CHAPTER 241 Early Management of Brachial Plexus Injuries
Surgical treatment of BP injury includes end-to-end neurotization, interpositional nerve grafting, intraplexus/extraplexus nerve transfer, and in rare instances, neurolysis alone. Secondary surgery is often performed at a later date and may include free muscle transfer, tendon transfers, and muscle/tendon releases. Management of BP injuries is complex, and there is still ongoing debate about the optimal timing of surgery, determination of whether surgery is indicated, and the type of repair to perform.1,2
Historical
The first surgical repair of the BP was attempted in the last decade of the 19th century after successful attempts at nerve suturing,3,4 experimental work on nerve grafts,5 and the introduction of this method to clinical surgery.6 The repair techniques commonly applied were neurolysis, direct repair with epineural sutures, and coaptations wrapped in a membrane. Over the years, surgeons have used different kinds of suture material for nerve repair, including animal and human hair, animal tendon, fascia, linen, silk, cotton, and polyester. Various aqueous or gel media have been used for adherence of nerve ends difficult to suture. BP lesions became a separate clinical entity among peripheral nerve lesions in the first half of the 19th century. At autopsy, Flaubert described rupture of the C5 spinal nerve with avulsion of C6 through T1 caused by reduction of a dislocated shoulder.7 Subsequently, BP management of 8 cases in wounded soldiers during the American Civil War8 and 24 European cases9 was reported.
Secondary suture of a traction injury to the BP 7 months after trauma was initially carried out in the beginning of the 20th century.10 The first nerve transfer consisted of implanting the distal stump of the damaged spinal nerve C5 into the healthy C6.11 Unfortunately, the results of these first surgical attempts at repair are not known. At that time, however, the results of surgery were inconsistent and often poor. BP surgery was performed only by a small group of surgeons,12–15 and several deaths related to surgery occurred.14,16 Before and during World War I, nerves were regarded as simple cord-like structures. Severed nerves were repaired by simply restoring continuity in the expectation that nerve regeneration would then restore function. Procedures to close large gaps under considerable tension were still preferred over nerve grafting. Throughout this period, infection remained a problem, and the results of nerve repair continued to be disappointing.
During the first half of the 20th century, reconstructive surgical procedures were developed to treat the sequelae of poliomyelitis and could also be applied to BP lesions. These musculotendinous transfers had the approval of surgeons because they produced more predictable results and were more readily available than the demanding, time-consuming nerve repair surgery. BP lesions remained uncommon before World War II because the violent trauma needed to cause avulsion or rupture often resulted in life-threatening lesions as well. During the 1930s, BP lesions became more common, and the role of motorcycle accidents as an important cause of BP lesions was recognized by Bonola.17
The Second World War produced a large number of patients with BP lesions who were thoroughly studied and operated on by the team of Sir Seddon (Barnes, Bonney, Brooks, and Yeoman). They reported on the use of autologous nerve grafts in five patients with success in two.18,19 The accumulation of World War II victims stressed the necessity of intensifying the search for better treatment modalities and led to a breakthrough in nerve repair. For the first time these patients were referred to special centers to receive specialist attention and to be available for thorough investigation and study. Antibiotics made effective control of wound infection possible. Studies at a basic level revealed the complexities of the internal structure of nerves. Then came the realization that restoration of continuity is just the first step in restoration of function. Disorderly regeneration and loss of regenerating axons at the suture line were recognized as important factors that complicated and adversely affected recovery. The central objective of nerve repair finally emerged, namely, to reduce the loss of axons that occurs during regeneration and to assist regenerating axons to reestablish useful functional connection with the periphery such that the new pattern of innervation approximated the original as closely as possible. Procedures and techniques were developed to create optimal conditions for regeneration and improve the repair. Among these was the suggestion that microsurgical techniques might be used to improve the repair of severed nerves.20 The significance of the data emerging from basic studies filtered through to the clinical level in the 1960s.
By the late 1950s and into the 1960s, intercostal nerve (ICN)–to–musculocutaneous nerve (MCN) transfers were performed.21 Several other surgical teams also contributed to the understanding and treatment of BP lesions.22–26 Unfortunately, during the 1960s, repair of traction injuries remained disappointing, and direct surgery was discontinued in favor of nonoperative treatment.27–32 Above- or below-elbow amputation was advised in many cases.33
Surgeons involved in reconstructive work on the upper extremity often explored the BP mainly to determine the diagnosis and obtain a prognosis. The discouragement widely felt culminated at a meeting of the International Society for Orthopaedic Surgery and Traumatology in Paris in 1966, where the following consensus was reached: (1) surgical exploration of BP injuries, particularly at the supraclavicular level, was of no real benefit to diagnosis and prognosis, and (2) repair was mostly impossible and, when performed, did not achieve substantial results.34 The introduction of microscopic magnification in the same period offered new opportunities in the surgical management of BP injuries.35 In the mid-1960s, a major step forward was made independently by Millesi and Narakas, who used microsurgical technique for nerve grafts coapted to ruptured trunks and cords. Both men were in disagreement with Fletcher, who favored amputation of the limb in severe cases and fitting of a prosthesis.36 Their first reports stirred much interest but were also met with skepticism.37–40 Gratifying results were achieved in a sufficient number of patients to encourage other surgeons to pursue early surgical reconstruction after injury. The efforts of Millesi and Narakas were soon complemented by those of other surgeons such as Allieu, Alnot, and Sedel in France, Brunelli in Italy, Kline in the United States, and Hudson in Canada.
Knowledge of nerve regeneration broadened as diagnostic tests became available, including electromyography (EMG)41 and cervical myelography.42 Refinements included the use of evoked nerve action potentials (NAPs)43 and the association of computed tomography (CT) and myelography.44 Seddon’s18 and Sunderland’s20 classifications of nerve injury could be applied with more clarity, and the distinction between patients who should or should not undergo surgery became less vague. In the 1970s, survival after motorcycle trauma increased because of the use of helmets and improvement in lifesaving procedures. From 1975 onward, regular exchange of knowledge was established between European teams motivated by the steady increase in the number of patients with BP lesions. In similar fashion, groups of peripheral nerve experts became established in various parts of the world and published articles on a variety of topics related to peripheral nerves. Surgical repair of the peripheral nervous system has become commonplace in most major medical centers. A comment made in 1951 by Sir Sydney Sunderland, “it is no longer a question of what can be done, but of establishing what should be done,”20 would appear to have been addressed.
History of Brachial Plexus Birth Injury Management
One of the he first description of a birth-related brachial plexus injury (BRBPI) was provided by the obstetrician Smellie in 1768.16,45–47 He reported on a spontaneously resolving paralysis of both arms lasting several days. In 1851, the first pathologic description of an 8-day-old infant was presented. At autopsy, extensive hemorrhagic infiltration was found in the entire BP that strongly suggested that a laceration had taken place.16 Duchenne described infants in 1872 and Erb described adults in 1874 with the distinct clinical entity of a flaccid palsy in which abduction and external rotation of the arm were paralyzed, together with absent elbow flexion and supination of the forearm.47,48 The condition is frequently referred to as Erb-Duchenne paralysis or Erb’s palsy. Other authors have probably described the occurrence of BRBPI at an earlier date than Duchenne and Erb did.16,47
The first nerve surgery for BRBPI was reported by Kennedy from the University of Glasgow in February 1903. Kennedy described 2 patients who were selected for surgery because of the absence of spontaneous recovery and muscle responses at the age of 2 months. One baby was treated surgically at 2 months and the other at 6 months of age.48 The surgical procedures were well documented with scarring of the superior trunk described. The upper trunk was resected, and three distal targets (suprascapular nerve [SSN], anterior division and posterior division) were sutured with a central suture of “fine chromotized catgut” to the fifth and sixth nerves. Because resection of the upper trunk resulted in a gap, the shoulder of the infant was pushed upward and the head tilted to the side operated on to perform a tension-free nerve suture. Kennedy claimed that 4 to 8 months after surgery, the infants had fairly good use of their arm.48 One year later, Kennedy had extended his surgical series to 5 infants with BRBPI.49 Only 2 months after Kennedy, on the other side of the Atlantic Ocean, the American surgeon Taylor performed his first surgical procedure in New York.16 The surgical technique that he and his colleagues described is approximately the same as Kennedy’s, although the actual nerve suture was done by “lateral sutures of fine silk involving the nerve sheaths only.” Taylor operated mainly on older children (4.5 to 11 years), but he did operate on some infants: the 3 youngest were 8, 16, and 25 months. Of these 3 infants, 2 died within days after the operation. In a 1921 paper, Taylor described his surgical experience in treating 76 patients with BRBPI. In his last 25 patients (surgically treated from 1914 to 1921), only 1 “died of hemorrhage on table,” and in 1 patient the surgery was stopped because of hemorrhage.50 Surgery for an obstetric BP injury was apparently performed quite commonly in those years. William Sharp (who was trained by Cushing) reported his neurosurgical procedures in 146 patients with BRBPI operated on between 1913 and 1923 in the New York Polyclinic Hospital.51 The length of surgery was short because of lack of proper anesthesia, adequate illumination, and magnification. The surgical results were, however, were quite good.
Today’s standard technique of autologous nerve grafting to bridge the defect was not commonly used in the early days of BRBPI surgery.52 Nerve transplantation had been performed in 1892: a dog’s sciatic nerve was sutured in place of a defect of the external popliteal nerve. The results of nerve transplantation were poor, and therefore nerve grafting was not widely applied in clinical practice. Other surgical techniques that were reported consisted of nerve transfer (which was then alternatively called nerve crossing), fascicular transfer, and end-to-side repair.52
In 1916, the orthopedic surgeon Sever downplayed the use of nerve surgery in a paper on 471 nonoperatively treated patients.53 He followed with a second publication along the same lines.47 He concluded that “In regard to the operation on the plexus in the usual upper arm type of case, it might be said that in the experience of this clinic it has not been found necessary…. It cannot be too strongly emphasized that no operation on the plexus will be of any great use in restoring functional activity to the arm, unless contracted and restricting muscles are divided, and careful after-treatment persisted in for a long period.”47 The neurosurgeon Sharpe added that “there is not one case of complete recovery of function” in his series of 146 patients.51 This belief was shared by Jepson a few years later: “There has been no case yet…which has shown an anatomic and physiologic cure from the plexus operation. Even marked improvement is usually lacking…. Many times the nerve is so badly damaged that it is beyond repair.”54 Both Sever and Jepson advised that orthopedic operations be performed for BRBPI.54 For improvement in shoulder function, Sever recommended release of the restricted shoulder mobility, followed by muscle and tendon transfers. Jepson performed a rotation osteotomy of the humerus. Modifications of these surgeries are still performed today.55
The high mortality rates and the limited results in the young infants may have contributed to the relative abandonment of BRBPI surgery.16 In the 1920s, even Taylor, who had considerable operative experience, preferred to wait as long as any improvement was taking place.56 Fifty years later, after development of the surgical microscope, improvements in surgical tools, creation of fine suture materials, and the use of autologous nerve grafts together with improved safety of anesthesia, nerve surgery for infants was reintroduced in 1978 by Alain Gilbert and associates.57 The field has progressed to the point that even with total or pan-BRBPI lesions, a useful hand can be obtained.58
Anatomy
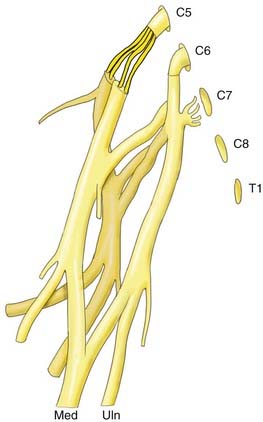
Although numerous variations in formation of the BP have been reported, the following common form can be taken as a point of departure. The BP is composed of five spinal nerves that join to form three trunks, each of which divides into an anterior and posterior division that lead to the three cords from which all the major nerves to the arm subsequently arise. The five spinal nerves are C5, C6, C7, C8, and T1. Commonly, there is a contribution to the BP from C4. The C5 and C6 spinal nerves merge (Erb’s point) to form the upper trunk. The C8 and T1 nerves merge to form the lower trunk, and the C7 nerve continues into the middle trunk. Each trunk terminates in an anterior and posterior division. The posterior divisions come together into the posterior cord. The anterior divisions of the upper and middle trunks form the lateral cord, and the anterior division of the lower trunk forms the medial cord. Each cord has two main terminal branches: the lateral cord provides the MCN and the lateral component of the median nerve, the medial cord leads to the ulnar nerve and the medial component of the median nerve, and the posterior cord provides the axillary nerve and the radial nerve. Over its entire trajectory, smaller nerves to the shoulder and upper limb muscles leave the plexus. Variations in anatomy may hamper clinical efforts to localize the site of a lesion.59
Pathophysiology and Classification
Despite the numerous ways to injure a nerve, the pathologic reactions are similar. After axonal injury, responses are at first degenerative and later regenerative. An increase in traction force applied to a peripheral nerve results in stepwise rupture of the peripheral nerve elements moving from inside to outside. Anatomic rupture begins with the axon or its coverings and then proceeds to the basal membrane, endoneurium, perineurium, and finally the epineurium. Based on these sequential events, the severity of the nerve lesion is graded in relation to the degree of neural damage. The most frequently applied classification scheme is Seddon’s classification of neurapraxia, axonotmesis, and neurotmesis.18 Sunderland introduced a classification based on five grades of progressive pathologic events.20
Clinical
The clinical history, physical examination, and adjuvant testing provide insight on localizing the injury and determining the extent of injury. The physical examination requires a detailed motor and sensory examination of the affected limb. The grading system of the Medical Research Council of Great Britain (MRC) dates back to the early treatment of poliomyelitis and war injuries and is at present the most commonly used strength-grading system.60,61 Evaluation of both passive and active range of motion of the joints may reveal contractures requiring management.
The term magnetic resonance neurography (MRN) applies to novel MRI techniques that have greatly improved the ability to visualize peripheral nerves.62 Thus far, this technique has been more valuable in evaluating compression syndromes and tumors than traumatic injury. Notwithstanding, with improvement in the resolution of MRN will come added ability to localize nerve injury and characterize the underlying pathophysiology.
Preoperative diagnosis of a preganglionic or root avulsion injury indicates the need for early surgery and use of nerve transfers. One standard for the diagnosis of root avulsion is laminectomy with direct visualization of the roots. This can be modified to a minimally invasive procedure using an endoscope63; however, it has not become a commonly used technique. Clinical findings consistent with root avulsion include lack of power in the proximal muscles, presence of SNAPs with EMG denervation, Horner’s syndrome, and lack of nerve roots on imaging. When compared with intradural inspection of roots, CT myelography with 1- to 3-mm axial slices enables accurate diagnosis of the integrity of the roots in 75% to 85% of patients.64 MRI with 3-mm axial slices provided an accurate diagnosis in just 52% of patients when compared with intradural inspection.64 The presence of a pseudomeningocele, although highly suggestive of root avulsion, is not conclusive evidence. In the acute situation, an intradural blood clot can prevent filling of a pseudomeningocele. The resolution of MRI has improved enough in some institutions to trace the presence or absence of intradural nerve roots and thus negate the need for CT myelography. MRI acquisitions timed to the respiratory and cardiac cycle can improve the resolution of fine structures in cerebrospinal fluid by reducing apparent motion, although scan time may be greatly increased.
Based on his experience in treating 1068 patients with BP injuries during an 18-year span, Narakas developed his rule of “seven seventies.” He reported that approximately 70% of traumatic BP injuries occur secondary to motor vehicle accidents; of these, approximately 70% involve motorcycles or bicycles. Of the cycle riders, approximately 70% had multiple injuries. Overall, 70% had supraclavicular lesions; of these, 70% had at least one root avulsed. At least 70% of patients with a root avulsion have avulsions of the lower roots (C7, C8, or T1). Finally, of patients with lower root avulsion, nearly 70% will experience persistent pain.65
Therapy/Management
Avulsion of nerve roots may lead to the development of severe pain in the distribution of the injured nerve root. The pain is often described as a burning or crushing pain with paroxysmal burning or shooting pain. Nonoperative treatment may require polypharmacy under the guidance of a pain center. Fortunately, the natural history of avulsion pain is that about half the patients become pain free or able to cope with their pain within 1 year and the majority are pain free within 3 years. Unfortunately, in some patients avulsion-related pain can become exceedingly severe. Avulsion pain can be surgically managed by making a series of lesions at the dorsal root entry zone of the traumatized spinal cord.66
When there is evidence of a Sunderland V (rupture) injury as a result of a stretch mechanism at the site of transection, the nerve ends often need to be resected back to a more healthy region. Demarcation of the injury can be difficult to appreciate acutely, and 2 to 3 weeks is generally required for the injury to declare itself. This delay allows more effective trimming of the stumps to healthy tissue and results in better outcomes.67,68 An alternative is to explore the wound, tag the nerve ends, and then re-explore in a few weeks. For gunshot wounds, a low-velocity projectile is often associated with Sunderland grade I injuries, whereas high-velocity projectiles cause more soft tissue disruption and higher grade Sunderland injury. Most gunshot wounds leave the plexus element in continuity. If there is no improvement on EMG in the first few months, exploration, NAP recordings, and if indicated, repair are necessary.
One factor that may limit recovery is the death of neurons after axotomy. Retrograde transport of neurotrophic factors potentially derived from target organs can support the survival and regeneration of both sensory and motor neurons.69 Some neuroprotection is afforded by the presence of a nerve graft, but the amount of cell loss already present at the time of repair remains.70 It is also apparent that over time, loss of Schwann cells in the distal nerve likewise has a negative impact on regeneration. These findings suggest that earlier repair of nerve injury is likely to be associated with improved outcome.
An argument against early surgery is that it does not allow the possibility of spontaneous recovery, which may bestow a better outcome than achieved with surgery. By waiting 3 months, there is the possibility of performing intraoperative electrophysiologic studies on a lesion to help determine whether regeneration is occurring. In contrast, early surgery is easier to perform because there is less scarring. Early surgery can allow visual inspection of the acute lesion and perhaps some prediction of outcome with or without nerve repair. Although there is some clinical evidence that early surgery may be beneficial to outcome, the debate rages on.71
Surgical management is divided into the initial, or primary, surgery and delayed, or secondary, surgery. Primary surgery is aimed at repairing the nerves and is time dependent. Examples include end-to-end nerve repair, end-to-side nerve repair, nerve grafting, nerve transfer, and neurolysis. Secondary surgery is performed when primary nerve repair is not likely to result in functional improvement or when recovery after nerve repair reaches a plateau still lacking in function. Secondary surgery is focused on maximizing function by performing muscle and tendon transfers and bone or joint work.72
The ability to conduct a compound NAP across a lesion indicates that preserved axons or significant regeneration must be present. Studies performed by Kline and colleagues suggest that the ability to conduct an NAP (small amplitude and slow conduction) across a neuroma-in-continuity indicates that recovery will occur after neurolysis alone without the need for additional treatment (e.g., neuroma resection and grafting). More than 90% of patients with a preserved NAP will gain clinically useful recovery.67,68 A postganglionic injury that does not show evidence of recovery should be repaired. The pathologic area is removed and either an end-to-end coaptation is performed, or more commonly, cable grafts are used to provide a tension-free repair.
Timing of and Selection for Surgery in Patients with Birth-Related Brachial Plexus Injuries
BRBPIs are most often caused by traction to the BP during labor. The severity of the traction injury may vary from neurapraxia or axonotmesis to neurotmesis and avulsion of rootlets from the spinal cord.73 Neurapraxia and axonotmesis eventually result in complete or nearly complete recovery. Neurotmesis and root avulsion, in contrast, result in permanent loss of arm function and, in time, development of skeletal malformations and cosmetic deformities.74–77 The prognosis of patients with BRBPI has generally been considered to be good, with complete or almost complete spontaneous recovery occurring in more than 90% of patients.78–83 However, this opinion is based on a limited number of series.75,84 Methodologic problems were discussed in a systematic literature review on the available natural history studies.85 The conclusion was that no study presented a prospective, population-based cohort that was scored with a proper scoring system and had an adequate follow-up time. Instead, from analysis of the most methodologically sound studies it was concluded that the proportion of children with residual deficits is 20% to 30%. A major problem is how to select those infants, shortly after birth, who will form the aforementioned 20% to 30% with a poor prognosis. A satisfactory test for such selection is not currently available. The second problem, also concerning the 20% to 30% with incomplete recovery, is how to predict whether function will be best after spontaneous nerve outgrowth or after nerve reconstructive surgery. The results achieved by surgery are claimed to be superior to those in nonoperatively treated subjects with equally severe lesions.86–88 However, this comparison relied on historical controls,89 and as yet no randomized controlled study has been conducted.90,91 Nerve surgery for neurotmetic lesions and root avulsions in which spontaneous restoration of function will not occur can offer functional recovery in the outflow of the reconstructed nerve elements.67 Unfortunately, the functional recovery pursued by nerve reconstructive surgery cannot be guaranteed for each individual case and the level of recovery is variable, so surgery is indicated for the more severe lesions.73 Determination of the lesion present may become apparent only over time, but time cannot be wasted because the interval between nerve trauma and reconstruction is inversely related to the outcome of reconstruction. Early surgery is therefore preferred over delayed surgery. At present, the earliest accepted time at which severe lesions can be determined is 2 to 3 months of age. Paralysis of the biceps muscle at 3 months, especially with wristdrop, is associated with a poor prognosis92 and is considered an indication for nerve surgery by some authors.86,93–96 However, biceps paralysis at the age of 3 months does not preclude satisfactory spontaneous recovery.87,97–99 Additionally, biceps muscle testing may not be reliable in infants.97,100,101 Alternative tests are complex or are done at an even later age.96,100,102
Ancillary EMG testing is not considered reliable for prognostication of BRBPI.86,103,104 In a paralytic biceps brachii muscle, the expected findings are absence of motor unit potentials (MUPs) and the presence of positive sharp waves or fibrillation potentials, or both. However, in a typical BRBPI patient, MUPs are present and denervation is absent in a paralytic biceps muscle at 3 months of age. This confusing finding has been noted by others105,106 and may have contributed to the opinion that EMG is not useful for BRBPI.82,107 We previously outlined several possible explanations for “inactive MUPs” (i.e., MUPs in a paralytic muscle),104 such as that the presence of inactive MUPs may depend on the time after injury because they reflect incomplete outgrowth of damaged axons and the formation of motor programs in the central nervous system. In one study, in 20 of 28 infants who had no biceps function at 3 months, biceps contraction had developed at 6 months.99 In addition, spontaneous recovery of useful extremity function has been observed in a carefully selected subset of patients without elbow flexion at 3 months of age.97 Together with our findings that MUPs can almost always be found in the biceps muscle at 3 months,108 this strongly suggests that the age of 3 months does not represent a stable state in BRBPI. In fact, outgrowing axons may well have only just arrived in the various muscles, and the central nervous system may not yet have learned to cope with the situation. In nerve lesions in adults, one may expect all motor programs to be ready and waiting for the restoration of peripheral connections. In BRBPI, axonal outgrowth may just be the starting point for restoration of function because formation of central nervous system motor programs may not commence until after enough axons have arrived to start exerting force. At the same time, forming such central motor programs may be more difficult and thus take longer than in healthy children because the central nervous system must somehow take aberrant outgrowth and the confusing feedback that it causes into account. Faced with a degree of inescapable co-contraction, it may not be easy to program effective elbow flexion, abduction, or rotation. In this hypothetic view, the age of 3 months may well be the very worst period imaginable to correlate EMG with clinical findings: it is late enough to show evidence of axonal outgrowth but too early for the brain to control contraction efficiently. This leaves the role of EMG for prognosis at 3 months undetermined at present.
In the Leiden University Medical Center, surgery for BRBPI is only rarely performed before 3 months of age, mainly for anesthesiology reasons, but almost always before the age of 7 months. In selecting infants for surgery we seek to identify all cases of neurotmesis or avulsion. Infants are selected for surgery when external shoulder rotation and elbow flexion with supination remain paralytic at 3 to 4 months. Impaired hand function is an absolute indication for nerve surgery as soon as the infant turns 3 months old.57 If there is doubt about the quality of shoulder and elbow joint movements, surgical exploration is performed in the hope that errors would consist of not finding neurotmesis or avulsion during surgery rather then letting such lesions go unoperated. At the Johns Hopkins BRBPI center, we make use of the Toronto active movement scale to help decide on the need for early surgical intervention.109,110 Preoperative ancillary investigations consist of ultrasound of diaphragm excursions to assess phrenic nerve function and CT myelography under general anesthesia to detect root avulsions.111,112
Surgical Exposure
Infraclavicular Exposure
In selected cases (e.g., those with an accompanying vascular lesion), horizontal transection of the clavicle with formation of a bone strip pedicle to the pectoralis major muscle can widen the exposure.113
Clavicular section is only occasionally necessary, and simple elevation of the intact clavicle will usually suffice. The suprascapular artery and vein beneath the clavicle can be isolated, ligated, and sectioned. The pectoralis major is then removed from the inferior edge of the clavicle while leaving a cuff of muscle to sew back to. Moistened elongated sponges or tape is passed around the clavicle to permit it to be shifted up or down.114,115
The infraclavicular portion of the plexus can also be exposed by division of the pectoral major near its humeral attachment and close to the clavicle. The clavicle can be sectioned three fingerbreadths away from the sternal end if divisions of the trunks are more focally injured.116 Sectioning allows rotation of the scapula, which brings the structures of the axilla forward. The pectoralis minor is divided close to the coracoid process and turned medially. Alternatively, the coracoid process can be extricated at the base to enable retraction of the tendinous insertions of the coracobrachialis muscle, the short head of the biceps brachii muscle, and the pectoralis minor muscle for exposure. The advantage of the latter two approaches is that the entire trajectory of the BP can be inspected in one view. Disadvantages are the risk for pseudarthrosis of the clavicle and more laborious approaches than with the transpectoral technique. Adequate fixation of the divided clavicle is a challenge even when compression plates are placed by an orthopedist.
Posterior Exposure
A posterior approach to the BP has been described by Kline’s group and is indicated in well-selected cases to expose the juxtaforaminal portion of the spinal nerves, an area difficult to reach with the anterior approach. The patient is positioned prone, and through a parascapular incision, the trapezius and rhomboid muscles are sectioned. The paraspinal muscles are retracted, and resection of the first rib and scalene muscles exposes the proximal spinal nerves. If necessary, the overlying facets can be removed to allow visualization of the spinal nerves, intraforaminally close to their dural exits.117
Nerve Transfer Surgery
In a nerve transfer, a functioning donor nerve is divided and the proximal end is coapted to the more denervated distal target nerve. Nerve transfer often places the repair site closer to the target than with nerve graft repair and thus lessens the time needed for regeneration and improves the prognosis. In the case of preganglionic injury, nerve transfer is the only repair that will probably result in functional recovery. Reimplantation of avulsed roots is a controversial topic but does show great promise.118
Oberlin and coworkers described transfer of a single ulnar nerve fascicle to the biceps innervation of the MCN as a means of providing elbow flexion.119 In doing so, they helped begin a new era in nerve repair. Earlier published transfers included the ICN to the MCN and the medial pectoral to the MCN, as well as the upper cervical spinal nerves to other plexus spinal nerves or trunks. See, for example, Tuttle’s early nerve paper.15 This technique has been broadened to encompass a variety of transfers, including median nerve fascicles to the biceps or brachialis nerve. The recent creative use of a variety of nerve transfers to repair BP injuries has been a major change in the discipline.
Nerve Transfer Schemes
Use of the phrenic nerve for transfer remains controversial. The phrenic nerve has approximately 800 myelinated fibers. We often transfer the phrenic nerve to the axillary nerve with an interpositional nerve graft. Although this repair will reliably provide some bulk to the deltoid, it results in only grade 3/5 power, but this is sufficient to prevent subluxation of the shoulder. Gu and associates have promoted transfer of the phrenic nerve to the MCN to provide useful elbow flexion.120
Spinal Accessory Transfer
The SAN has approximately 1500 myelinated axons and is often used as a transfer to innervate the SSN or, less commonly, the MCN. The intention is to perform a direct end-to-end transfer of the SAN to the SSN. It has also been used for neurotization of the axillary, radial, and facial nerves.121 We have used the SAN to innervate a free gracilis muscle transfer.
The SAN exits deep to the lateral border of the sternocleidomastoid muscle and crosses the posterior triangle of the neck. The nerve exits under the sternocleidomastoid at approximately the junction of the upper and lower two thirds of the muscle. As it crosses the posterior triangle, it can form a plexus with branches from the C3 and C4 nerves. The SAN passes deep to the upper border of the trapezius muscle approximately two fingerbreadths above the lateral portion of the clavicle. The standard supraclavicular incision is carried laterally to cross the trapezius at this point and allow exposure of the SAN for transfer to the SSN. An unfortunate iatrogenic complication of using the SAN for transfer is denervation of the trapezius muscle with its own attendant issues. A major concern has been where to divide the SAN to prevent denervation of the upper trapezius muscle. The nerve should be traced well under the trapezius for at least 2 cm and then divided. A dorsal approach to the SAN can be taken to expose the nerve well distal to innervation of the upper trapezius.122 The infraclavicularly displaced SSN stump is neurolysed back to the original supraclavicular region as far up as possible. Frequently, internal neurolysis of the upper trunk provides additional length for the SSN. The nerve is divided as far proximal as possible and passed through the fascia colli medial to the lateral neck region. Both stumps are than coapted with 10-0 sutures or fibrin glue, or with both.
Intercostal Transfer
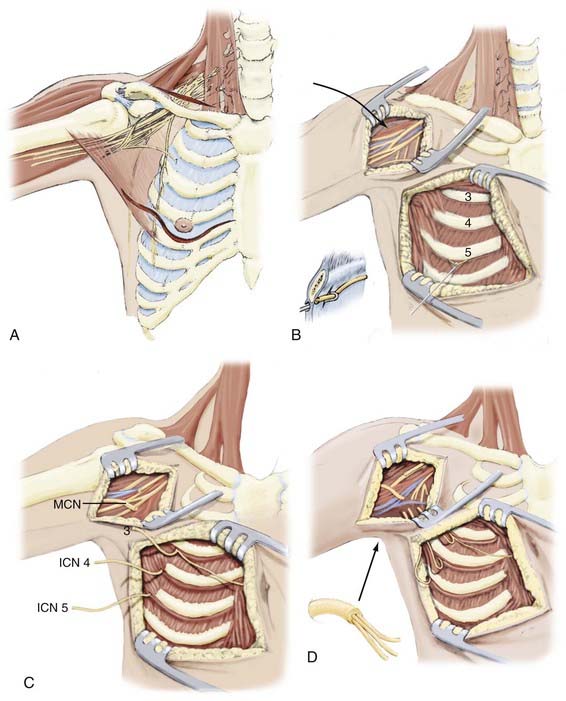
Use of the ICN as a donor nerve remains a popular choice for many surgeons.1 An ICN has approximately 1200 axons. Two to four nerves are used, often T3, T4, and T5 because these nerves can be mobilized for end-to-end transfer to the MCN. We have not been impressed with our own success at providing functional elbow flexion, but the literature contains many reports of reasonable success with this transfer.123 The sensory component of an ICN can also be used for transfer to the lateral cutaneous nerve or median nerve in an attempt to provide some protective sensation to the extremity.
The third through fifth ICNs are dissected free with an incision starting at the inferior border of the major pectoral muscle, continuing beneath the nipple (or breast in females), and extending medially toward the costosternal junction (Fig. 241-1). The inferior part of the major pectoral muscle is shifted upward, which sometimes necessitates partial cutting of its sternal insertion. The ICN should be dissected free over its entire anterior course from the midaxillary line to the parasternal level. Motor responses are assessed by using electrical nerve stimulation. If feasible, sensory branches are identified by their course toward the skin and left intact or used for transfer to gain sensory function. The ICNs are then transected as close as possible to the sternum to obtain sufficient length for direct coaptation to the MCN and are subsequently tunneled to the axilla.
Contralateral C7
Gu first proposed using the entire contralateral C7 nerve for transfer in 1986, a rather radical approach to BP injury.124 Either the complete C7 spinal nerve or the posterior portion is coapted to a graft that is brought across the neck to innervate some portion of the injured BP. There are several variations of this technique, including the use of a vascularized or nonvascularized graft and the particular course to take across the neck.125 Various target nerves have been used, including the upper trunk.1 A major concern in performing this procedure has been the possibility of iatrogenic injury to the normal upper extremity; however, such injury has seldom been the case.125 In general, we have found this technique to be useful in children. Adults have lacked the neural plasticity to independently control the two upper extremities.
Triceps Transfer
Functional restoration of shoulder abduction can be achieved by transferring a nerve branch of the radial nerve innervation of the triceps muscle to the axillary nerve.126 Targeting can be directly to the anterior division of the axillary nerve or more proximal on the main axillary nerve. The donor nerve can come from the long head, lateral head, or medial head of the triceps muscle. Taking a single triceps branch does not sacrifice elbow extension. We have found that the nerve to the medial head of the triceps can provide increased length for the transfer with a better likelihood of direct end-to-end coaptation to the axillary nerve.
Elbow Flexion
Transfer of a single fascicle of the ulnar nerve to the biceps innervation was described by Oberlin and colleagues.119 Internal neurolysis of the ulnar nerve is performed and the dominant fascicle to the flexor carpi ulnaris is identified by electrical stimulation. This fascicle is divided and the proximal portion rotated toward the biceps muscle for a direct end-to-end coaptation to the biceps branch. An alternative is to use a fascicle of the median nerve as a donor nerve.
Restoration of elbow flexion has often focused on the biceps muscle, but it should be noted that this muscle is a forearm supinator, as well as an elbow flexor. The brachialis muscle is the primary muscle of elbow flexion. To provide the best possible outcome, we try to target both muscles. In the first scheme, the medial pectoral nerve is transferred to the MCN, and an ulnar nerve fascicle is transferred to the biceps branch innervation. The lateral cutaneous nerve is divided, rotated proximally, and buried in the biceps or brachialis muscle. In the second scheme, an ulnar fascicle is transferred to the biceps nerve and a median nerve fascicle is transferred to the brachialis muscle. We have found that both schemes provide functional results. Mackinnon and coauthors reported M4 or better strength of elbow flexion in six patients in their series of dual transfers for elbow flexion.127
In a similar fashion of providing two muscles for elbow flexion, dual transfer is also possible for shoulder abduction. Such transfers to restore shoulder function (to the SSN and axillary nerve) have yielded significantly improved results when compared with single-nerve transfer. One can achieve 90 degrees or more of shoulder abduction.128 Further along these lines, including direct repairs by grafts, element to element, whenever possible, along with nerve transfers, appears to give results superior to those of nerve transfers alone.129
Novel nerve transfer techniques are being used in an attempt to provide functional forearm muscle.130 We have had some success with transfer of the brachialis nerve to the median nerve. Targeting innervation of the intrinsic muscles of the hand after BP injury has not met with success.
As a rule of thumb, it is assumed that for a functional result, muscle needs to be reinnervated by 2 years after injury. In the delayed manifestation, rather than attempting primary nerve repair, secondary surgery with tendon transfers can be performed. An alternative is the use of a free muscle transfer. A gracilis muscle can be harvested from the leg and placed in the arm via microanastomosis of vessels. An ulnar nerve fascicle or the SAN can serve as a donor to the free muscle. This technique is useful for restoring elbow flexion. It has also been used for restoring forearm function, but with much less impressive results.131
Clinical Outcome after Surgical Treatment of Brachial Plexus Injury
Extensive data on the outcomes of more than 2500 repairs for BP injury have been reported recently by Kim and colleagues in the updated edition of Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors.68 The authors have divided outcome reporting into separate sections involving lacerations of the plexus,132,133 gunshots to the BP,134,135 and stretch injuries.136,137
For lacerations caused by knives, glass, automobile metal, fan blades, chain saws, and animal bites, the authors reported 71 patients with 201 BP elements damaged.132 These injuries included 83 that were sharply transected and 61 that the authors described as “bluntly transected.” In an additional 57 plexus elements covered, some continuity was maintained despite a lacerating injury (i.e., partial transection).
Outcome data from stretch injuries136,137 are more complex. The reporting was based on 2500 BP injuries in patients seen between 1968 and 1998 at Louisiana State University, about 1000 of whom underwent surgery. Of these, the authors reported about 509 classified as stretch injuries. Follow-up data are limited, in part as a result of the difficulty of arranging for long-term follow-up visits with patients who were referred from a wide variety of locations around the United States and from many other countries. Even more local patients were often lost to follow-up for various socioeconomic reasons. The authors segmented their reported outcomes according to anatomic location, extent, and initial severity. Management varied across time such that outcomes from more recent work that included more extensive use of nerve transfer might be better than outcomes from procedures carried out as long as 40 years before the date of the publication.
The outcome data for gunshot wounds to the BP were based on 118 patients with injuries to 293 plexus elements.134 Of the 118 patients, 51 did not require surgery. For 202 elements with complete loss of function, successful outcomes (LSUHC grade 3 or better) occurred in 91% of the 46 elements that could be treated by neuroplasty only, in 67% of the 21 elements with direct suture anastomosis, and in 54% of the 135 elements requiring a graft. In 91 elements with incomplete loss of function, functional levels of LSUHC grade 3 or above were reported for 94% of the 82 elements that could be treated by neuroplasty alone, for 83% of the 6 elements requiring partial direct suture repair, and for 54% of the 138 elements requiring some grafting.
Outcomes after Treatment of Brachial Plexus Birth Injury
Numerous reports have been published concerning the results of nerve reconstructive surgery for BRBPI.80,87,93,95,101,138–142 It is well documented that improvement in outcome can be achieved after direct repair. There is no consensus about how the results of nerve reconstructive surgery for patients with BRBPI should be evaluated and documented. Ideally, outcome should be described in terms of quality of life or with a functional scale rather than simply the power of individual muscles or a joint. Patients with a BRBPI have significantly lower global and upper extremity function than do their healthy, age-matched peers as measured by the Pediatric Outcomes Data Collection Instrument (PODCI). The PODCI is a patient- or parent-derived outcome instrument.143 Although not ideal, physician-derived measures can be used to predict ultimate functional outcomes. The main focus of nerve reconstructive surgery for BRBPI lesions is restoration of shoulder function and elbow flexion and, with severe lesions, recovery of hand function.58,142
Physician-derived measures that have been widely used thus far consist of the following. The Mallet classification has been used to characterize shoulder function in infants and children.144 The limitation of the Mallet classification is that it does not differentiate between trick movements or true glenohumeral external rotation.142 Elbow flexion based on biceps function can be documented with the MRC 0 to 5 grading system or the LSUHSC grading system.68 For evaluation of hand function, the Raimondi scale is used.145
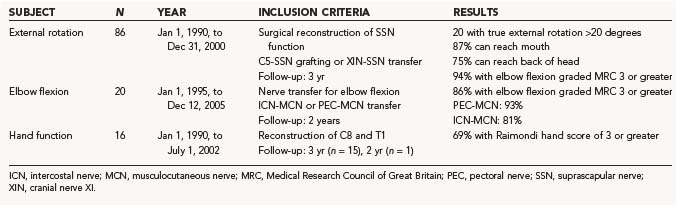
Shoulder Function
The results of nerve repairs to improve shoulder function, published in a number of series, suggest that fairly good recovery of global shoulder function is attainable.87,93,95,139,146 We performed a study (N = 86) that focused on recovery of true glenohumeral external rotation as a solitary movement to determine specific factors affecting recovery after neurotization of the SSN.142 During the neurological evaluation of these children, trick movements were eliminated for a clean comparison of two surgical techniques and prognostic factors. We found that only 20% of the patients gained greater than a 20-degree range of true external rotation and that restoration of true glenohumeral external rotation failed in as many as 41% of all patients. We found no difference between C5-SSN grafting and transfer of the accessory nerve (XI) to the SSN. In contrast to this disappointing result of true external rotation, functional evaluation showed that 87% of patients could reach their mouth and 75% of children could reach the back of their head. This illustrates the great ability of infants to compensate for their limited true external rotation by thoracoscapular movements. Recently, a novel approach was introduced to regain external rotation in delayed fashion. SAN transfers to the SSN were performed at a mean age of 22 months in children who had otherwise spontaneously recovered their upper trunk function with the exception of external rotation. Patient outcomes were excellent.147 The failure of voluntary active external rotation to develop is thought to be the consequence of secondary, disorderly development of central nervous system connections and motor programs rather than the lack of potentially functional peripheral tools such as the SSN and spinatus muscles. There are certainly pitfalls in the conclusions of the paper. First, some of these patents with partial recovery may eventually have had good recovery of incomplete lesions. Second, in patients who did not recover useful external rotation spontaneously, there are good alternatives, such as derotational humeral osteotomy or latissimus dorsi transfer, that can yield reproducible and controllable results. Third, there was some risk associated with sectioning the SSN, including loss of the abduction provided almost entirely by the supraspinatus in some patients. This would seem risky, particularly in some of the older patients in this group.148
Elbow Flexion
In our published series, biceps muscle force against gravity or greater (MRC 4/5) was gained in 92% of patients after nerve grafting.142 Recently, we analyzed 30 consecutive patients (1995 to 2005) in whom nerve transfers for biceps reanimation had been performed. From 1995 to 2000, only ICN-MCN transfers were performed; from 2001 to 2005, a pectoral-MCN transfer was preferentially applied (the C8/T1 trajectory to the inferior trunk was intact). In 15 of 16 ICN-MCN transfers, 3 ICNs were coapted directly to the MCN; in 1 patient, a 1-cm graft proved necessary. In all patients with pectoral-MCN transfers we were able to perform a direct coaptation. Elbow flexion of MRC 3 or greater was achieved in 87% of patients after a mean follow-up of 40 months. The results in the pectoral-MCN group were somewhat better than in the ICN-MCN group (93% versus 81% response rate), which may be explained by the more severe BP lesions that were included in the ICN-MCN group (8 of 16 patients in the ICN-MCN group had a flail arm). Our results correspond well to other reports in literature. Kawabata and coauthors reported the results of 31 ICN-MCN transfers in BRBPI patients: 94% reached MRC 3 or greater.141 For pectoral-MCN transfer, a success rate of 88% MRC 3 or greater was reported by Blaauw and Slooff.138 After satisfactory results of the Oberlin technique in adults were published, transfer of a single fascicle of the ulnar nerve to the biceps motor branch has been proposed as an alternative transfer.149,150
Recovery of Hand Function
The objective of nerve repair in an infant with a flail arm is significantly different from that in adult BP surgery. The main objective for an infant is to establish the ability to use the affected hand to assist in bimanual activity. In addition to good elbow flexion, strong finger flexion is mandatory for a supportive role in the bimanual execution of daily life tasks. Without reanimation of the hand, the maximal function that can be obtained is use of the affected limb as a “hook.” Reanimation of hand function in adults with a total BP lesion has been attempted but has not resulted in useful function.151 Because of the enhanced nerve regeneration and neural plasticity in infants as compared with adult patients, restoration of hand function in infants with BRBPI is feasible (Fig. 241-2). We analyzed 16 patients with a flail arm in whom discontinuity of outflow of spinal nerves C7, C8, and T1 was present as a result of avulsion injury or Sunderland V injury. Neurotization of the C8/T1/inferior trunk or the median nerve was performed.57 Useful reanimation of the hand was achieved in 69% of the patients (Raimondi score ≥3) (Table 241-1). Gilbert reported that 76% achieved good recovery of hand function, but secondary surgery (i.e., tendon transfers) had also been performed on a number of patients in this series.140 In Haerle and Birch’s series of 47 patients with repair of C8/T1, 57% regained Raimondi 4 or greater and 93% regained Raimondi 3 or greater.93,152 Anand and Birch noted that sensory recovery is better than motor recovery.153 Other authors report abnormal Semmes-Weinstein filament findings in the ulnar dermatome in about three quarters of patients with total injury; a third of the hands do not have protective sensation, and stereognosis appeared to be abnormal in 40% of the patients.154
Contralateral C7 Transfer and End-to-Side Transfers
Transfer of the contralateral C7 root to different nerves has been performed in adults and children with BRBPI. The absence of complete disconnection between donor and acceptor limits functional use of the arm in daily life. The greater potential for central plastic changes in young patients than in adults makes this an enticing transfer technique. Gu’s group reported on 12 pediatric C7 transfers and noted that improvements in strength and sensation were accompanied by little synchronous motion and changes in sensibility in the donor limb in 7 children. In these 7 patients, the repaired nerves were those innervating the shoulder or elbow, or both, or both the MCN and median nerve.155 Further publication of different authors’ results is required for this procedure to be endorsed.
End-to-side nerve repair has been used and evaluated for severe BRBPI.156 This technique seems to be safe in that the functions supplied by the donor nerve do not decline. Some patients showed return of function, but the exact source of reinnervation could not be unequivocally contributed to the end-to-side repair. In the majority of patients, only limited or no function returned. There have been recent reports of consistently useful recovery if small nerves with one main function are used as donors and recipients. The results are not as good as with end-to-end coaptation, and therefore end-to-side coaptation should be applied only in cases in which end-to-end coaptation is impossible.157 Much like the contralateral C7 transfer, there is no substantial literature to support use of the end-to-side nerve repair technique for the reconstruction of BRBPIs.
Belzberg AJ, Dorsi MJ, Storm PB, et al. Surgical repair of brachial plexus injury: a multinational survey of experienced peripheral nerve surgeons. J Neurosurg. 2004;101:365-376.
Birch R, Ahad N, Kono H, et al. Repair of obstetric brachial plexus palsy: Results in 100 children. J Bone Joint Surg Br. 2005;87:1089-1095.
Carlstedt T. Nerve root replantation. Neurosurg Clin N Am. 2009;20:39-50. vi
Clarke HM, Al-Qattan MM, Curtis CG, et al. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97:974-982.
Curtis C, Stephens D, Clarke HM, et al. The active movement scale: an evaluative tool for infants with obstetrical brachial plexus palsy. J Hand Surg Am. 2002;27:470-478.
Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299-309.
Gilbert A, Khouri N, Carlioz H. Birth palsy of the brachial plexus—surgical exploration and attempted repair in twenty one cases [author’s transl]. Rev Chir Orthop Reparatrice Appar Mot. 1980;66:33-42.
Gilbert A, Tassin JL. Surgical repair of the brachial plexus in obstetric paralysis. Chirurgie. 1984;110:70-75.
Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7:36-43.
Kim DH, Midha R, Murovic JA, et al. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed. Philadelphia: Elsevier; 2008.
Kline D. Surgical repair of brachial plexus injury [editorial]. J Neurosurg. 2004;101:361-364.
Kline DG, Hackett ER, May PR. Evaluation of nerve injuries by evoked potentials and electromyography. J Neurosurg. 1969;31:128-136.
Mackinnon SE, Novak CB, Myckatyn TM, et al. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978-985.
Midha R. Stretch injuries to brachial plexus. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. Philadelphia: Elsevier; 2008:325-362.
Millesi H. On the problem of overbridging defects of the peripheral nerves. Wien Med Wochenschr. 1968;118:182-187.
Narakas AO. The treatment of brachial plexus injuries. Int Orthop. 1985;9:29-36.
Oberlin C, Beal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
Pondaag W, Malessy MJ, van Dijk JG, et al. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138-144.
Shenaq SM, Berzin E, Lee R, et al. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25:527-536.
Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. New York: Churchill Livingstone; 1991.
Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516.
Terzis JK, Papakonstantinou KC. Management of obstetric brachial plexus palsy. Hand Clin. 1999;15:717-736.
Walker AT, Chaloupka JC, de Lotbiniere AC, et al. Detection of nerve rootlet avulsion on CT myelography in patients with birth palsy and brachial plexus injury after trauma. AJR Am J Roentgenol. 1996;167:1283-1287.
Xu L, Gu Y, Xu J, et al. Contralateral C7 transfer via the prespinal and retropharyngeal route to repair brachial plexus root avulsion: a preliminary report. Neurosurgery. 2008;63:553-558.
Yeoman PM, Seddon HJ. Brachial plexus injuries: treatment of the flail arm. J Bone Joint Surg Br. 1961;43:493-500.
1 Belzberg AJ, Dorsi MJ, Storm PB, et al. Surgical repair of brachial plexus injury: a multinational survey of experienced peripheral nerve surgeons. J Neurosurg. 2004;101:365-376.
2 Kline D. Surgical repair of brachial plexus injury [editorial]. J Neurosurg. 2004;101:361-364.
3 Houel M. Resection et suture du nerf median par M. nelaton. Gaz Hop (Paris). 1864;77-79:307-310.
4 Laugier P. Note sur la suture du nerf median. Gaz Hop (Paris). 1864;75:297-299.
5 Phillipeaux JM, Vulpian A. Note sur les essais de greffe d’un troncon de nerf lingual entre les deux bouts de l’hypoglosse. Arch Physiol Norm Pathol. 1870;3:618-620.
6 Albert E. Einige operationen an nerven. Wien Med Presse. 1885;26:1285-1289.
7 Flaubert AC. Memoires sur plusieurs cas de luxation dans lesquels les efforts pour la reduction ont ete suivis d’accidents graves. Rev Gen Anat Physiol Pathol. 1827;3:55-79.
8 Mitchell SW. Injuries of Nerves and Their Consequences. Philadelphia: JB Lippincott; 1872.
9 Secretan H. Contribution a l’Etude des Paralysies Radiculaires du Plexus Brachial. Paris. 1885.
10 Thorburn W. Secondary suture of brachial plexus. Br Med J. 1900;1:1073-1075.
11 Harris W, Low VW. On the importance of accurate muscular analysis in lesions of the brachial plexus and the treatment of Erb’s palsy and infantile paralysis of the upper extremity by cross-union of nerve roots. Br Med J. 1903;2:1035-1038.
12 Fairbank HAT. Birth palsy: subluxation of the shoulder joint in infants and young children. Lancet. 1913;1:1217-1223.
13 Lange W. Schultergelenksdistorsion und entbindungslahmung. Verh Dtsch Ges Orthop. 1912;11:348-354.
14 Taylor A. Brachial birth palsy and injuries of similar type in adults. Surg Gynecol Obstet. 1920;30:494-502.
15 Tuttle H. Exposure of the brachial plexus with nerve transplantation. JAMA. 1912;61:15-17.
16 Clark LP, Taylor AS, Prout TP. Study on brachial birth palsy. Am J Med Sci. 1905;130:670-707.
17 Bonola A. La paralisi del plesso brachiale da traumi di motociclette. Chir Organi Mov. 1936;22:309-313.
18 Seddon H. Three types of nerve injury. Brain. 1943;66:237-288.
19 Seddon H. The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br J Surg. 1947;35:151-167.
20 Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491-516.
21 Yeoman PM, Seddon HJ. Brachial plexus injuries: treatment of the flail arm. J Bone Joint Surg Br. 1961;43:493-500.
22 Fantis A, Slezak K. Kotazce chirurgicke cecby poraneni brachialneho plexu. Acta Chir Orthop Trauma (Cech). 1967;34:301-309.
23 Leffert RD, Seddon H. Infraclavicular brachial plexus injuries. J Bone Joint Surg Br. 1965;47:9-22.
24 Lurje A. Concerning surgical treatment of traumatic injury of the upper division of the brachial plexus (Erb’s-type). Ann Surg. 1948;127:317-326.
25 Merle d’Aubigne R, Deburge A. Etiologie, evolution et pronostic des paralysies traumatiques du plexus brachial.
26 Scaglietti O. Einzelheiten ueber Operationstechnik bei Verletzungen der Wurzeln des Plexus brachialis durch Schusswaffen. Zentralbl Neurochir. 1942;7:129-132.
27 Barnes R. Traction injuries of the brachial plexus in adults. J Bone Joint Surg Am. 1949;31B(1):10-16.
28 Bonney G. Prognosis in traction lesions of the brachial plexus. J Bone Joint Surg Br. 1959;41-B(1):4-35.
29 Brooks DM. Open wounds of the brachial plexus. J Bone Joint Surg Am. 1949;31:17-33.
30 Krenkel W. Die technik der Nervenoperationen unter besonderer Beruecksichtigung der Verletzungen des Plexus brachialis. Hefte Unfallheilk. 1965;81:267-271.
31 Lange M. Die Behandlung der irreparablen peripheren Nervenletzungen. Wiederherstellungschir Traum. 1953;1:240-262.
32 Schuler C. Zur Behandlung der geschlossenen Verletzungen des Plexus brachialis. Schweiz Med Wochenschr. 1958;88:801-806.
33 Seddon HJ. Nerve grafting. J Bone Joint Surg Br. 1963;45:447-461.
34 Narakas AO. Brachial plexus injuries. In: McCathy JG, editor. Plastic Surgery. 7th ed. Philadelphia: WB Saunders; 1990:4776-4816.
35 Smith JW. Microsurgery of peripheral nerves. Plast Reconstr Surg. 1964;33:317-329.
36 Fletcher I. Traction lesions of the brachial plexus. Hand. 1969;1:129-136.
37 Millesi H. On the problem of overbridging defects of the peripheral nerves. Wien Med Wochenschr. 1968;118:182-187.
38 Millesi H, Ganglberger L, Berger A. Erfahrungen mit der Mikrochirurgie peripherer Nerven. Chir Plast Reconstr. 1967;3:47-55.
39 Narakas AO. Plexo braquial. Terapeutica quirurgica directa, technica, indicacion operatoria, resultados. Cirugia de los nervios perifericos. Rev Ortop Traumatol. 1972;16:521-528.
40 Narakas A, Verdan C. Nerve grafts. Z Unfallmed Berufskr. 1969;62:137-152.
41 Hodes R, Larrabee MG, German W. The human electromyogram in response to nerve stimulation and the conduction velocity of motor axons; studies on normal and on injured peripheral nerves. Arch Neurol Psychiatry. 1948;60:340-365.
42 Murphey F, Hartung W, Kirklin JW. Myelographic demonstration of avulsing injury of the brachial plexus. AJR Am J Roentgenol. 1947;58:102-108.
43 Kline DG, Hackett ER, May PR. Evaluation of nerve injuries by evoked potentials and electromyography. J Neurosurg. 1969;31:128-136.
44 Marshall RW, De Silva RD. Computerised axial tomography in traction injuries of the brachial plexus. J Bone Joint Surg Br. 1986;68:734-738.
45 Ashhurst AP. Birth injuries of the shoulder. Ann Surg. 1918;67:25-50.
46 Harrenstein RJ. De Geboorteverlamming van den Arm. Ned Tijdschr Geneeskd. 1922;66:1435-1447.
47 Sever JW. Obstetric paralysis. Its cause and treatment. Can Med Assoc J. 1920;10:141-161.
48 Kennedy R. Suture of the brachial plexus in birth paralysis of the upper extremity. Br Med J. 1903;1:298-301.
49 Kennedy R. Suture of the brachial plexus in birth paralysis of the upper extremity. Br Med J. 1904;2:1065-1068.
50 Taylor AS. So-called congenital dislocation of the shoulder posterior subluxation. Ann Surg. 1921;74:368-375.
51 Sharpe W. End results in neuro-surgery: impressions during decade 1913-1923. Ann Surg. 1925;82:684-697.
52 Sherren J. Some points in the surgery of the peripheral nerves. Glasgow Med J. 1910:297-332.
53 Sever JW. Obstetric paralysis. An orthopedic problem. J Bone Joint Surg Am. 1916;s2:456-475.
54 Jepson PN. Obstetrical paralysis. Ann Surg. 1930;91:724-730.
55 Mehlman CT, Koepplinger ME. Hyphenated history: the Sever-L’episcopo procedure. J Pediatr Orthop. 2007;27:533-536.
56 Morison JE. Peripheral brachial paralysis in infants and children. Arch Dis Child. 1938;13:310-332.
57 Gilbert A, Khouri N, Carlioz H. Birth palsy of the brachial plexus—surgical exploration and attempted repair in twenty one cases [author’s transl]. Rev Chir Orthop Reparatrice Appar Mot. 1980;66:33-42.
58 Pondaag W, Malessy MJ. Recovery of hand function following nerve grafting and transfer in obstetric brachial plexus lesions. J Neurosurg. 2006;105(suppl 1):33-40.
59 Venieratos D, Anagnostopoulou S. Classification of communications between the musculocutaneous and median nerves. Clin Anat. 1998;11:327-331.
60 Seddon HJ. Methods of investigating nerve injuries. Spec Rep Ser Med Res Counc (G B). 1954;282:1-15.
61 Seddon HJ. The treatment of the early stages of poliomyelitis. Trans Med Soc Lond. 1954;70:22-41.
62 Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299-309.
63 Monsivais JJ, Narakas AO, Turkof E, et al. The endoscopic diagnosis and possible treatment of nerve root avulsions in the management of brachial plexus injuries. J Hand Surg Br. 1994;19:547-549.
64 Carvalho GA, Nikkhah G, Matthies C, et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86:69-76.
65 Narakas AO. The Treatment of brachial plexus injuries. Int Orthop. 1985;9:29-36.
66 Friedman AH, Nashold BSJr, Bronec PR. Dorsal root entry zone lesions for the treatment of brachial plexus avulsion injuries: a follow-up study. Neurosurgery. 1988;22:369-373.
67 Kline DG, Hudson AR. Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. Philadelphia: WB Saunders; 1995.
68 Kim DH, Midha R, Murovic JA, et al. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed. Philadelphia: Elsevier; 2008.
69 Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236-250.
70 Jivan S, Novikova LN, Wiberg M, et al. The effects of delayed nerve repair on neuronal survival and axonal regeneration after seventh cervical spinal nerve axotomy in adult rats. Exp Brain Res. 2006;170:245-254.
71 Jivan S, Kumar N, Wiberg M, et al. The influence of pre-surgical delay on functional outcome after reconstruction of brachial plexus injuries. J Plast Reconstr Aesthet Surg. 2009;62:472-479.
72 Carlsen BT, Bishop AT, Shin AY. Late reconstruction for brachial plexus injury. Neurosurg Clin N Am. 2009;20:51-64. vi
73 Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. New York: Churchill Livingstone; 1991.
74 Adler JB, Patterson RLJr. Long-term results of treatment in eighty-eight cases. Erb’s palsy. J Bone Joint Surg Am. 1967;49;:1052-1064.
75 Gordon M, Rich H, Deutschberger J, et al. The immediate and long-term outcome of obstetric birth trauma. I. Brachial plexus paralysis. Am J Obstet Gynecol. 1973;117:51-56.
76 Pearl ML, Edgerton BW. Glenoid deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:659-667.
77 Pollock AN, Reed MH. Shoulder deformities from obstetrical brachial plexus paralysis. Skeletal Radiol. 1989;18:295-297.
78 Bradley WG, Daroff RB, Feniche GM, et al. Neurology in Clinical Practice. Boston: Butterworth-Heinemann; 1996.
79 Greenberg MS. Handbook of Neurosurgery. New York: Thieme; 2001.
80 Laurent JP, Lee RT. Birth-related upper brachial plexus injuries in infants: operative and nonoperative approaches. J Child Neurol. 1994;9:111-117.
81 Painter MJ, Bergman I. Obstetrical trauma to the neonatal central and peripheral nervous system. Semin Perinatol. 1982;6:89-104.
82 Shenaq SM, Berzin E, Lee R, et al. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25:527-536.
83 Terzis JK, Papakonstantinou KC. Management of obstetric brachial plexus palsy. Hand Clin. 1999;15:717-736.
84 Walle T, Hartikainen-Sorri AL. Obstetric shoulder injury: associated risk factors, prediction and prognosis. Acta Obstet Gynecol Scand. 1993;72:450-454.
85 Pondaag W, Malessy MJ, van Dijk JG, et al. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138-144.
86 Gilbert A, Tassin JL. Surgical repair of the brachial plexus in obstetric paralysis. Chirurgie. 1984;110:70-75.
87 Waters PM. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81:649-659.
88 Xu J, Cheng X, Gu Y. Different methods and results in the treatment of obstetrical brachial plexus palsy. J Reconstr Microsurg. 2000;16:417-420.
89 Kline DG. Different methods and results in the treatment of obstetrical brachial plexus palsy [letter]. J Reconstr Microsurg. 2000;16:420-422.
90 Bodensteiner JB, Rich KM, Landau WM. Early infantile surgery for birth-related brachial plexus injuries: justification requires a prospective controlled study. J Child Neurol. 1994;9:109-110.
91 Kay SP. Obstetrical brachial palsy. Br J Plast Surg. 1998;51:43-50.
92 Tassin JL. Paralysies obstetricales du plexus brachial. evolution spontanée; resultats des interventions reparatrices prococes (thèse) [dissertation]. 1983.
93 Birch R, Ahad N, Kono H, et al. Repair of obstetric brachial plexus palsy: results in 100 children. J Bone Joint Surg Br. 2005;87:1089-1095.
94 Clarke HM, Al-Qattan MM, Curtis CG, et al. Obstetrical brachial plexus palsy: results following neurolysis of conducting neuromas-in-continuity. Plast Reconstr Surg. 1996;97:974-982.
95 Kawabata H, Masada K, Tsuyuguchi Y, et al. Early microsurgical reconstruction in birth palsy. Clin Orthop Relat Res. 1987;215:233-242.
96 Waters PM. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop B. 2005;14:233-244.
97 Fisher DM, Borschel GH, Curtis CG, et al. Evaluation of elbow flexion as a predictor of outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2007;120:1585-1590.
98 Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675-680.
99 Smith NC, Rowan P, Benson LJ, et al. Neonatal brachial plexus palsy: outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86:2163-2170.
100 Borrero JL, Pawlikowski W. Obstetrical brachial plexus palsy [dissertation]. Lima. 2005.
101 Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563-580.
102 Bisinella GL, Birch R, Smith SJ. Neurophysiological prediction of outcome in obstetric lesions of the brachial plexus. J Hand Surg Br. 2003;28:148-152.
103 van Dijk JG, Malessy MJ, Stegeman DF. Why is the electromyogram in obstetric brachial plexus lesions overly optimistic? Muscle Nerve. 1998;21:260-261.
104 van Dijk JG, Pondaag W, Malessy MJ. Obstetric lesions of the brachial plexus. Muscle Nerve. 2001;24:1451-1461.
105 Vredeveld JW, Blaauw G, Slooff BA, et al. The findings in paediatric obstetric brachial palsy differ from those in older patients: a suggested explanation. Dev Med Child Neurol. 2000;42:158-161.
106 Zalis OS, Zalis AW, Barron KD, et al. Motor patterning following transitory sensory-motor deprivations. Arch Neurol. 1965;13:487-494.
107 Grossman JA. Early operative intervention for birth injuries to the brachial plexus. Semin Pediatr Neurol. 2000;7:36-43.
108 Malessy MJ, Pondaag W, Hofstede-Buitebhuis SM, et al. Severe obstetric brachial plexus injuries can be identified easily and reliably at one month of age. Proceedings of the Annual Scientific Meeting of the American Society for Peripheral Nerve, 2007.
109 Fisher DM, Borschel GH, Curtis CG, et al. Evaluation of elbow flexion as a predictor of outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2007;120:1585-1590.
110 Curtis C, Stephens D, Clarke HM, et al. The active movement scale: an evaluative tool for infants with obstetrical brachial plexus palsy. J Hand Surg Am. 2002;27:470-478.
111 Chow BC, Blaser S, Clarke HM. Predictive value of computed tomographic myelography in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2000;106:971-977.
112 Walker AT, Chaloupka JC, de Lotbiniere AC, et al. Detection of nerve rootlet avulsion on CT myelography in patients with birth palsy and brachial plexus injury after trauma. AJR Am J Roentgenol. 1996;167:1283-1287.
113 Thomeer RT, Malessy MJ. Surgical repair of brachial plexus injury. Clin Neurol Neurosurg. 1993;95(suppl):S65-S72.
114 Tender GC, Kline DG. Anterior supraclavicular approach to the brachial plexus. Neurosurgery. 58(suppl 2), 2006.
115 Tender GC, Kline DG. The infraclavicular approach to the brachial plexus. Neurosurgery. 2008;62(suppl 1):180-184.
116 MacCarty CS. Surgical exposure of the brachial plexus. Surg Neurol. 1984;21:593-596.
117 Dubuisson AS, Kline DG, Weinshel SS. Posterior subscapular approach to the brachial plexus: report of 102 patients. J Neurosurg. 1993;79:319-330.
118 Carlstedt T. Nerve root replantation. Neurosurg Clin N Am. 2009;20:39-50. vi
119 Oberlin C, Beal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg Am. 1994;19:232-237.
120 Gu YD, Wu MM, Zhen YL, et al. Phrenic nerve transfer for treatment of root avulsion of the brachial plexus. Chin Med J (Engl). 1990;103:267-270.
121 Bonnard C, Narakas A. Neurotization using the spinal accessory nerve in the brachial plexus lesions. In: Alnot JY, Narakas A, editors. Traumatic Brachial Plexus Injuries. Paris: Expansion Scientifique Française; 1996:156-166.
122 Guan SB, Chen DS, Fang YS, et al. An anatomic study of the descending branch of the spinal accessory nerve transfer for the repair of suprascapular nerve to restore the abduction function of the shoulder through the dorsal-approach. Chin J Hand Surg (Chin). 2004;20:55-57.
123 Chuang DC, Yeh MC, Wei FC. Intercostal nerve transfer of the musculocutaneous nerve in avulsed brachial plexus injuries: evaluation of 66 patients. J Hand Surg Am. 1992;17:822-828.
124 Gu YD. Cervical nerve root transfer from the healthy side in the treatment of brachial plexus root avulsion. Zhonghua Yi Xue Za Zhi. 1989;69:563-565. 38
125 Xu L, Gu Y, Xu J, et al. Contralateral C7 transfer via the prespinal and retropharyngeal route to repair brachial plexus root avulsion: a preliminary report. Neurosurgery. 2008;63:553-558.
126 Bertelli JA, Santos MA, Kechele PR, et al. Triceps motor nerve branches as a donor or receiver in nerve transfers. Neurosurgery. 2007;61(5 suppl 2):333-338.
127 Mackinnon SE, Novak CB, Myckatyn TM, et al. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978-985.
128 Merrell GA, Barrie KA, Katz DL, et al. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the english literature. J Hand Surg Am. 2001;26:303-314.
129 Kline D, Tiel R. Direct plexus repair by grafts supplemented by nerve transfers. Hand Clin. 2005;21:55-69.
130 Zheng XY, Hou CL, Gu YD, et al. Repair of brachial plexus lower trunk injury by transferring brachialis muscle branch of musculocutaneous nerve: anatomic feasibility and clinical trials. Chin Med J (Engl). 2008;20(121):99-104.
131 Doi K, Hattori Y, Yamazaki H, et al. Importance of early passive mobilization following double free gracilis muscle transfer. Plast Reconstr Surg. 2008;121:2037-2045.
132 Kim DH, Murovic JA. Lacerations of the brachial plexus. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. Philadelphia: Elsevier; 2008:305-312.
133 Kim DH, Murovic JA, Tiel RL, et al. Lacerations to the brachial plexus: surgical techniques and outcomes. J Reconstr Microsurg. 2005;21:435-440.
134 Kim DH. Gunshot wounds to the brachial plexus. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. Philadelphia: Elsevier; 2008:313-324.
135 Kim DH, Murovic JA, Tiel RL, et al. Gunshot wounds involving the brachial plexus: surgical technique and outcomes. J Reconstr Microsurg. 2006;22:67-72.
136 Kim D, Cho Y, Tiel R, et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
137 Midha R. Stretch injuries to brachial plexus. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. Philadelphia: Elsevier; 2008:325-362.
138 Blaauw G, Slooff AC. Transfer of pectoral nerves to the musculocutaneous nerve in obstetric upper brachial plexus palsy. Neurosurgery. 2003;53:338-341.
139 Gilbert A, Brockman R, Carlioz H. Surgical treatment of brachial plexus birth palsy. Clin Orthop Relat Res. 1991;264:39-47.
140 Haerle M, Gilbert A. Management of complete obstetric brachial plexus lesions. J Pediatr Orthop. 2004;24:194-200.
141 Kawabata H, Shibata T, Matsui Y, et al. Use of intercostal nerves for neurotization of the musculocutaneous nerve in infants with birth-related brachial plexus palsy. J Neurosurg. 2001;94:386-391.
142 Pondaag W, de Boer R, van Wijlen-Hempel MS, et al. External rotation as a result of suprascapular nerve neurotization in obstetric brachial plexus lesions. Neurosurgery. 2005;57:530-537.
143 Bae DS, Waters PM, Zurakowski D. Correlation of pediatric outcomes data collection instrument with measures of active movement in children with brachial plexus birth palsy. J Pediatr Orthop. 2008;28:584-592.
144 Mallet J. Obstetrical paralysis of the brachial plexus. II. Therapeutics, treatment of sequelae, priority for the treatment of the shoulder, method for the expression of results. Rev Chir Orthop Reparatrice Appar Mot. 1972;58(suppl 1):166-168.
145 Raimondi P. Evaluation of results in obstetric brachial plexus palsy, the hand. International Meeting on Obstetric Brachial Plexus Palsy, Heerlen, The Netherlands, 1993.
146 Laurent JP, Lee R, Shenaq S, et al. Neurosurgical correction of upper brachial plexus birth injuries. J Neurosurg. 1993;79:197-203.
147 van Ouwerkerk WJ, Uitdehaag BM, Strijers RL, et al. Accessory nerve to suprascapular nerve transfer to restore shoulder exorotation in otherwise spontaneously recovered obstetric brachial plexus lesions. Neurosurgery. 2006;59:858-867.
148 Malessy MJ, Spinner RJ. Accessory nerve to suprascapular nerve transfer to restore shoulder exorotation in otherwise spontaneously recovered obstetric brachial plexus lesions [comment]. Neurosurgery. 2006;59:868-869.
149 Al-Qattan MM. Oberlin’s ulnar nerve transfer to the biceps nerve in Erb’s birth palsy. Plast Reconstr Surg. 2002;109:405-407.
150 Noaman HH, Shiha AE, Bahm J. Oberlin’s ulnar nerve transfer to the biceps motor nerve in obstetric brachial plexus palsy: indications, and good and bad results. Microsurgery. 2004;24:182-187.
151 Narakas AO, Allieu Y, Alnot JY, et al. Complete supraclavicular paralysis. Surgical possibilities and results. In: Alnot JN, Narakas AO, editors. Paralysis of the Brachial Plexus. Paris: Expansion Scientifique Francaise; 1989:162.
152 Birch R, Bonney G, Wynn Parry CB. Surgical Disorders of the Peripheral Nerves. Edinburgh: Churchill Livingstone; 1998.
153 Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125:113-122.
154 Kirjavainen M, Remes V, Peltonen J, et al. The function of the hand after operations for obstetric injuries to the brachial plexus. J Bone Joint Surg Br. 2008;90:349-355.
155 Chen L, Gu YD, Hu SN, et al. Contralateral C7 transfer for the treatment of brachial plexus root avulsions in children—a report of 12 cases. J Hand Surg Am. 2007;32:96-103.
156 Pondaag W, Gilbert A. Results of end-to-side nerve coaptation in severe obstetric brachial plexus lesions. Neurosurgery. 2008;62:656-663.
157 Millesi H, Schmidhammer R. Nerve fiber transfer by end-to-side coaptation. Hand Clin. 2008;24:461-483. vii