11 Ebstein’s Anomaly
I. CASE
A. Fetal echocardiography findings
1. The fetal echo reveals situs solitus of the atria, levocardia, left aortic arch, and heart rate of 112 bpm.
2. There are frequent conducted and nonconducted premature atrial beats (PACs), abnormal cardiac axis (left), and massive cardiomegaly (cardiothoracic ratio = 0.55).
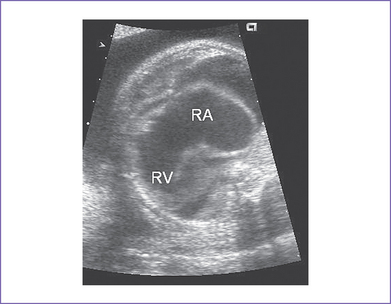
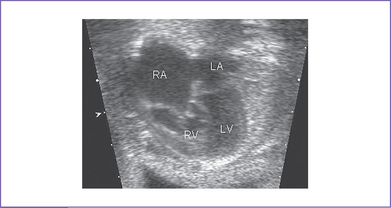
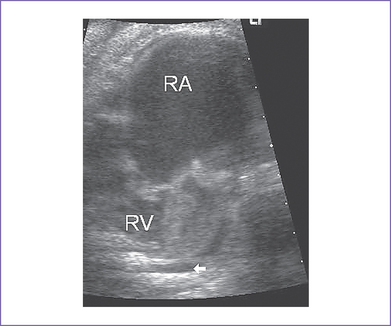
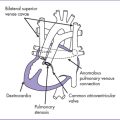
3. The four-chamber view reveals marked dilation of the right atrium (RA) and atrialization of the right ventricle (RV) (Fig. 11-1).
4. The septal and posterior leaflets of the tricuspid valve are displaced into the RV cavity (the essence of Ebstein’s malformation).
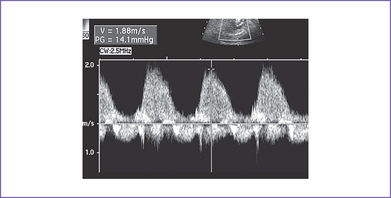
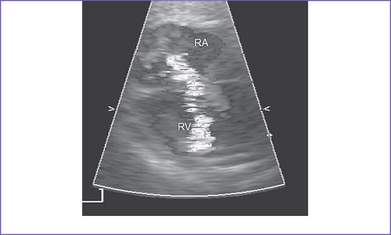
5. There is 4+ tricuspid valve regurgitation with peak velocity of 3.3 m/s and a decreased dP/dt (change in pressure per change in time) of 550 mm Hg per second (Fig. 11-2).
6. The pulmonary valve is atretic and the proximal main pulmonary artery is severely hypoplastic, with reversal of flow in the ductus arteriosus (aorta to pulmonary).
7. The branch pulmonary artery Doppler velocity is decreased, with a negative response to the maternal hyperoxygenation test, suggesting severe pulmonary hypoplasia and pulmonary arteriolar underdevelopment.
8. The left ventricle (LV) is compressed by the dilated atrialized RV.
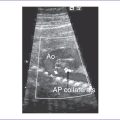
9. The LV outflow tract is normal, as are the aortic Doppler flow values, pattern, and velocity through it.
10. The aortic arch is leftward and the ductal and aortic arches are of similar size.
11. The flow though the aortic arch is antegrade, and flow through the ductal arch is retrograde, filling the pulmonary arteries.
12. The foramen ovale is large and patent, and the flow is unrestricted and mainly right to left.
13. The pulmonary venous flow pattern is normal.
14. The RV Tei index (myocardial performance index) is elevated (0.6) and the LV Tei index is normal.
D. Fetal management and counseling
The baby has a severe form of congenital heart disease combining Ebstein’s malformation of the tricuspid valve with massive tricuspid regurgitation and pulmonary atresia. There is a 10% to 15% chance for the baby to survive after birth, assuming a term delivery with normal chromosomes and no other abnormalities present. With hydrops and low peak velocity of the tricuspid valve regurgitation, the prognosis is poor (see Fig. 11-2).
1. Amniocentesis could not be offered at this gestation, and it is usually not productive in this lesion.
a. Parents should be aware that the disease can progress to a more severe form.
b. Serial antenatal fetal echocardiography studies are performed at weekly intervals.
c. Consider treatment with digoxin to support the LV function and attempt to prevent catecholamine excess.
E. Delivery
1. If the fetus remains well compensated, normal vaginal delivery can occur at term in a tertiary care center.
2. The true degree of pulmonary stenosis (atresia) after birth and after ductus arteriosus closure may be difficult to predict.
3. In the presence of heart failure or hydrops, delivery should be by cesarean section, which will in effect add the risk of prematurity to that of severe circulatory failure in a fetus already endangered by a major cardiac malformation.
4. Parents should be made aware that if the fetus survives to delivery, the risk of neonatal mortality is high because many of these babies cannot be adequately ventilated.
F. Neonatal management
b. General treatment, especially for anatomical pulmonary atresia.
c. Closure of ductus arteriosus for functional pulmonary atresia.
d. Congestive heart failure (CHF) requires anticongestive measures with digoxin and diuretics.
a. Indications for surgical intervention vary from one institution to the other. Common indications for surgery include:
b. There is controversy concerning the surgical procedure of choice.
c. Palliative and corrective options.
G. Follow-up
1. Arrhythmias can persist after surgery (10%-20%), requiring follow-up.
2. Subacute bacterial endocarditis (SBE) prophylaxis is recommended when indicated.
3. Limited activities and abstaining from competitive or strenuous sports may be indicated.
4. Survival after birth will be related to:
a. The extent of tricuspid valve disease.
I. Outcome of this case
1. In the next perinatal visit, the fetus developed sustained SVT and hydrops fetalis. The mother was started on PO digoxin and sotalol in hopes of controlling the arrhythmia and hence the heart failure.
2. Because of fetal distress, the baby was delivered at 33 weeks by emergency cesarean section.
a. The Apgar scores were poor: 7 at 1 minute and 5 at 5 minutes.
c. Moderate cyanosis was noted at birth: Pulse oximeter reading was 77%.
d. Intravenous PGE1 was started.
3. Postnatal echocardiography confirmed the diagnosis of severe Ebstein’s anomaly, massive cardiomegaly, severe tricuspid regurgitation, poor cardiac function, rudimentary RV, anatomical pulmonary atresia, ductus-dependant pulmonary circulation, pericardial effusion, large ASD (ASD II, mainly right-to-left shunting), and a patent ductus arteriosus with bidirectional shunting.
4. The baby was resuscitated and ventilated. Because of severe pulmonary hypertension, high-frequency ventilation was used with inhaled nitric oxide (up to 80 ppm).
5. The baby developed atrial fibrillation, and direct-current shock was applied.
6. The baby died at 12 hours of age due to failing heart, severe pulmonary hypertension, and lung prematurity (hyaline membrane disease).
II. YOUR HANDY REFERENCE
A. Prevalence
1. Ebstein’s anomaly is a rare diagnosis with an estimated incidence of 0.3% to 0.5% of congenital cardiac malformations. However, it forms a larger fraction of prenatal series of congenital cardiac diseases than would be expected from postnatal series.
2. Male and female fetuses are equally affected.
3. Exposure to lithium during early pregnancy has been associated with the anomaly.
B. Outcome
1. Most cases diagnosed during fetal life are at the severe end of the spectrum and are likely to have a poor prognosis.
2. Patients with Ebstein’s anomaly are prone to develop cardiac arrhythmia, which is a risk for sudden death later in life.
3. The 1991 fetal series reported by Sharland and colleagues included 54 cases of Ebstein’s malformation.
b. Survival: Only 14 survived.
4. Celermajer and colleagues (1994) reviewed 220 cases of Ebstein’s anomaly manifesting from fetal to adult life, with 1 to 34 years of follow-up.
a. Actuarial survival for all live-born patients was 67% at 1 year and 59% at 10 years.
b. There were 58 deaths, including 26 from heart failure, 19 perioperative, and 8 sudden.
c. Predictors of death included:
d. Morbidity was mainly related to arrhythmias and late hemodynamic deterioration.
e. Of 155 survivors, 129 (83%) were in functional class 1 and 104 (67%) were receiving no medical therapy.
f. Surgery was undertaken at some stage in 86 (39%) of the 220 patients.
C. Associated syndromes and extracardiac anomalies
1. Ebstein’s anomaly was rarely associated with extracardiac lesions in Sharland and colleagues’ series reported in 1991.
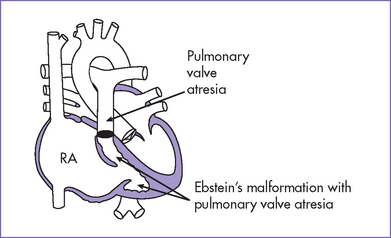
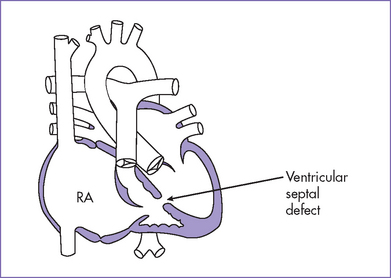
2. Other series have reported associated malformations and syndromes (Figs. 11-3 and 11-4) including Apert’s syndrome, Noonan’s syndrome, trisomy 18, and CHARGE association (coloboma, heart anomalies, choanal atresia, retardation of growth and development, and genital and ear anomalies).
D. Differential diagnosis
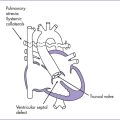
1. The differential diagnosis must include tricuspid valve dysplasia.
2. Here the tricuspid valve inserts normally and there is abnormal coaptation of the leaflets and significant tricuspid valve regurgitation (Fig. 11-5).
E. Clues to fetal sonographic diagnosis
1. Displacement of the septal leaflets of the tricuspid valve into the RV.
2. Long anterior tricuspid leaflet.
3. Variable degrees of RA enlargement.
a. The LV may be compressed by the dilated RA.
b. This should not be mistaken for a hypoplastic LV (apex forming) (Fig. 11-6).
5. Pulmonary stenosis or atresia (common).
a. Functional pulmonary atresia may be impossible to differentiate from anatomic atresia in the absence of pulmonary regurgitation.
b. Flow reversal in the ductus arteriosus is detectable in both situations.
6. Rare associated cardiac defects.
7. Inferiorly displaced origin of tricuspid valve regurgitation on color Doppler imaging (Fig. 11-7).
F. Predictors of poor outcome
1. Increased cardiothoracic ratio.
2. Degree of displacement (ratio of the distance from the tricuspid annulus to the apex over the functional opening to the apex).
4. Tethered distal attachments of the anterior-superior leaflet.
5. Severity of tricuspid regurgitation.
6. Presence and type of RV outflow obstruction.
7. Flow pattern through the main pulmonary artery and the ductus arteriosus (retrograde systolic and/or diastolic flows)
8. Small foramen ovale (0.3 cm ratio to interatrial septum): The size of the foramen ovale might influence fetal adaptation to increased LV volume.
10. LV compression resulting in inability to redistribute flow from the RA to the LA and LV.
11. Sustained arrhythmia (atrial or ventricular) or even premature beats in a compromised fetus.
H. Progression in utero
1. Cardiomegaly can progress, thus impairing normal lung development.
2. Pulmonary arteries might not grow at the normal rate.
3. Pulmonary outflow tract obstruction can progress, presumably as a result of reduced forward main pulmonary arterial blood blow.
4. LV dysfunction can progress, as manifested by increased LV Tei index.
a. When this occurs, there is no way for the heart to compensate.
b. This is likely a primary cause of heart failure and even acute demise among affected fetuses (Inamura et al, 2005).
c. Earlier delivery in the third trimester might lead to survival of some of these babies.
I. Postnatal management
1. For patients without a prenatal diagnosis of Ebstein’s anomaly.
a. After birth the clinical presentation of Ebstein’s anomaly is variable.
b. In Celermajer and colleagues’ 1994 review of 220 cases of Ebstein’s anomaly, the most common presentation in each age group was:
c. Early presentation was often associated with other cardiac lesions, usually pulmonary stenosis or atresia.
2. For patients with other complex forms of Ebstein’s anomaly, the medical and surgical management of this anomaly in the newborn period remains a significant challenge. Most data are devoted to different surgical techniques beyond the neonatal period (acceptable low mortality rates of 5%-10%).
a. Conversion of the patient to tricuspid atresia with the construction of an aorta-to-pulmonary shunt has been tried at some risk.
b. If the RV can generate some pressure and the pulmonary valve itself is patent, a newer strategy is to limit ductus arteriosus patency.
J. Pathophysiology
Ebstein’s anomaly is a spectrum of diseases.
1. The salient anatomical feature in diagnosis is finding the hinge point of the septal and mural leaflets of the valve within the inlet component of the RV rather than at the AV junction.
2. The septal and mural leaflets show a varying degree of distal displacement from their annular attachment, and there is usually some dysplasia of the leaflets.
3. Mildly affected patients usually have only the septal leaflet and have a higher attachment than usual. These patients might not have symptoms, and the anomaly can go undetected during life, with the late onset of cyanosis.
4. In severe forms, both the septal and mural leaflets may be tethered down into the apex of the RV.
a. This results in the incorporation of the inlet part of the RV into the RA (atrialization of the RV).
b. Atrialization of the RV reduces the functional size of the RV, and the RA may be grossly dilated.
5. Usually, the foramen ovale is patent and has a variable degree of right-to-left shunt.
a. The right-to-left shunt normally observed through the foramen ovale is significantly increased in the setting of severe tricuspid regurgitation.
b. The foramen is usually large and nonrestrictive, thus allowing an increase in combined ventricular output secondary to an increase in LV stroke volume.
6. In severe tricuspid regurgitation, the pulmonary circulation is maintained by retrograde flow through the ductus arteriosus, even though the RV outlet (pulmonary artery) may be patent.
7. Ebstein’s anomaly can occur in the setting of AV discordance and ventriculoarterial discordance (corrected transposition). This often results in significant systemic valve (functional mitral) regurgitation.
III. TAKE-HOME MESSAGE
A. Diagnosis
1. During four-chamber screening of the heart, a dilated RA will raise the suspicion of Ebstein’s anomaly.
a. In the incomplete form, only the septal leaflets have a lower attachment than usual, and in this case the RA cavity might appear normal.
b. In the complete form of Ebstein’s anomaly, early in the second trimester during screening the diagnosis could be missed because the right atrium might not be significantly enlarged. The lack of mobility of the leaflets and the abnormal opening of the tricuspid valve leaflets might be the only clue to diagnosis.
2. Antegrade flow through the main pulmonary artery depends on many factors, including:
a. The severity of the tricuspid regurgitation.
b. The functional capacity of the remaining portion of the RV.
c. The possible presence of valvar or subvalvar pulmonary stenosis.
3. In prenatal life, the patency of the RVOT can be difficult to assess with the current Doppler technique, because the entire RV ejection fraction could flow backward through the leaking tricuspid valve. This valve offers lower resistance than the pulmonary valve, which opens against pulmonary and systemic resistance.
B. Prognosis
1. In Ebstein’s anomaly, fetal and neonatal presentation is associated with a poor outcome and can be predicted by the echocardiographic appearance and presence of associated lesions. In older children and adults, incidental findings and arrhythmia are common, and the long-term outcome is superior.
2. Ebstein’s anomaly predisposes to right bundle branch block, preexcitation, and an increased risk of sudden cardiac death. Atrial fibrillation occurs in about one third of patients with Ebstein’s anomaly.
3. The risk of postnatal death in decompensated babies with Ebstein’s anomaly is high. The major challenge is to establish efficient oxygenation through the lungs, which are usually compressed in utero by the grossly dilated heart.
Allan LD, Desai G, Tynan MJ. Prenatal echocardiographic screening for Ebstein’s anomaly for mothers on lithium therapy. Lancet. 1982;2(8303):875-876.
Bader RS, Perrin D, Yoo SJ. Congenitally corrected transposition of the great arteries with Ebstein malformation and hypoplasia of the aortic arch in a fetus. Fetal Pediatr Pathol. 2004;23(4):257-263.
Bruckheimer E, Bulbul Z, Pinter E, et al. Inhaled nitric oxide therapy in a critically ill neonate with Ebstein’s anomaly. Pediatr Cardiol. 1998;19(6):477-479.
Celermajer DS, Bull C, Till JA, et al. Ebstein’s anomaly: Presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23(1):170-176.
Hornberger LK, Sahn DJ, Kleinman CS, et al. Tricuspid valve disease with significant tricuspid insufficiency in the fetus: Diagnosis and outcome. J Am Coll Cardiol. 1991;17(1):167-173.
Huhta JC. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol. 2004;25(3):274-286.
Inamura N, Taketazu M, Smallhorn JF, Hornberger LK. Left ventricular myocardial performance in the fetus with severe tricuspid valve disease and tricuspid insufficiency. Am J Perinatol. 2005;22(2):91-97.
Knott-Craig CJ, Overholt ED, Ward KE, et al. Repair of Ebstein’s anomaly in the symptomatic neonate: An evolution of technique with 7-year follow-up. Ann Thorac Surg. 2000;73(6):1786-1792.
Pavlova M, Fouron JC, Drblik SP, et al. Factors affecting the prognosis of Ebstein’s anomaly during fetal life. Am Heart J. 1998;135(6 Pt 1):1081-1085.
Sharland GK, Chita SK, Allan LD. Tricuspid valve dysplasia or displacement in intrauterine life. J Am Coll Cardiol. 1991;17(4):944-949.
Wald RM, Adatia I, Van Arsdell GS, Hornberger LK. Relation of limiting ductal patency to survival in neonatal Ebstein’s anomaly. Am J Cardiol. 2005;96(6):851-856.
Weinstein MR, Goldfield M. Cardiovascular malformations with lithium use during pregnancy. Am J Psychiatry. 1975;132(5):529-531.