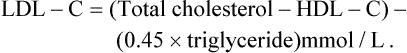24 Dyslipidaemia
Epidemiology
The ideal serum lipid profile is unknown and varies between different populations, even across Europe, and also within a given population. For practical purposes the values presented in Table 24.1 represent the target levels for TC and LDL-C in the UK for adults receiving treatment for secondary prevention of CVD. For completeness, the values for triglycerides and HDL-C are also presented, although the benefit of achieving the stated targets is less clear.
| Total cholesterol (TC)a | <4.0 mmol/L |
| LDL cholesterol (LDL-C)a | <2.0 mmol/L |
| Triglyceridesb | <1.7 mmol/L |
| HDL cholesterol (HDL-C) | >1.0 mmol/L in men |
| >1.2 mmol/L in women |
a Target levels in individuals with established atherosclerotic disease, coronary heart disease, stroke, peripheral arterial disease, diabetes mellitus or where there is a cardiovascular disease risk >20% over 10 years. In these identified individuals, the aim is to achieve the value stated in the table or a 25% reduction in total cholesterol and a 30% reduction in LDL-C from their baseline levels should these set target levels lower than those stated in the table.
Despite a 50% reduction in the death rate from CVD over the past 25 years, it remains the leading cause of premature death and morbidity in the UK (British Heart Foundation, 2008), and the higher the levels of TC in an individual the greater the chance of developing CVD. At the individual level there appears no level below which a further reduction of TC or LDL-C is not associated with a lower risk of CVD. The death rate from CVD is threefold higher in males than females, but because women live longer and are at increased risk of stroke after the age of 75 years their lifetime risk of disease is greater (National Institute of Health and Clinical Excellence, 2008a).
Lipid transport and lipoprotein metabolism
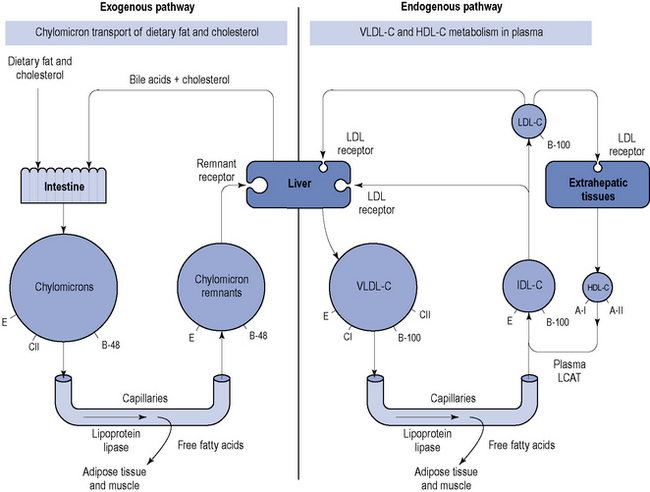
The protein components of lipoproteins are known as apoproteins (apo), of which apoproteins A-I, E, C and B are perhaps the most important. Apoprotein B exists in two forms: B-48, which is present in chylomicrons and associated with the transport of ingested lipids, and B-100, which is found in endogenously secreted VLDL-C and associated with the transport of lipids from the liver (Fig. 24.1).
Reverse cholesterol transport pathway
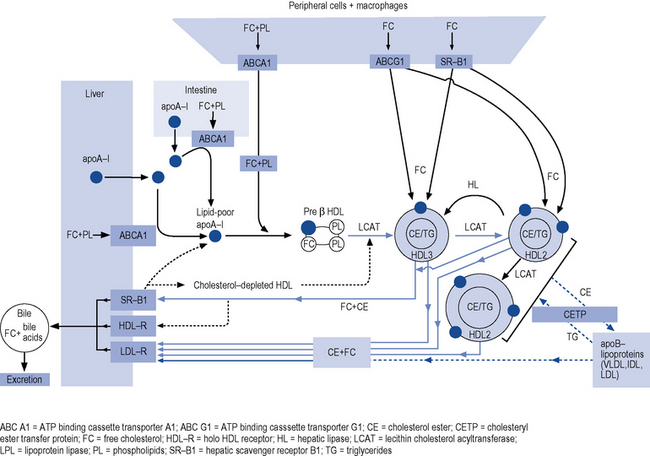
The reverse cholesterol transport pathway (Fig. 24.2) controls the formation, conversion, transformation and degradation of HDL-C and is the target site for a number of new, novel drugs and has recently been described (Chapman et al., 2010).
Triglycerides
The role of hypertriglyceridaemia as an independent risk factor for coronary heart disease (CHD) is unclear because triglyceride levels are confounded by an association with low HDL-C, hypertension, diabetes and obesity, and a synergistic effect with LDL-C and/or low HDL-C. An isolated elevation of triglyceride may be the consequence of a primary disorder of lipid metabolism, it may be secondary to the use of medicines or it may be a component of the metabolic syndrome or type 2 diabetes mellitus. Many individuals have a mixed dyslipidaemia that includes elevated levels of triglycerides and LDL-C, but reduction of LDL-C normally remains the primary focus of treatment. A recent analysis, in over 73,000 individuals, of a genetic variant which regulates triglyceride concentrations has demonstrated a causal association between triglycerides and CHD (Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, 2010).
Aetiology
Primary dyslipidaemia
Familial hypercholesterolaemia
Heterozygous familial hypercholesterolaemia (often referred to as FH) is an inherited metabolic disease that affects approximately 1 in 500 of the population. In the UK, this represents about 110,000 individuals. Familial hypercholesterolaemia is caused by a range of mutations, which vary from family to family, in genes for the pathway that clear LDL-C from the blood. The most common mutation affects the LDL receptor gene. Given the key role of LDL receptors in the catabolism of LDL-C, patients with FH may have serum levels of LDL-C two to three times higher than the general population. It is important to identify and treat these individuals from birth, otherwise they will be exposed to high concentrations of LDL-C and will suffer the consequences. Familial hypercholesterolaemia is transmitted as a dominant gene, with siblings and children of a parent with FH having a 50% risk of inheriting it. It is important to suspect FH in people that present with TC >7.5 mmol/L, particularly where there is evidence of premature CV disease within the family. Guidance on diagnosis, identifying affected relatives and management are available but it is important to seek specialist advice for this group of high-risk patients (National Institute of Health and Clinical Excellence, 2008b).
Lipoprotein(a)
Concentrations of Lp(a) above 0.3 g/L occur in about 20% of caucasians and increase the risk of coronary atherosclerosis and stroke. Under a wide range of circumstances, there are continuous, independent, and modest associations of Lp(a) concentration with the risk of CHD and stroke (Emerging Risk Factors Collaboration, 2009).
Secondary dyslipidaemia
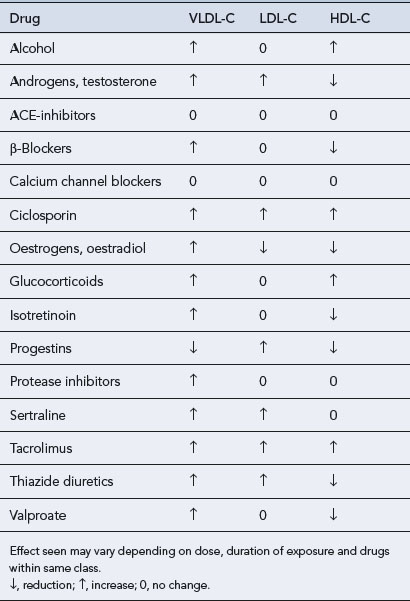
Dyslipidaemias that occur secondary to a number of disorders (Box 24.1), dietary indiscretion or as a side effect of drug therapy (Table 24.2) account for up to 40% of all dyslipidaemias. Fortunately, the lipid abnormalities in secondary dyslipidaemia can often be corrected if the underlying disorder is treated, effective dietary advice implemented or the offending drug withdrawn.
Some of the more common disorders that cause secondary dyslipidaemias include the following.
Diabetes mellitus
Obesity
Chronic, excessive intake of calories leads to increased concentrations of triglycerides and reduced HDL-C. Obesity per se can exacerbate any underlying primary dyslipidaemia. Individuals with central obesity appear to be at particular risk of what has become known as the metabolic or DROP (dyslipidaemia, insulin resistance, obesity and high blood pressure) syndrome which represents a cluster of risk factors. Obesity and sedentary lifestyle coupled with inappropriate diet and genetic factors interact to produce the syndrome (Kolovou et al., 2005).
Risk Assessment
Primary prevention
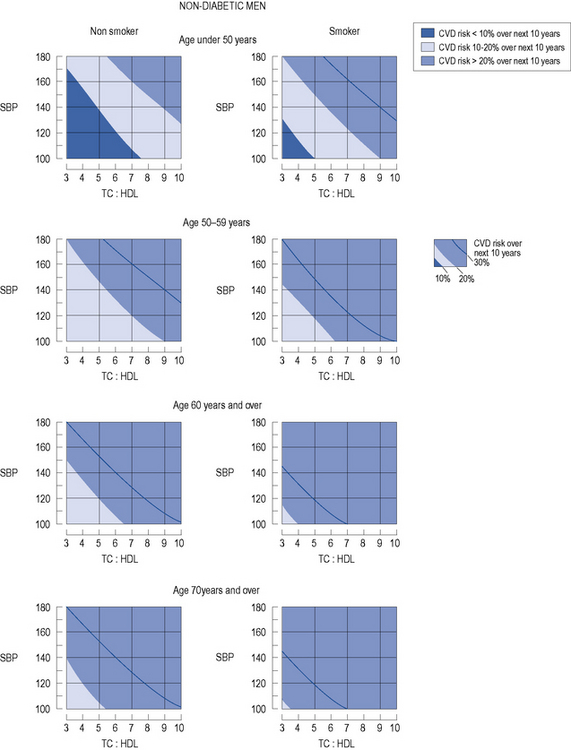
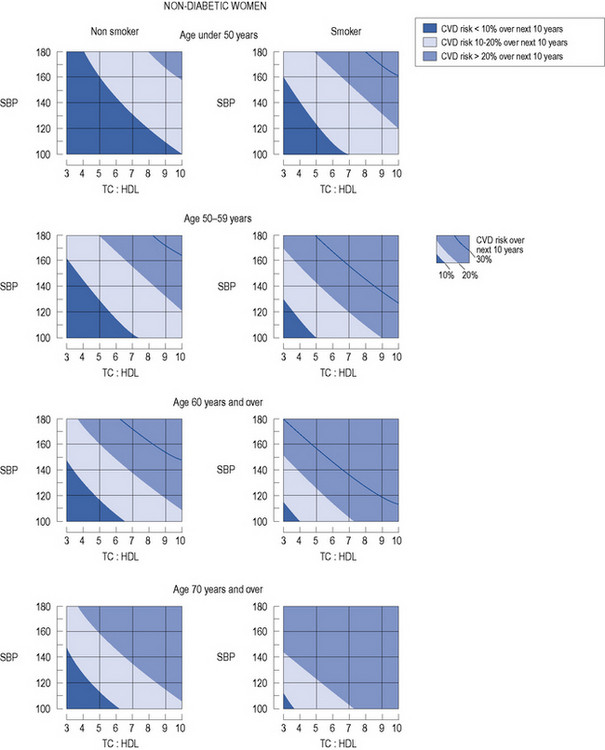
In patients with no evidence of CHD or other major atherosclerotic disease, there are a number of CVD risk prediction charts, including those produced by the Joint British Societies (JBS2) (British Hypertension Society, 2009) for males (Fig. 24.3) and females (Fig. 24.4). JBS2 recommends that all adults from the age of 40 years, with no history of CVD or diabetes, and not receiving treatment for raised blood pressure or dyslipidaemia, should receive opportunistic screening every 5 years in primary care. The cardiovascular risk calculated using the JBS2 charts is based on the number of cardiovascular events expected over the next 10 years in 100 women or men with the same risk factors as the individual being assessed. Those with a cardiovascular risk >20% over 10 years are deemed to require treatment according to current national and international guidelines, although individuals with a risk as low as 8% over 10 years will gain some benefit, this will be small.
Box 24.2 Criteria that indicate familial hypercholesterolaemia
Individuals with type 2 diabetes aged over 40 years and with an additional cardiovascular risk factor are considered to be at greater than 20% risk over 10 years and eligible for treatment. In those who are 40 years of age or older but without any additional risk factor, a specific risk engine is available (http://www.dtu.ox.ac.uk/riskengine/) based on data from the United Kingdom Prospective Diabetes Study.
Assign
This is a risk calculator based on data from a Scottish population and includes many of the variables utilised in the Framingham-based model. It also takes into account social status, determined by postcode of residence in Scotland, and family history of CVD (Woodward et al., 2007). The calculator can be found at: http://www.assign-score.com.
Secondary prevention
As in the situation with primary prevention outlined above, if an individual is to receive a lipid-lowering agent as part of a secondary prevention strategy, the possibility of a familial dyslipidaemia and the need to assess other family members must not be overlooked (National Institute of Health and Clinical Excellence, 2008b).
Treatment
Lifestyle
When a decision is made to start treatment with a lipid-lowering agent, other risk factors must also be tackled as appropriate, such as smoking, obesity, high alcohol intake and lack of exercise (Box 24.3). Underlying disorders such as diabetes mellitus and hypertension should be treated as appropriate. Issues around body weight, diet and exercise will be briefly covered in the following sections.
Box 24.3 Lifestyle targets
Drugs
Primary prevention
In primary prevention, dyslipidaemia should not be treated in isolation and management must be embarked upon with clear goals. In addition to lifestyle advice, this will not only address management of dyslipidaemia but will also seek to optimise use of antihypertensive agents, other cardiovascular protective therapies and achieve tight blood glucose control as appropriate. In patients without evidence of arterial disease, treatment must be considered if the risk of CVD is >20% or more over 10 years. Although some dispute the benefit of statins in primary prevention (Kausik et al., 2010), treatment will normally include:
Secondary prevention
In individuals diagnosed with CVD or other occlusive arterial disease, treatment should include:
Lipid-lowering therapy
There are five main classes of lipid-lowering agents available:
Agents such as soluble fibre and fish oils have also been used to reduce lipid levels. A number of new agents are also under investigation for their novel effect on different parts of the cholesterol biosynthesis pathway (Table 24.3).
Table 24.3 Mechanism of lipid-lowering agents under investigation
| Drug group | Mechanism |
|---|---|
| Acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibitors | ACAT esterifies excess intracellular cholesterol. Inhibition of ACAT prevents transport of cholesterol into the arterial wall and thereby prevents atheroma developing. Lowers VLDL-C and triglycerides |
| Bile acid sequestrants | Related to first-generation resins but improved patient tolerance. Sequester bile acids and prevent re-absorption. Reduce LDL-C while HDL-C and triglycerides increase or remain unchanged |
| Cholesteryl ester transfer protein (CETP) inhibitors | CETP is responsible for the transfer of cholesteryl ester from HDL-C to the atherogenic LDL-C and VLDL-C |
| Lipoprotein lipase (LPL) activity enhancers | LPL is responsible for VLDL-C catabolism with subsequent loss of triglycerides and increase in HDL-C. Protects against atherosclerosis |
| Microsomal triglyceride transfer protein (MTP) inhibitors | Inhibit absorption of lipid and reduce hepatic secretion of lipoproteins, thereby reducing atherosclerotic plaque formation |
| Peroxisome proliferator-activated receptor (PPAR) activators | PPAR-α and -γ regulate the expression of genes involved in lipid metabolism and inhibit atherosclerotic plaque rupture. They reduce entry of cholesterol into cells, lower LDL-C and triglycerides, and increase HDL-C |
| Squalene synthase inhibitors | Inhibit squalene synthase, upregulate LDL receptor activity and enhance removal of LDL-C |
Statins
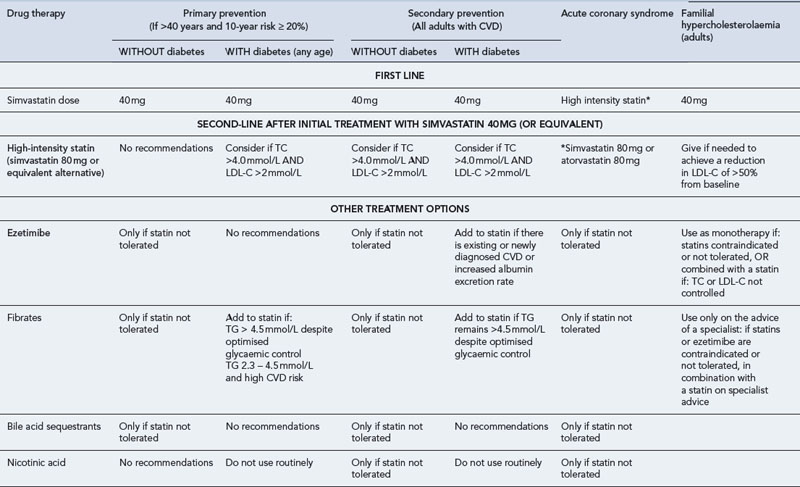
There is much debate around the statin of choice. Simvastatin is currently the preferred agent because of its relatively low cost, safety profile and evidence of efficacy (see Table 24.4). Perhaps more important is the need to identify patients who need treatment, ensure they receive an appropriate, effective dose of a statin and adhere to treatment. Despite overwhelming evidence of benefit, effectiveness is frequently compromised by poor adherence with up to 50% of patients discontinuing treatment within 12 months and 75% within 3 years. Patient factors that influence this include perception of risk, side effects of medication, expected treatment duration and socio-demographic factors.
Table 24.4 Typical recommendations for use of lipid-lowering agents (UKMI, 2009) (LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; TC, total cholesterol)
Adverse effects
The statins are a heterogeneous group metabolised by different CYP450 isoenzymes. Simvastatin, atorvastatin and lovastatin are metabolised by CYP3A4, fluvastatin is metabolised by CYP 2C9, and pravastatin and rosuvastatin are eliminated by other metabolic routes and less subject to interactions with CYP450 isoenzymes than other members of the family. Nevertheless, caution is still required as a 5- to 23-fold increase in pravastatin bioavailability has been reported with ciclosporin. Simvastatin and atorvastatin do not alter the activity of CYP3A4 themselves, but their serum levels are increased by known inhibitors of CYP3A4 (Table 24.5). Advice has been published for the prescribing of simvastatin and atorvastatin with inhibitors of CYP3A4 (Table 24.6).
Table 24.5 Examples of drug interactions involving statins and the cytochrome P450 enzyme pathway
| CYP 450 isoenzyme | Inducers | Inhibitors |
|---|---|---|
| CYP3A4 | ||
| Atorvastin | Phenytoin | Ketoconazole |
| Lovastatin | Barbiturate | Itraconazole |
| Simvastatin | Rifampicin | Fluconazole |
| Dexamethasone | Erythromycin | |
| Cyclophosphamide | Clarithromycin | |
| CarbamazepineOmeprazole | Tricyclic antidepressants | |
| Nefazodone | ||
| Venlafaxine | ||
| Fluoxetine | ||
| Sertraline | ||
| Ciclosporin | ||
| Tacrolimus | ||
| Diltiazem | ||
| Verapamil | ||
| Protease inhibitors | ||
| Midazolam | ||
| Corticosteroids | ||
| Grapefruit juice | ||
| Tamoxifen | ||
| Amiodarone | ||
| CYP2C9 | ||
| Fluvastatin | Rifampicin | Ketoconazole |
| Phenobarbitone | Fluconazole | |
| Phenytoin | Sulfaphenazole | |
Table 24.6 Advice for prescribing simvastatin or atorvastatin with inhibitors of CYP3A4
| Avoid simvastatin with potent inhibitors of CYP3A4: | HIV protease inhibitors, azole, antifungals, erythromycin, clarithromycin, telithromycin |
| Do not exceed the following doses: | Simvastatin 10 mg daily with ciclosporin, gemfibrozil or niacin (>1 g/day) |
| Simvastatin 20 mg daily with verapamil or amiodarone | |
| Simvastatin 40 mg daily with diltiazem | |
| Avoid grapefruit juice when taking simvastatin | |
| Atorvastatin to be used cautiously with CYP3A4 inhibitors: | Additional care required at high doses of atorvastatin; avoid drinking large quantities of grapefruit juice |
Fibrates
In patients with diabetes, the typical picture of dyslipidaemia is one of raised triglycerides, reduced HDL-C and near normal LDL-C. Despite the effect of fibrates to reduce triglycerides and increase HDL-C, statins are first-line lipid-lowering agent in most guidelines because of a lack of clear evidence that fibrates prevent CVD in diabetes. It was hoped that a 5-year study of fenofibrate in individuals with type 2 diabetes (FIELD Investigators, 2005) would clarify the issue. However, in the final analysis the results provided little convincing evidence to change from recommending a statin, although they did confirm the safety of using a combination of a statin and fenofibrate. In contrast, gemfibrozil should not be used with a statin.
Adverse effects
Fibrates have been implicated in a number of drug interactions (Table 24.7), of which two in particular are potentially serious. Fibrates are known to significantly increase the effect of anticoagulants, while concurrent use with a statin is associated with an increased risk of myositis and, rarely, rhabdomyolysis. Concurrent use of cerivastatin and gemfibrozil was noted to cause rhabdomyolysis and this contributed to the withdrawal of cerivastatin from clinical use in 2001.
Table 24.7 Typical drug interactions involving bile acid binding agents and fibratesa
| Drug group | Interacting drug | Comment |
|---|---|---|
| Bile acid binding agents Colestyramine/colestipol | All medication should be taken 1 h before or at least 4 h after colestyramine/colestipol to reduce absorption caused by binding in the gut | |
| Acarbose | Hypoglycaemia enhanced by colestyramine | |
| Digoxin | Absorption reduced | |
| Diuretics | Absorption reduced | |
| Levothyroxine | Absorption reduced | |
| Mycophenolate mofetil | Absorption reduced | |
| Paracetamol | Absorption reduced | |
| Raloxifene | Absorption reduced | |
| Valproate | Absorption reduced | |
| Statins | Absorption reduced | |
| Vancomycin | Effect of oral vancomycin antagonised by colestyramine | |
| Warfarin | Increased anticoagulant effect due to depletion of vitamin K or reduced anticoagulant effect due to binding or warfarin in gut | |
| Colesevelam | All medication should be taken at least 4 h before or 4 h after colesevelam to reduce absorption caused by binding in the gut | |
| Ciclosporin | Absorption reduced | |
| Digoxin | Absorption unchanged | |
| Glyburide | Absorption reduced | |
| Levothyroxine | Absorption reduced | |
| Oral contraceptive | Absorption reduced | |
| Statins | Absorption unchanged | |
| Valproate | Absorption unchanged | |
| Warfarin | Absorption unchanged. Increased anticoagulant possible due to depletion of vitamin K | |
| Fibrates | Antidiabetic agents | Improvement in glucose tolerance |
| Ciclosporin | Increased risk of renal impairment | |
| Colestyramine/colestipol | Reduced bioavailability of fibrate if taken concomitantly | |
| Statin | Increased risk of myopathy | |
| Warfarin | Increased anticoagulant effect |
Bile acid binding agents
Adverse effects
Colestyramine, colestipol and colesevelam are known to interact with many drugs primarily by interfering with absorption (Table 24.7). Whether these absorption-type interactions are qualitatively and quantitatively similar between the different agents is unclear and the picture is confused when the absorption of a given drug is known to interact with one bile acid binding agent but has not been tested with other members of the group.
Cholesterol absorption inhibitors
Ezetimibe should be prescribed either with a statin, a fibrate or a nicotinic acid derivative and rarely by itself, and then only in statin intolerant individuals. Although apparently well tolerated, no long-term trials (Kastelein et al., 2008; Rossebø et al., 2008) have demonstrated an additional reduction in cardiovascular morbidity or mortality that could be attributed to ezetimibe.
Cholesterol ester transfer protein (CETP) inhibitors
Answers
Answers
As this patient is being treated with a statin for primary prevention, National Institute of Health and Clinical Excellence (2008a) guidelines suggest that only generic statin agents are cost-effective and, therefore, pravastatin should be considered as a first-line alternative for this patient.
Questions
Answers
Answers
Answers
British Heart Foundation. Coronary Heart Disease Statistics Book. London: BHF; 2008.
British Hypertension Society. Proposed Joint British Societies Cardiovascular Disease Risk Assessment Charts. Available at: http://www.bhsoc.org/Cardiovascular_Risk_Prediction_Chart.stm, 2009.
Chapman M.J., Le Goff W., Guerin M., et al. Cholestery ester transfer protein: at the heart of the action of lipid modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur. Heart J.. 2010;31:149-164.
Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. J. Am. Med. Assoc.. 2009;302:412-423.
FIELD Investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849-1861.
Kastelein J.J., Akdim F., Stroes E.S., et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. The ENHANCE study. N. Engl. J. Med.. 2008;358:1431-1443.
Kausik R.K., Sreenivasa R.K.S., Erqou S., et al. Statins and all-cause-mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med.. 2010;170:1024-1031.
Kolovou G.D., Anagnostopoulou K.K., Cokkinos D.V. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad. Med. J.. 2005;81:358-366.
National Institute of Health and Clinical Excellence. Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: NICE; 2008. Available at: http://www.nice.org.uk/CG067
National Institute of Health and Clinical Excellence. Identification and Management of Familial Hypercholesterolaemia. London: NICE; 2008. Available at: http://guidance.nice.org.uk/CG71/Guidance/pdf/English
Rossebø A.B., Pedersen T.R., Boman K., for the SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med.. 2008;359:1343-1356.
Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634-1639.
UKMI. Lipid Modification. Liverpool: North West Medicines Information Service; 2009. Available at: http://www.nelm.nhs.uk/en/ NeLM-Area/Health-In-Focus/NICE-Bites–August-0908/
Woodward M., Brindle P., Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart. 2007;93:172-176.
Brinton E.A. Does the addition of fibrates to statin therapy have a favourable risk to benefit ratio. Curr. Atherosclerosis Rep.. 2008;10:25-32.
Cannon C.P., Steinberg B.A., Murphy S.A., et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J. Am. Coll. Cardiol.. 2006;48:438-445.
Genest J., McPherson R., Frohlich J., et al. Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidaemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can. J. Cardiol.. 2009;25:567-579.
Kearney P.M., Blackwell L., Collins R., et al. Cholesterol Treatment Trialists’ (CTT) collaborators: efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
Mills E.J., Rachlis B., Wu P., et al. Primary prevention of cardiovascular mortality and events with statin treatment. J. Am. Coll. Cardiol.. 2008;52:1769-1781.
Ray K.K., Seshasai S.R., Erqou S., et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med.. 2010;170:1024-1031.