CHAPTER 49 DUODENAL INJURIES
Duodenal injuries are uncommon, but not so rare as to preclude a comprehensive understanding of treatment strategies by general surgeons. A 6-year statewide review in Pennsylvania documented a 0.2% incidence of blunt duodenal injury (206 of 103,864 trauma registry entries), and only 30 of these patients had full-thickness duodenal injuries.1 Blunt duodenal injuries are the result of a direct blow to the epigastria, which in adults is usually from a steering wheel injury in an unrestrained driver, and in children is the result of a direct blow from a bicycle handlebar, fist, or similar mechanism. Penetrating wounds are more common causes of duodenal injury, with about 75% of patients in published reports sustaining penetrating trauma.2 This figure may primarily be a reflection of the experience of urban trauma centers where penetrating mechanism are more prevalent, and of academic centers that publish their results. Penetrating duodenal wounds are usually rapidly diagnosed as part of a laparotomy and evaluation of the tract of the offending agent. But blunt duodenal injuries are often more insidious in their presentation, making the initial diagnosis difficult. Despite this well-known observation, delays in the diagnosis of duodenal trauma continue to plague trauma surgeons and seriously compromise patient care.3
DETERMINANTS OF OUTCOME
Directly attributable duodenal mortality ranges from 2%–5%, and is the result of the common complications of wound dehiscence, sepsis, and multiple organ failure.4–11 Associated causes of mortality in patients with a duodenal injury can be garnered from large series of duodenal injuries reported during the late 20th century. These reports demonstrated an average mortality in patients with a duodenal injury of 18%, but with great individual report variability, ranging from 6%–29%.6,12–14 Morbidity rates after duodenal injury range from 30%–63%, although only about a third of these are directly related to the duodenal injury itself.6,9,12 Reasons for this variability in morbidity and mortality statistics include the mechanism of injury, associated injuries, and time to initial diagnosis. For example, Ivatury and colleagues’ review of 100 consecutive penetrating duodenal injuries documented a 25% mortality rate,6 compared with mortality rates of 12%–14% in patients with blunt injury mechanisms.3,9
Early death from a duodenal injury, particularly with penetrating wounds, is caused by exsanguination from associated vascular, liver, or spleen injuries.15,16 The proximity of the duodenum to other vital structures makes isolated injuries uncommon, but not unheard of. While exsanguinating hemorrhage and associated injuries are responsible for early deaths, infection and multiple-system organ failure are responsible for most late deaths. Up to one-third of patients who survive the first 48 hours develop a complication related to the duodenal injury. Anastomotic breakdown, fistula, intra-abdominal abscess, pneumonia, septicemia, and organ failure are the common complications. Late deaths in patients with a duodenal injury typically occur 1–2 weeks or more after the injury, with about one-third of the late deaths attributable to the injury itself.9,12
The time from injury to definitive treatment is also an important factor in the development of late complications and subsequent mortality. Roman and colleagues17 identified 10 patients in whom the diagnosis of duodenal injury was delayed over 24 hours; 4 of the 10 died, and 3 of the 10 had duodenal fistulas. In a true trauma classic report, Lucas and Ledgerwood18 demonstrated the remarkable importance (and frequency) of a delay in diagnosis of duodenal injury. In their report, a delay in diagnosis of more than 12 hours occurred in 53% of their patients, and a delay of more than 24 hours in 28%; mortality was 40% among the patients in whom the diagnosis was delayed greater than 24 hours, as opposed to 11% in those undergoing surgery within 24 hours. Snyder and coworkers9 confirmed these observations, noting that of the four patients with blunt duodenal trauma in their series in whom the diagnosis was delayed, two died and the other two developed duodenal fistula. Cuddington and associates3 also noted that 100% of the deaths directly attributable to duodenal injury occurred in patients in whom there was a delay in diagnosing such injury.
The implication of these observations is that the first priority in managing duodenal trauma should be control of hemorrhage. The next priority is limiting bacterial contamination from colon or other bowel injury to prevent late infections. A clear identification of the extent of the duodenal injury should follow as the next priority, with an emphasis on determining the status of the pancreas as well, as this affects definitive treatment plans.13,19
ANATOMY AND PHYSIOLOGY
The duodenum is the first portion of the small intestine, beginning just to the right of the spine at the level of the first lumbar vertebra and extending from the pyloric ring to the duodenojejunal flexure, commonly known as the ligament of Treitz. The duodenum is named from the Latin word duodeni, which means “twelve each,” because it is in total 25 to 30 cm, or about 12 fingerbreadths, in length. For convenience of description, the duodenum is arbitrarily divided into four divisions, differentiated by the alteration in direction of the organ.20 The superior or first portion of the duodenum passes backward and upward toward the neck of the gallbladder, and most of this portion is intraperitoneal. The descending (vertical) or second portion forms an acute angle with the first portion and descends 7–8 cm. It contains the bile and pancreatic duct openings. This portion (and the remainder of the duodenum) is entirely retroperitoneal; this is the segment mobilized by a Kocher maneuver. The transverse or third portion of the duodenum runs 12 cm horizontally to the left in front of the ureter, inferior vena cava, lumbar column, and aorta, and ends at just at the left edge of the third lumbar vertebra. The superior mesenteric artery runs downward over the anterior surface of the third portion of the duodenum. The ascending or fourth portion of the duodenum runs upward and slightly to the left for only a short distance (2–3 cm) alongside the spine to the duodenal suspensory ligament of Treitz.
The arterial blood supply of the duodenum is derived from the pancreaticoduodenal artery. The superior branch comes off the hepatic artery, and the inferior branch from the superior mesenteric artery. These two arteries run in a groove between the descending (second) and transverse (third) portions of the duodenum and the head of the pancreas, with well-developed collateralization via a continuous marginal artery. The venous drainage parallels the arterial supply, with the posterosuperior arcade draining into the portal vein and the anteroinferior arcade draining into the gastrocolic trunk.21
DIAGNOSTIC ADJUVANTS
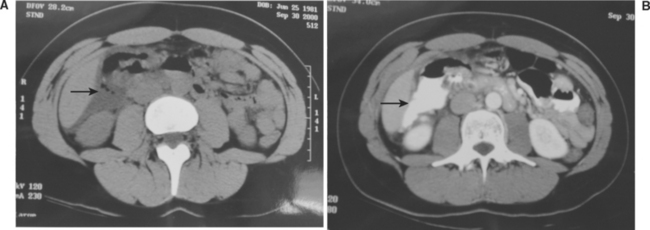
The radiologic signs of duodenal injury on the initial plain abdominal or upright chest radiograph are often quite subtle, with mild spine scoliosis or obliteration of the right psoas muscle being occasionally all that suggests a retroperitoneal duodenal injury. The presence of air in the retroperitoneum is a clear sign of duodenal injury, but this is often difficult to distinguish from the overlying transverse colon. Computed tomography (CT) at the current time is the best method of early diagnosis of a duodenal injury, but it is not infallible. In a 1997 report describing a 6-year statewide experience with duodenal injuries, Ballard et al.1 reported that of 30 documented blunt duodenal injuries, the initial CT scan missed 27%. The (CT) scan must be performed with both oral and intravenous contrast (Figure 1). The exam must be interpreted with great suspicion for injury, and uncertainty in interpretation is adequate justification for operative exploration. False-negative exams are known to occur.22 In one careful study of the accuracy of CT in diagnosing duodenal and other small bowel injuries, only 59% (10 of 17) scans were prospectively (preoperatively) interpreted as suggestive for bowel injury, which increased to 88% (15 of 17 injuries) when evaluated retrospectively.23 These investigators emphasized that using CT for the diagnosis of blunt bowel rupture requires careful inspection and technique to detect the often-subtle findings.
A more cumbersome alternative to CT is upper gastrointestinal series with water-soluble contrast medium followed by barium if the initial exam is negative. Some have advocated this study if the initial CT is difficult to interpret, but I would argue that subtle findings on CT are adequate justification for operative exploration. In a series of 96 patients with CT findings suspicious for duodenal injury, the sensitivity of a subsequent duodenography was 54% with a specificity of 98%.24 For those injuries requiring operative repair, the sensitivity was only 25%, with a 25% false-negative rate. Allen et al.25 demonstrated that 83% of the patients with a delay in the diagnosis of blunt duodenal injury had subtle CT findings, including pneumoperitoneum, unexplained fluid, and unusual bowel morphology that were dismissed. These authors emphasized the point that subtle findings of duodenal injury on abdominal CT should mandate laparotomy.
Diagnostic peritoneal lavage (DPL) is unreliable in detecting isolated duodenal and other retroperitoneal injuries. Nevertheless, DPL is often helpful because approximately 40% of patients with a duodenal injury have associated intra-abdominal injuries that will result in a positive peritoneal lavage. The findings of amylase or bile in the lavage effluent are more specific indicators of possible duodenal injury. Serum amylase levels are nondiagnostic as well, but if elevated additional investigation (CT or celiotomy) for the possibility of pancreatic or duodenal injury is warranted. At celiotomy, the presence of any central upper abdominal retroperitoneal hematoma, bile staining, or air mandates visualization and a thorough examination of the duodenum. Asensio and colleagues26 have published the technical details of exposing the entire duodenum and pancreas.
TREATMENT
Treatment principles are governed by the severity of duodenal injury and the likelihood of postrepair complications. Approximately 70% of duodenal wounds can be safely repaired primarily, and the remaining 30% are “severe” injuries that require more complex procedures. Snyder and colleagues9 are credited with cataloging the factors that determine whether a duodenal wound can be primarily repaired. In their review of 247 patients treated for duodenal trauma, they reported an overall duodenal fistula rate of 7% and a mortality rate of 10.5% in the 228 patients surviving for greater than 72 hours. These investigators felt that five of the factors listed in Table 1 most significantly correlate with the severity of duodenal injury and subsequent morbidity and mortality. A more recent addition, as noted in the table, is the presence of a pancreatic injury, a significant predictor of late morbidity and mortality. Each of these factors, either individually or in combination, has been used to develop a variety of duodenal injury classification systems. Snyder and colleagues9 demonstrated that patients with “mild” duodenal trauma had 0% mortality and 2% duodenal fistula rate, as compared with 6% mortality and 10% fistula rate among those with severe duodenal injuries. In general, patients with a “mild” duodenal injury and no pancreatic injury can be primarily repaired. Patients with more severe duodenal injuries may require more complex treatment strategies. A useful algorithm approach to the management of duodenal injuries is provided in Figure 2.27 A classification system for all organ injuries has been developed by the American Association for the Surgery of Trauma (AAST) and is known as the Organ Injury Scale (OIS).28 This classification for duodenal wounds is listed in Table 2, with duodenal injuries graded from I to V, minor to major injury. In a review of 164 duodenal trauma patients managed at eight trauma centers and to whom the AAST/OIS classification scheme was applied, there were 38 class I, 70 class II, 48 class III, four class IV, and four class V injuries. Primary repair alone was performed in 71% (117) of all cases.4 Primary duodenal repair was performed in 90 of 108 patients with class I or II injuries. More complex duodenal treatment strategies, including pyloric exclusion, duodenoduodenostomy, duodenojejunostomy, or pancreatoduodenectomy, were employed in 26 of 56 (46%) patients with class III to V injuries, or 29% of the total population.
| Mild | Severe | |
|---|---|---|
| Determinants of Injury Severity | ||
| Agent | Stab | Blunt or missile |
| Size | <75% wall | ≥75% wall |
| Duodenal site | 3, 4 | 1, 2 |
| Injury-repair interval (hr) | <24 | ≥24 |
| Adjacent injury | No CBD | CBD |
| No pancreatic injury | Pancreatic injury | |
| Outcome | ||
| Mortality (%) | 6% | 16% |
| Duodenal morbidity (%) | 6% | 14% |
CBD, Common bile duct.
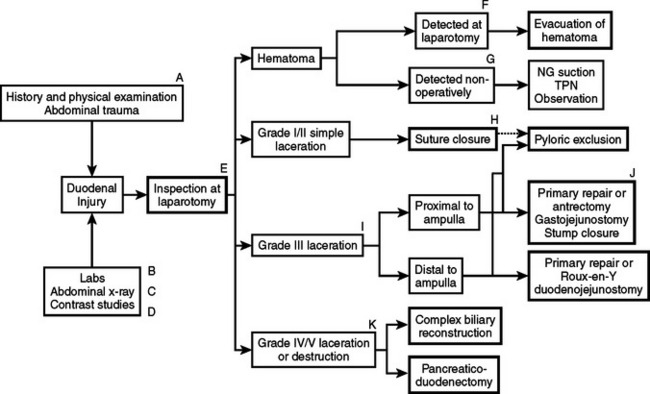
Figure 2 Algorithm for the management of duodenal injuries.
(From Jurkovich G: Duodenal injury. In McIntyre R, Van Stiegmann G, Eiseman B, editors: Surgical Decision Making, 5th ed. Philadelphia, Elsevier, 2004, pp. 512–513.)
Table 2 AAST-OIS Grading of Duodenal Injury Severity
| Grade | Type | Description |
|---|---|---|
| I | Hematoma | Single portion of duodenum |
| Laceration | Partial thickness | |
| II | Hematoma | More than one portion |
| Laceration | <50% circumference | |
| III | Laceration | 50%–75% D2 |
| 50%–100% D1, D3, D4 | ||
| IV | Laceration | ≥75% D2 |
| Involves ampulla or distal CBD | ||
| V | Laceration | Massive disruption of duodenopancreatic complex |
| Devascularization |
AAST-OIS, American Association for the Surgery of Trauma Organ Injury Severity scoring system.
Modified from American Association for the Surgery of Trauma (AAST).
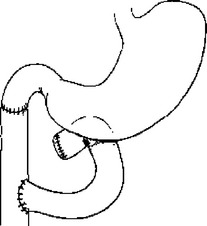
Primary repair is usually the simplest, fastest, and most appropriate way to manage duodenal injuries.29 Primary repair is appropriate for complete transection of the duodenum if there is little tissue loss, if the ampulla is not involved, and if the mucosal edges can be debrided and closed without tension. The repair is done as with any small bowel repair. I prefer a two-layer closure, but a watertight, serosa-approximating single layer repair is equally acceptable. However, if adequate mobilization for a tension-free repair is impossible, or if the injury is very near the ampulla and mobilization risks common bile duct injury, a Roux-en-Y jejunal limb anastomosis to the proximal duodenal injury with oversewing of the distal duodenal injury is the most reasonable option (Figure 3). Mucosal jejunal patch repair as depicted in Figure 4 is rarely, if ever, used, and was not used in any patient in the most recent multicenter trial in which only five patients (3%) had duodenoduodenostomy or duodenojejunostomy repairs.4 While historically utilized, a 1985 report by Ivatury et al.30 demonstrates why this is generally a poor solution to managing a duodenal wound. In this report of 60 patients with penetrating duodenal injuries, there was a 64% incidence of abdominal sepsis and a 27% death rate in 11 patients with duodenal gunshot wounds, compared with a 7% abdominal sepsis and 0% mortality in 30 patients who had either primary repair of Roux-en-Y anastomotic repair of similar injuries. In 17 patients from that same series with duodenal stab wounds, the complication rate was also higher if the “sucker patch” repair of a duodenal wound was used. It is usually preferable to fully debride the wound and transect the duodenum, mobilize the edges, and perform a direct end-to-end duodeno–duodeno anastomosis. Because the duodenal mesentery is short, this is usually readily accomplished. Pancreatoduodenectomy is only required for duodenal injuries if there is uncontrollable pancreatic hemorrhage or combined duodenal and distal common bile duct or pancreatic duct injury. Most often this represents the completion of a debridement initiated by the injury forces.
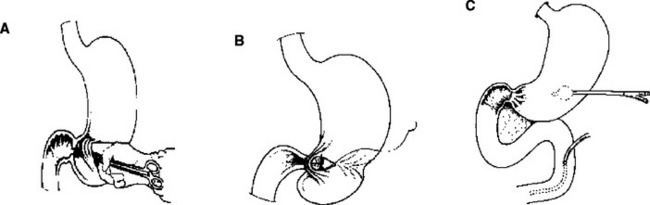
Several techniques may help protect a tenuous duodenal repair. Buttressing the repair with omentum (my preference) or a “serosal patch” from a loop of jejunum seems logical, although the benefit of such techniques is unproven.6,31 Diversion of gastric contents is another option, most commonly accomplished by the Vaughan/Jordan pyloric exclusion technique.32 Probably first described by Summers in 1904 as an adjunct to treatment of duodenal wounds,33 pyloric exclusion is a less disruptive procedure than true duodenal “diverticulization” advocated by Berne and Donovan and colleagues34,35 (Figure 5).
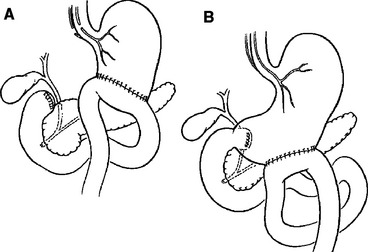
Figure 5 (A) Berne diverticulization and (B) pyloric exclusion.
(From Jurkovich GJ: Duodenum and pancreas. In Moore EE, Feliciano DV, Mattox K, editors: Trauma, 5th ed. New York, McGraw-Hill, 2004, p. 717.)
Duodenal “diverticulization” employs primary closure of the duodenal wound, antrectomy, vagotomy, end-to-side gastrojejunostomy, T-tube common bile duct drainage, and lateral tube duodenostomy. The concept is to completely divert both gastric and biliary contents away from the duodenal injury, provide enteral nutrition via the gastrojejunostomy, and convert a potential uncontrolled lateral duodenal fistula to a controlled fistula. A less formidable and less destructive alternative is the “pyloric exclusion,” which does not employ antrectomy, biliary diversion, or vagotomy32,34,36,37 (Figure 6). This procedure is performed through a gastrotomy and consists of grasping the pylorus with a Babcock clamp and suturing closed the pylorus with absorbable size 0 polyglycolic acid or polyglactin, or Maxon® (polyglyconate) or PDS® (polydioxanone) suture and construction of loop gastrojejunostomy. This diverts gastric flow away from the duodenum for several weeks while the duodenal and pancreatic injuries heal. The pylorus eventually opens (2 weeks to 2 months) and the gastrojejunostomy functionally closes. Fang and colleagues38 at Chang-Gung Memorial Hospital in Taiwan have described a technical method of a controlled release of the pyloric exclusion knot and thereby timing the opening of the pyloric occlusion. Marginal ulceration at the site of gastrojejunostomy has been reported in 5%–33% of patients, prompting some to add truncal vagotomy to the procedure.14,32,36,38 Most surgeons do not add a truncal vagotomy to pyloric exclusion, however, because nearly all of the pyloric closures open within a few weeks, regardless of the type of suture material used, and the occasional marginal ulcer can be medically managed in the interim. The data supports the use of pyloric exclusion and gastrojejunostomy in “severe” duodenal injuries7,14 or in cases of delayed diagnosis,36 although no prospective, randomized trial has proven the true benefit of gastric diversion. In addition, the added operating time and the extra anastomosis suggest a good deal of selectivity should be applied to its use.
Mortality directly related to the duodenal injury is the result of duodenal dehiscence, uncontrolled sepsis, and subsequent multiple-system organ failure. Knowledge of the lethal nature of duodenal dehiscence and duodenal fistula certainly tempts the operating surgeon to add pyloric exclusion, anastomosis buttressing, and duodenostomy to the repair of class III or IV duodenal injuries. As noted previously, concomitant pancreatic injury should also be included as a high-risk confounder that might warrant pyloric exclusion added to the duodenal repair. In one report of 40 patients with penetrating duodenal injuries, there were 14 patients with combined duodenal and pancreatic wounds. Five patients with this combination of injuries had primary duodenal repair alone, and two incurred duodenal leaks. Three of the patients with combined injuries had pyloric exclusion as a treatment adjunct, and none had duodenal leaks.39
An alternative or addition to gastric diversion is duodenal decompression via retrograde jejunostomy. Stone and Fabian10 reported a fistula rate of less than 0.5% (1 in 237 patients) in a variety of duodenal injuries all treated by retrograde jejunostomy tube drainage, in contrast to a 19.3% incidence of duodenal complications when decompression was not used. Retrograde duodenodenal drainage is preferred to lateral duodenostomy. Direct drainage with a tube through the suture line results in a high dehiscence or fistula rate of 23%. Hasson and colleagues40 reviewed the literature up to 1984 on penetrating duodenal trauma and tube duodenostomy, evaluating eight retrospective series and over 550 patients. They reported overall mortality of 19.4% and a fistula rate of 11.8% without decompression, compared with 9% mortality and 2.3% fistula rate with decompression. They too concluded that tube drainage should be performed either via stomach or retrograde jejunostomy, as these methods had a lower fistula rate and less overall mortality than lateral tube duodenostomy. Nonetheless, as is the case of pyloric exclusion for gastric diversion, there has been no prospective, randomized analysis of the efficacy of tube duodenal drainage techniques, and not all surgeons support use of decompression techniques.
In very massive injuries of the proximal duodenum and head of the pancreas, destruction of the ampulla and proximal pancreatic duct or distal common bile duct may preclude reconstruction. In addition, because the duodenum and the head of the pancreas have a common arterial supply, it is essentially impossible to entirely resect one without making the other ischemic. In this situation, a pancreatoduodenectomy is required. Between 1961 and 1994, 184 Whipple procedures were reported for trauma, with 26 operative deaths (14%) and 39 delayed deaths, for a 64% overall survival rate.41 With appropriate selection criteria, pancreatoduodenectomy for injury can be performed with similar morbidity and mortality as described in resections done for cancer.42–44
DUODENAL HEMATOMA
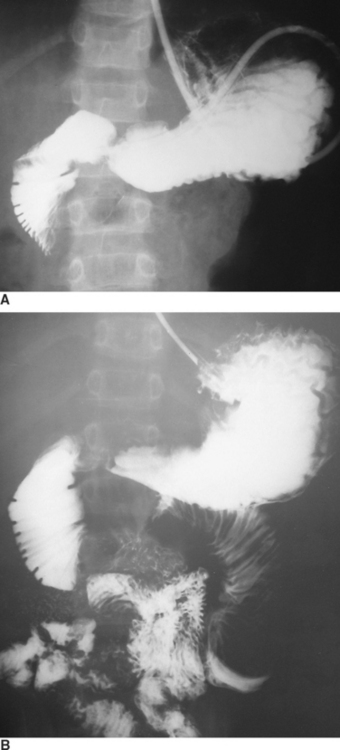
Duodenal hematoma is generally considered an injury of childhood play or child abuse, but can occur in adults as well. In one report, 50% of the cases of duodenal hematoma in children resulted from child abuse.45 Remarkably, the duodenum is the fourth most commonly injured intra-abdominal organ after blunt abdominal trauma, occurring in 2%–10% of children.46 Nearly one-third of the patients present with obstruction of insidious onset at least 48 hours after injury, presumably the result of fluid shift into the hyperosmotic duodenal hematoma. Duodenal hematoma in general represents a nonsurgical injury, in that the best results are obtained with conservative or nonsurgical management.47 It can be diagnosed either by contrast-enhanced CT scan or upper gastrointestinal (UGI) study (Figure 7). The initial water-soluble contrast (meglumine diatrizoate) exam should be followed by barium to provide the greater detail needed to detect the so-called “coiled spring” or “stacked coin” sign. Although characteristic of intramural duodenal hematoma, this finding is present in only approximately one-quarter of patients with hematoma.
Although the initial treatment is nonoperative, associated injuries should be excluded, particularly pancreatic injury. Desai et al.46 reported that 42% of pediatric patients with a duodenal injury (perforation or hematoma) had a concomitant pancreatic injury, and Jewett et al.47 found a 20% incidence of pancreatic injury in patients with a duodenal hematoma. Continuous nasogastric suction should be employed and total parenteral nutrition begun. The patient should be re-evaluated with UGI contrast studies at 5–7-day intervals if signs of obstruction do not spontaneously abate. Ultrasound has also been used to follow a resolving duodenal hematoma.48 Percutaneous drainage of an unresolving duodenal hematoma has been reported,49,50 but operative exploration and evacuation of the hematoma is usually recommended after 2 weeks of conservative therapy to rule out stricture, duodenal perforation, or injury to the head of the pancreas as factors that might be contributing to the obstruction.51 One review of six cases of duodenal and jejunal hematomas resulting from blunt trauma demonstrated resolution with nonoperative management in five of the six patients, with an average hospital stay of 16 days (range, 10–23 days), and total parenteral nutrition of 9 days (range, 4–16 days). The sixth case had evidence of complete bowel obstruction on UGI series, which failed to resolve after 18 days of conservative management. Laparotomy revealed jejunal and colonic strictures with fibrosis, which were successfully resected.52 Another report included 19 cases of duodenal hematoma in children, 17 (89%) managed nonoperatively and 2 patients in operative incision and drainage occurred within the first 24 hours and never attempted nonoperative management.46 Nasogastric decompression and total parenteral nutrition were employed for an average of 9.3 (±7.7) days (range, 2–29 days), with an average hospital stay of 16.4 (±17.8) days (range, 2–37 days).
1 Ballard RB, Badellino MM, Eynon CA, Spott MA, Staz CF, Buckman RFJr. Blunt duodenal rupture: a 6-year statewide experience. J Trauma. 1997;43(2):229-232. discussion 33
2 Asensio J, Feliciano D, Britt L, Kerstein M. Management of duodenal injuries. Curr Probl Surg. 1993;11:1021.
3 Cuddington G, Rusnak C, Cameron R, Carter J. Management of duodenal injuries. Can J Surg. 1990;33(1):41-44.
4 Cogbill T, Moore E, Feliciano D, et al. Conservative management of duodenal trauma: a multicenter perspective. J Trauma. 1990;30(22):1469-1475.
5 Flint L, McCoy M, Richardson J, Polk H. Duodenal injury: analysis of common misconceptions in diagnosis and treatment. Ann Surg. 1980;191(6):697-702.
6 Ivatury R, Nallathambi M, Gaudino J, Rohman M, Stahl W. Penetrating duodenal injuries: an analysis of 100 consecutive cases. Ann Surg. 1985;202(2):154-158.
7 Kashuk J, Moore E, Cogbill T. Management of the intermediate severity duodenal injury. Surgery. 1982;92:758-764.
8 Levison M, Petersen S, Sheldon G. Duodenal trauma: experience of a trauma center. J Trauma. 1984;24(6):475-480.
9 Snyder W, Weigelt J, Watkins W, Bietz D. The surgical management of duodenal trauma. Arch Surg. 1980;115:422-429.
10 Stone H, Fabian T. Management of duodenal wounds. J Trauma. 1979;19(5):334-339.
11 Vasquez JC, Coimbra R, Hoyt DB, Fortlage D. Management of penetrating pancreatic trauma: an 11-year experience of a level-1 trauma center. Injury. 2001;32(10):753-759.
12 Shorr R, Greaney G, Donovan A. Injuries of the duodenum. Am J Surg. 1987;154(7):93-98.
13 Jurkovich GJ, Bulger E. Duodenum and pancreas. In: Moore EE, Feliciano DV, Mattox K, editors. Trauma. 5th ed. New York: McGraw-Hill; 2004:709-733.
14 Martin T, Feliciano D, Mattox K, Jordon G. Severe duodenal injuries: treatment with pyloric exclusion and gastrojejunostomy. Arch Surg. 1983;118:631-635.
15 Heitsch R, Knutson C, Fulton R, et al. Delineation of critical factors in the treatment of pancreatic trauma. Surgery. 1976;80(4):523-529.
16 Sukul K, Lont H, Johannes E. Management of pancreatic injuries. Hepatogastroenterology. 1992;39:447-450.
17 Roman E, Silva Y, Lucas C. Management of blunt duodenal injury. Surg Gynecol Obstet. 1971;132:7-14.
18 Lucas C, Ledgerwood A. Factors influencing outcome after blunt duodenal injury. J Trauma. 1975;15(10):839-846.
19 Smego DR, Richardson JD, Flint LM. Determinants of outcome in pancreatic trauma. J Trauma. 1985;25(8):771-776.
20 Anatomy and physiology of the duodenum. In: Shackelford R, Zuidema G, editors. Surgery of the Alimentary Tract. Philadelphia: WB Saunders; 1981:38-45.
21 Edwards E, Malone P, MacArthur J. Operative Anatomy of Abdomen and Pelvis. Philadelphia: Lea & Febiger, 1975.
22 Sherck J, Oakes D. Intestinal injuries missed by computed tomography. J Trauma. 1990;30(1):1-5.
23 Mirvis S, Gens D, Shanmuganathan K. Rupture of the bowel after blunt abdominal trauma: diagnosis with CT. Am J Roentgenol. 1992;159(6):1217-1221.
24 Timaran CH, Daley BJ, Enderson BL. Role of duodenography in the diagnosis of blunt duodenal injuries. J Trauma. 2001;51(4):648-651.
25 Allen G, Moore F, Cox CJ, Mehall J, Duke J. Delayed diagnosis of blunt duodenal injury: an avoidable complication. J Am Coll Surg. 1998;187:393-399.
26 Asensio JA, Demetriades D, Berne JD, et al. A unified approach to the surgical exposure of pancreatic and duodenal injuries. Am J Surg. 1997;174(1):54-60.
27 Jurkovich G. Duodenal injury. In: McIntyre R, Van Stiegmann G, Eiseman B, editors. Surgical Decision Making. 5th ed. Philadelphia: Elsevier; 2004:512-513.
28 Moore E, Cogbill T, Malangoni M, et al. Organ injury scaling II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30(11):1427-1429.
29 Asensio J, Feliciano D, Britt L, Kerstein M. Management of duodenal injuries. Curr Probl Surg. 1993;11:1021-1100.
30 Ivatury R, Gaudino J, Ascer E, Nallathambi M, Ramirez-Schon G, Stahl W. Treatment of penetrating duodenal injuries: primary repair vs. repair with decompressive enterostomy/serosal patch. J Trauma. 1985;25(4):337-341.
31 McInnis W, Aust J, Cruz A, et al. Traumatic injuries of the duodenum: a comparison of primary closure and the jejunal patch. J Trauma. 1975;15:847-858.
32 Vaughan G, Grazier O, Graham D, et al. The use of pyloric exclusion in the management of severe duodenal injuries. Am J Surg. 1977;134:785-790.
33 Summers JJ. The treatment of posterior perforations of the fixed portions of the duodenum. Ann Surg. 1904;39:727.
34 Berne C, Donovan A, White E, et al. Duodenal “diverticulization” for duodenal and pancreatic injury. Am J Surg. 1974;127:503-505.
35 Donovan A, Hagen W, Berne D. Traumatic perforations of the duodenum. Am J Surg. 1966;111:341-350.
36 Buck JR, Sorensen VJ, Fath JJ, Horst HM, Obeid FN. Severe pancreatico-duodenal injuries: the effectiveness of pyloric exclusion with vagotomy. Am Surg. 1992;58(9):557-560. discussion 561
37 Cogbill T, Moore E, Kashuk J. Changing trends in the management of pancreatic trauma. Arch Surg. 1982;117:722-728.
38 Fang JF, Chen RJ, Lin BC. Controlled reopen suture technique for pyloric exclusion. J Trauma. 1998;45(3):593-596.
39 McKenney MG, Nir I, Levi DM, Martin L. Evaluation of minor penetrating duodenal injuries. Am Surg. 1996;62(11):952-955.
40 Hasson J, Stern D, Moss G. Penetrating duodenal trauma. J Trauma. 1984;24(6):471-474.
41 Delcore R, Stauffer J, Thomas J, Pierce G. The role of pancreatogastrostomy following pancreatoduodenectomy for trauma. J Trauma. 1994;37(3):395-400.
42 Heimansohn DA, Canal DF, McCarthy MC, Yaw PB, Madura JA, Broadie TA. The role of pancreaticoduodenectomy in the management of traumatic injuries to the pancreas and duodenum. Am Surg. 1990;56(8):511-514.
43 McKone T, Bursch L, Scholten D. Pancreaticoduodenectomy for trauma: a life saving procedure. Am Surg. 1988;54(6):361-364.
44 Oreskovich M, Carrico C. Pancreaticoduodenectomy for trauma: a viable option? Am J Surg. 1984;147(5):618-623.
45 Wooley M, Mahour G, Sloan T. Duodenal hematoma in infancy and childhood. Am J Surg. 1978;136:8-14.
46 Desai KM, Dorward IG, Minkes RK, Dillon PA. Blunt duodenal injuries in children. J Trauma. 2003;54(4):640-645. discussion 645–646
47 Jewett TJ, Caldarola V, Karp M, Allen J, Cooney D. Intramural hematoma of the duodenum. Arch Surg. 1988;123(1):54-58.
48 Megremis S, Segkos N, Andrianaki A, et al. Sonographic diagnosis and monitoring of an obstructing duodenal hematoma after blunt trauma: correlation with computed tomographic and surgical findings. J Ultrasound Med. 2004;23(12):1679-1683.
49 Gullotto C, Paulson EK. CT-guided percutaneous drainage of a duodenal hematoma. Am J Roentgenol. 2005;184(1):231-233.
50 Kortbeek JB, Brown M, Steed B. Percutaneous drainage of a duodenal haematoma. Injury. 1997;28(5–6):419-420.
51 Touloukian R. Protocol for the nonoperative treatment of obstructing intramural duodenal hematoma. Am J Surg. 1983;145:330-335.
52 Czyrko C, Weltz C, Markowitz R, O’Neill J. Blunt abdominal trauma resulting in intestinal obstruction: when to operate? J Trauma. 1990;30(12):1567-1571.