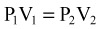133 Dysbarisms, Dive Injuries, and Decompression Illness
• Decompression illness describes diving-related injuries, including decompression sickness and arterial gas embolism.
• Any neurologic symptoms that occur in a diver immediately or soon after resurfacing are highly suspicious for arterial gas embolism and require urgent medical attention.
• A thorough neurologic examination is crucial with any suspicion of decompression illness.
• Dive injuries are best differentiated into the following categories: disorders of descent, disorders at depth, disorders of ascent, and disorders that occur after resurfacing.
• Decompression illness is treated on the scene by rapid implementation of high-flow oxygen and intravenous hydration; definitive treatment is recompression with hyperbaric oxygen therapy.
• One should delay flying after diving for at least 12 hours following a single no-decompression dive and at least 24 hours for dives involving decompression stops.
• Consultation with a diving physician or the Divers Alert Network (or both) is needed for a patient with any symptoms associated with recent diving.
Epidemiology
Dysbarisms refer to the pathophysiologic effects of changes in ambient (surrounding) pressure on the body. Decompression illness (DCI) includes decompression sickness (DCS or “the bends”) and arterial gas embolism (AGE). DCI occurs during or after ascent (decompression) when dissolved gases come out of solution, form bubbles, and then become lodged in various tissues (instead of being filtered by the lungs). Diagnosis of DCI in the emergency department (ED) is vital because delayed treatment or missed cases can have permanent sequelae (Box 133.1).
With the advent of “extreme sports” that involve water contact, sport diving, and the increasing number of people engaging in breath-hold diving, a sharp increase in diving injuries has been seen in EDs (Box 133.2). Deaths from breath-hold diving alone have almost doubled in the last 5 years, thus illustrating the potential for injury associated with this form of diving.1 With acute DCI, rapid assessment and treatment are the foundation of management. Three keys to successful ED treatment are having a high index of suspicion (DCI may have nonspecific findings), performing a thorough neurologic examination, and obtaining hyperbaric medicine consultation when DCI is suspected.
Box 133.2
Fatality Statistics for Diving
Deaths: 120 diving fatalities in the United States and Canada (2007)
Death analyses: men (85%); median age 50 years (men) and 43 years (women)
Obesity: 76% of deaths involved overweight or obese divers
Causes of death: drowning (86%); acute heart condition (9%); arterial gas embolism (3%); decompression sickness (1%)
Activities: 66% of deaths during pleasure of sight-seeing diving
Data from Divers Alert Network. DAN report on decompression illness, diving fatalities and project dive exploration, 2008. Available at www.diversalertnetwork.org/.
Pathophysiology
Bubble Physiology
Knowledge of bubble mechanics and the effects of bubbles in various tissues is critical to understanding the pathophysiology and treatment of DCI. Venous bubbles are not usually problematic because the lungs can filter large gas loads. Bubbles have damaging effects when they remain within tissues or embolize. Bubbles can pass from the venous circulation to the arterial circulation via a right-to-left shunt (patent foramen ovale or arteriovenous malformation). Bubbles can grow from “nucleation sites” within body tissues, such as the joint spaces, tendon sheaths, periarticular sheaths, and peripheral nerves.2 Once inside these areas, bubbles can act as emboli and block perfusion of distal tissues or act as foreign bodies with resultant vascular damage through activation of the inflammatory and clotting cascades. Interestingly, scientists are now evaluating a possible biologic marker of DCI. As gas emboli within the circulation induce decompression stress, endothelial cells release microparticles in response to cellular activation or cell death. These microparticles may, in the future, reflect a biologic marker of decompression stress that can be used to gauge the extent of disease, efficacy of treatment, or prophylaxis.3
Principles of Gas Laws and Dysbarism
Boyle’s Law
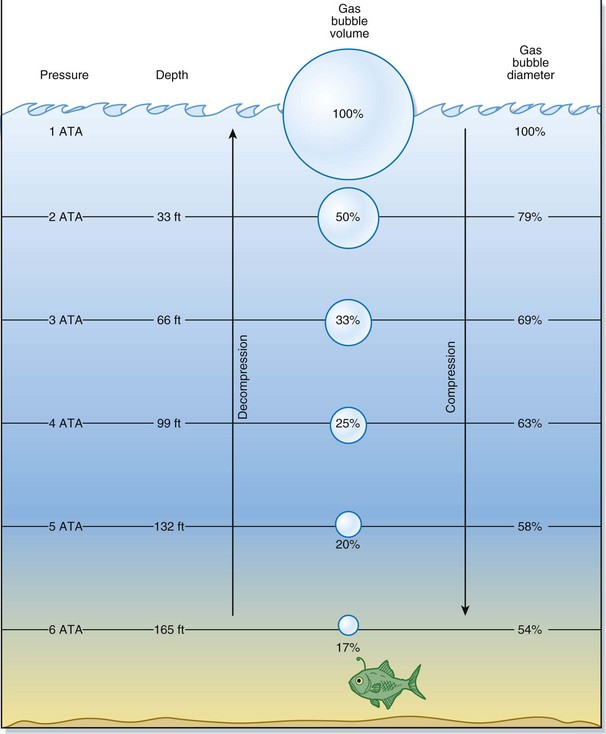
Usually, as a diver descends deeper, the surrounding pressure increases in linear fashion. Water pressure at the surface is referenced as 1 ATA. In general, for every 33 fsw that the diver descends, there is an increase in pressure of 1 ATA. Therefore, a descent to 33 fsw would equal 2 ATA, a descent to 66 fsw would equal 3 ATA, and so on. Furthermore, for the first 33 fsw descended, the volume of gas present is reduced by half the original amount of gas at the surface. At 66 fsw, the volume is one third the original volume. The greatest changes in volume occur closest to the water surface and represent a significant vulnerability to injury (Fig. 133.1).
Presenting Signs and Symptoms
Barotrauma
Barotrauma is sustained from failure to equalize the pressure of an air-containing space with that of the surrounding environment. The most common examples of barotrauma occur during air travel and scuba diving.1,4 Barotrauma occurs only in gas-containing (compressible) body spaces. More than 95% of the body is composed of water (incompressible). Typical gas-filled spaces include the sinuses, middle and inner ears, air-filled areas within carious or filled teeth, and hollow viscous organs such as the intestines and lungs. Barotrauma incurred during descent is called a “squeeze.” Barotrauma incurred during ascent is called a “reverse squeeze,” “reverse block,” or expansion injury.
Differential Diagnosis and Medical Decision Making
Table 133.1 lists the differential diagnosis for dive injuries based on the time of onset of symptoms.
Table 133.1 Differential Diagnosis of Dive Injuries Based on the Onset of Symptoms
| SYMPTOM ONSET | INJURIES TO CONSIDER |
|---|---|
| Descent |
Ear Barotrauma
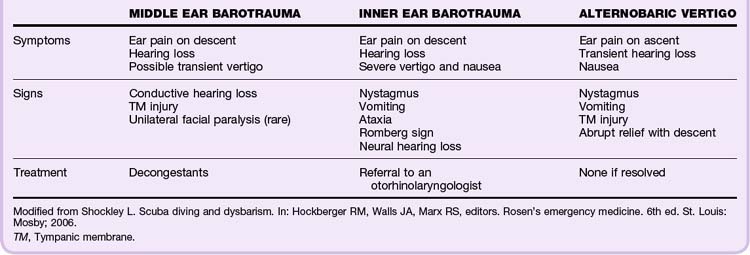
With an intact tympanic membrane (TM), the only communication for equilibration of pressure between the middle ear and the ambient atmosphere is through the eustachian tube (ET).5 Divers typically perform Valsalva maneuvers during decent to equalize pressure in the middle ear. Failure to equalize leads to pain and damage from injury to the middle or inner ear and results in TM edema, rupture, or hemorrhage, as well as rupture of the oval or round window (may lead to a perilymphatic fistula).5 Table 133.2 summarizes the types of ear barotrauma.
External Ear Barotrauma (“Squeeze”)
During diving, water replaces the air in the external ear canal. Obstructions such as wax, a bony growth, or earplugs can create an unvented air space that changes in volume in response to changes in ambient pressure. During descent, the increased pressure “squeezes” this space and causes the TM to bulge outward toward the canal with resultant pain, small hemorrhages, or TM blebs.4 Prevention consists of cleaning the external canal and removing any foreign bodies.
Middle Ear Barotrauma (“Squeeze”)
Middle ear barotrauma (middle ear squeeze, barotitis media) is the most common disorder in divers and hyperbaric medicine patients. It usually occurs during descent as a result of an inability to equalize pressure across the TM. It occurs in 30% of novice divers and 10% of experienced divers.6 When water exerts pressure on the external TM and pushes it inward, a diver can usually equalize the pressure in the ears by swallowing, yawning, performing a Valsalva maneuver, or blowing against closed nostrils. Divers are often unable to clear their ears because of anatomic variability of the ET, inflammation, a viral infection, or upper aerodigestive dysfunction. Without equalization of the pressure, the TM ruptures. As little as 100 mm Hg (5 fsw) can create a pressure differential large enough to rupture the TM.7 Symptoms of middle ear barotrauma are ear pain, pressure, and muffled hearing. If the TM is ruptured, vertigo may occur because of the effects of cold water on the middle ear or TM. Treatment consists of decongestants, rest from diving, and follow-up with an otorhinolaryngologist (refractory cases). In general, these injuries are self-limited. However, if a patient needs hyperbaric medicine treatments, pressure equalization tubes may be placed to prevent middle ear barotrauma. Any form of TM rupture, placement of a pressure equalization tube, or myringotomy would be a contraindication to wet water diving because water comes in direct contact with the middle ear.
Alternobaric Facial Palsy
Alternobaric facial palsy, a complication of middle ear barotrauma after diving, is a syndrome consisting of unilateral facial nerve palsy, ataxia, vertigo, nausea, and vomiting. The symptoms can be confused with those of AGE or DCS; however, the mechanism is elevated middle ear pressure pressing against the facial nerve and causing ischemic neurapraxia.7 Alternobaric palsy is also observed in those who fly after diving, fly at high altitude in unpressurized airplanes, and experience explosive decompression in flight.7 Though uncomfortable, the symptoms usually resolve within minutes once middle ear pressures equilibrate.
Sinus Barotrauma
Sinus barotrauma (“sinus squeeze”) is the second most common disorder in divers, but it is significantly less common than middle ear barotrauma, with only 1% of divers affected.5 Symptoms include sinus pain on descent and bloody nasal discharge on ascent.4 Treatment consists of decongestants, antiinflammatory agents, and rest from diving.
Pulmonary Barotrauma and Pulmonary Overpressurization Syndromes
Arterial Gas Embolism
AGE, the most lethal result of pulmonary barotrauma, is a common cause of death in recreational divers.8 Its incidence is underestimated because many in-water deaths are classified as drowning. When rupture of the lung parenchyma leads to intravascular bubbles, these bubbles can embolize and cause end-organ damage. Sadly, this disorder is often seen in inexperienced divers who panic at depth and shoot to the surface without exhaling slowly. The symptoms are dramatic, usually LOC immediately or within minutes of the diver reaching the surface. Death is common. The arterial emboli are most deadly when they travel to the coronary or cerebral circulation. Cerebral AGE is manifested very much like a stroke and results in headache, confusion, agitation, hemiplegia, or sudden LOC. Air embolism can also block blood flow through the coronary circulation and lead to cardiac ischemia, dysrhythmias, shock, and death.
Definitive treatment of AGE consists of high-flow oxygen on site and immediate hyperbaric recompression. The sooner patients are recompressed, the less likely they are to have permanent neurologic injury. Full recovery is common when recompression is available immediately. It is important to remember that AGE can occur in shallow water while breathing compressed gas or during breath-hold diving. This is in contrast to DCS, which usually occurs following a deep or prolonged dive when high nitrogen partial pressure develops.9 AGE also occurs in the hospital setting as a result of iatrogenic errors (central line manipulation, hemodialysis), but it can occur with any procedure or trauma that can entrain gas into the bloodstream.
Decompression Sickness
Type I (“Mild” Symptoms)
Type I DCS describes mild symptoms such as joint pain (most common), dermatologic manifestations, and lymphatic-associated swelling and edema as a result of the effects of gas bubbles in the tissues (Box 133.3). Bubble formation in joints is due to the greater negative pressure that exists in the joint spaces.10 Pain is most often felt in the shoulders or knees but can appear in any joint. The pain is gradual and aching and varies in intensity, usually worsening with time. Limb pain in divers affects the upper extremities three times more often than the lower extremities, and its distribution is often asymmetric.11 Caisson workers, however, are affected more often in their lower limbs.8 Merritt makes the point that type I DCS usually involves pain in the extremities whereas type II DCS usually involves central structures.7
Box 133.3
Type I Decompression Sickness
Definitive Treatment
Adapted from Bove AA, Davis J. Bove and Davis’ diving medicine. 4th ed. Philadelphia: Saunders; 2004; and U.S. Department of Commerce: NOAA navy diving manual. 5th ed. Flagstaff, Ariz: Best Publishing; 2005.
Dermatologic DCI (“skin bends”) can have several manifestations. A diver can experience itching alone (without a rash) in localized or generalized areas of the arms, legs, face, or trunk (“fleas”). This form is commonly thought to follow dry dives, appears shortly after resurfacing, and lasts only a few minutes to a few hours.12 Other dermatologic manifestations of DCS are mottling (cutis marmorata) and rindlike skin (peau d’orange).
Type II (“Severe” Symptoms)
Type II DCS causes more severe symptoms that have a high risk of leading to major disability or death. Cardiopulmonary, neurologic, and inner ear manifestations predominate (Box 133.4). All patients with type II DCS symptoms require emergency recompression in a hyperbaric chamber. The sooner they are recompressed, the more likely they are to have a full recovery. However, even if treatment is delayed several days, recompression can still be helpful. Multiple treatments may be necessary to maximize recovery.
Box 133.4 Type II Decompression Sickness
Type II is associated with a higher risk for permanent disability or death than type I.
Neurologic DCS
Type III Decompression Sickness
Type III includes both type II symptoms and AGE.
Tips and Tricks
Limb pain as a result of DCS usually persists even at rest, whereas the pain of a traumatic injury often improves with rest or nonuse.
Resolution of the pain at a joint when a blood pressure cuff is inflated is a strong indicator of joint DCS.
Neurologic examination of an injured diver is best performed with the patient out of the stretcher or bed to unmask any neurologic deficits.
Serious diving injuries are much more common in recreational divers than in commercial or military divers.
Abdominal pain may be an early signal of spinal cord injury in divers with DCS.
Neurologic Examination
The importance of performing a thorough neurologic examination cannot be understated. Unless an obvious neurologic deficit is noted, many dive-injured patients do not realize that they have an injury. However, if “hard” neurologic symptoms are observed, the patient requires immediate recompression with hyperbaric oxygen therapy without delay (Box 133.5). The neurologic examination should include a diver’s mental status, cranial nerves, balance, coordination, sensory testing, deep tendon reflexes, pathologic reflexes, and both fine and gross motor skills. Preprinted neurologic examination forms enable the physician to maximize diagnosis and treatment, as well as to standardize findings.
Treatment
Prehospital Management and Air Evacuation from the Site
Prehospital treatment of a dive injury is similar to that given in the ED. However, the hyperbaric team should be notified early that the patient is incoming to minimize delays (Box 133.6).
Box 133.6 Prehospital Treatment and Air Evacuation Instructions for Dive-Injured Patients
1. Maintain the ABCs of resuscitation and evaluate the patient’s serum glucose level.
2. Use ACLS protocols to stabilize the patient.
3. Administer high-flow oxygen, establish intravenous lines, and begin fluid administration.
4. Keep the patient flat; avoid the Trendelenburg position. If aspiration is a risk, lay the patient on the left side or in the right lateral position.
6. If air evacuation is being performed, ensure that the cabin of the airplane is pressurized or have the pilot fly at altitudes below 1000 feet.
7. Transport the patient along with all gear (it will have to be examined later).
8. Remember that the other members of the diving party might also need transport and evaluation for decompression sickness; they should accompany the patient to the ED.
9. Alert the consultant dive physician and hyperbaric center ahead of time that a dive injury has occurred and the patient is being brought to the ED.
Emergency Department Evaluation and Treatment
ED treatment of dive-injured patients must follow a standard approach. In addition to ordering routine laboratory tests, oxygen, and intravenous fluids, obtaining a thorough history is critical to treatment (Boxes 133.7 and 133.8).
Box 133.7 Emergency Department Diagnostic Work-up for Dive-Injured Patients
Box 133.8 Dive History Interview
Questions to Ask Dive-Injured Patients
1. Where did the dive occur (e.g., ocean, river, pool)?
2. When was the onset of the patient’s symptoms (on resurfacing, during descent or ascent, at the bottom)?
3. How deep did the patient go? Was a dive computer being used?
4. Was the patient intoxicated or dehydrated?
5. What type of diving equipment was used? What type of gas was used (compressed air, mixed gas, enriched air)? What was the source of the gas?
6. Did the patient perform heavy exertion or work during the dive?
7. Did the dive approach or exceed decompression limits?
8. How many dives did the patient perform and what were the depths, bottom times, total times, and resurface intervals for all dives in the previous days preceding symptoms (the dive “profiles”)?
9. Were decompression stops missed? Was in-water recompression attempted?
10. What was the time delay from the last dive to the time of air travel or travel to altitude such as a mountain range?
11. Did the patient experience ear or sinus problems on this dive or in the past?
12. Does the patient have any other medical problems? What medications does the patient take?
Follow-Up, Next Steps in Care, and Patient Education
Although each patient is unique, many patients with DCI can be safely discharged from the hospital. However, any diver with serious DCS symptoms should be admitted. All patients who have experienced DCS or embolic dive injuries should be transferred immediately to the closest emergency hyperbaric facility. The physician should err on the side of caution; any patient with concerning symptoms should not be discharged until a hyperbaric physician has been consulted (Boxes 133.9 and 133.10).
![]() Patient Teaching Tips
Patient Teaching Tips
Barratt M, Harch P, Van Meter K. Decompression illness in divers: a review of the literature. Neurologist. 2002;8:186–202.
Bove AA, Davis J. Bove and Davis’ diving medicine, 4th ed. Philadelphia: Saunders; 2004.
U.S. Department of Commerce. NOAA navy diving manual, 5th ed. Flagstaff, Ariz: Best Publishing; 2005.
1 Divers Alert Network. DAN report on decompression illness, diving fatalities and project dive exploration. www.diversalertnetwork.org/, 2008. Available at
2 Piantadosi C, Brown S. Diving medicine and near drowning. Hall JB, Schmidt GA, Wood LDH. Principles of critical care, 3rd ed, New York: McGraw-Hill, 2005.
3 Laden G, Madden L, Purdy G, et al. Endothelial damage as a marker of decompression stress. Undersea and Hyperbaric Medical Society Abstracts. http://archive.rubicon-foundation.org/1611, 2004. Available at
4 Bookspan J. Diving and hyperbaric medicine review for physicians. Kensington, Md: Undersea and Hyperbaric Medical Society; 2000.
5 Bove AA, Davis J. Bove and Davis’ diving medicine, 4th ed. Philadelphia: Saunders; 2004.
6 Shockley L. Scuba diving and dysbarism. Hockberger RM, Walls JA, Marx RS, et al. Rosen’s emergency medicine, 6th ed, St. Louis: Mosby, 2006.
7 Merritt D. Mending the bends—assessment, management, and recompression therapy. Flagstaff, Ariz: Best Publishing; 2006.
8 U.S. Department of Commerce. NOAA navy diving manual, 5th ed. Flagstaff, Ariz: Best Publishing; 2005.
9 Rutkowski D. UHMS diving accident and management manual. Undersea and Hyperbaric Medical Society. Flagstaff, Ariz: Best Publishing; 1989.
10 Chandy D, Weinhouse G. Complications of scuba diving. UpToDate www.uptodate.com/, 2007. Available at
11 Barratt M, Harch P, Van Meter K. Decompression illness in divers: a review of the literature. Neurologist. 2002;8:186–202.
12 . London Diving Chamber and Hyperbarics website. Available at www.londondivingchamber.co.uk/