37 Watson H. Electrode catheters and the diagnosis of Ebstein’s anomaly of the tricuspid valve. Br Heart J. 1966;28:161-171.
38 Obioha-Ngwu O., Milliez P., Richardson A., Pittaro M., Josephson M.E. Ventricular tachycardia in Ebstein’s anomaly. Circulation. 2001;104:E92-E94.
39 Lo H.M., Lin F.Y., Jong Y.S., Tseng Y.Z., Wu T.L. Ebstein’s anomaly with ventricular tachycardia: evidence for the arrhythmogenic role of the atrialized ventricle. Am Heart J. 1989;117:959-962.
40 Tede N.H., Shivkumar K., Perloff J.K., et al. Signal-averaged electrocardiogram in Ebstein’s anomaly. Am J Cardiol. 2004;93:432-436.
41 Bialostozky D., Horwitz S., Espino-Vela J. Ebstein’s malformation of the tricuspid valve. A review of 65 cases. Am J Cardiol. 1972;29:826-836.
42 Samanek M. Boy:girl ratio in children born with different forms of cardiac malformation: a population-based study. Pediatr Cardiol. 1994;15:53-57.
43 Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence. An international co-operative study of 505 cases. Br Heart J. 1974;36:417-427.
44 Flores Arizmendi A., Fernandez Pineda L., Quero Jimenez C., et al. The clinical profile of Ebstein’s malformation as seen from the fetus to the adult in 52 patients. Cardiol Young. 2004;14:55-63.
45 Balaji S., Dennis N.R., Keeton B.R. Familial Ebstein’s anomaly: a report of six cases in two generations associated with mild skeletal abnormalities. Br Heart J. 1991;66:26-28.
46 Donegan C.C.Jr, Moore M.M., Wiley T.M.Jr, Hernandez F.A., Green J.R.Jr, Schiebler G.L. Familial Ebstein’s anomaly of the tricuspid valve. Am Heart J. 1968;75:375-379.
47 Lo K.S., Loventhal J.P., Walton J.A.Jr. Familial Ebstein’s anomaly. Cardiology. 1979;64:246-255.
48 Mcintosh N., Chitayat D., Bardanis M., Fouron J.C. Ebstein anomaly: report of a familial occurrence and prenatal diagnosis. Am J Med Genet. 1992;42:307-309.
49 Pierard L.A., Henrard L., Demoulin J.C. Persistent atrial standstill in familial Ebstein’s anomaly. Br Heart J. 1985;53:594-597.
50 Emanuel R., O’brien K., Ng R. Ebstein’s anomaly. Genetic study of 26 families. Br Heart J. 1976;38:5-7.
51 Uyan C., Yazici M., Uyan A.P., Akdemir R., Imirzalioglu N., Dokumaci B. Ebstein’s anomaly in siblings: an original observation. Int J Cardiovasc Imaging. 2002;18:435-438.
52 Gollob M.H., Seger J.J., Gollob T.N., et al. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030-3033.
53 Park J.M., Sridaromont S., Ledbetter E.O., Terry W.M. Ebstein’s anomaly of the tricuspid valve associated with prenatal exposure to lithium carbonate. Am J Dis Child. 1980;134:703-704.
54 Zalzstein E., Koren G., Einarson T., Freedom R.M. A case-control study on the association between first trimester exposure to lithium and Ebstein’s anomaly. Am J Cardiol. 1990;65:817-818.
55 Hong Y.M., Moller J.H. Ebstein’s anomaly: a long-term study of survival. Am Heart J. 1993;125:1419-1424.
56 Gentles T.L., Calder A.L., Clarkson P.M., Neutze J.M. Predictors of long-term survival with Ebstein’s anomaly of the tricuspid valve. Am J Cardiol. 1992;69:377-381.
57 Celermajer D.S., Bull C., Till J.A., et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23:170-176.
58 Paranon S., Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237-243.
59 Hsieh Y.Y., Lee C.C., Chang C.C., Tsai H.D., Yeh L.S., Tsai C.H. Successful prenatal digoxin therapy for Ebstein’s anomaly with hydrops fetalis. A case report. J Reprod Med. 1998;43:710-712.
60 Pavlova M., Fouron J.C., Drblik S.P., et al. Factors affecting the prognosis of Ebstein’s anomaly during fetal life. Am Heart J. 1998;135:1081-1085.
61 Rossi L., Thiene G. Mild Ebstein’s anomaly associated with supraventricular tachycardia and sudden death: clinicomorphologic features in 3 patients. Am J Cardiol. 1984;53:332-334.
62 Fitzsimmons P.J., Mcwhirter P.D., Peterson D.W., Kruyer W.B. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142:530-536.
63 Cabin H.S., Wood T.P., Smith J.O., Roberts W.C. Structure–function correlations in cardiovascular and pulmonary diseases (CPC): Ebstein’s anomaly in the elderly. Chest. 1981;80:212-214.
64 Makous N., Vander Veer J.B. Ebstein’s anomaly and life expectancy. Report of a survival to over age 79. Am J Cardiol. 1966;18:100-104.
65 Harris R.H. Ebstein’s anomaly: discovered in a 75-year-old subject in the dissecting laboratory. Can Med Assoc J. 1960;83:653-655.
66 Seward J.B., Tajik A.J., Feist D.J., Smith H.C. Ebstein’s anomaly in an 85-year-old man. Mayo Clin Proc. 1979;54:193-196.
67 Donnelly J.E., Brown J.M., Radford D.J. Pregnancy outcome and Ebstein’s anomaly. Br Heart J. 1991;66:368-371.
68 Oki T., Fukuda N., Tabata T., et al. The ‘sail sound’ and tricuspid regurgitation in Ebstein’s anomaly: the value of echocardiography in evaluating their mechanisms. J Heart Valve Dis. 1997;6:189-192.
69 Crews T.L., Pridie R.B., Benham R., Leatham A. Auscultatory and phonocardiographic findings in Ebstein’s anomaly. Correlation of first heart sound with ultrasonic records of tricuspid valve movement. Br Heart J. 1972;34:681-687.
70 Pocock W.A., Tucker R.B., Barlow J.B. Mild Ebstein’s anomaly. Br Heart J. 1969;31:327-336.
71 Fontana M.E., Wooley C.F. Sail sound in Ebstein’s anomaly of the tricuspid valve. Circulation. 1972;46:155-164.
72 Willis P.W.T., Craige E. First heart sound in Ebstein’s anomaly: observations on the cause of wide splitting by echophonocardiographic studies before and after operative repair. J Am Coll Cardiol. 1983;2:1165-1168.
73 Koiwaya Y., Narabayashi H., Koyanagi S., et al. Early closure of the tricuspid valve in a case of Ebstein’s anomaly with type B Wolff-Parkinson-White Syndrome. Circulation. 1979;60:446-450.
74 Perloff J.K. Physical examination of the heart and circulation, 4th ed. Shelton, Connecticut: People’s Medical Publishing House; 2009.
75 Macruz R., Tranchesi J., Ebaid M., Pileggi F., Romero A., Decourt L.V. Ebstein’s disease. Electrovectorcardiographic and radiologic correlations. Am J Cardiol. 1968;21:653-660.
76 Kastor J.A., Goldreyer B.N., Josephson M.E., et al. Electrophysiologic characteristics of Ebstein’s anomaly of the tricuspid valve. Circulation. 1975;52:987-995.
77 Kaushik M.L., Sharma M., Kashyap R. ‘Himalayan’ p wave. J Assoc Physicians India. 2007;55:856.
78 Ho S.Y., Goltz D., Mccarthy K., et al. The atrioventricular junctions in Ebstein malformation. Heart. 2000;83:444-449.
79 Price J.E., Amsterdam E.A., Vera Z., Swenson R., Mason D.T. Ebstein’s disease associated with complete atrioventricular block. Chest. 1978;73:542-544.
80 Bialostozky D., Medrano G.A., Munoz L., Contreras R. Vectorcardiographic study and anatomic observations in 21 cases of Ebstein’s malformation of the tricuspid valve. Am J Cardiol. 1972;30:354-361.
81 Follath F., Hallidie-Smith K.A. Unusual electrocardiographic changes in Ebstein’s anomaly. Br Heart J. 1972;34:513-519.
82 Smith W.M., Gallagher J.J., Kerr C.R., et al. The electrophysiologic basis and management of symptomatic recurrent tachycardia in patients with Ebstein’s anomaly of the tricuspid valve. Am J Cardiol. 1982;49:1223-1234.
83 Surawicz B. Brugada syndrome: manifest, concealed, “asymptomatic,” suspected and simulated. J Am Coll Cardiol. 2001;38:775-777.
84 Wichter T., Matheja P., Eckardt L., et al. Cardiac autonomic dysfunction in Brugada syndrome. Circulation. 2002;105:702-706.
85 Priori S.G., Napolitano C., Gasparini M., et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342-1347.
86 Wolff L., Parkinson J., White P.D.. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. 1930. Ann Noninvasive Electrocardiol, 11. 2006:340-353.
87 Durrer D., Roos J.P. Epicardial excitation of the ventricles in a patient with Wolff-Parkinson-White syndrome (type B). Circulation. 1967;35:15-21.
88 Tao M.S., Partridge J., Radford D. The plain chest radiograph in uncomplicated Ebstein’s disease. Clin Radiol. 1986;37:551-553.
89 Ammash N.M., Warnes C.A., Connolly H.M., Danielson G.K., Seward J.B. Mimics of Ebstein’s anomaly. Am Heart J. 1997;134:508-513.
90 Ahmed S., Nanda N.C., Nekkanti R., Pacifico A.D. Transesophageal three-dimensional echocardiographic demonstration of Ebstein’s anomaly. Echocardiography. 2003;20:305-307.
91 Osler W. The principles and practice of medicine, 6th ed. New York: Appleton-Century-Crofts; 1905.
92 Segall H.N. Parchment heart (Osler). Am Heart J. 1950;40:948-950.
93 Uhl H.S. A previously undescribed congenital malformation of the heart: almost total absence of the myocardium of the right ventricle. Bull Johns Hopkins Hosp. 1952;91:197-209.
94 Reeve R., Macdonald D. Partial absence of the right ventricular musculature—partial parchment heart. Am J Cardiol. 1964;14:415-419.
95 Vecht R.J., Carmichael D.J., Gopal R., Philip G. Uhl’s anomaly. Br Heart J. 1979;41:676-682.
96 Bharati S., Ciraulo D.A., Bilitch M., Rosen K.M., Lev M. Inexcitable right ventricle and bilateral bundle branch block in Uhl’s disease. Circulation. 1978;57:636-644.
97 Hoback J., Adicoff A., From A.H., Smith M., Shafer R., Chesler E. A report of Uhl’s disease in identical adult twins: evaluation of right ventricular dysfunction with echocardiography and nuclear angiography. Chest. 1981;79:306-310.
98 Ouyang F., Fotuhi P., Goya M., et al. Ventricular tachycardia around the tricuspid annulus in right ventricular dysplasia. Circulation. 2001;103:913-914.
99 Marcus F.I., Fontaine G.H., Guiraudon G., et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384-398.
100 Nava A., Thiene G., Canciani B., et al. Familial occurrence of right ventricular dysplasia: a study involving nine families. J Am Coll Cardiol. 1988;12:1222-1228.
101 Virmani R., Robinowitz M., Clark M.A., Mcallister H.A.Jr. Sudden death and partial absence of the right ventricular myocardium: a report of three cases and a review of the literature. Arch Pathol Lab Med. 1982;106:163-167.
102 Pinamonti B., Pagnan L., Bussani R., Ricci C., Silvestri F., Camerini F. Right ventricular dysplasia with biventricular involvement. Circulation. 1998;98:1943-1945.
103 Mccrohon J.A., John A.S., Lorenz C.H., Davies S.W., Pennell D.J. Images in cardiovascular medicine. Left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2002;105:1394.
104 Boulos M., Lashevsky I., Reisner S., Gepstein L. Electroanatomic mapping of arrhythmogenic right ventricular dysplasia. J Am Coll Cardiol. 2001;38:2020-2027.
105 Baran A., Nanda N.C., Falkoff M., Barold S.S., Gallagher J.J. Two-dimensional echocardiographic detection of arrhythmogenic right ventricular dysplasia. Am Heart J. 1982;103:1066-1067.
106 Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507-513.
107 Ichida F., Tsubata S., Bowles K.R., et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103:1256-1263.