Chapter 100 Early and late complications of liver transplantation
Overview
Liver transplantation has evolved from a risky procedure with high morbidity and mortality to a standard treatment for patients with liver failure. Patients who undergo successful liver replacement have 5-year survival rates that exceed 70% (Muraji et al, 1997; see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ). Despite this dramatic improvement in outcome, a significant percentage of patients experience life-threatening complications that can result in the need for reoperation. As expertise in the procedure grows, surgeons are willing to attempt liver transplantation in patients who previously were considered poor candidates for surgery. Recipient portal vein thrombosis (PVT) was considered an absolute contraindication to surgery, but now transplantation is commonly performed in the presence of PVT (Shaked & Busuttil, 1991; Stieber et al, 1991).
As surgeons continue to develop new surgical procedures, such as transplantation of reduced-size and split-liver grafts and living-donor liver transplantation (LDLT), a new series of complications unique to these procedures is emerging (Broelsch et al, 2000; Emond et al, 1993). The incidence of hepatic artery thrombosis, bile leaks, and stricture is at least two times higher in patients who receive living-donor grafts compared with those who receive cadaveric grafts (Malago et al, 2003). Despite a higher morbidity rate in recipients of living-donor grafts, patient and graft survival are similar or superior to those observed with deceased donors (Fan et al, 2002; Lo et al, 2002; Pomposelli et al, 2006). This chapter reviews common early and late complications encountered during and after liver transplantation. Because complications after liver transplantation represent a continuum, most can occur at any time after surgery.
Procurement Injury to the Graft
To increase the number of organs available, donation after cardiac death (DCD) is a new form of donation that is increasing in incidence (Chin et al, 2002). In this form of donation, a patient who is deemed hopeless but has not met criteria of brain death is allowed to die naturally after removal of supportive measures. After a period of usually 5 minutes of asystole, organs can be procured for transplantation. Despite the various periods of hypotension and warm ischemia that develop, outcomes with donation after cardiac death have been acceptable (Chin et al, 2002; Cooper et al, 2004). In liver grafts procured from DCD donors, increased biliary strictures and worse long-term graft survival have been suggested (D’Alessandro et al, 2004).
The most common injury during liver procurement is aberrant hepatic artery ligation and division. This injury occurs by failure to recognize a replaced or accessory right or left hepatic artery during hilar dissection or by unintentional division during organ removal. Such injuries are serious, because segments of the liver are not perfused during recovery and usually require reconstruction on the back table before reimplantation. An additional anastomosis on the back table prolongs ischemic time, increases the chances of thrombosis, and increases the need for retransplantation. Prolonged ischemic time, especially longer than 12 hours, increases the risk for graft loss and bile duct necrosis (Mor et al, 1993; Quiroga et al, 1991). To minimize dissection-related injuries, some authors prefer to use the en bloc method of removing abdominal organs, with back-table dissection (Imagawa et al, 1996). Regardless of the technique used, attention to detail and identification of the appropriate anatomy help avoid graft procurement injury.
Intraoperative Hemorrhage and Coagulopathy (See Chapter 70B)
Improvements in surgical techniques and better patient selection have led to the performance of liver transplantation without the need for blood transfusion in selected patients. The advent of the transjugular intrahepatic portosystemic shunt (TIPS) can significantly lower portal hypertension preoperatively and may help to reduce blood loss during transplantation (Forster et al, 1994); however, a misplaced TIPS in the vena cava or portal vein can be a life-threatening complication during liver transplantation. In these situations, TIPS removal is difficult and may lead to massive hemorrhage, if vascular control of the native vessels cannot be achieved.
In the presence of severe portal hypertension and underlying coagulopathy, the infusion of fresh frozen plasma and antifibrinolytic agents is the mainstay of therapy during liver transplantation (Palareti et al, 1991). These modalities cannot supplant the need for sound surgical technique with adequate control of all surgical bleeding sites. Bleeding observed after reperfusion may be related to poor initial graft function but also has been related to the release of heparin and heparin-like substances from the graft. Kettner and colleagues (1998) used heparinase-modified thromboelastography (TEG) to identify patients who developed bleeding secondary to the release of heparin-like substances. Such screening methods may help stratify patients at particular risk for the development of reperfusion fibrinolysis and may offer future therapeutic strategies to control coagulopathy encountered immediately after reperfusion (Kettner et al, 1998).
Primary Graft Dysfunction or Nonfunction
Numerous conditions can interfere with the initial function of the allograft after transplantation, including donor-related, procurement-related, and recipient-related factors. Donor-related factors that can affect graft function adversely include hemodynamic instability, poor nutritional status, extremes of age, drug toxicity, and steatosis (D’Alessandro et al, 2004; Marsman et al, 1996; Washburn et al, 1996).
Although no uniform definition exists, the severity and prognosis for graft dysfunction vary considerably. The most ominous syndrome is primary graft nonfunction, which usually requires immediate retransplantation. In such instances, a progressive increase is typically seen in serum transaminase levels (>8000 IU/L) within the first 24 to 48 hours in association with diminished bile and urine output. In our practice, postoperative trends in the prothrombin time (PT) have been the most reliable predictor of graft function and outcome. Laboratory studies drawn immediately after surgery serve as the baseline: the PT should plateau and then trend toward normalization in a matter of days with good graft function; any increase in the PT portends a worse prognosis and may represent primary graft nonfunction, especially if this occurs rapidly. An elevated PT that neither increases nor decreases may suggest primary graft dysfunction; recovery usually occurs with time, if no additional insults occur, such as infection or rejection. In these situations, infusion of prostaglandin E1 may be beneficial in resolving or preventing renal or graft dysfunction (Chavin et al, 1996; Klein et al, 1996).
In addition to trends in the PT, clinical assessment can be helpful in identifying patients with graft dysfunction or nonfunction. Resolution of hepatic encephalopathy, adequate urine production, and absence of metabolic acidosis are reassuring in the early postoperative period. In patients who have received significant quantities of blood products, the development of metabolic alkalosis may be a sensitive indicator of early graft function (Driscoll et al, 1987). Such indicators are based on the ability of the liver graft to process citrate in the administered blood products to bicarbonate; failure to metabolize citrate to bicarbonate may reflect early allograft dysfunction.
Vascular Complications
Hepatic Artery Thrombosis
Vascular complications are a major source of morbidity and graft loss in liver transplant patients. Arterial complications, of which hepatic arterial thrombosis (HAT) is the most common, account for 64% to 82% of the vascular complications encountered (Bell et al, 1990; Leonardi et al, 2004). The overall incidence of HAT is 1.6% to 8% in various adult series, but it can be 15% to 26% in pediatric patients (Busuttil et al, 1991; Mazzaferro et al, 1989; Tan et al, 1988). The incidence of HAT in LDLT varies widely and is influenced by the type of graft, surgeon experience, and donor anatomy.
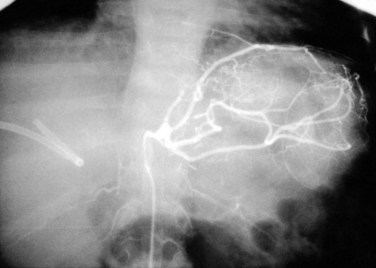
Doppler ultrasound (US) is the best screening method and should be used liberally in the first 2 weeks after transplantation with any change in graft function or significant elevation in bilirubin or transaminases. Because collateral blood flow through the gastroduodenal artery can result in a false-negative result, care must be taken to establish arterial flow within the hepatic parenchyma. In cases of suspected HAT revealed by US, confirmation should be made by celiac arteriography (Fig. 100.1) or multiphase computed tomography (CT). Failure to make a rapid diagnosis can result in hepatic necrosis and graft loss.
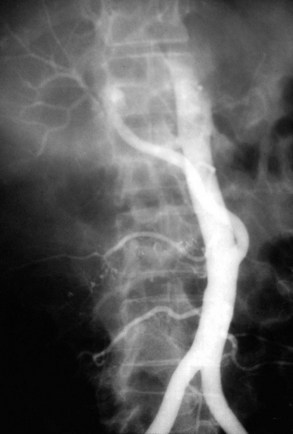
Intimal dissection of the recipient hepatic artery down to the origin of the celiac axis is a common cause of intraoperative HAT. In these cases, immediate reconstruction with a donor iliac allograft is indicated (Fig. 100.2). Under no circumstances should the patient leave the operating room without a completely revascularized graft. When a donor iliac allograft is unusable or unavailable, an autogenous saphenous vein graft should be used. Rarely, an artificial conduit made from Dacron or expanded polytetrafluoroethylene can be used.
The clinical presentation of HAT observed postoperatively ranges from a completely asymptomatic patient with minimal alterations in liver function to a critically ill patient with fulminant hepatic necrosis. A more common presentation of HAT is with postoperative biliary complications, including leaks and stricture formation (Margarit et al, 1998; Orons et al, 1995). Treatment for HAT in the early postoperative period is the same, whether symptoms are present or not: rapid reestablishment of arterial inflow should be attempted, which generally requires urgent operation with arterial reconstruction using a donor iliac artery allograft or autogenous graft material. Some authors have attempted thrombolysis using tissue plasminogen activator and urokinase, but this can be expected to be successful only when no technical factors are contributing to the thrombosis (Hidalgo et al, 1989). Hepatic artery intimal dissection is not amenable to thrombolytic therapy, and attempts at systemic thrombolysis waste valuable time and resources and worsen outcome. In most cases, 50% to 70% of patients ultimately require retransplantation (Langnas et al, 1991).
Portal Vein Thrombosis
Reconstitution of portal flow usually can be obtained through portal vein thrombectomy in most cases or by using a donor iliac vein allograft anastomosed between the superior mesenteric vein and liver allograft portal vein (Davidson et al, 1994; Shaked & Busuttil, 1991). Living-donor grafts that have relatively short portal vein segments can be difficult to reconstruct, and approximately 10% of institutions consider PVT an absolute contraindication to LDLT (Kadry et al, 2002).
PVT observed after transplantation is a rare complication that can occur in the immediate postoperative period, usually for technical reasons, such as incomplete thrombectomy or twisting of the anastomosis. PVT observed several months to years after transplantation usually results from intimal hyperplasia with gradual cavernous transformation with collaterals. A high index of suspicion is needed to make the diagnosis. Accumulation of ascites, splenomegaly, or the presence of varices after transplantation should prompt investigation. Early thrombosis is best treated with reoperation, thrombectomy, and systemic coagulation. The treatment of late thrombosis is more controversial, because direct repair is difficult. Transhepatic angioplasty with the placement of metal stents has been successful in some patients, whereas other patients have responded to selective shunting procedures to control variceal hemorrhage in the setting of adequate liver function (Jenkins et al, 1999; Raby et al, 1991).
Inferior Vena Cava Obstruction
Inferior vena cava (IVC) obstruction is a rare complication that occurs in 1% to 2% of patients after liver transplantation (Wozney et al, 1986). Most surgeons prefer to anastomose the donor IVC to the suprahepatic and infrahepatic IVC. A growing trend is to perform a so-called piggyback transplantation, with the anastomosis of the donor suprahepatic IVC to the confluence of the recipient middle and left hepatic veins, leaving the recipient IVC in situ (Neuhaus & Platz, 1994; Tzakis et al, 1989).
Treatment of an IVC thrombosis usually depends on the cause. Direct surgical removal is difficult in a critically ill patient and requires extensive mobilization of the right colon and small bowel mesentery to facilitate exposure. Medical management of IVC thrombosis was successful in our experience and that reported by others (Kraus et al, 1992). Our experience suggests that a more conservative management algorithm that employs invasive radiologic procedures and systemic anticoagulation can treat this complication satisfactorily (Kraus et al, 1992). Thrombolytic therapy can also be adjunctive in resolving “fresh” thrombus formation.
Biliary Complications
Biliary tract complications related to bile duct reconstruction previously were considered the Achilles heel of liver transplantation, but improvements in operative technique have reduced these complications markedly. Nevertheless, bile duct obstruction and leaks are the cause of approximately half of all technical failures after transplantation and require reoperation in 10% to 20% of patients (Lerut et al, 1987). In general, recipients of living-donor grafts have an incidence of biliary complications approximately two times higher than recipients of cadaveric grafts (Pomfret et al, 2001). HAT is associated with biliary complications and may explain the increased incidence among living-donor graft recipients.
Biliary Leaks
The reported incidence of biliary leaks after liver transplantation varies widely from 10% to 50% (Rabkin et al, 1998; Reichert et al, 1998). Leaks are observed most commonly at the site of choledochal anastomosis or the choledochal T-tube insertion site. Leaks at the choledochal tube insertion site are observed most commonly at the time of tube removal and occur in 25% to 40% of patients (O’Connor et al, 1995). Most of these patients can be managed conservatively with a short course of analgesics and antibiotics. To minimize this risk, some surgeons prefer not to place stents.
The bile ducts of living-donor or split-liver grafts can be reconstructed with a duct-to-duct (choledochocholedochostomy) or Roux-en-Y hepaticojejunostomy. The leak rate is similar with both types of reconstructions (Gondolesi et al, 2004). Signs and symptoms related to biliary leaks include bilious fluid in drains (biliary fistula), abdominal or shoulder pain or both, increased serum bilirubin, nausea, vomiting, and fever. Diagnosis can be confirmed by cholangiography if a choledochal tube is in place; otherwise, endoscopic retrograde cholangiography (ERC) or percutaneous transhepatic cholangiography (PTC) can be performed. We favor ERC, because treatment with endoscopic stent placement can be readily achieved without the risk associated with the indwelling transhepatic catheters used during PTC. Large biliary leaks resulting in bile collections adjacent to the liver require percutaneous drainage.
Biliary Stricture or Obstruction
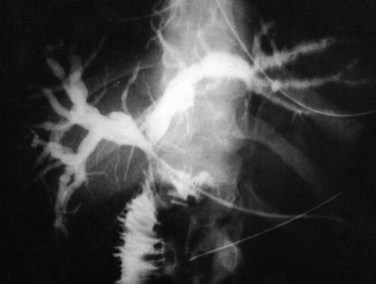
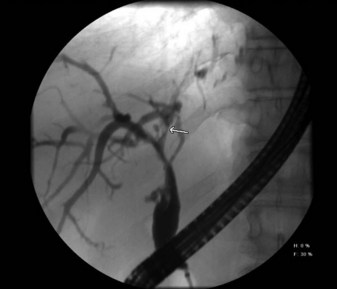
Biliary obstruction occurs in approximately 7% to 15% of patients after liver transplantation (Klein et al, 1991; Lerut et al, 1987). As with leaks, the site of obstruction aids in determining the cause. Anastomotic stricture accounts for 50% of obstruction cases and can occur early in the postoperative period, secondary to edema, or later, as a result of compromised blood supply (Fig. 100.3). Biliary strictures usually present within weeks but may occur years after transplantation. Two types of biliary obstruction usually are found: anastomotic and nonanastomotic. Nonanastomotic obstruction or stricture can be caused by or associated with bile duct ischemia or sludge and debris that can accumulate in the biliary system after transplanting (Fig. 100.4).
The diagnosis of biliary stricture or obstruction is implied by an obstructive pattern on routine liver function tests. Commonly, patients are seen with constitutional symptoms of rigor, fever, headache, and fatigue. Occasionally, patients present with severe symptoms of cholangitis and sepsis. Because patients with “mild” signs and symptoms also may reflect the presence of any number of serious conditions—acute rejection, HAT, cytomegalovirus (CMV) infection, or recurrent disease—diagnosis can be difficult. To ascertain the correct diagnosis rapidly, a series of diagnostic studies that include abdominal US, cholangiography, and liver biopsy is obtained immediately after the onset of abnormal clinical signs and symptoms (Kuo et al, 1994).
Regardless of the method used to reestablish the biliary continuity, the most common site of a biliary stricture in the posttransplantation setting is at the biliary anastomosis. Technical error during reconstruction is an important causative factor, but the patency of the hepatic artery also should be assessed, particularly in pediatric recipients. Other factors that have been implicated in the development of biliary stricture include ABO incompatibility, prolonged preservation times, chronic rejection, CMV infections, and recurrence of primary ductal disease, such as sclerosing cholangitis (Feller et al, 1996; Greif et al, 1994; Sebagh et al, 1995).
Biliary reconstruction after LDLT is by Roux-en-Y hepaticojejunostomy or duct-to-duct anastomosis (Kawachi et al, 2002). Anastomotic leak or stricture is generally two times more common after living-donor versus cadaveric liver transplantation (Miller et al, 2001). Nonanastomotic strictures can occur in the hilar region or intrahepatically. As with anastomotic strictures, thrombosis of the hepatic artery or one of its branches should be suspected. Nonanastomotic strictures also have been reported in association with chronic ductopenic rejection, ABO blood group incompatibility, and as a result of ischemia-reperfusion injury associated with allograft preservation (Sanchez-Urdazpal et al, 1992).
After appropriate fluid resuscitation and antibiotic coverage, we recommend ERC to confirm the diagnosis and to implement treatment with immediate stenting if possible. Failure to cross the stricture through endoscopic means requires PTC or surgical revision with Roux-en-Y hepaticojejunostomy. Intrahepatic strictures are best treated with percutaneous balloon dilation. Rarely, stents are needed to achieve a satisfactory outcome (Colonna et al, 1992).
Renal Dysfunction
Renal dysfunction is observed to some degree in almost every patient who undergoes liver transplantation (Baliga et al, 1992; Lam et al, 2004). Early renal dysfunction usually is characterized by a period of oliguria with a transient increase in serum creatinine but can also be manifested as anuria with acute renal failure. Risk factors include preexisting renal dysfunction and primary graft nonfunction. In patients with normal preoperative serum creatinine and good initial graft function, the usual mechanism is prerenal azotemia secondary to periods of hypotension and hypovolemia during the operative procedure. An additional insult to renal function can be incurred by the administration of nephrotoxic agents, especially the calcineurin inhibitors (CNIs) cyclosporine and tacrolimus; renal failure induced by CNIs occurs in approximately 5% of transplanted patients (Myers et al, 1986).
Sirolimus (rapamycin) is a macrocyclic antibiotic originally developed as an anticandidal and antitumor agent (Vezina et al, 1975). It is often used for renal sparing but has the disadvantage of increasing the risk for dyslipidemia and impaired wound healing. The impairment of wound healing may be related to alterations in vascular endothelial growth factor (VEGF) and may explain the apparent benefit of sirolimus in reducing cancer formation in patients (Fuereder et al, 2010). Because sirolimus has been associated with early hepatic artery thrombosis (HAT) after liver transplantation, the manufacturer recommends waiting at least 4 weeks after transplantation before initiating therapy.
Fluid and Electrolyte Disturbances
The early development of metabolic alkalosis secondary to metabolism of citrate in blood products is a favorable sign of graft function but can be serious if alkalemia develops (Driscoll et al, 1987). In this situation, judicious replacement of chloride in the form of hydrogen chloride is warranted.
Excess total body water and excess sodium are best treated with gentle diuresis with furosemide. Careful repletion of potassium should be instituted, but it must be monitored closely in the setting of medications such as tacrolimus, which tends to increase serum potassium levels (Oishi et al, 2000). Derangements in serum calcium, magnesium, and phosphorus are common in patients with cirrhosis and should be repleted to avoid neurologic, skeletal, and cardiac muscle dysfunction.
Acute Cellular Rejection
In the early days of liver transplantation, the importance of rejection was overshadowed by technical complications. As surgical technique has evolved, with concomitant improvement in graft preservation, rejection has taken on greater clinical importance. Acute cellular rejection is defined as an acute deterioration in allograft function associated with specific histologic changes in the liver allograft. These changes include a mixed inflammatory cell infiltrate, predominantly lymphocytes, that involves the portal triads and disrupts the biliary, hepatic artery, and portal venous endothelia (endotheliitis) (Sedivy et al, 1998).
The incidence of acute rejection is approximately 45% (range, 24% to 80%), depending on the series reported. Although acute rejection has little to no impact on mortality, significant morbidity results in increased hospitalization and higher overall costs (Bucuvalas et al, 2001; Khettry et al, 2002). In the early stage, most patients are asymptomatic, but a variety of clinical signs and symptoms may develop that include fever, abdominal pain, malaise, fatigue, and poor appetite.
The earliest laboratory indicator of acute rejection is elevated bilirubin level, which may be associated with a modest increase in alkaline phosphatase and aminotransferase levels. PT and serum albumin levels are usually unaffected. Because laboratory measurements are neither sensitive nor specific for acute cellular rejection, liver histology obtained from a percutaneous biopsy specimen remains the standard for the diagnosis of cellular rejection (Khettry et al, 2002).
The initial treatment for acute cellular rejection includes high-dose pulse steroids, which is successful in approximately 80% to 90% of cases (Klintmalm et al, 1989). OKT3, a murine monoclonal antibody to the CD3 antigen–receptor complex, is reserved for the approximately 10% to 20% of patients whose acute cellular rejection cannot be not reversed with high-dose corticosteroid therapy. The major risks posed by using this treatment for acute cellular rejection include increased susceptibility to infection and the association between OKT3 use and posttransplantation lymphoproliferative disease (PTLD; Deschler et al, 1995). In addition, a severe systemic inflammatory response can be initiated from OKT3 infusion that can lead to pulmonary edema, hypotension, and shock.
Chronic ductopenic rejection affects approximately 10% of liver transplant patients. Ductopenic rejection rarely occurs during the first 2 months after liver transplantation; it is defined as loss of bile ducts in more than 50% of portal tracts, when 20 or more portal tracts are available for evaluation, and is diagnosed on the basis of histologic criteria (van Hoek et al, 1992). In addition, arteriopathy has been described that affects large and medium-sized arteries, characterized by foam-cell infiltration of the intima. The most important manifestation of chronic rejection, the term ductopenic rejection is used synonymously with the histologic description vanishing bile duct syndrome. No treatment exists with the exception of retransplantation (Koukoulis et al, 2001).
Infection
With few exceptions, all patients who undergo transplantation are committed to lifelong immunosuppression therapy to prevent graft rejection. Inadequate immunosuppression can result in graft loss, whereas injudicious use of immunosuppression can result in life-threatening infection or the development of PTLD. A tenuous balance exists between the proper amount of immunosuppression to prevent rejection and minimization of the risk for nosocomial and opportunistic infection. Despite better understanding of the immune response and proliferation of more selective immunosuppressive agents, approximately two thirds of transplant patients experience at least one episode of serious infection, which accounts for more than half of the observed mortality associated with liver transplantation (Kibbler, 1995).
Risk factors for postoperative infection after liver transplantation include those incurred from the donor, recipient, and intraoperative course: donor and recipient viral status, underlying medical comorbidities, and nutritional status all can contribute. Prolonged surgery with massive blood loss, prolonged ischemia time, and violation of the gastrointestinal tract also are risk factors for nosocomial infection (George et al, 1992).
Bacterial infections tend to occur within the first month after liver transplantation and vary from center to center (range, 35% to 68%) (Kibbler, 1995; Kusne et al, 1992). Early risk factors include prolonged operating time, indwelling catheters, biliary obstruction, PVT, and poor graft function. In addition, vascular ischemia, recurrent hepatitis C virus (HCV) infection, patient exposure to resistant organisms, and chronic rejection can contribute to nosocomial infection (Singh et al, 1997). Common bacterial pathogens include gram-negative organisms found in the bile (Escherichia coli, Enterobacter spp., Pseudomonas spp.) and gram-positive organisms (S. aureus, coagulase-negative staphylococci, and group D streptococci). Rarely, S. aureus can result in the development of toxic shock syndrome in the early postoperative period. Listeria, Nocardia, and Legionella are uncommon but significant pathogens (George et al, 1992).
Cytomegalovirus (CMV) infection is the single most important infection observed in organ transplant patients. An asymptomatic infection in the general population, CMV attains significant potential severity in immunosuppressed transplant recipients and is the most important pathogen in clinical transplantation (Kusne & Shapiro, 1997). CMV disease usually occurs 30 to 50 days after transplantation, with clinical manifestations that include fever, malaise, arthralgia, leukopenia and thrombocytopenia, hepatitis, interstitial pneumonitis, enterocolitis, and disseminated disease. Differentiation between CMV disease and CMV infection is clinically important: CMV disease is defined as a histologically evident invasive CMV infection or a positive CMV culture from deep tissue specimens—liver biopsy, endoscopic mucosal biopsy or brushing, bronchoscopic mucosal biopsy or brushing—in the setting of clinical manifestation. The presence of positive blood, body fluid, or serologic tests is insufficient to establish the diagnosis of CMV disease. Liver biopsy with immunostaining using a monoclonal antibody against CMV antigen enables early diagnosis. Common histologic findings include hepatocyte necrosis, parenchymal microabscesses, and a magenta-colored intranuclear inclusion surrounded by a clear halo, the so-called owl’s eye nucleus (Everson & Kam, 1997).
Risk factors for the development of CMV disease include a seronegative recipient who received an organ from a seropositive donor, the use of antilymphocyte antibody therapy (particularly OKT3), and retransplantation. In general, the level of immunosuppressive therapy influences manifestation of CMV infection (Furukawa et al, 1996). Chronic CMV infection with persistent CMV replication within hepatocytes is associated with cholestatic hepatitis and vanishing bile duct syndrome.
Prophylaxis against CMV disease using oral and intravenous ganciclovir after transplantation has been effective. Other prophylaxis regimens using intravenous gancyclovir plus oral acyclovir for 3 months after transplantation or oral acyclovir used alone have been less successful (Nakazato et al, 1993; Oldakowska-Jedynak et al, 2003). More recently, prevention and treatment of mild CMV infection using oral valganciclovir has been demonstrated to be as effective as intravenous ganciclovir (Eid et al, 2010). Because of the effectiveness of oral valganciclovir, most transplant centers have switched to prophylactic regimens that range from 3 to 6 months of daily oral treatment (450 or 900 mg, depending on renal function). Side effects are minimal, but leukopenia can occur. Late-onset CMV disease remains a potential problem, especially in patients on higher doses of immunosuppression and in high-risk groups, whose donor was CMV positive when the recipient was CMV negative (CMV D+/R−).
Epstein-Barr virus (EBV) belongs to the herpes family, and infection in a transplant patient is characterized by a mononucleosis-like syndrome that differs from that observed in the normal host by the absence of a heterophil antibody response and the infrequency of pharyngitis or splenomegaly (Alshak et al, 1993). The clinical significance of EBV infection in a liver transplant patient has to do with its role in the pathogenesis of PTLD (Manez et al, 1997), the development of which is thought to reflect the unrestricted proliferation of B cells stimulated by EBV infection. The incidence and treatment for established PTLD are discussed later.
Opportunistic infections also are commonly encountered. The incidence of clinically significant fungal infection is 20% to 25% in liver transplant recipients. Risk factors for fungal infection include poor nutrition status, retransplantation, bacterial infection with prolonged antibiotic use, high doses of immunosuppression, and biliary reconstruction using Roux-en-Y hepaticojejunostomy (Castaldo et al, 1991).
Disseminated fungal infection with Candida or Aspergillus can be difficult to diagnose and is associated with a high mortality rate in these patients. If the suspicion of invasive fungal infection is high, every effort should be made to obtain histologic or culture evidence to establish early diagnosis. Aspergillus, the second most frequent fungal infection in transplant patients after Candida, is acquired by inhalation and colonizes the airways before causing invasive disease. Aspergillus is angioinvasive and tends to disseminate to the central nervous system (CNS) and can cause infarcts and cavitation in the lungs. The incidence of invasive aspergillosis is approximately 1% in liver transplant recipients, with a mortality rate close to 100% despite adequate treatment (Kusne et al, 1992).
Infection by Cryptococcus neoformans usually occurs months to years after transplantation and affects approximately 0.25% of liver transplant patients (Patel et al, 1996). Because signs and symptoms may be subtle, delayed diagnosis is common. Symptoms that bring the patient to medical attention include changes in mental status, headache, and fever. In our experience, one patient was seen initially with lesions in the CNS and large cavitating masses in the thorax that required operative removal.
Posttransplantation Lymphoproliferative Disorder
PTLD is a life-threatening complication of chronic immunosuppression. Lymphoproliferative disorders have been strongly associated with the replication of EBV in B cells induced by enhanced immunosuppression (Manez et al, 1997; Newell et al, 1996); this has been observed primarily in patients who have received more than one course of polyclonal antilymphocyte globulin or monoclonal OKT3 (Davis et al, 1995). An association with CMV infection also has been noted. The incidence of PTLD varies from 1% to 3% among liver transplant recipients, and prognosis depends on the histologic characteristics of the tumor. Polyclonal PTLD is treatable with discontinuation of immunosuppression with relatively low risk of rejection (Hurwitz et al, 2004). Monoclonal PTLD is more difficult to treat and can result in death. Antibody against CD20 represents a novel approach in treating monoclonal PTLD, with favorable outcome (Yedibela et al, 2003; Zompi et al, 2000). The clinical presentation of PTLD varies and includes fever, malaise, and lymphadenopathy with or without tonsillitis. In addition, gastrointestinal bleeding, perforation, or obstruction; hepatocellular dysfunction; and CNS manifestations, such as seizures, mental status changes, and focal neurologic symptoms also have been described.
Lymphoproliferative disorders occurring after transplantation have characteristics distinct from the lymphoproliferative disorders that occur in the general population. Non-Hodgkin lymphoma accounts for 65% of lymphomas in the general population, compared with 93% in transplant recipients. These tumors are mostly large-cell lymphomas, and most are of the B cell type. Extranodal involvement is common and occurs in approximately 70% of cases (Zompi et al, 2000).
Treatment of polyclonal PTLD consists of reduction of immunosuppressive medications and antiviral therapy (Starzl et al, 1984). Patients with monoclonal PTLD and patients with polyclonal disease that does not respond to reduced immunosuppression have been treated with radiation, chemotherapy, and occasionally surgical resection. Therapy using monoclonal antibody against CD20 shows promising results for patients with monoclonal PTLD (Dotti et al, 2001; Zompi et al, 2000).
Acute Immunosuppressive Drug Toxicity
In addition to PTLD, chronic immunosuppression has been associated with an increased frequency of many other neoplasms, including basal skin cancer, non-Hodgkin lymphoma, Kaposi sarcoma, uterine cervical carcinoma, and carcinomas of the external genitalia (Busuttil et al, 1991). As with PTLD, patients who received repeated doses of antilymphocyte antibody have a particularly high incidence of neoplasms (Millis et al, 1995). Long-term use of steroids, particularly at high doses, is associated with obesity, hypertension, bone disease, glucose intolerance, pancreatitis, muscle weakness, hirsutism, and fluid and sodium retention; in children, growth retardation is a major problem (Busuttil et al, 1991).
The side-effect profiles of cyclosporine and tacrolimus are similar and include gastrointestinal disturbances, headache, and tremor. Gingival hyperplasia and hirsutism are encountered frequently during cyclosporine and steroid treatment, whereas glucose intolerance is reported more often with tacrolimus treatment than with cyclosporine therapy. Hyperkalemia, hyperuricemia, hypophosphatemia, and hypomagnesemia are manifestations of renal tubular dysfunction and usually can be controlled by adjusting the dose according to drug levels. Nephrotoxicity is the most clinically significant adverse effect of both drugs and is manifested as acute azotemia. This effect is largely reversible after reducing the dose of the drug and providing adequate hydration. Occasionally, progressive chronic renal disease can develop, which is usually irreversible. In such a situation, dialysis or kidney transplantation is required. Other renal effects of cyclosporine include chronic tubular dysfunction and, rarely, hemolytic uremic syndrome (Cohen et al, 2002).
Recurrent Hepatitis (See Chapter 64)
In patients who develop cirrhosis secondary to chronic hepatitis B virus (HBV) infection, the recurrence rate as evidenced by signs of viral replication (HBV e-antigen–positive or positive titers of HBV DNA) is approximately 80% to 90% in the first year. Given the almost universal recurrence of HBV antigenemia in the early postoperative period, some authors have questioned the utility of liver replacement in these patients. With the advent of hepatitis B immunoglobulin (HBIg) and other adjuvant therapies, such as interferon and lamivudine, to prevent recurrent disease, transplantation now is routinely offered to patients with chronic HBV infection, with excellent results (Dodson et al, 2000; Grellier et al, 1996).
Recurrent HCV infection is universal after liver transplantation (Gordon et al, 1997). In 90% of patients, hepatitis can be confirmed on routine biopsy. Of these, 60% have mild hepatitis, whereas 30% develop a more severe pattern seen on histologic examination. In patients with severe recurrent HCV, 20% go on develop to cirrhosis within 5 years (Gordon et al, 1997). Of 100 patients with HCV infection who are transplanted, approximately 6 will require retransplantation (Gordon et al, 1997). Prophylaxis with ribavirin and interferon has been shown to be beneficial in reducing viral loads to zero, with significant prolongation of graft survival (Ahmad et al, 2001). Given the epidemic growth in HCV infection, it is likely to dominate the indications for liver replacement for years to come.
Bone Disease
Almost all patients who undergo liver transplantation have some degree of hepatic osteodystrophy. The mechanism seems to vary according to the underlying disease. The causes are multifactorial and include corticosteroid therapy, bed rest, and cholestasis. Osteoporosis is particularly common 3 to 6 months after transplantation; however, by the end of the first year after transplantation, patients start gaining bone density. This late improvement is probably because of a reduction in corticosteroid therapy and the resolution of the pretransplantation condition that was deleterious to skeletal health. Atraumatic bone fractures are more frequent within the first 6 months after transplantation as a result of the extensive bone loss that occurs during this period (Navasa et al, 1996). Avascular necrosis and vertebral body collapse may also occur.
Hypertension and Hyperlipidemia
Common sequelae of immunosuppression are the development of hypertension and hyperlipidemia in the posttransplantation period (Fernandez-Miranda et al, 1998). As patient survival improves, the consequences of these conditions have greater importance on long-term prognosis. Hypertension is observed in approximately 70% of liver transplant recipients at 1 year, and nearly 40% of patients develop sustained hypercholesterolemia and hypertriglyceridemia during the same time period. The pathophysiology of posttransplantation hyperlipidemia is complex. Rapamycin immunosuppression does not cause glucose intolerance, but it can lead to hyperlipidemia (Trotter, 2003). Omega-3 fatty acids found in fish oil can reduce hypertriglyceridemia significantly. In our experience, oral supplementation with over-the-counter fish oil capsules (4 to 6 g/day) has shown a dose-response reduction in serum triglycerides. Given the additional risk of development of coronary artery and peripheral vascular disease, aggressive therapy—with dietary changes, exercise, and medication—is indicated.
Ahmad J, et al. Recurrent hepatitis C after liver transplantation: a nonrandomized trial of interferon alfa alone versus interferon alfa and ribavirin. Liver Transpl. 2001;7:863-869.
Alshak NS, et al. Epstein-Barr virus infection in liver transplantation patients: correlation of histopathology and semiquantitative Epstein-Barr virus DNA recovery using polymerase chain reaction. Hum Pathol. 1993;24:1306-1312.
Baliga P, et al. Preoperative risk factor assessment in liver transplantation. Surgery. 1992;112:704-711.
Bell R, et al. Vascular complications following orthotopic liver transplantation. Aust N Z J Surg. 1990;60:193-198.
Broelsch CE, et al. Living donor liver transplantation in adults: outcome in Europe. Liver Transpl. 2000;6(Suppl 2):S64-S65.
Bucuvalas JC, et al. Predictors of cost of liver transplantation in children: a single center study. J Pediatr. 2001;139:66-74.
Busuttil RW, et al. Liver transplantation in children. Ann Surg. 1991;213:48-57.
Castaldo P, et al. Clinical spectrum of fungal infections after orthotopic liver transplantation. Arch Surg. 1991;126:149-156.
Chavin KC, et al. The effects of prostaglandin E1 on hepatic allograft vascular inflow: a prospective randomized double-blind study. Am Surg. 1996;62:184-187.
Chin LT, et al. Liver transplantation at the University of Wisconsin. Clin Transplant. 2002:207-213.
Cohen AJ, et al. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002;8:916-921.
Colonna JO2nd, et al. Biliary strictures complicating liver transplantation: incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344-352.
Cooper JT, et al. Donation after cardiac death: the University of Wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490-1494.
D’Alessandro AM, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9:68-71.
Davidson BR, et al. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation. 1994;57:1174-1177.
Davis CL, et al. Antiviral prophylaxis and the Epstein-Barr virus–related post-transplant lymphoproliferative disorder. Clin Transplant. 1995;9:53-59.
Deschler DG, et al. Posttransplantation lymphoproliferative disorder in patients under primary tacrolimus (FK 506) immunosuppression. Arch Otolaryngol Head Neck Surg. 1995;121:1037-1041.
Dodson SF, et al. Lamivudine after hepatitis B immune globulin is effective in preventing hepatitis B recurrence after liver transplantation. Liver Transpl. 2000;6:434-439.
Dotti G, et al. Anti-CD20 antibody (rituximab) administration in patients with late occurring lymphomas after solid organ transplant. Haematologica. 2001;86:618-623.
Driscoll DF, et al. Development of metabolic alkalosis after massive transfusion during orthotopic liver transplantation. Crit Care Med. 1987;15:905-908.
Eid AJ, et al. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70(8):965-981.
Emond JC, et al. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet. 1993;176:11-17.
Everson GT, Kam I. Liver transplantation: current status and unresolved controversies. Adv Intern Med. 1997;42:505-553.
Fan ST, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676-683.
Feller RB, et al. Biliary strictures after liver transplantation: clinical picture, correlates and outcomes. J Gastroenterol Hepatol. 1996;11:21-25.
Fernandez-Miranda C, et al. Lipoprotein abnormalities in long-term stable liver and renal transplanted patients: a comparative study. Clin Transplant. 1998;12:136-141.
Forster J, et al. The role of transjugular intrahepatic portosystemic shunts in the management of patients with end-stage liver disease. Am J Surg. 1994;168:592-597.
Fuereder T, et al. mTOR inhibition by everolimus counteracts VEGF induction by sunitinib and improves anti-tumor activity against gastric cancer in vivo. Cancer Lett. 2010;296(2):249-256.
Furukawa H, et al. Effect of CMV serology on outcome after clinical intestinal transplantation. Transplant Proc. 1996;28:2780-2781.
George DL, et al. Patterns of infection after pediatric liver transplantation. Am J Dis Child. 1992;146:924-929.
Gondolesi GE, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842-1848.
Gordon FD, et al. Relationship between hepatitis C genotype and severity of recurrent hepatitis C after liver transplantation. Transplantation. 1997;63:1419-1423.
Greif F, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45.
Grellier L, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212-1215.
Hidalgo EG, et al. High-dose intra-arterial urokinase for the treatment of hepatic artery thrombosis in liver transplantation. Hepatogastroenterology. 1989;36:529-532.
Hurwitz M, et al. Complete immunosuppressive withdrawal as a uniform approach to post-transplant lymphoproliferative disease in pediatric liver transplantation. Pediatr Transplant. 2004;8:267-272.
Imagawa DK, et al. Rapid en bloc technique for pancreas-liver procurement: improved early liver function. Transplantation. 1996;61:1605-1609.
Jenkins RL, et al. Distal splenorenal shunt: role, indications, and utility in the era of liver transplantation. Arch Surg. 1999;134:416-420.
Kadry Z, et al. Living donor liver transplantation in patients with portal vein thrombosis: a survey and review of technical issues. Transplantation. 2002;74:696-701.
Kawachi S, et al. Biliary complications in adult living donor liver transplantation with duct-to-duct hepaticocholedochostomy or Roux-en-Y hepaticojejunostomy biliary reconstruction. Surgery. 2002;132:48-56.
Kettner SC, et al. Endogenous heparin-like substances significantly impair coagulation in patients undergoing orthotopic liver transplantation. Anesth Analg. 1998;86:691-695.
Khettry U, et al. Centrilobular histopathologic changes in liver transplant biopsies. Hum Pathol. 2002;33:270-276.
Kibbler CC. Infections in liver transplantation: risk factors and strategies for prevention. J Hosp Infect. 1995;30(Suppl):209-217.
Klein AS, et al. Reduction of morbidity and mortality from biliary complications after liver transplantation. Hepatology. 1991;14:818-823.
Klein AS, et al. Prostaglandin E1 administration following orthotopic liver transplantation: a randomized prospective multicenter trial. Gastroenterology. 1996;111:710-715.
Klintmalm GB, et al. Rejection in liver transplantation. Hepatology. 1989;10:978-985.
Koukoulis GK, et al. Cholangiocytic apoptosis in chronic ductopenic rejection. Hum Pathol. 2001;32:823-827.
Kraus TW, et al. Successful treatment of complete inferior vena cava thrombosis after liver transplantation by thrombolytic therapy. Br J Surg. 1992;79:568-569.
Kuo PC, et al. A comparison of operation, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography in biliary complications after hepatic transplantation. J Am Coll Surg. 1994;179:177-181.
Kusne S, Shapiro R. Surgical infections in immunocompromised patients: prevention and treatment. Adv Surg. 1997;31:299-331.
Kusne S, et al. Infections during a randomized trial comparing cyclosporine to FK 506 immunosuppression in liver transplantation. Transplant Proc. 1992;24:429-430.
Lam P, et al. The efficacy and limitations of sirolimus conversion in liver transplant patients who develop renal dysfunction on calcineurin inhibitors. Dig Dis Sci. 2004;49:1029-1035.
Langnas AN, et al. Hepatic allograft rescue following arterial thrombosis: role of urgent revascularization. Transplantation. 1991;51:86-90.
Leonardi MI, et al. Late hepatic artery thrombosis after liver transplantation: clinical setting and risk factors. Transplant Proc. 2004;36:967-969.
Lerut J, et al. Biliary tract complications in human orthotopic liver transplantation. Transplantation. 1987;43:47-51.
Lo CM, et al. Ten-year experience with liver transplantation at Queen Mary Hospital: retrospective study. Hong Kong Med J. 2002;8:240-244.
Malago M, et al. Right living donor liver transplantation: an option for adult patients: single institution experience with 74 patients. Ann Surg. 2003;238:853-863.
Manez R, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462-1467.
Margarit C, et al. Biliary complications secondary to late hepatic artery thrombosis in adult liver transplant patients. Transpl Int. 1998;11(Suppl 1):S251-S254.
Marsman WA, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246-1251.
Mazzaferro V, et al. Hepatic artery thrombosis after pediatric liver transplantation: a medical or surgical event? Transplantation. 1989;47:971-977.
Miller CM, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301-312.
Millis JM, et al. Liver transplantation at the University of Chicago. Clin Transpl. 1995:187-197.
Mor E, et al. Roux-en-Y anastomotic bleeding following orthotopic liver transplantation: experience in our first 300 patients. Transplant Proc. 1993;25:1925-1926.
Muraji T, et al. Biliary atresia: current management and outcome. Tohoku J Exp Med. 1997;181:155-160.
Myers BD. Cyclosporine nephrotoxicity. Kidney Int. 1986;30:964-974.
Nakazato PZ, et al. Viral prophylaxis in hepatic transplantation: preliminary report of a randomized trial of acyclovir and gancyclovir. Transplant Proc. 1993;25:1935-1937.
Navasa M, et al. Quality of life, major medical complications and hospital service utilization in patients with primary biliary cirrhosis after liver transplantation. J Hepatol. 1996;25:129-134.
Neuhaus P, Platz KP. Liver transplantation: newer surgical approaches. Baillieres Clin Gastroenterol. 1994;8:481-493.
Newell KA, et al. Posttransplant lymphoproliferative disease in pediatric liver transplantation: interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370-375.
O’Connor TP, et al. Biliary tract complications after liver transplantation. Arch Surg. 1995;130:312-317.
Oishi M, et al. A case of hyperkalemic distal renal tubular acidosis secondary to tacrolimus in living donor liver transplantation. Transplant Proc. 2000;32:2225-2226.
Oldakowska-Jedynak U, et al. Cytomegalovirus infection as a common complication following liver transplantation. Transplant Proc. 2003;35:2295-2297.
Orons PD, et al. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. AJR Am J Roentgenol. 1995;165:1145-1149.
Palareti G, et al. Coagulation and fibrinolysis in orthotopic liver transplantation: role of the recipient’s disease and use of antithrombin III concentrates. S. Orsola Working Group on Liver Transplantation. Haemostasis. 1991;21:68-76.
Patel R, et al. Risk factors of invasive Candida and non-Candida fungal infections after liver transplantation. Transplantation. 1996;62:926-934.
Pomfret EA, et al. Live donor liver transplantation. J Hepatol. 2001;34:613-624.
Pomposelli JJ, et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: program experience and lessons learned. Am J Transplant. 2006;6(3):589-598.
Quiroga J, et al. Cause and timing of first allograft failure in orthotopic liver transplantation: a study of 177 consecutive patients. Hepatology. 1991;14:1054-1062.
Rabkin JM, et al. Biliary tract complications of side-to-side without T tube versus end-to-end with or without T tube choledochocholedochostomy in liver transplant recipients. Transplantation. 1998;65:193-199.
Raby N, et al. Stenoses of vascular anastomoses after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;157:167-171.
Reichert PR, et al. Biliary complications of reduced-organ liver transplantation. Liver Transpl. 1998;4:343-349.
Sanchez-Urdazpal L, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49-53.
Sebagh M, et al. Sclerosing cholangitis following human orthotopic liver transplantation. Am J Surg Pathol. 1995;19:81-90.
Sedivy R, et al. Apoptotic hepatocytes in rejection and vascular occlusion in liver allograft specimens. Histopathology. 1998;32:503-507.
Shaked A, Busuttil RW. Liver transplantation in patients with portal vein thrombosis and central portacaval shunts. Ann Surg. 1991;214:696-702.
Singh N, et al. Intensive care unit management in liver transplant recipients: beneficial effect on survival and preservation of quality of life. Clin Transplant. 1997;11:113-120.
Starzl TE, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583-587.
Stieber AC, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg. 1991;213:199-206.
Tan KC, et al. Hepatic artery thrombosis in pediatric liver transplantation. J Pediatr Surg. 1988;23:927-930.
Trotter JF. Sirolimus in liver transplantation. Transplant Proc. 2003;35(3 Suppl):193S-200S.
Tzakis A, et al. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649-652.
van Hoek B, et al. Severe ductopenic rejection following liver transplantation: incidence, time of onset, risk factors, treatment, and outcome. Semin Liver Dis. 1992;12:41-50.
Vezina C, et al. Rapamycin (AY-22,989), a new antifungal antibiotic: I. Taxonomy of the producing streptomycere and isolation of the active principle. J Antibiotic. 1975;28:721-732.
Washburn WK, et al. Graft function and outcome of older (> or = 60 years) donor livers. Transplantation. 1996;61:1062-1066.
Wozney P, et al. Vascular complications after liver transplantation: a 5-year experience. AJR Am J Roentgenol. 1986;147:657-663.
Yedibela S, et al. Anti-CD20 monoclonal antibody treatment of Epstein-Barr virus–induced intrahepatic lymphoproliferative disorder following liver transplantation. Transpl Int. 2003;16:197-201.
Zompi S, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with clonal lymphoproliferative disorders after orthotopic liver transplantation: a report of three cases. J Hepatol. 2000;32:521-527.