).
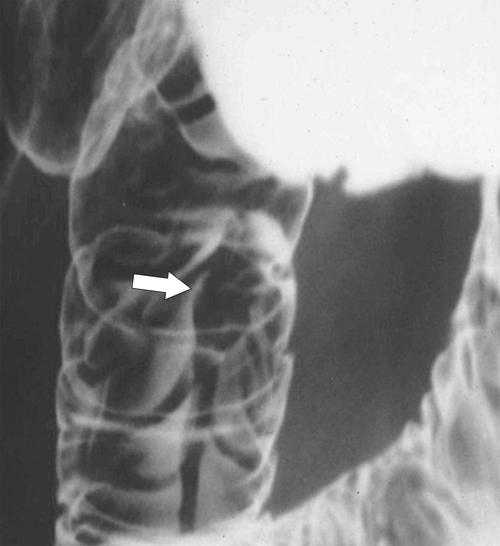
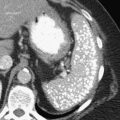
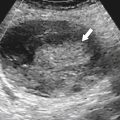
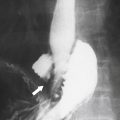
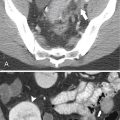
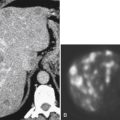
Figure 3-1 Normal ampulla of Vater (arrow).
Imaging Evaluation of the Duodenum
Historically visualization of the duodenum involved UGI contrast studies, but this has now been largely replaced by esophagogastroduodenoscopy (EGD). UGI contrast studies are now usually reserved to evaluate postoperative situations rather than disease de novo. The CT evaluation of the duodenum, though, has become more important. At fluoroscopy and UGI, the duodenal bulb had typically been the area of greatest interest, mainly because of the propensity of duodenal ulcers in the past. These ulcers are now far less common because of effective medications targeting gastric acid production and antibiotic treatment for Helicobacter pylori bacteria (see later in chapter). Good single-contrast or double-contrast technique is required to fully evaluate the duodenal bulb. The double-contrast technique, like optimized evaluation of the stomach, is somewhat of an art, and considerable expertise is required to assess patients with the variable anatomy, disease, or both.
The duodenal bulb is typically situated in a posterolateral axis (this can readily be appreciated through observation of its position at axial computed tomography [CT]), knowledge of which is required to fully evaluate bulb disease. The bulb will likely fill adequately with contrast once the patient is positioned in the right side down partial decubitus position. Once filled with barium, the bulb should be brought en face (perpendicular) to the x-ray beam, which usually requires positioning the patient right side up (right anterior oblique). At this point, the bulb should be adequately coated and distended with gas as a double-contrast view. Sometimes the bulb projects directly posteriorly and can be hard to view en face, but rotation of the patient into various positions (e.g., lateral) should bring the bulb en face, sometimes with the patient in a semiprone rather than semisupine position. Other times, the bulb can only be best viewed with the patient standing, which permits the weight of the gastric antrum to “fall away,” exposing the relatively fixed duodenal bulb.
Duodenal folds are usually best evaluated in the semisupine (right side up) position, and compression paddles may be required to move the gastric antrum away. In this semisupine position, ulcers may be seen as the pooling of barium on the posterior wall or radiolucent areas on the anterior wall (because the denser barium is now dependent on the posterior wall). Conversely, with the patient in a semiprone position (left side up or left anterior oblique), the barium will pool into ulcers on the anterior wall and not the posterior wall. As with any contrast fluoroscopic technique, any identified disease must be viewed tangentially, and multiple spot views must be taken. Ulcers may not be seen within duodenal folds, unless they are seen tangentially as they protrude outside the duodenal mucosa.
Hypertonic duodenography is a valuable tool for the evaluation of duodenal disease, including the bulb, but is now rarely performed. Hypertonic duodenography is achieved through temporary duodenal spasm after the administration of antispasmodics (glucagon or anticholinergics). Glucagon is relatively contraindicated in diabetics, and patients who are receiving anticholinergic agents should not drive vehicles for up to 4 hours because of the loss of visual accommodation. With appropriate bulb positioning, distortions in the duodenal wall, usually from ulcer disease, should be readily appreciated. If an abnormality is identified, views in multiple planes must be obtained to extract the most diagnostic information from the imaging examination. The second, third, and fourth parts of the duodenum are usually evaluated with single-contrast studies, although hypertonic duodenography should also temporarily distend at least the second part of the duodenum.
CT is not the primary modality for investigating the duodenum but can provide an excellent visualization of gross duodenal disease, including some congenital anomalies, duodenitis, and mural neoplastic lesions, particularly malignant masses. Positron emission tomography/computed tomography (PET/CT) is rarely used unless metastatic spread of duodenal malignancies is being evaluated, in which case it can identify small metastatic nodes that might otherwise be considered indeterminate by CT.
Congenital Duodenal Anomalies
Duodenal Atresia and Web
Duodenal atresia presents very early after birth because of the complete obstruction of the duodenum from the intrauterine failure of duodenal cannulation, which is often associated with Down∗ syndrome. Patients vomit their feeding, and on plain image, a characteristic “double bubble” is identified representing a gas-filled duodenum proximal to the complete obstruction and an enlarged, gas-filled stomach (Fig. 3-2). Duodenal webs are a form of duodenal atresia, although the duodenal obstruction is not complete; rather there is a diaphragm or membrane or web that causes duodenal stenosis (Fig. 3-3). These may persist into adulthood and present with an intraluminal diverticulum. The thin membrane or web can become stretched and propelled toward the distal duodenal lumen by constant peristalsis, creating the so-called windsock deformity (Fig. 3-4).
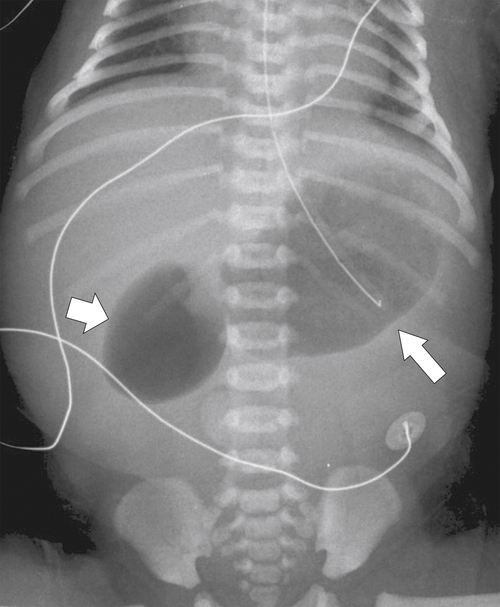
Figure 3-2 Plain abdominal film in a neonate with dilatation of the stomach (large arrow) and duodenum (small arrow) creating the double bubble sign caused by duodenal atresia.
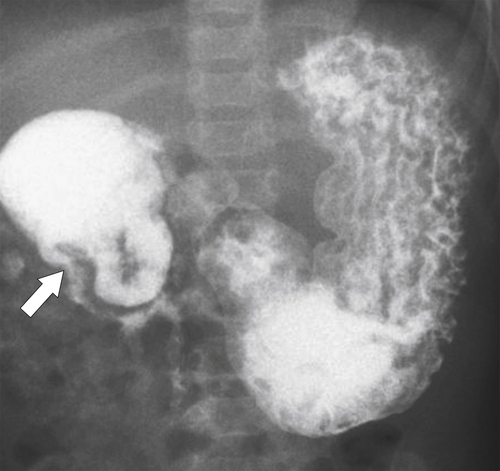
Figure 3-3 UGI swallow in a neonate demonstrating a duodenal obstruction (arrow) from a congenital duodenal web.
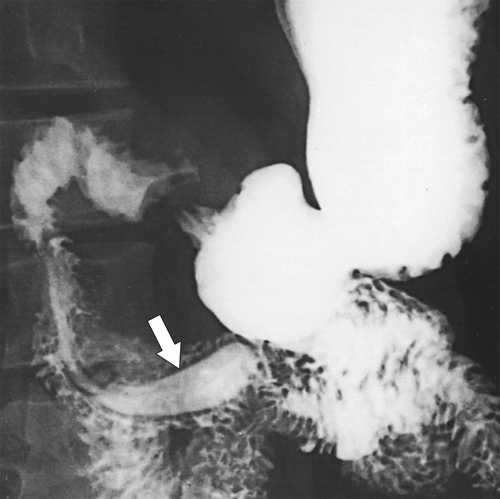
Figure 3-4 UGI series demonstrates a tubular filling defect (windsock) in the duodenum due to an intraluminal diverticulum (arrow).
Duodenal Duplication
Congenital duplication of the duodenum can be cystic or tubular and may or may not communicate with the lumen. Most are cystic and noncommunicating and located along the medial second part of the duodenum. Patients can have duodenal obstruction, and peptic ulceration or pancreatitis can also occur. At UGI series the affected duodenum is narrowed and displaced in the noncommunicating type, but contrast may fill the cyst when the duplication is communicating. The duplication may be more easily appreciated when it is extraluminal, often identified as a cystic structure medial to the duodenal C-sweep. CT or magnetic resonance imaging (MRI) will usually demonstrate the duplication better (Fig. 3-5). Other diagnoses, including choledochocele, pancreatic pseudocyst, and duodenal diverticulum, may have similar imaging appearances.
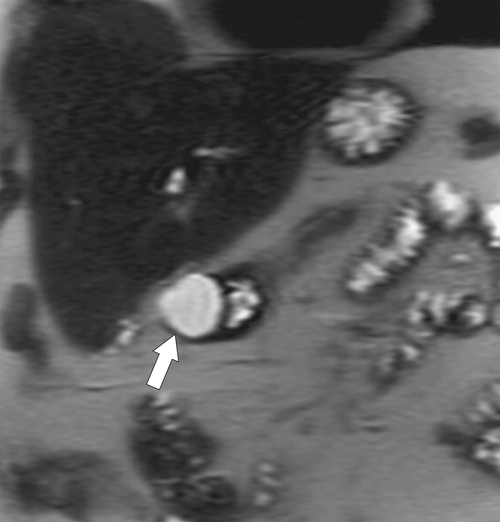
Figure 3-5 Coronal T2-weighted MRI in a 34-year-old woman with a hyperintense, smooth, rounded submucosal duodenal mass (arrow) due to duodenal duplication.
Midgut Malrotation
Midgut malrotation results from malrotation in utero as the duodenum rotates. It is the result of incomplete rotation that is recognized by the ligament of Treitz (represents the junction of the duodenum and jejunum) not migrating to its normal position to the left of the spine. Depending on the degree of malrotation, the ligament of Treitz will be located more inferiorly and to the right. In complete malrotation, it will be located to the right of the spine (Fig. 3-6). The cecal position will vary, usually in the left side of the abdomen with complete malrotation. The intestinal tract is susceptible to volvulus due to abnormal mesenteric anatomy (Fig. 3-7).
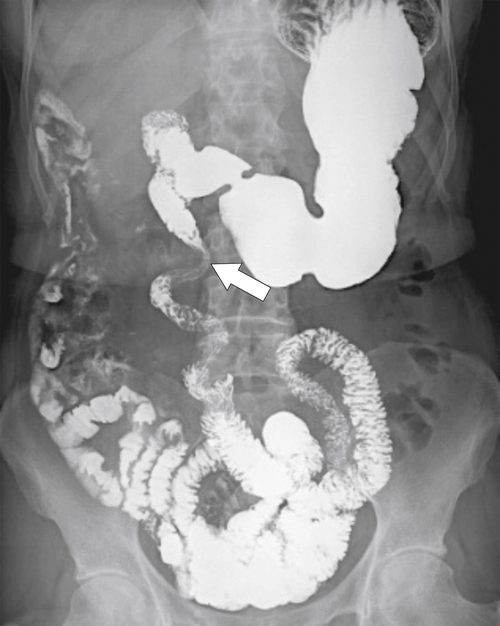
Figure 3-6 UGI series and follow-though demonstrating midgut malrotation. The duodenum fails to cross over the midline (arrow).
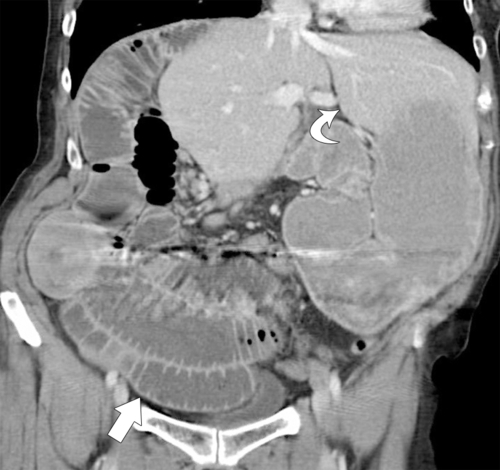
Figure 3-7 Coronal contrast-enhanced CT in a 33-year-old man demonstrating a left-sided liver (curved arrow) and right-sided bowel (arrow). The third and fourth parts of the duodenum do not cross the midline. Volvulus of the small bowel has also occurred, and there is small bowel obstruction.
Midgut Volvulus
Midgut volvulus is usually diagnosed in childhood and rarely in adulthood. It is an incomplete rotation of the midgut in utero that renders patients susceptible to a volvulus around a congenitally abnormal small bowel mesentery. At UGI series, there is an abrupt tapering of the third part of the duodenum, which is obstructed. Imaging is now usually performed with CT, which identifies a corkscrew or spiral appearance of the mesentery, with the duodenal-jejunal junction positioned inferiorly and to the right of its normal position at the ligament of Treitz (Fig. 3-8). The normal positioning of the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) are frequently reversed (normally the SMA lies to the left of the SMV). UGI series can also demonstrate the corkscrew appearance (Fig. 3-8). An alternative cause of congenital volvulus is a Ladd∗ band, which is an abnormal fibrous band of tissue at the root of the small bowel mesentery. This abnormality of the mesenteric root also predisposes the patient to malrotation.
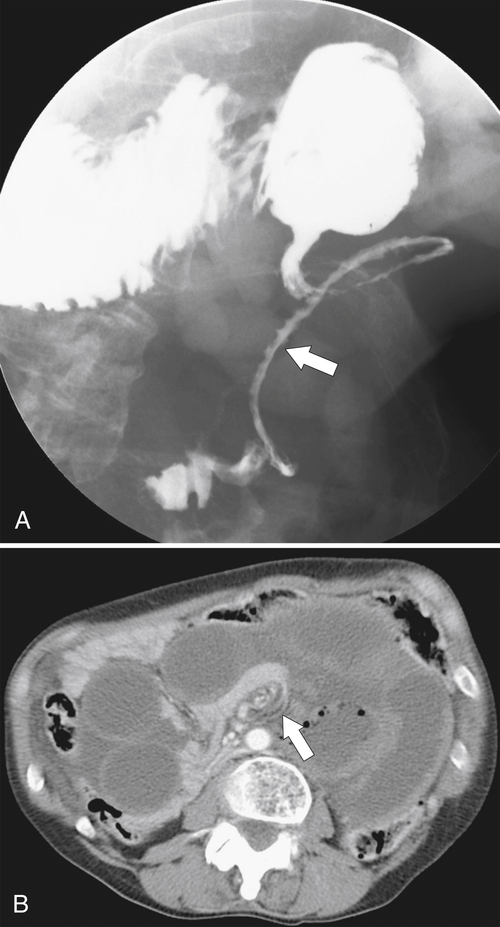
Figure 3-8 UGI series (A) and axial contrast-enhanced CT (B) in a 54-year-old woman with a midgut volvulus (arrows) caused by Ladd bands.
Paraduodenal Hernia
Paraduodenal hernias result from congenitally incomplete peritoneal fixation onto the posterior abdominal cavity, creating abnormal peritoneal spaces into which the duodenum and other small bowel can herniate. Therefore the small bowel can be seen in abnormal locations. If it is on the right, the second, third, and fourth parts of the duodenum can be displaced to the right. Left-sided paraduodenal hernias are more common, however, and can sequester large parts of the small bowel into the left upper quadrant, leaving only a small loop of bowel to connect to a normally positioned cecum.
Duodenal Diverticulum
Extraluminal diverticula are not true diverticula because they represent mucosal herniation through the muscular wall, but they are very common and are frequently identified incidentally at CT or UGI series, occurring mostly on the medial aspect of the second part of the duodenum at or just beyond the periampullary region (Figs. 3-9 and 3-10). Sometimes they can be very large (Fig. 3-11). Less commonly they are in the third and fourth parts of the duodenum (Fig. 3-12). They typically fill with fluid, gas, or both, and knowledge of their frequency, appearance, and location should avoid an erroneous diagnosis of a periduodenal mass or abscess. They are usually insignificant, but there is a risk of inadvertent perforation by endoscopy or feeding-tube placement. Diverticulitis can develop in large diverticula, resulting in fever and upper abdominal pain.
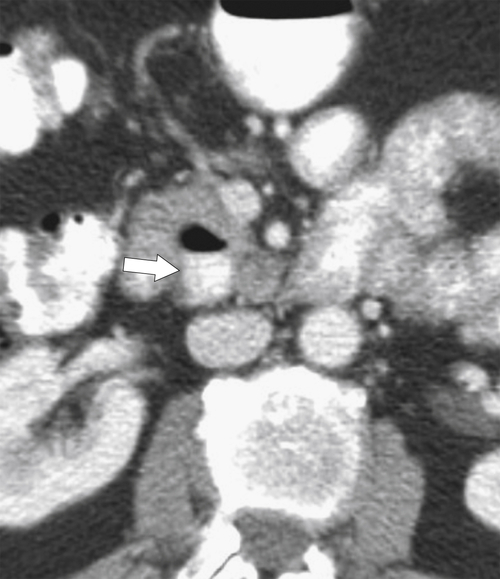
Figure 3-9 Axial contrast-enhanced CT in a 61-year-old man with a duodenal diverticulum (arrow) with an air/barium fluid level.
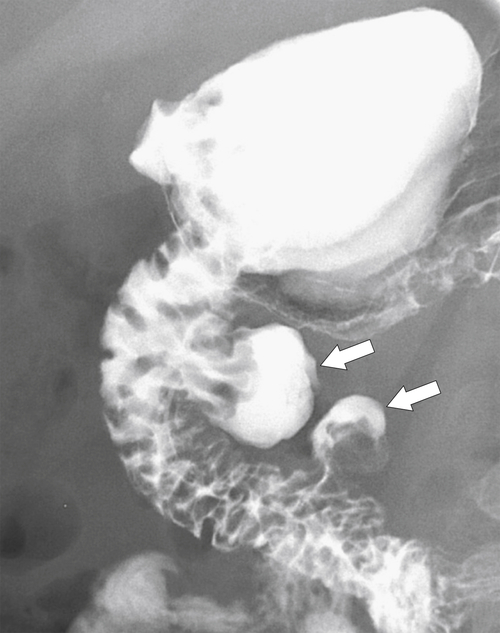
Figure 3-10 UGI series in a 38-year-old man with two medial duodenal diverticula (arrows).
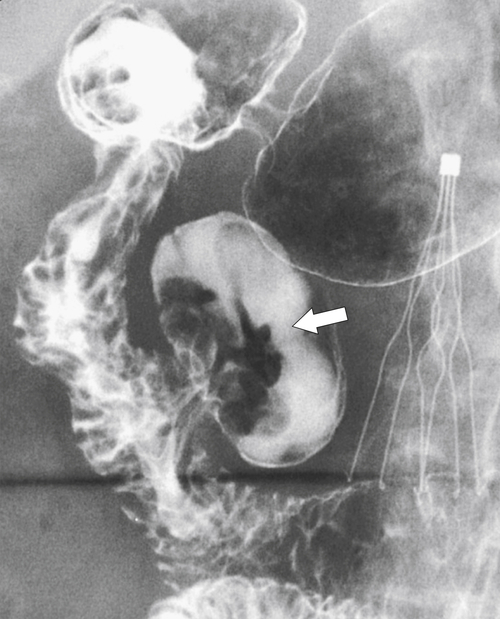
Figure 3-11 UGI series in a 65-year-old man with a large medial duodenal diverticulum (arrow). Duodenal folds can be seen entering the diverticulum. There is an incidental inferior vena cava filter in situ.
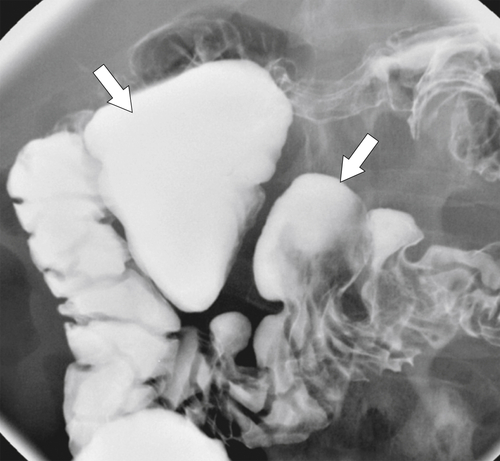
Figure 3-12 UGI series in a 61-year-old woman with multiple duodenal diverticula (arrows).
Pancreatic Rests
Also known as ectopic pancreas, pancreatic rests are congenital remnants of pancreatic tissue that are most commonly found in the first and second part of the duodenum, although they have also been reported elsewhere in the small bowel and stomach. They are clinically insignificant and usually asymptomatic. They are identified at UGI series or EGD, where they appear as a smooth round or lobulated mucosal filling defect approximately 1 to 2 cm in diameter. A classic central dimple filled with barium, representing the pancreatic ductule remnant, may or may not be present, but it is diagnostic if it is present (Fig. 3-13).
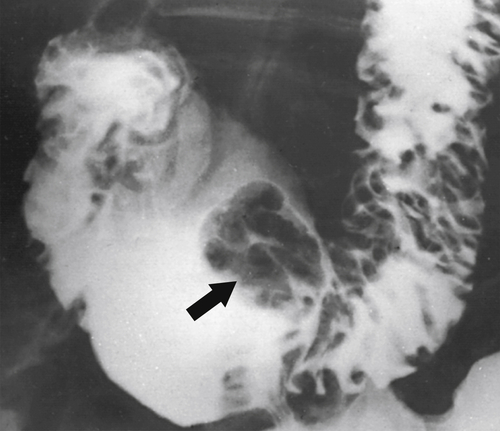
Figure 3-13 UGI series demonstrating a small duodenal bulb mass with a central barium collection caused by a pancreatic rest (arrow).
Annular Pancreas
Annular pancreas is an embryological abnormality of pancreatic development that results in the narrowing of the second part of the duodenum because of circumferential constriction by the pancreas. Normal embryological pancreatic development involves a single dorsal and two ventral buds (which fuse early). With normal intestinal rotation at about 7 weeks of gestation, the fused ventral bud rotates behind the duodenum from right to left to fuse with the dorsal bud and forms part of the pancreatic head and the uncinate. Failure of the ventral bud to rotate normally leaves the duodenum encircled by pancreatic tissue. It may remain asymptomatic, particularly with incomplete forms, but complete forms, which may not become symptomatic until adulthood, may require a gastroenterostomy rather than the simple release of the pancreatic annular tissue. At UGI series there is a characteristic narrowed, band-like, and uniform circumferential stricture in the midportion of the second part of the duodenum (Fig. 3-14). CT readily identifies the circumferential pancreatic tissue (Fig. 3-15).
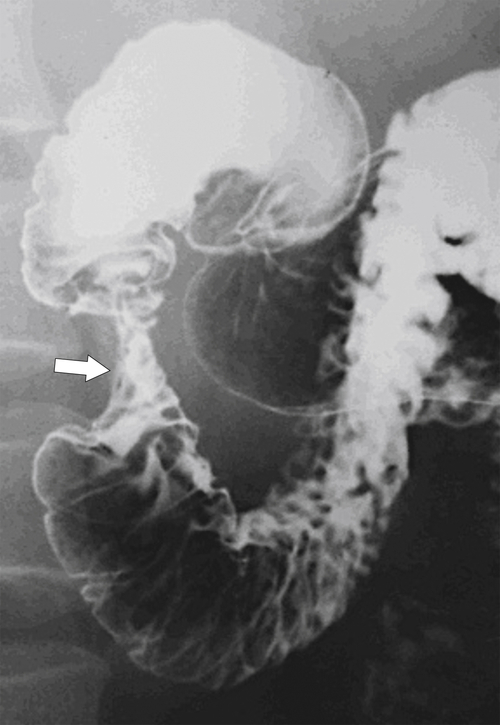
Figure 3-14 UGI series in a 45-year-old woman with a stricture of the second part of the duodenum (arrow) caused by an annular pancreas.
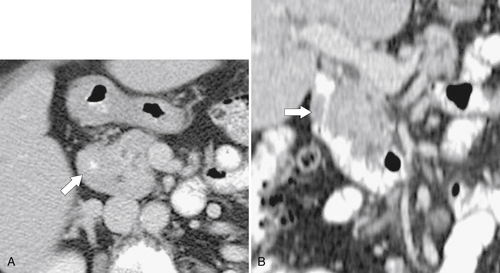
Figure 3-15 Axial (A) and coronal (B) contrast-enhanced CT in a 73-year-old woman with pancreatic annular pancreas (arrows) that almost completely occludes the duodenal lumen.
Superior Mesenteric Artery Syndrome
Although SMA syndrome is a congenital abnormality, it usually presents in adulthood, often after significant weight loss from severe acute illness. The theory is that as the retroperitoneal fat is metabolized, the angle between the proximal SMA and aorta becomes more acute, thereby compressing the duodenum that passes left to right immediately beneath this angle. Duodenal and gastric distention then occurs near the obstruction. The diagnosis is made in the correct clinical setting with contrast-enhanced CT demonstrating the features of a dilated duodenum, marked narrowing of the duodenum as it passes behind the SMA, and a relative paucity of retroperitoneal fat (Fig. 3-16). Duodenal narrowing at the level of the SMA, though, can also be a normal finding, and correlation with clinical symptoms is required.
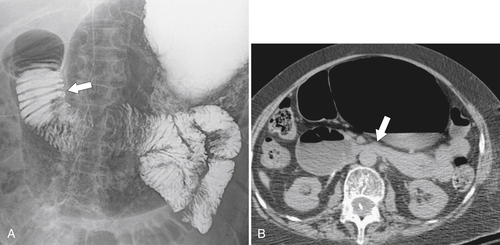
Figure 3-16 UGI (A) and axial (B) contrast-enhanced CT in a 65-year-old woman demonstrating gastric and duodenum dilatation (arrow in A) and stricture formation (arrow in B) due to superior mesenteric artery syndrome.
Duodenal Bulb Disease
There are a finite number of duodenal bulb diseases, most of which are benign (Box 3-1). Malignant lesions are rare. Normal anatomical structures can impress upon the duodenum (colon and gallbladder), which creates a smooth effacement of the duodenal bulb (Fig. 3-17).
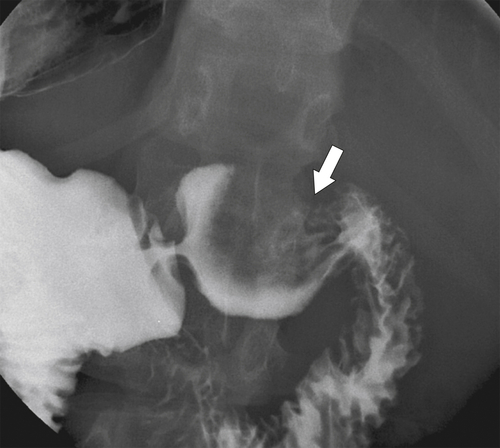
Figure 3-17 UGI series in a 39-year-old woman with smooth effacement of the duodenal bulb (arrow) caused by duodenal compression by the gallbladder.
Heterotopic Gastric Mucosa
Heterotopic gastric mucosa represents ectopic gastric mucosa most often situated in the duodenal bulb, although it can occur in the esophagus, jejunum, and even ileum. It can be recognized usually only at double-contrast UGI (or EGD) as small, plaque-like filling defects in a mosaic-like pattern (Fig. 3-18).
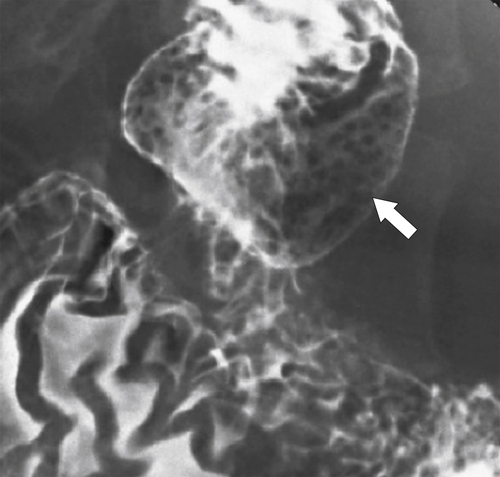
Figure 3-18 UGI in a 61-year-old woman with multiple small filling defects in the duodenal bulb (arrow) caused by ectopic gastric mucosa.
Brunner Gland Hyperplasia
Brunner glands secrete alkali, protecting the proximal small intestine from the hyperacidity produced by the gastric mucosa. The cause of their enlargement is uncertain, but multiple Brunner glands can be seen in the duodenal bulb, extending for a short distance into the second part of the duodenum. They are visualized on UGI series, particularly with double-contrast views, which demonstrate multiple small (<5 mm) nodular filling defects (strawberry-like) in the distended duodenal bulb (Fig. 3-19). When there is only one, it is known as Brunner gland adenoma or hamartoma. This can have an appearance similar to flexural pseudotumor if situated at the inferior margin between the duodenal bulb and second part of the duodenum.
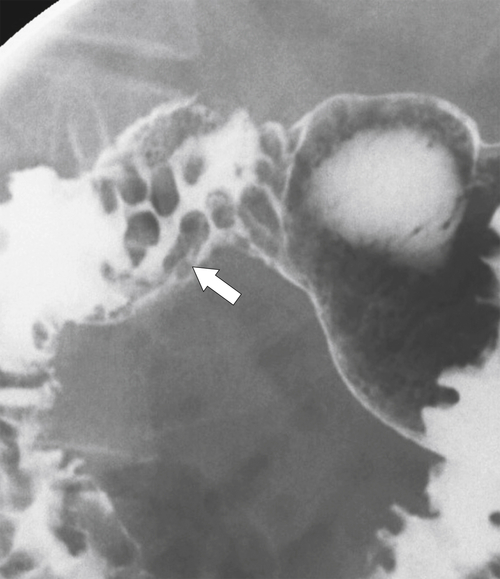
Figure 3-19 UGI series in a 55-year-old man with multiple nodular filling defects in the duodenal bulb caused by Brunner gland hypertrophy (arrow).
Duodenal Flexural Pseudotumor or Pseudopolyp
Duodenal flexural pseudotumor or pseudopolyp is a normal variant in which the angle between the duodenal apex and the descending duodenum is particularly acute, and it is more common in thin, asthenic individuals. The duodenal mucosa on the inner (inferior) aspect of this abrupt angle is sometimes heaped up into the duodenal lumen, giving the spurious appearance of a mass (Fig. 3-20). Repositioning the patient on the fluoroscopy table, though, should diminish the abnormality or make it disappear altogether, which clarifies the diagnosis. At CT, it can appear as a definitive duodenal mass or polyp.
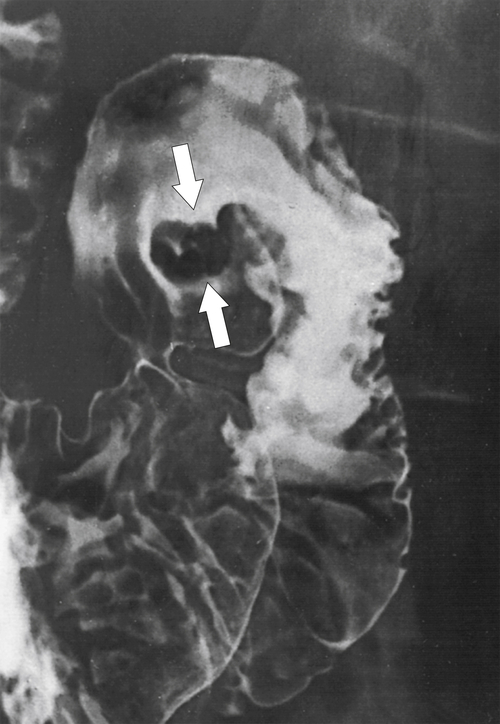
Figure 3-20 UGI series demonstrating a smooth duodenal polyp, identified as a duodenal flexural pseudopolyp (arrows).
Duodenitis
Most causes of gastritis also cause duodenitis because of the duodenum’s proximity to the stomach (see Chapter 2). The imaging features also mimic features of gastritis, including fold thickening and erosions (Fig. 3-21). More severe ulceration can progress to a duodenal ulcer. At CT, there is duodenal wall thickening and thickened folds, but these findings are nonspecific (Fig. 3-22).
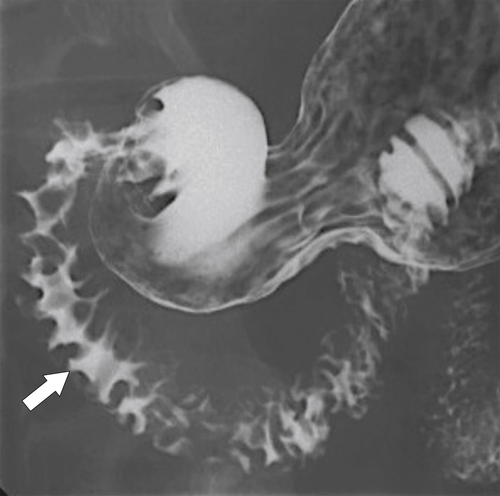
Figure 3-21 UGI in a 22-year-old woman with antral and duodenal mucosal thickening caused by gastritis (arrow) and secondary duodenitis.
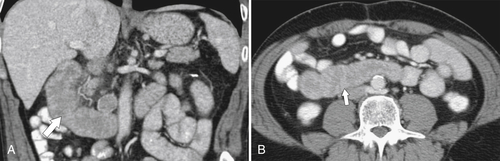
Figure 3-22 Coronal (A) and axial (B) contrast-enhanced CT in a 49-year-old woman with duodenal wall thickening (arrows) caused by duodenitis.
Duodenal Ulcer
Although it still an important disease, duodenal ulcer is far less common now that H. pylori is known as a causative agent and its treatment is well understood. Furthermore, most patients are now evaluated by EGD, so radiologists will now be unlikely to see many duodenal ulcers in the course of their clinical work.
Duodenal ulcers are two to three times more common than gastric ulcers, but unlike gastric ulcers, almost all duodenal ulcers are benign. Approximately 95% occur in the duodenal bulb, with 5% being postbulbar. Ulcers arise from gastric hyperacidity or other caustic agents such as alcohol or drugs, especially aspirin and nonsteroidal antiinflammatory agents. The disease is frequently associated with the features of peptic disease in the stomach (concomitant gastric fold thickening is common).
Their appearance is best evaluated with single-contrast and double-contrast UGI series, where a persistent pooling of contrast material within the ulcer crater can be observed, especially when the ulcer crater is in the dependent position. There are smooth folds that converge on the acute ulcer (Fig. 3-23). Some ulcers form beyond the bulb and are termed postbulbar ulcers (Fig. 3-24). Most ulcers cannot be seen with CT, but deep ones are occasionally identified (Fig. 3-25). Postbulbar ulcers are more common in patients with severe gastric hyperacidity such as Zollinger-Ellison syndrome (see Chapter 2). The hyperacidity can be so severe that duodenitis continues into the fourth part of the duodenum and even into the jejunum (Fig. 3-26).
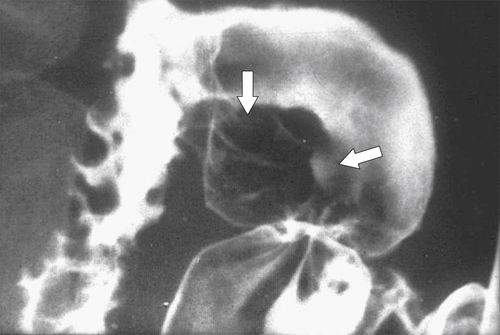
Figure 3-23 UGI series in a 44-year-old man with thickened folds (vertical arrow) radiating toward an ulcer crater (horizontal arrow) from an acute duodenal ulcer.
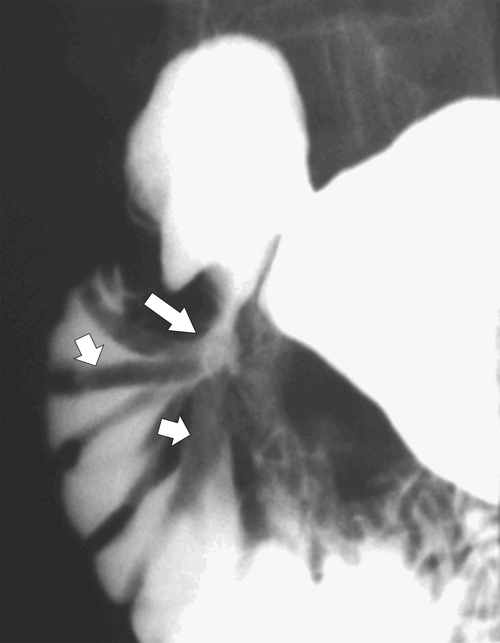
Figure 3-24 UGI series in a 61-year-old woman with a postbulbar ulcer (large arrow) and radiating thickened duodenal folds (small arrows).
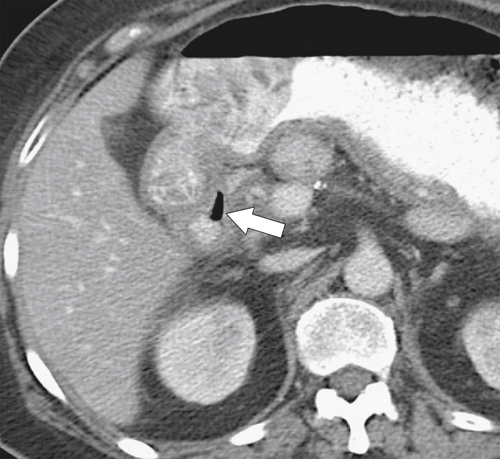
Figure 3-25 Axial contrast-enhanced CT in a 46-year-old woman with a perforated duodenal ulcer. There is wall thickening of the proximal duodenal wall, mild surrounding edema, and a sliver of extraluminal gas (arrow).
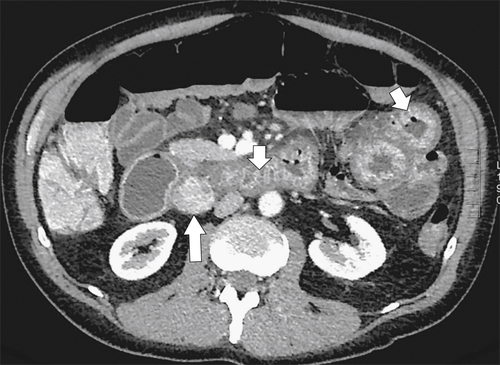
Figure 3-26 Axial contrast-enhanced CT in a 36-year-old man with a duodenal gastrinoma (large arrow) and Zollinger-Ellison syndrome with marked duodenal and jejunal mucosal thickening (small arrows).
The ulcer typically heals by fibrosis distorting the normal architecture of the duodenal bulb, although both acute ulcers and chronic findings can be seen at the same time (Fig. 3-27). Occasionally the ulcers can be very large, known as giant duodenal ulcers, and may mimic the configuration of the bulb itself, potentially leading the radiologist to think that the ulcer is a normal bulb. There should, however, be no duodenal folds within the ulcer itself, and there is persistent pooling of contrast rather than the contrast passing on down the duodenum from the normal peristaltic waves.
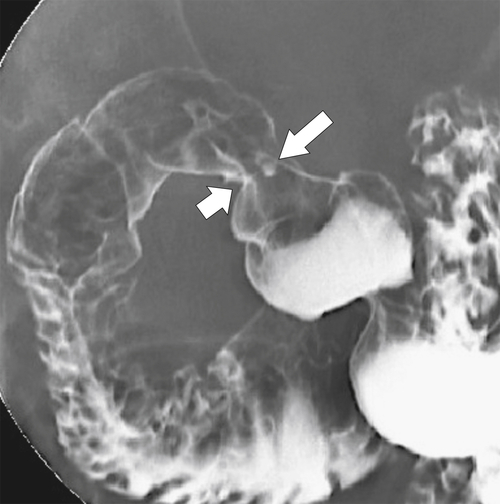
Figure 3-27 UGI series in a 66-year-old woman with proximal duodenal narrowing and distortion (large arrow) caused by healing from chronic duodenal ulcer disease. A tiny acute ulcer is also present (small arrow).
The most serious complication is perforation caused by transmural penetration of the ulcer, which mainly occurs into the peritoneum because most (95%) bulbar ulcers are on the anterior wall. Less commonly, retroperitoneal perforation occurs from the rarer posterior wall ulcers. Subtle perforation may require CT analysis with lung “window” contrast settings; otherwise, subtle extraluminal gas may be missed. Perforation can also cause severe hemorrhage with profuse hematemesis or melena. The ulcer itself is often not identified at CT, but the diagnosis is strongly suggested by duodenal mucosal thickening and periduodenal inflammation (stranding), fluid, and gas (Fig. 3-28). Sometimes there is profuse gas that can be both intraperitoneal and extraperitoneal (Fig. 3-29).
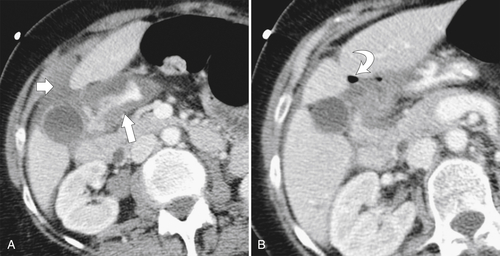
Figure 3-28 Axial CT in a 60-year-old woman with duodenal perforation as evidenced by mucosal thickening (A; arrow) and periduodenal fluid (A; small arrow) and pockets of extraluminal gas (B; curved arrow).
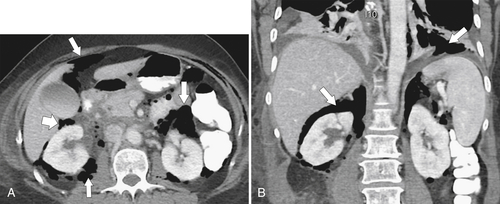
Figure 3-29 Axial (A) and coronal (B) contrast-enhanced CT in a 62-year-old woman with a perforated duodenal ulcer and diffuse retroperitoneal and peritoneal gas (arrows).
Thickened Duodenal Folds
Duodenal mucosal thickening is generally not confined to the bulb unless it is due to peptic ulcer disease. There are several disparate causes, most of which are responsible for mucosal thickening of the gastrointestinal tract elsewhere (Box 3-2). Many intrinsic or extrinsic inflammatory conditions can affect a part of or the entire duodenum. The second part of the duodenum surrounds the pancreatic head, so diseases of the pancreas can have a direct effect on the duodenal wall and mucosa. Pancreatitis frequently causes gastric and duodenal wall inflammation and is usually visualized by CT or UGI series (Figs. 3-30 and 3-31). At UGI series, the medial wall can appear tethered on its medial wall by the inflammatory process, causing medial spiculation that has been described as a “reverse 3” pattern that is also recognized with pancreatic adenocarcinoma. More chronic pancreatic pseudocyst changes can result in duodenal stricture formation (Fig. 3-32). Groove pancreatitis (see Chapter 9), occurring in the duodenal-pancreatic groove, will inevitably cause duodenal thickening (Fig. 3-33). Other adjacent anatomical structures, including the gallbladder (cholecystitis) and colon (diverticulitis), can cause the same effect (Fig. 3-34). Adjacent malignant infiltration may cause nonspecific duodenal thickening early on, but as further duodenal invasion occurs, the duodenal lumen and mucosa will be distorted (Fig. 3-35).
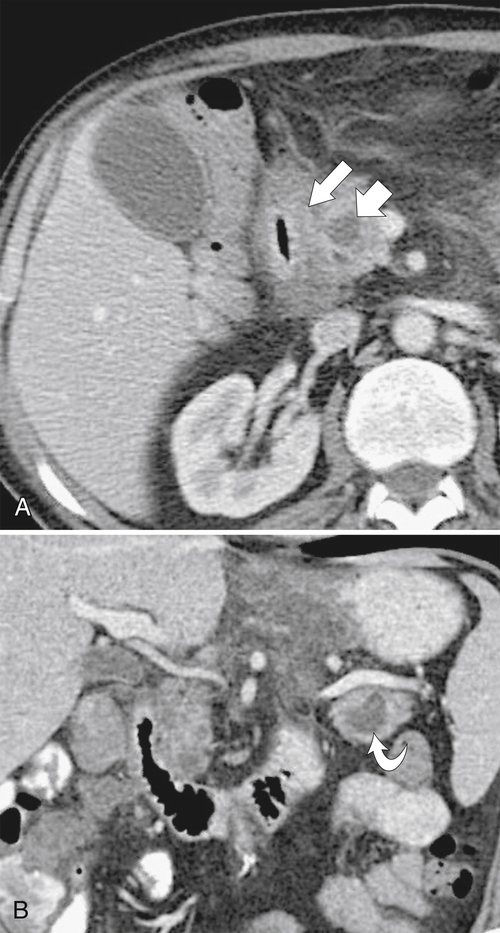
Figure 3-30 Axial (A) and coronal (B) contrast-enhanced CT in a 52-year-old man with duodenal wall thickening (large arrow) and luminal narrowing due to acute pancreatitis. There are small fluid collections (small arrow) in the pancreatic head and tail (curved arrow).
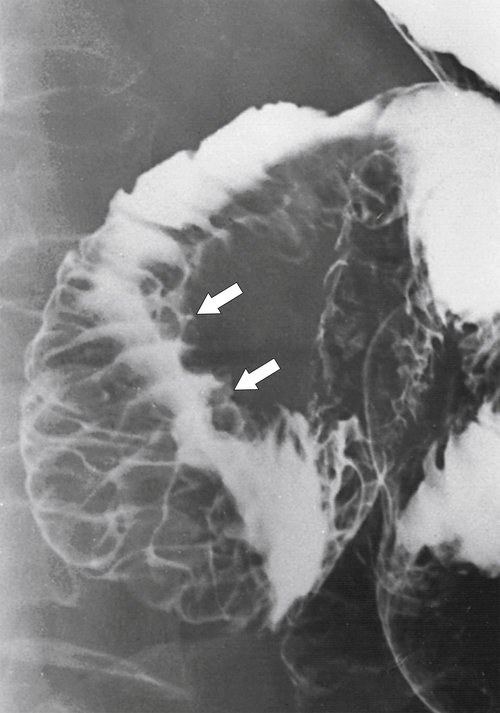
Figure 3-31 UGI series demonstrating marked duodenal wall and mucosal thickening caused by acute pancreatitis (arrows). There is a “reverse 3” pattern.
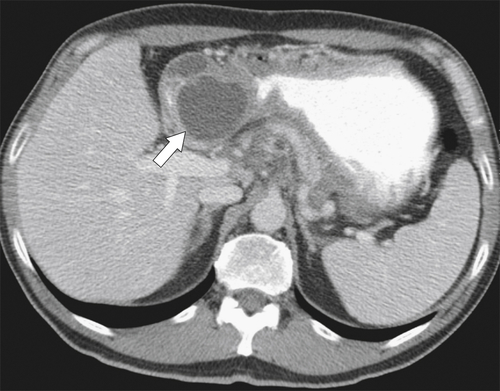
Figure 3-32 Axial contrast-enhanced CT in a 52-year-old man with pancreatic pseudocyst (arrow) and compression of the second part of the duodenum.
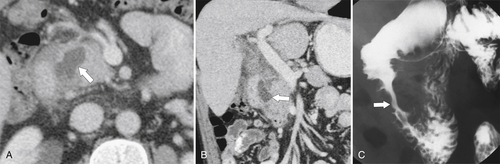
Figure 3-33 Axial (A) and coronal (B) CT and UGI series (C) in a 44-year-old man with inflammation and cystic formation in the pancreatic-duodenal space caused by “groove” pancreatitis (arrows).
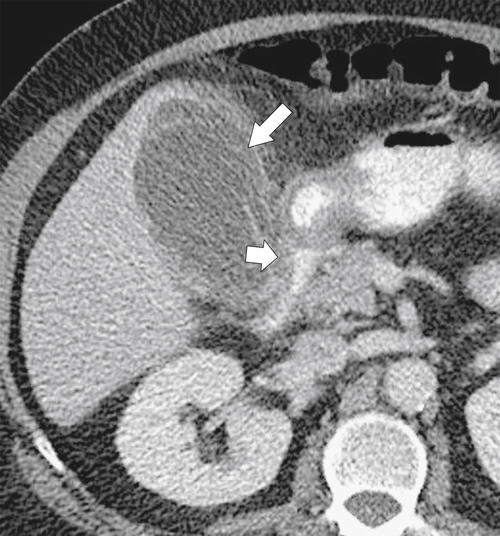
Figure 3-34 Axial contrast-enhanced CT in 79-year-old woman with acute cholecystitis (large arrow) with duodenal wall thickening and narrowing (small arrow).
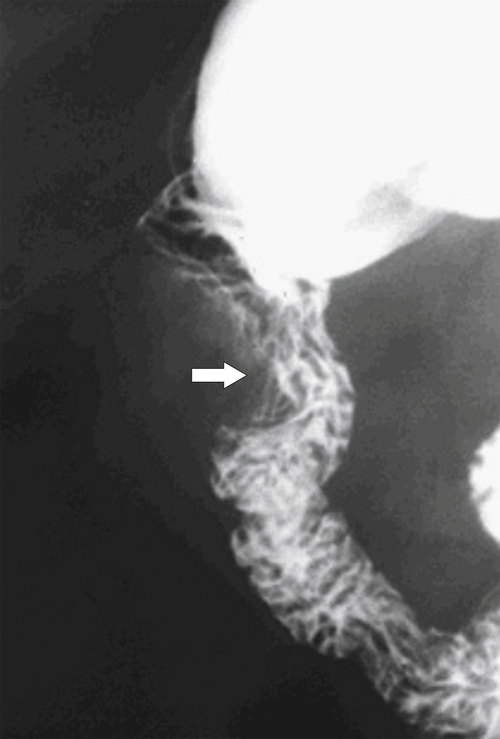
Figure 3-35 UGI series in a 63-year-old man with duodenal distortion (arrow) but general preservation of mucosal folds caused by early metastatic invasion by an adjacent hepatic flexure colon cancer.
Infections
Tuberculosis (see Chapters 2, 4, and 5), as elsewhere in the GI tract, can produce acute disease with mucosal thickening and ulceration. It also typically heals with fibrosis and stricture formation, leading to proximal duodenal dilatation.
Many parasites can infest the duodenum, in particular, Strongyloides stercoralis and Ancylostoma duodenale (hookworm) and cause markedly thickened duodenal folds. Ascaris lumbricoides more often affects the distal small bowel, but because of its endemic infestation in many developing countries, it is not uncommonly identified in the duodenum. It is also purported to be one of the most common causes worldwide of pancreatitis as a result of the worm tunneling into the pancreatic and/or bile duct and occluding pancreatic secretions from reaching the duodenum. The pancreatitis itself will further contribute to the duodenal fold thickening. The worms are recognized as tubular, elongated filling defects at UGI series or CT (Fig. 3-36).
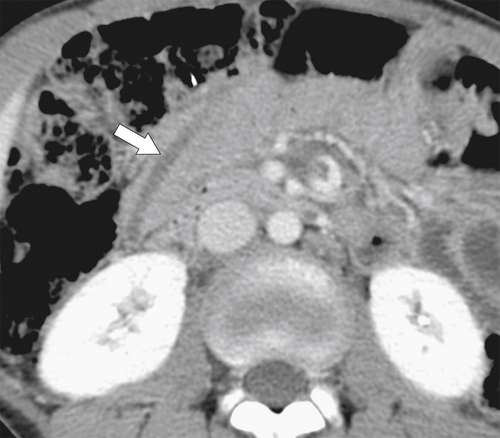
Figure 3-36 Axial contrast-enhanced CT in a 12-year-old boy with Ascaris worm in the second part of the duodenum (arrow).
Giardia lamblia is another common worldwide parasite that infests much of the local population. The protozoan leads to diffuse duodenal and jejunal mucosal thickening, usually with hypersecretion and hypermotility and associated lymphoid hyperplasia (see Chapter 4) (Figs. 3-37 and 3-38).
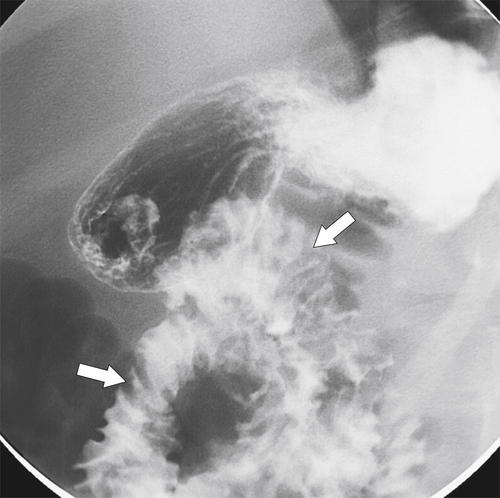
Figure 3-37 UGI series in a 10-year-old boy with diffuse duodenal and jejunal thickening (arrows) caused by giardiasis.
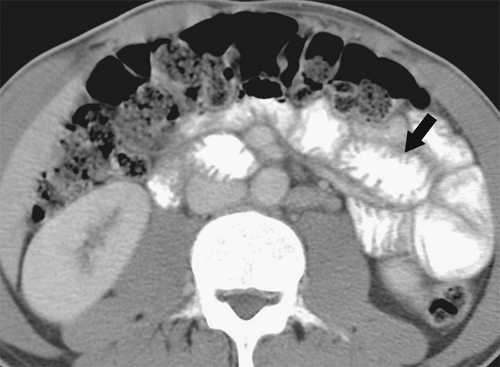
Figure 3-38 Axial contrast-enhanced CT in a 29-year-old woman with giardiasis. There is duodenal and jejunal mucosal thickening (arrow).
Caustic Ingestion
Large volumes of caustic ingestion can reach the duodenum and will cause a profound duodenitis. Perforation may occur, but this is usually more proximal in the esophagus or stomach. Fibrotic healing can lead to fixed strictures (see Fig. 3-31).
Crohn Disease (see Chapter 4)
Crohn disease that involves the duodenum usually manifests as thickened folds and ulceration rather than stricture and fistula formation, although these are well-recognized complications (Fig. 3-39). There is usually associated involvement of the terminal ileum, the colon, or both.
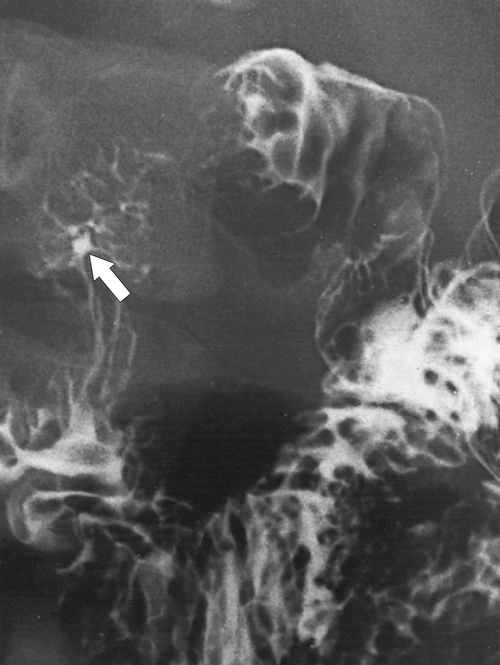
Figure 3-39 UGI series in a patient with duodenal Crohn disease demonstrating proximal fold irregularity and ulceration (arrow).
Duodenal Varices
Duodenal varices are rare and result from marked portal hypertension but are a recognized cause of duodenal mucosal thickening, which may be serpiginous in appearance as the dilated veins course through the duodenum.
Portal Hypertension
Any cause of portal hypertension (cirrhosis, portal vein thrombus) can cause duodenal mucosal thickening because of elevated venous pressure (Fig. 3-40).
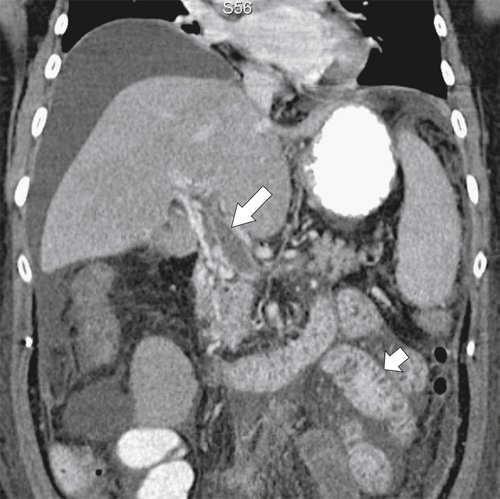
Figure 3-40 Coronal contrast-enhanced CT in a 68-year-old woman with a portal vein thrombus (large arrow) and diffuse duodenal and jejunal mucosal thickening (small arrow). There is associated ascites.
Lymphoid Hyperplasia
Lymphoid hyperplasia is recognized as multiple tiny filling defects within the duodenum and can be a normal variant in children. When seen in adults, though, it is usually associated with giardiasis and hypogammaglobulinemia.
Duodenal Papillitis
When inflamed, the duodenal papilla can protrude into the duodenal lumen with the appearance of a smooth-walled mass. It is usually a result of an impacted gallstone or pancreatic stone.
Duodenal Hemorrhage
Hemorrhage into the duodenal wall can result spontaneously from trauma, in patients with bleeding diatheses or those taking anticoagulants, and from Henoch-Schönlein purpura (see Chapter 4). The findings can present as either an intramural mass or thickened mucosal folds (Fig. 3-41). Traumatic duodenal injury can result in marked hemorrhage, particularly as the duodenum is relatively fixed in the retroperitoneum and susceptible to acceleration/deceleration forces (Fig. 3-42). At imaging there are thickened and crowded mucosal folds due to the intramural hemorrhage, which at UGI series are thought to resemble a picket fence (see Chapter 4). CT features may even demonstrate a hyperdense duodenal wall, particularly if the patient has been imaged without oral and intravenous contrast material.
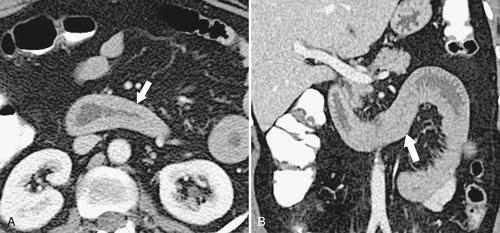
Figure 3-41 Axial (A) and coronal (B) contrast-enhanced CT in a 43-year-old woman with duodenal hemorrhage and diffuse duodenal thickening (arrows).
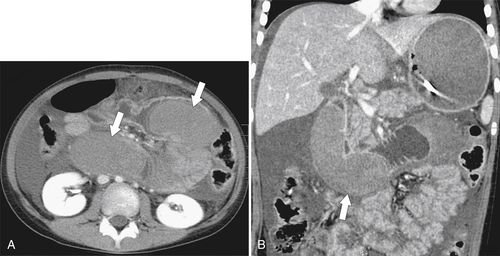
Figure 3-42 Axial (A) and coronal (B) contrast-enhanced CT in a 22-year-old woman after a motor vehicle accident, demonstrating duodenal widening with luminal hemorrhage (arrows), mucosal thickening, and ascites.
Duodenal Masses
Most benign and malignant duodenal masses are common to other lesions elsewhere in the GI tract, although there are some that are unique to the duodenum (Table 3-1).
Table 3-1
Duodenal Masses
| Congenital | Pancreatic rest Polyposis syndromes |
| Normal variant | Flexural pseudopolyp Brunner gland hypertrophy |
| Neoplasia | |
| Intrinsic | Mesenchymal: GIST, neurofibroma, hemangioma, lipoma Polyps (adenoma, hamartoma) Carcinoid Carcinoma Kaposi sarcoma |
| Extrinsic | Metastases Lymphoma Pancreatic, gallbladder, colonic, renal, adrenal extension |
| Inflammatory | Pancreatitis including fluid collections |
| Extrinsic compression | Gallbladder, hepatic, choledochal cyst, retroperitoneal lymphadenopathy |
GIST, Gastrointestinal stromal tumor.
Submucosal Mesenchymal Duodenal Tumors
Gastrointestinal Stromal Tumor (see Chapter 2)
The duodenum is the second most common site for gastrointestinal stromal tumor (GIST) after the stomach and has similar features to gastric GISTs. These tumors are usually exophytic and eccentric and spare the mucosa, although larger tumors may cause mucosal ulceration. These larger tumors tend to also undergo central necrosis (Fig. 3-43).
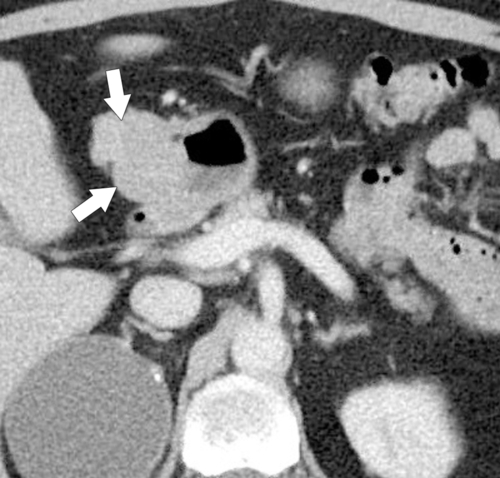
Figure 3-43 Axial contrast-enhanced CT in a 56-year-old man with a benign GIST of the first part of the duodenum (arrows). The mass is mainly exophytic.
Other Benign Submucosal Duodenal Lesions
Duodenal lipomas are relatively common submucosal tumors, and given their malleability, they often appear intraluminal. They are readily characterized at CT because of their fat density (Fig. 3-44). Other rare mesenchymal tumors include hemangioma, lymphangioma, fibroma, and neurofibroma, all of which usually demonstrate features of submucosal lesions (Fig. 3-45).
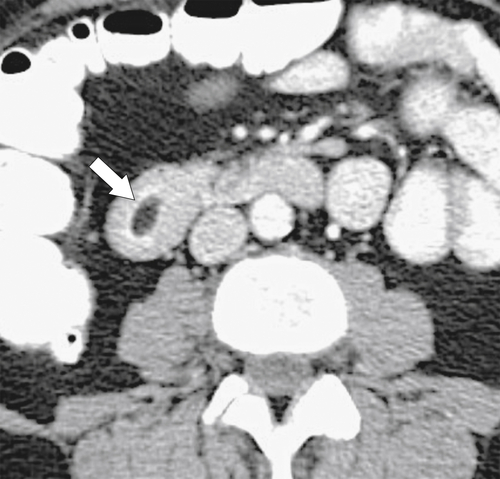
Figure 3-44 Axial contrast-enhanced CT in a 44-year-old man with a duodenal lipoma. CT confirms the mass with fat density (arrow).
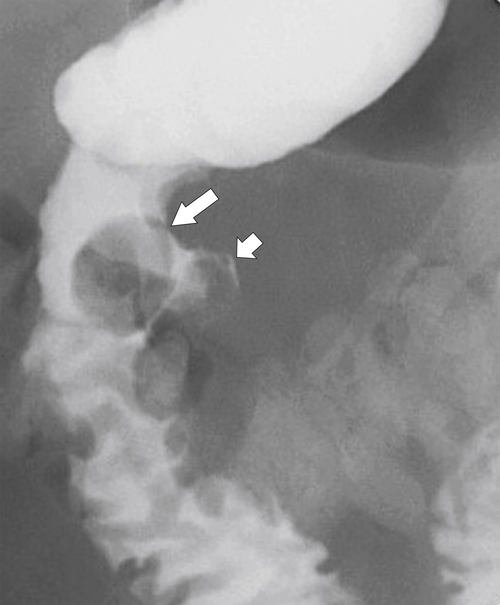
Figure 3-45 UGI in a 63-year-old man with a submucosal mass (large arrow) caused by a duodenal lymphangioma. There is an associated microperforation (small arrow) after recent upper endoscopy and biopsy of the mass.
Duodenal Carcinoid
These are submucosal (intramural), usually situated close to the duodenal ampulla, and can range from smooth polyps to irregular invasive masses (Fig. 3-46). In general, they do not arise from enterochromaffin cells, unlike their jejunal and ileal counterparts, and therefore rarely produce serotonin and carcinoid syndrome. Most are gastrin cell (G cell) tumors and therefore some manifest as Zollinger-Ellison syndrome. They are associated with multiple endocrine neoplasia (MEN-1) and neurofibromatosis (NF-1).
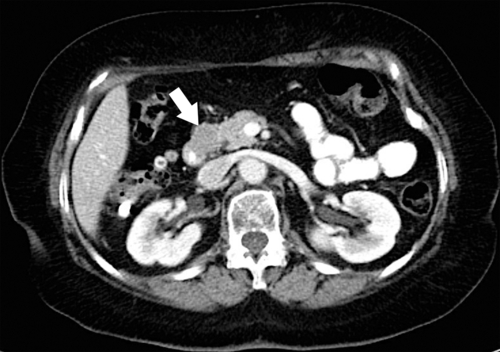
Figure 3-46 Axial contrast-enhanced CT in a 42-year-old man with an eccentric 2.5-cm duodenal mass (arrow) caused by carcinoid.
Duodenal Polyps
Adenomatous polyps are similar to those elsewhere in the small and large bowel, and these are premalignant, especially if greater than 2 cm. Most are tubular with tubulovillous and villous being less common (Fig. 3-47). Patients usually have GI bleeding or obstructive symptoms from intussusception. Adenomatous polyps are more readily recognized at UGI rather than at CT as an irregular polypoid mass, usually close to the duodenal bulb or medial aspect of the second part of the duodenum (Fig. 3-48). Occasionally they appear as a more solid mass (Fig. 3-49).
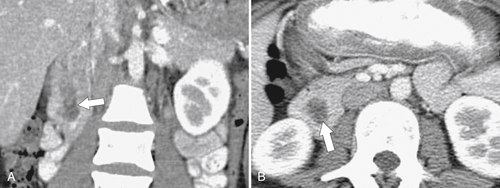
Figure 3-47 Coronal (A) and axial (B) contrast-enhanced CT in a 72-year-old man with a filling defect in the second part of the duodenum (arrows) caused by a duodenal villous adenoma.
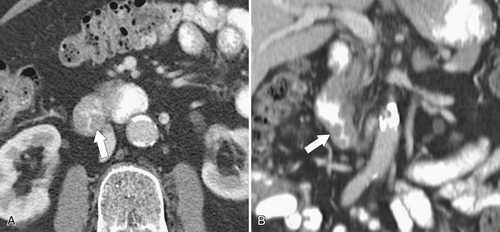
Figure 3-48 Axial (A) and coronal (B) contrast-enhanced CT in a 55-year-old woman with circumferential duodenal wall thickening (arrows) due to an adenoma.
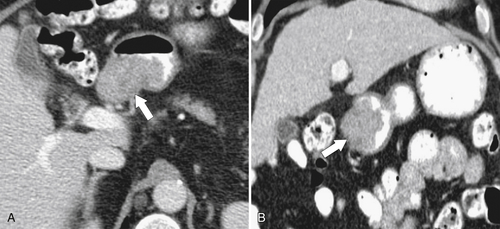
Figure 3-49 Axial (A) and coronal (B) contrast-enhanced CT in a 59-year-old man with an eccentric duodenal mass (arrows) due to an adenoma.
Hyperplastic polyps are rare benign epithelial sessile polyps, usually small and multiple within the duodenum. They are far less common than gastric hyperplastic polyps.
Familial polyposis, Peutz-Jeghers syndrome (hamartomatous polyps), Cronkhite-Canada syndrome, and Cowden’s disease (see Chapter 5) can all demonstrate duodenal polyposis, although at contrast fluoroscopy, it is not possible to distinguish them. Usually a family history or other manifestations of the disease (e.g., mucocutaneous pigmentation in Peutz-Jeghers syndrome) are a clue to the diagnosis.
Duodenal Carcinoma
Duodenal carcinomas are rare, although they are the most common malignant duodenal tumors. They develop from the adenoma-carcinoma sequence (see Chapter 5), so all duodenal adenomas should be considered premalignant. Other predisposing factors include familial polyposis syndromes, particularly Gardner syndrome. They present with upper abdominal pain, nausea, vomiting, and malignant symptoms of weight loss and anemia. At imaging, they have characteristic features similar to adenocarcinoma elsewhere in the GI tract. Most are in the mid to distal duodenum and present with an irregular intraluminal mass (with or without ulceration) or an apple core appearance, especially if presenting late (Figs. 3-50 and 3-51). Malignancy is confirmed if there is associated regional lymphadenopathy.
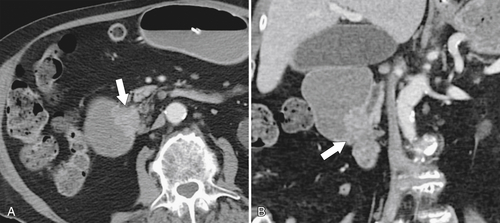
Figure 3-50 Axial (A) and coronal (B) contrast-enhanced CT in a 55-year-old woman with a duodenal malignant stricture (arrow in A) caused by duodenal adenocarcinoma and resulting in duodenal obstruction. There is also common bile duct dilatation secondary to the mass that involves the ampulla (arrow in B).
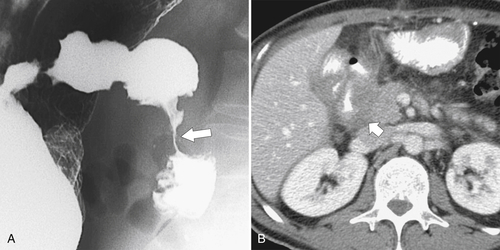
Figure 3-51 UGI (A) and axial (B) contrast-enhanced CT in a 65-year-old woman with duodenal adenocarcinoma and a malignant duodenal stricture, a circumferential duodenal wall mass (large arrow) that invades the pancreatic head (short arrow).
Malignant Gastrointestinal Stromal Tumor (see Chapter 2)
A malignant GIST, like one elsewhere in the GI tract, presents as a predominantly soft tissue mass that may become necrotic as it enlarges (Fig. 3-52). Some are predominantly eccentric to the affected GI tract. It frequently metastasizes to the liver, often as cystic metastases (see Chapter 6). Surgery to remove the primary tumor is the principal treatment, but c-kit tyrosine kinase inhibitors (i.e., imatinib) can produce dramatic therapeutic responses in patients with metastatic disease, sometimes within days.
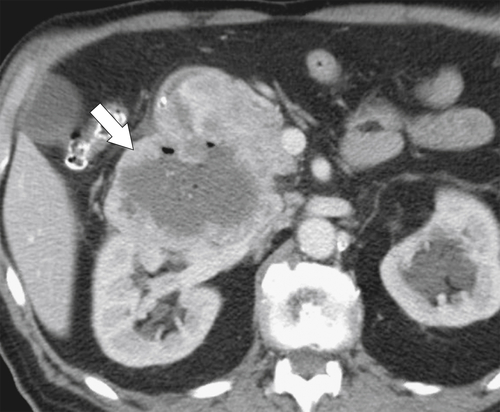
Figure 3-52 Contrast-enhanced CT in a 56-year-old man with a 9-cm heterogeneous and partially necrotic mass (arrow) caused by a duodenal malignant GIST.
Duodenal Lymphoma
Duodenal lymphoma is usually associated with lymphoma elsewhere and only very rarely involves the duodenum as primary non-Hodgkin disease. Either there is a diffuse polypoid mass, infiltrating mucosal thickening without mass formation, or it appears as an irregular, ill-defined, infiltrating mass (Figs. 3-53, 3-54, and 3-55). As elsewhere in the GI tract, the lymphomatous mass does not usually cause luminal obstruction.
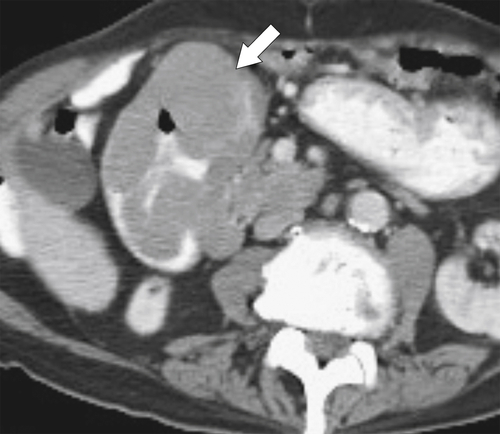
Figure 3-53 Axial contrast-enhanced CT in a 77-year-old woman with a circumferential lobulated mass (arrow) in the second part of the duodenum caused by duodenal lymphoma.
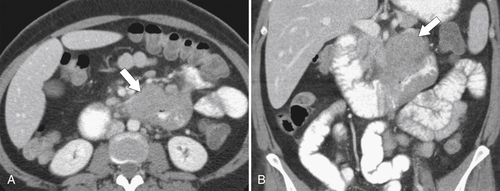
Figure 3-54 Axial (A) and coronal (B) contrast-enhanced CT in a 47-year-old woman with duodenal stricture (arrows) (but no obstruction) caused by lymphoma.
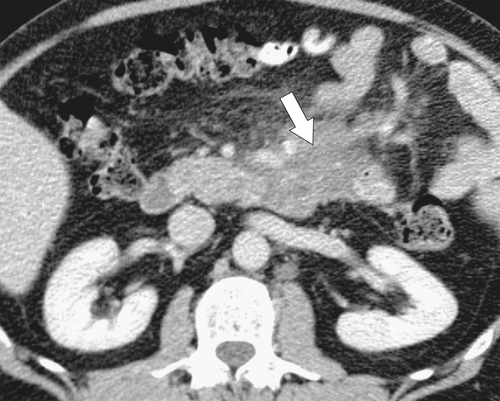
Figure 3-55 Axial contrast-enhanced CT in a 55-year-old man with duodenal lymphoma and an ill-defined irregular mass (arrow) surrounding the third part of the duodenum.
Kaposi Sarcoma
Kaposi sarcoma usually involves the stomach or more distal small bowel, but occasionally the duodenum is involved, demonstrating multiple small rounded polyps, often associated with ulceration. Distinguishing these lesions from other causes of polyps can be difficult by imaging, but a clinical history of acquired immune deficiency syndrome (AIDS) and the presence of cutaneous violaceous lesions are indicators to the diagnosis.
Metastases
Most commonly, metastases are a result of direct extension from adjacent malignancies, particularly pancreatic carcinoma and, less often, colon and gallbladder cancer (Fig. 3-56). Less commonly, metastatic disease is hematogenous (melanoma, breast, lung), producing submucosal masses that may ulcerate and produce a bull’s-eye lesion or “target” lesion, similar to those seen in the stomach (Fig. 3-57).
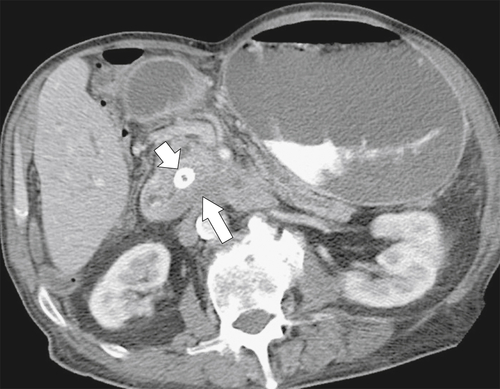
Figure 3-56 Axial contrast-enhanced CT in a 74-year-old man with duodenal obstruction (leading to gastric dilatation) from direct duodenal invasion from pancreatic carcinoma (large arrow). There is a biliary stent in situ (small arrow).
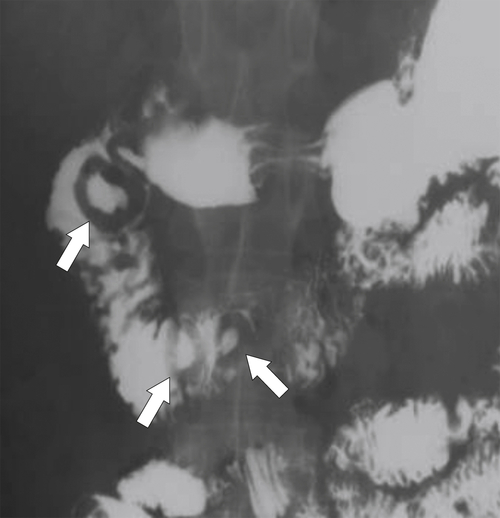
Figure 3-57 UGI series in a 47-year-old man with a melanoma metastasis in the second part of the duodenum (arrows) with bull’s-eye–appearing lesions.
Dilated Duodenum
Similar to the GI tract elsewhere, dilated duodenum can be mechanical or functional (Table 3-2). Functional causes rarely result in absolute obstruction; rather, the duodenum distends and transit time is prolonged (Table 3-3).
Table 3-2
Mechanical Duodenal Obstruction
| Congenital | Atresia Webs Duplication Diverticulum Annular pancreas Midgut volvulus |
| Inflammatory | Peptic ulcer disease Crohn disease Pancreatitis Cholecystitis |
| Infectious | Tuberculosis |
| Vascular | Hemorrhage (bleeding diatheses, Henoch-Schönlein purpura) Superior mesenteric artery (SMA) syndrome Aorticoduodenal fistula |
| Neoplastic | Primary (carcinoma, lymphoma) Secondary Local (pancreas, renal, gallbladder, colon) Distant (melanoma, breast) |
Table 3-3
Functional Duodenal Dilatation
| Neuromuscular | Scleroderma, idiopathic intestinal pseudoobstruction, diabetes, Chagas disease |
| Drugs (anticholinergics) | Narcotic, anticholinergics |
| Sprue | Dilatation and delayed transit |
| Severe illness | Burns, shock, trauma |
| Adynamic ileus | Postsurgical |
Duodenal Trauma
Most noniatrogenic duodenal injuries occur in the second or third part of the duodenum because of its fixation by the retroperitoneum. These injuries are either blunt (motor vehicle accidents) or penetrating (stab or gunshot). The investigation of choice is contrast-enhanced CT, which demonstrates intramural duodenal hematoma and extravasation of contrast material from the gastroduodenal artery if the trauma is severe (see Fig. 3-42). There may be duodenal rupture with extraluminal extravasation of oral contrast media (Fig. 3-58). Hepatic, pancreatic, and splenic lacerations are frequently associated.
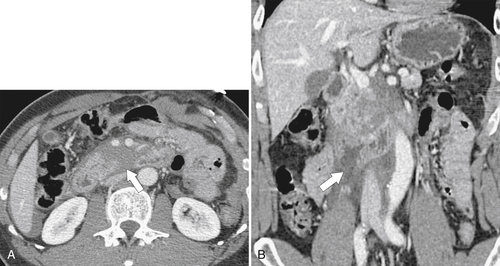
Figure 3-58 Axial (A) and coronal (B) contrast-enhanced CT in a 29-year-old man with duodenal rupture after a motor vehicle accident. There is periduodenal fluid and blood due to duodenal rupture (arrows).
Traumatic duodenal injuries are a well-recognized complication of EGD and ERCP, particularly in patients with unusual anatomy (e.g., duodenal diverticula, annular pancreas), as this makes the procedure more challenging. Perforation either is blunt by the endoscopic maneuvers or results from biopsy procedures. The injury is usually in the second part of the duodenum, and perforation will therefore be retroperitoneal (Fig. 3-59). Other trauma can result from ingested foreign bodies (Fig. 3-60).
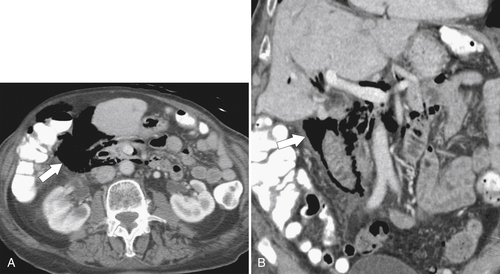
Figure 3-59 Axial (A) and coronal (B) contrast-enhanced CT in a 62-year-old man with duodenal perforation after upper endoscopy. There is retroperitoneal gas surrounding the duodenum (arrows).
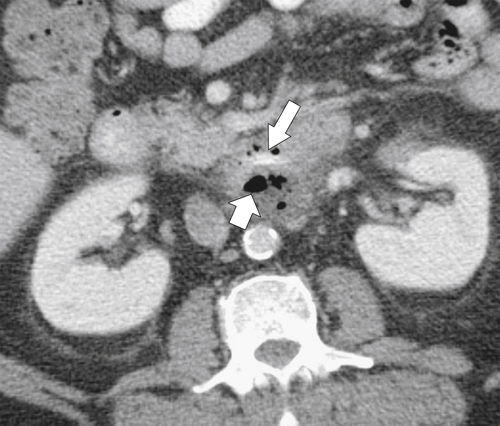
Figure 3-60 Axial contrast-enhanced CT in a 58-year-old man with duodenal perforation caused by a chicken bone (large arrow). There is an extraluminal collection of fluid and gas (small arrow).
Aorticoduodenal Fistula
This fistula is a life-threatening condition that can present insidiously with relatively minor UGI bleeding or rectal bleeding/melena only to be followed some days/weeks later with a massive, often fatal, hemorrhage. It is caused by either aortic (aneurysms, infectious aortitis, postsurgical) or duodenal disease (peptic ulcer, malignancy, radiation change). It is diagnosed by the recognition of gas and inflammatory change between the aorta and duodenum (most often after surgical aneurysmal repair) at CT (Fig. 3-61). Almost all of these fistulas are in the duodenum, but some have been recognized in the jejunum, ileum, stomach, and colon.
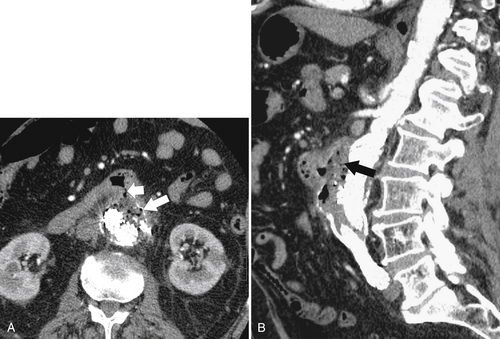
Figure 3-61 Axial (A) and sagittal (B) contrast-enhanced CT in an 81-year-old woman with aortoenteric fistula between an aortic graft and the duodenum. There are gas, fluid, and inflammatory change (large white and black arrows) between the duodenum and aorta and a fistula (small arrow) into the duodenum.
Postoperative Duodenum
Most procedures of the duodenum result from gastric procedures that create duodenal afferent loops and are discussed in Chapter 2, in conjunction with the postoperative stomach. Nonsurgical palliation of duodenal obstruction (e.g., from pancreatic adenocarcinoma) can be achieved by endoscopic or fluoroscopic placement of metallic stents to bypass the stricture. These are often temporary, though, and are prone, ultimately, to obstruction due to continued invasion and overgrowth by the primary tumor (Fig. 3-62).
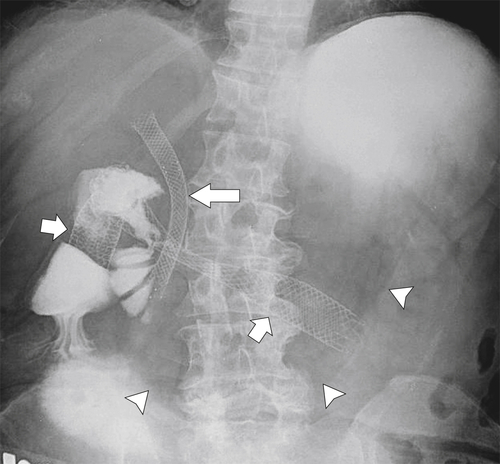
Figure 3-62 UGI series with water-soluble contrast media in a 69-year-old woman with biliary (large arrow) and duodenal (small arrows) metallic stents. There is gross gastric distention (arrowheads) caused by an obstructed duodenal stent.
Suggested Readings
Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol 2007;41(1):62–65.
Small-bowel masses found and missed on capsule endoscopy for obscure bleeding. Scand J Gastroenterol 2007;42(9):1127–1132.
Early duodenal cancer: detection on double-contrast upper gastrointestinal radiography. AJR 2000;174:1564–1566.
Duodenal abnormalities at MR small-bowel follow-through. AJR 2008;191:1082–1092.
Hypotonic MR duodenography with water ingestion alone: feasibility and technique. Eur Radiol 2009;19(7):1731–1735.
Radiologic evaluation of gastritis and duodenitis. AJR 1999;173:357–361.
Secondary aortoduodenal fistula. World J Gastroenterol 2008;14(3):484–486.
GI/liver/biliary/pancreas. AJR 2007;188(5):A125–A140.
Best cases from the AFIP: villous duodenal adenoma. Radiographics 2010;30(1):295–299.
CT of the duodenum: an overlooked segment gets its due. Radiographics 2001;21:S147–S160: Spec No.
Gastrointestinal stromal tumors of the duodenum: CT and barium study findings. AJR AM J Roentgenol 2004;183(2):415–419.
Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology 2005;237(3):967–972.
Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics 2008;28(6):1591–1602.
The duodenal wind sock sign. Radiology 2001;218(3):749–750.
Aorto-enteric fistula: CT findings. Abdom Imaging 2007;32(3): 339–.
Giant duodenal ulcers. World J Gastroenterol 2008;14(32):4995–4999.
64-Slice multidetector computed tomography evaluation of gastrointestinal tract perforation site: detectability of direct findings in upper and lower GI tract. Eur Radiol 2010;20(6):1396–1403.
Brunner’s gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation. AJR Am J Roentgenol 2006;187(3):715–722.
CT findings in duodenal diverticulitis. AJR 2006;187:W392–W395.
Intramural fat in the duodenum and proximal small intestine in patients with eeliac disease. AJR 2007;189:786–790.
Early infectious complications of percutaneous metallic stent insertion for malignant biliary obstruction. AJR 2010;194:261–265.
Non-ampullary duodenal adenocarcinoma: factors important for relapse and survival. J Surg Oncol 2009;100(2):144–148.
Periampullary tumors: high-spatial-resolution MR imaging and histopathologic findings in ampullary region specimens. Radiology 2004;231(3):767–774.
Groove pancreatitis: a diagnostic challenge. Eur Radiol 2009;19(7):1736–1743.
Extension of air into the right perirenal space after duodenal perforation: CT findings. Radiology 2009;250(3):740–748.
∗ Va´clav Treitz (1819-1872), Czech pathologist.
† Johann Conrad Brunner (1653-1727), Swiss anatomist.
† Giovanni Domenico Santorini (1681-1737), Italian anatomist.
§ Abraham Vater (1684-1751), German anatomist.
∗ John L. Down (1828-1896), British physician.
∗ William E. Ladd (1880-1967), American pediatrician.