Drug Therapy in Pregnancy
Perspective
Major birth defects affect 3 to 5% of all live births.1 Most are of unknown etiology, but 1 to 3% of these are thought to be due to pharmaceutical or environmental agents.1,2 A teratogen is any chemical, pharmacologic, environmental, or mechanical agent that can cause deviant or disruptive development of the conceptus.1,2 Included in this definition are functional impairment, growth restriction, and congenital malformations.2 These may range from subtle neurobehavioral effects to devastating physiologic effects and physical deformities, including fetal death.1–3 Why one pregnancy would be affected and not another remains to be elucidated. Highly teratogenic medications seem to be few in number, estimated at well below 50 agents (Box 180-1).3,4
The process of establishing teratogenicity is tedious and often flawed. Animal research, although valuable in determining risk initially, is not always applicable to humans,1,2,5 and controlled prospective human studies are generally not performed for ethical reasons. As a result, much of our current knowledge on teratogenicity has been derived from case reports, case-control studies, or cohort studies, which are inherently weak in establishing a causal relationship.1,2,5,6 These reports are often complicated by a multitude of confounding factors, making the determination of a causal link between a specific exposure and malformations difficult. The genetic background of the fetus, timing and duration of the exposure, environmental factors, multiple exposures, nutritional deficits, maternal illness, and illicit drug use all contribute to the outcome of pregnancy.1,2,5,6 Large population studies are needed to understand the connection between the outcome of a pregnancy and an associated in utero exposure.6 Finally, as in the case of diethylstilbestrol, teratogenicity may not be apparent for years after birth.
Classification of Teratogenic Risk
To aid physicians in determining the teratogenic potential of a particular medication, the U.S. Food and Drug Administration (FDA) assigns one of five letters, A, B, C, D, and X, to the drug, depending on the strength of evidence for its safety or teratogenicity (Box 180-2). This classification system has been criticized as overly simplistic and perhaps inaccurate.1,2,5–7 Many clinicians believe that the classification system conveys the incorrect impression that there is a gradation of reproductive risk from exposure across categories (i.e., that risk increases from A to B to C to D to X) and that the drugs within a given category present similar reproductive risks.7 Yet more than 90% of newly introduced drugs in the United States are assigned to class C, an undetermined teratogenic risk.8 The FDA has acknowledged these problems, and in 2008 it proposed new rules for drug labeling during pregnancy. The proposed rules recommend giving a narrative description of the risks and the likelihood of developmental abnormalities and are awaiting the final stages of clearance.9 Currently, a number of clinical teratology resources that assign risk are available online. These include TERIS, REPROTOX, and Micromedex REPRORISK (Shepard’s Catalog of Teratogenic Agents).
Principles of Disease
Drugs may affect the fetus through a variety of mechanisms. Some alter the availability of substrates, such as vitamins, glucose, oxygen, and amino acids, needed for normal nutrition and growth.1–3 Others directly affect cellular growth and differentiation. The age at which fetal exposure occurs is crucial in determining its impact on the pregnancy. The fetus is most vulnerable to toxic insults during the time of organogenesis (days 21-56 of fetal life).1–3 Exposure during this period may result in major anatomic defects. Exposure after the period of organogenesis may affect the growth and development of the fetus.1–3 Functional development of the central nervous system (CNS) is affected when it is exposed to a CNS teratogen during the 10th to 17th weeks of pregnancy.1–3
Drug Transfer across the Placenta
Drug transfer across the placenta occurs most commonly by simple passive diffusion or by protein transport.1–3,10 A thin layer of trophoblastic cells is all that separates maternal from fetal circulation.1–3,10 The degree to which a drug gains access to fetal circulation depends on molecular size, ionic state, lipid solubility, and extent of protein binding. Drugs with a molecular mass of less than 5 kDa readily diffuse.1–3,10 Anionic forms diffuse through the lipid layer more readily than ionized forms.1–3,10 Free drug diffuses more readily than a drug that is bound to plasma proteins.1–3,10 Because fetal pH is slightly more alkalotic than maternal pH, weak organic acids (e.g., salicylate) may become ion trapped in the fetal circulation, increasing fetal exposure.1–3,10
Drug Transfer during Lactation
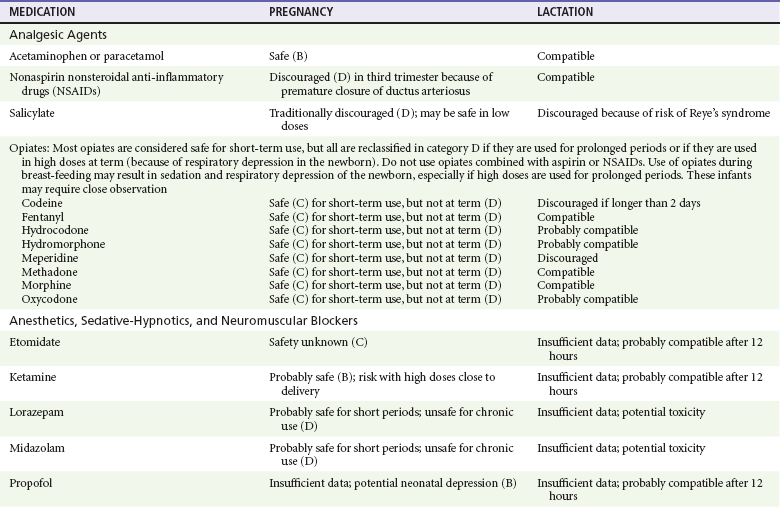
For the most part, drugs and substances that are ingested or injected by the mother diffuse passively into milk and then back into the maternal circulation for excretion.11 The amount of drug diffusing into milk depends on many factors. Lipid-soluble and nonionic substances diffuse more readily, and highly protein bound substances diffuse less readily.11 Whether a substance is concentrated in maternal milk or not, the neonate generally is able to detoxify it with no adverse effects, and only a few drugs pose a serious danger to a breast-feeding infant.11–13 The interruption of breast-feeding should not be advocated except in rare situations of known drug toxicity to the infant and in all cases of maternal critical illness.11,12 Table 180-1 summarizes the compatibility of medications and their effects in pregnancy and lactation.
Drug Therapy during Pregnancy
Analgesic Agents
Acetaminophen (paracetamol) is widely used during pregnancy and has not been associated with congenital malformations.13–15 In fact, in a population-based case-control study, acetaminophen was associated with a decreased risk of certain craniofacial malformations when it was used for febrile illnesses in the first trimester.13,15 Acetaminophen is safe during lactation because only a small amount is excreted into breast milk, and the amount involved is tolerated by the neonate’s sulfhydration pathway.11–13 However, in overdose situations, there may be an increase in the incidence of spontaneous abortion and fetal demise, especially when antidote treatment with N-acetylcysteine is delayed.13,16
Aspirin
Early studies linked aspirin to an increased risk of perinatal and neonatal bleeding, increased risk of postmaturity, prolonged labor, low birth weight, neonatal hypoglycemia, neonatal metabolic acidosis, and neonatal death.13,14 However, a number of meta-analyses in humans failed to demonstrate a teratogenic effect of aspirin,13,14,17,18 and in the Perinatal Antiplatelet Review of International Studies (PARIS) Collaboration, low doses of aspirin were actually found to be beneficial and to reduce the risk of preeclampsia, premature birth, and adverse perinatal outcomes.19 There was a trend, however, toward a slightly increased incidence of gastroschisis (a defect in the abdominal wall through which abdominal contents protrude) with use of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin in the first trimester.13,18–20 Aspirin is excreted into breast milk, and because of its association with Reye’s syndrome, its use is therefore discouraged in breast-feeding.11–13
Nonsteroidal Anti-Inflammatory Drugs
Prostaglandin synthesis inhibitors such as NSAIDs have been shown to block blastocyst implantation and may therefore inhibit conception.20 Their use in the first trimester has been linked to an increased risk of spontaneous abortions and a slight increase in cardiac septal defects, oral clefts, and gastroschisis.13,14,20–22 When they are used in the third trimester, NSAIDs inhibit labor and have been used as tocolytic agents for premature labor.13,14,21 When they are used in the latter part of pregnancy, NSAIDs have been linked to a number of negative effects on the neonate, most notably premature closure of the ductus arteriosus, leading to neonatal pulmonary hypertension and death.13,14,21,23 An increased incidence of fetal periventricular hemorrhages, fetal nephrotoxicity, oligohydramnios, and neonatal gastrointestinal hemorrhage has also been reported.13,14,21,23 Their use in the latter part of pregnancy is therefore discouraged. NSAIDs in general appear to be safe during lactation.12,13
Opiate Analgesics
In general, short-term, episodic use of opiates such as oxycodone, hydrocodone, morphine, and fentanyl appears to be safe in pregnancy.13,14 Their use near term, however, may result in severe respiratory depression of the neonate.13,14 In addition, prescribing of narcotics for long periods can lead to fetal addiction, low birth weight, and neonatal abstinence syndrome.13,14,24 Neonatal abstinence syndrome is characterized by CNS hyperirritability and autonomic nervous system dysfunction and a higher mortality in the offspring.24
The short-term use of opiates during lactation appears to be safe, but nursing infants should be closely monitored for respiratory depression.12,13 A case report of the death of a nursing infant whose mother was taking codeine for pain13,25 and a review of complications of codeine use in nursing mothers prompted the FDA to issue an advisory for codeine use in lactation.
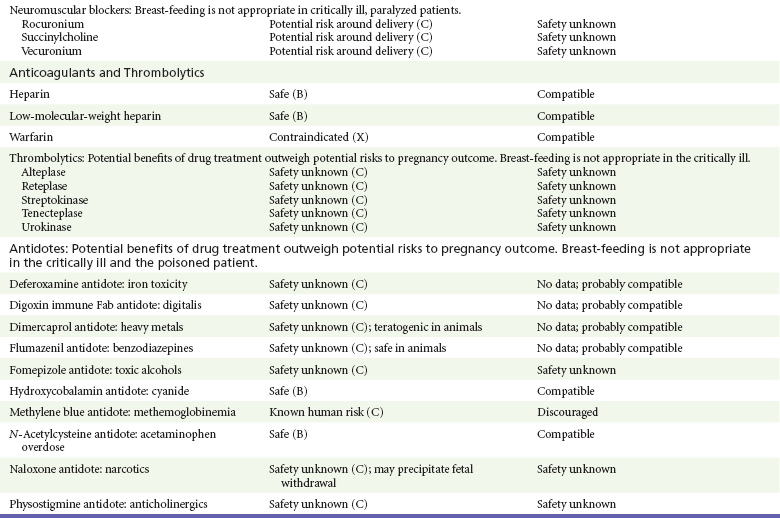
Anesthetics, Sedative-Hypnotics, and Neuromuscular Blockers
General anesthetics, sedative-hypnotics, and neuromuscular blockers are indicated for rapid sequence intubation (RSI). Some anesthetics and sedative-hypnotics are also used for procedural sedation. Most of the data regarding the use of these agents during pregnancy have been obtained from animal studies and from retrospective human data. None of the agents has been consistently associated with congenital malformations.26–28
Anesthetics
Etomidate.: Etomidate is an ultra-short-acting hypnotic agent that is commonly used for procedural sedation or RSI. It has not been linked to congenital defects in animals even when high doses were used.13,14,26,27 Similarly, it is not associated with an increase in congenital anomalies in humans.13,14,26,27 However, newborns of mothers undergoing cesarean section with etomidate were found to have significant reductions in serum cortisol concentrations 1 hour after delivery.13,14,27,28 The significance of this effect remains unclear. Because etomidate is excreted in milk in very small amounts and decreases rapidly, it is most likely compatible with breast-feeding.13,14,28
Ketamine.: Ketamine is a rapidly acting dissociative anesthetic that is commonly used in pediatric procedural sedation and may be used in RSI. It is frequently used in obstetrics and has not been associated with fetal developmental malformations.13,14,26,27 Ketamine has a dose-dependent oxytocic effect and in high doses (>2 mg/kg) has been associated with uterine tetany.13,14,26,27 It may also result in increased maternal blood pressure and heart rate and an increased neonatal muscle tone.13,14 Neonatal depression has also been reported.13,14 Whereas there are no published data on the effects of maternal ketamine use on the nursing infant, the amount of ketamine in the mother’s plasma should be undetectable after 12 hours, and the American Academy of Pediatrics (AAP) considers it compatible with breast-feeding when it is used after this time.12–14
Midazolam.: Midazolam is a short-acting benzodiazepine commonly used in procedural sedation or RSI. It has not been linked to congenital defects in most laboratory animals even when high doses are used.13,14,26–28 In humans, there are no data available on its use in the first and second trimesters of pregnancy, but benzodiazepines do not appear to result in malformations in human observational studies.13,14,26–28 When it is used at term, midazolam has been associated with neonatal respiratory depression and decreased muscle tone.13,14,26–28 The AAP considers midazolam use during lactation of concern because there are few data addressing the issue.12 However, in a number of studies, the concentration of midazolam in breast milk was found to be negligible 8 hours after maternal ingestion.13,14,29
Propofol.: Propofol is a rapidly acting sedative anesthetic that is commonly used in RSI and procedural sedation. No data are available regarding its use in the first and second trimesters of pregnancy in humans, but it does not result in malformations in animal studies.13,14,26,27 Its use at term also appears to be safe, although high doses have been linked to neonatal respiratory and CNS depression.13,14,26–28 Propofol is thought to be compatible with breast-feeding because only negligible amounts are found in breast milk 24 hours after its use.13,14,26–29
Thiopental.: Thiopental is an ultra-short-acting barbiturate that may be used during RSI or for status epilepticus. It has not been linked to congenital defects in laboratory animals even when high doses are used.13,14,26–28 Similarly, it has not been associated with an increase in congenital anomalies in humans.13,14,26–28 Thiopental is excreted in milk in concentrations that are too low to be pharmacologically significant and is considered compatible with breast-feeding.12–14
Neuromuscular Blockers
Nondepolarizing Neuromuscular Blocking Agents.: Nondepolarizing neuromuscular blocking agents, such as rocuronium and vecuronium, are used in RSI. The effects of these agents on organogenesis in humans are not known, but these agents are not thought to pose a significant teratogenic risk because very little of the maternal dose crosses the placenta.13,14,26–28 Furthermore, according to the manufacturers, there was no increase in congenital anomalies noted when small laboratory animals were injected with rocuronium daily.13,14,26–28 Because of their chemical properties, very little of either drug is excreted in milk.13,14,26–28 Women are not expected to breast-feed while taking neuromuscular blockers.
Depolarizing Neuromuscular Blocking Agents.: Succinylcholine is a depolarizing neuromuscular blocking agent used in RSI for its rapid onset of action and short duration of paralysis. It has not been associated with congenital defects, although there is limited experience with its use in early pregnancy in humans.13,14,26–28 In addition, it does not appear to have any effects on the newborn, except in rare cases of neonates with pseudocholinesterase deficiency.13,14,26–28 As occurs in adults with the same condition, newborns with cholinesterase deficiency exhibit prolonged respiratory depression and paralysis.13,14,26–28 Succinylcholine in lactation has not been studied; however, it is probably safe because it is hydrolyzed quickly.13,14
Sedative-Hypnotics
Benzodiazepines.: Benzodiazepines are commonly used for the sedation of the acutely agitated patient, in the treatment of acute seizures and alcohol withdrawal, and as a sedative during RSI. The short-term use of benzodiazepines during pregnancy appears to be safe. Case reports have linked their use during the first trimester of pregnancy to increased risk of oral clefts,10,13,14 but in an observational study based on the Swedish medical birth registry, no such association was found.13,14,30 Neonates exposed to benzodiazepines may exhibit signs of toxicity, including apnea, cyanosis, unresponsiveness, hypotonia, poor feeding, and withdrawal symptoms characterized by irritability and tremulousness.10,13,14 Because of the reported risk of apnea, it is recommended that neonates exposed to benzodiazepines through breast-feeding be monitored closely.10,12–14
Anticoagulants and Thrombolytics
Warfarin (Coumadin) is a known human teratogen and affects 4 to 5% of exposed fetuses. The risk from exposure is greatest during 6 to 9 weeks of gestation and seems to be dose dependent.14,15,31 The fetal warfarin syndrome is associated with multiple abnormalities, such as hypoplasia of the nasal bones, midline dysplasia including agenesis of the corpus callosum, optic atrophy and blindness, mental retardation, seizures, and stippling of the bones with scoliosis and shortening of limbs.10,14,15,31,32 Because warfarin is so highly protein bound, only a little is secreted into milk, and use by breast-feeding mothers is acceptable.12–14,31 Caution should be used in breast-feeding of premature infants because they may be at increased risk for intraventricular hemorrhage.13,31
Heparin
Unfractionated heparin is a highly charged heterogeneous molecule with a molecular mass between 5 and 35 kDa.13 It does not cross the placenta and does not present a direct risk to the fetus.13,14 Early reports on the use of heparin for the prevention or treatment of venous thromboembolism during pregnancy noted an increased risk of prematurity, stillbirth, and fetal hemorrhage.14 However, these risks were recently attributed to the underlying maternal condition rather than to heparin.14,15,31,33 When anticoagulation during pregnancy is required, heparin is considered the agent of choice.13,14,33 Its use is sometimes associated with maternal osteopenia and immune-mediated thrombocytopenia and maternal hemorrhage at delivery.13,14,31,33 Patients need to be carefully monitored for these adverse effects. Because of its high molecular weight, heparin is not excreted in breast milk and is compatible with breast-feeding, but critically ill patients are not expected to breast-feed.12–14,31
Low-molecular-weight heparin may be used during pregnancy and in the postnatal period for therapeutic or prophylactic anticoagulation.14,15,31,33 All currently available low-molecular-weight heparin products have been used safely during pregnancy.14,15,31,33 Data are limited, however, because of their relatively recent introduction.
Thrombolytic Agents
Alteplase, reteplase, urokinase, and streptokinase have been used successfully in pregnant women in cases of life-threatening pulmonary embolus34 or myocardial infarction. Experience with these agents during pregnancy, however, remains limited. To date, no teratogenic effects have been reported in humans, but intrapartum maternal hemorrhage has been reported with alteplase and urokinase.14,15,31 Most thrombolytics are thought to be compatible with breast-feeding because of their short half-life.13,14
Antidotes
N-Acetylcysteine is a mucolytic agent that has been used successfully and without untoward effects in pregnant women who have overdosed on acetaminophen.13–16,35 No teratogenic effects have been reported, and pregnant patients who overdose on acetaminophen should be treated in the same manner as nonpregnant patients are.13–16,35 Although there are no reports of N-acetylcysteine use during lactation, it is most likely safe because it has been used in neonates without untoward effects.13,14,35
Deferoxamine
Deferoxamine is indicated for iron toxicity occurring from iron overdose or from multiple transfusions in thalassemia patients. It has been associated with developmental effects on ossification in some animal species.13,14,35,36 Experience in humans is limited, but it does not appear to affect the fetus.13,14,35,36 The effects of deferoxamine on the nursing infant are not known, but it is probably compatible.13,14
Digoxin Immune Fragment
Digoxin immune fragment (Fab) therapy is indicated for life-threatening digitalis overdose and is being studied for treatment of preeclampsia.13,14,35 There are very few case reports of the use of digoxin immune Fab during pregnancy, and a conclusion on its effects on the offspring cannot be made.13,14 Thus in cases of life-threatening digitalis overdose with arrhythmias, the benefits of treatment of the mother outweigh the risk to the fetus. Digoxin immune Fab is not likely to be excreted in large amounts in milk, and it is probably safe for use during lactation.12–14
Dimercaprol
Dimercaprol or British antilewisite is a chelating agent that is used as an antidote for acute mercury, lead, arsenic, and gold poisoning.13,14,35,37 It has also been used in Wilson’s disease.13,14 It is teratogenic in mice and has been associated with increased mortality, growth restriction, cleft facial features, cerebral herniation, and abnormal digits, but experience in humans is limited.13,14,35,37 In general, in cases of heavy metal poisoning, the maternal benefits of treatment will outweigh the potential risks to the unborn fetus. Breast-feeding is contraindicated in patients poisoned by heavy metals.13,14
Flumazenil
Flumazenil is a benzodiazepine antagonist. No teratogenic effects have been reported in animals, and data on humans are limited.13,14,35 Its use in pregnancy and lactation depends on the potential maternal benefit compared with possible risks to the fetus and nursing infant. Because it has a short half-life, breast-feeding may resume after a few hours.13,14
Fomepizole
Fomepizole is a competitive inhibitor of alcohol dehydrogenase indicated in cases of methanol and ethylene glycol poisoning. Its use during pregnancy has not been studied in animals or humans. Its safety during pregnancy is not known.13,14,35 In cases of toxic alcohol poisoning, the benefits of treatment of the mother outweigh the possible risks to the fetus or nursing infant. Use of ethyl alcohol in these situations may be considered.13,14 Breast-feeding is not expected to continue during acute toxic alcohol poisoning.
Hydroxycobalamin
Hydroxycobalamin is a vitamin B12 derivative that is indicated in the treatment of cyanide toxicity. The effects of hydroxycobalamin on human pregnancy have not been studied, but benefits of its use in cyanide poisoning outweigh any risk to the fetus. Studies in animals do not reveal an association with any developmental abnormality.13,14 Breast-feeding is not expected to continue during cyanide poisoning, but hydroxycobalamin, like its related compounds, is considered compatible with breast-feeding.12–14
Methylene Blue
Methylene blue is used in the treatment of methemoglobinemia. In the past, it was injected into the amniotic sac to identify twins and to detect rupture of the membranes, but these practices were associated with hemolytic disease in the newborn, hyperbilirubinemia, and deep blue staining of the newborn.13,14,35,38 Methylene blue in pregnancy has also been associated with an increased incidence of intestinal obstruction and atresia in the newborn.13,14,35,38 Breast-feeding is not expected to continue during acute severe methemoglobinemia, but the effects of methylene blue on the nursing infant are expected to be minimal.13,14
Naloxone
Naloxone, used to reverse severe respiratory depression in opiate overdose, readily crosses the placenta. Although it has not been associated with reproductive abnormalities,13,14,35 its use during pregnancy results in increased fetal wakefulness, increased fetal movement, and increased heart rate, effects attributable to antagonism of fetal endorphins.13,14,35 In addition, its use in opiate-addicted mothers may precipitate withdrawal in both mother and term fetus.13,14,39 It is compatible with breast-feeding.12–14
Physostigmine
Physostigmine is an anticholinesterase agent indicated in cases of severe anticholinergic poisoning associated with delirium. Experience with the medication during pregnancy is limited, and its effects on the developing fetus are unknown.13,14,35 Use of physostigmine at term was associated with only mild decreases of Apgar scores at 1 and 5 minutes.13,14,40 Breast-feeding is not expected to continue during anticholinergic poisoning, but physostigmine is thought to be probably compatible with breast-feeding.13,14
Pralidoxime
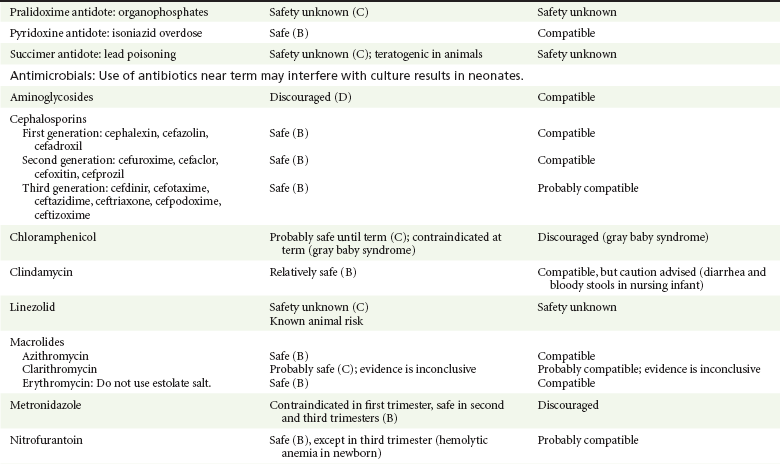
Pralidoxime is indicated for organophosphate/cholinergic poisoning because it is able to reactivate cholinesterase. Experience with pralidoxime in pregnancy is limited, and its effects on fetal development are not known.13,14,35,40 In cases of organophosphate poisoning, the benefits to the mother generally outweigh the possible risk to the fetus. Breast-feeding is not expected to continue during cholinergic poisoning.
Pyridoxine
Pyridoxine is a vitamin required for good maternal health and good fetal development. It is indicated in isoniazid poisoning and in gyromitrin mushroom poisoning. Its use has been advocated by some for nausea and vomiting of pregnancy, gestational hypertension, and diabetes.13,14 It has not been associated with any adverse developmental effects, and it is safe in lactation.13,14
Succimer
Succimer is a heavy metal chelator that is indicated in lead poisoning. It has also been used in arsenic, mercury, and cadmium poisoning. It has been linked to congenital defects in animal models, possibly because of its negative effects on zinc and copper metabolism.13,14,35 Experience with the use of succimer in human pregnancy is limited to case reports, which did not shed any light on its teratogenicity in humans.13,14,35,37,41 There are no reports of succimer use during lactation, but breast-feeding is contraindicated in heavy metal poisoning.13,14
Antimicrobial Agents
Infections in the pregnant woman have the potential to adversely affect pregnancy outcome as well as fetal development. When they occur in the first trimester, they are a common cause of spontaneous abortion, and when they occur in the second or third trimester, they are the most common cause of preterm labor and delivery. Antimicrobial agents also may adversely affect the pregnancy. Aminoglycosides, for example, may be nephrotoxic and ototoxic in both the mother and her offspring,13,14,41,42 tetracyclines may result in dental staining of the developing fetus,13,14,41,42 and lincosamides may be skeletotoxic.13,14 The penicillins, cephalosporins, and macrolide antibiotics remain the drugs of choice for infections during pregnancy.41,42 Other classes of antibiotics are prescribed only if these have failed to control the infection or in cases of severe maternal intolerance to these drugs.13,14,41,42 The choice of antimicrobial therapy will depend on the age of the pregnancy, severity of infection, and maternal tolerance for the drug used. Many drugs also are secreted into breast milk. Potential problems for the neonate include direct effects on the neonate, changes in bowel flora, diarrhea, and potential interference with culture results.12–14
Antibiotics
Aminoglycosides.: The association of aminoglycosides such as gentamicin, streptomycin, tobramycin, and neomycin with nephrotoxicity and ototoxicity is well known in the literature and in practice.13,14,41,42 Aminoglycosides, however, do not appear to have any structural teratogenic effect in humans,43 but kanamycin and streptomycin have been reported to cause ototoxicity in the mother and her offspring.13,14 There are no reports definitively linking in utero exposure to gentamicin, streptomycin, tobramycin, and neomycin to either ototoxicity or nephrotoxicity.13,14 Aminoglycosides are secreted in small amounts in breast milk and are poorly absorbed from the gastrointestinal tract.12–14 They are probably compatible with breast-feeding.12–14
Cephalosporins.: The first- to fourth-generation cephalosporins appear to be safe for use during pregnancy, although there are no controlled studies examining their safety.* Some cephalosporins are excreted into breast milk and may interfere with culture results in the workup of neonatal sepsis.13
Chloramphenicol.: Chloramphenicol is a broad-spectrum antibiotic with excellent blood-brain barrier penetration. It remains the drug of choice for meningitis in many developing countries, but it has been associated with bone marrow suppression and aplastic anemia,13,14,42 which may be fatal. Outside of these complications, its use during pregnancy appears to have no effects on the developing fetus.13,14,31 However, when it is used at term, chloramphenicol has been associated with cardiovascular collapse in the neonate (the “gray baby” syndrome),13,14,31,41,42 prompting its contraindication at term. The safety of chloramphenicol during breast-feeding is unknown; however, because of its potential for bone marrow depression and its association with gray baby syndrome, it is not recommended for use during lactation.12–14
Clindamycin.: Clindamycin has not been associated with birth defects in humans, and animal studies have failed to link clindamycin to congenital abnormalities in the offspring.13,31,41,42 The AAP considers clindamycin to be compatible with breast-feeding.12–14 However, this recommendation is not echoed by other agencies because of its rare association with bloody diarrhea in the nursing infant.13,14
Fluoroquinolones.: Fluoroquinolones have been linked to numerous toxic effects on bone and cartilage growth in animal models and are discouraged from use during pregnancy, particularly during the first trimester.† A number of observational studies, however, have failed to demonstrate such a toxic effect on the human fetus.13,14,31 Furthermore, a recent meta-analysis did not reveal any increase in the rates of spontaneous abortions, birth defects, prematurity, or low birth weight in women exposed to fluoroquinolones in the first trimester.44 The AAP considers ciprofloxacin to be compatible with breast-feeding because breast-fed infant plasma levels are low.12–14 Data are inconsistent for other quinolones, and they are best avoided in lactation.
Linezolid.: Linezolid is effective in the treatment of methicillin-resistant Staphylococcus aureus infection. It has been linked to embryonic death, decreased weight, and abnormalities in cartilage and ossification in animal studies,13,14 but there is no information about its effects in humans. Its use in pregnant women should be limited to cases in which the maternal benefits outweigh possible risks to the fetus.13,14 Linezolid is excreted into breast milk, but the amount that reaches the nursing infant appears to be small, and it is likely to be compatible with breast-feeding.13,14
Macrolides.: Erythromycin, azithromycin, and clarithromycin are considered to be safe for use in pregnancy and compatible with breast-feeding, although there are no well-controlled studies examining their effects on the fetus.13,14,31,41,42 Some reports have linked erythromycin to pyloric stenosis, but the studies were not controlled.13,14,31,41,42 The estolate salt of erythromycin has also been associated with the development of hepatotoxicity in pregnant women and should be avoided during pregnancy.13,14,31 Clarithromycin has been associated with an increased risk of fetal and embryonic death as well as with congenital malformations in animal species.13,14 To date, however, this has not been shown in humans. For example, in a prospective controlled multicenter observational study comparing the outcomes of pregnancies exposed to new macrolides (including clarithromycin) with matched controls, no difference in the types or patterns of malformations between the groups was found.13,14,45 Azithromycin is poorly concentrated in breast milk and may be the preferred agent in lactating mothers.12–14
Metronidazole.: Metronidazole is indicated for the treatment of anaerobic infections, amebiasis, Clostridium difficile infection, and bacterial vaginoses. It is mutagenic and carcinogenic in mice and rats.13,14 In humans, a number of studies failed to demonstrate a clear association between metronidazole use and congenital malformations when it was used in the first trimester of pregnancy.13,14,35,46 Metronidazole has also been frequently used during the second and third trimesters to treat bacterial vaginosis with no untoward effects.13,14 However, because of its effects in mice, many physicians avoid prescribing it at all during pregnancy.13 Use of metronidazole during lactation is discouraged because of its potential mutagenic and carcinogenic effects in rats and its slow elimination from infants.12–14
Nitrofurantoin.: Nitrofurantoin is commonly used for the treatment of uncomplicated urinary tract infections and has traditionally been considered safe for use throughout pregnancy except near term. However, a population-based multicenter case-control study linked its use in the first trimester to a number of congenital abnormalities, including anencephaly, microcephaly, hypoplastic left heart, atrial septal defects, and craniofacial clefts.42 Its use near term has been associated with hemolytic anemia in the newborn.13,14,31,47
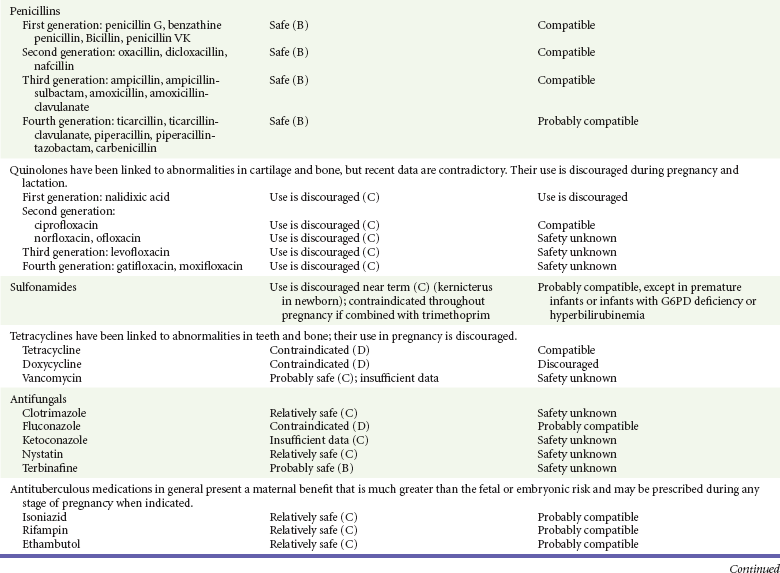
Penicillins.: The first- to fourth-generation penicillins and their derivatives (including procaine, benzathine, clavulanate, sulbactam, and tazobactam) are considered safe for use in pregnancy, as is oral probenecid.13,14,31,41,42 Penicillins are considered safe during breast-feeding, but their use may interfere with culture results if workup is required for a neonatal fever.12,13
Sulfonamides.: Sulfonamides are used in the treatment of uncomplicated urinary tract infections and are indicated in the treatment of Pneumocystis jiroveci (formerly known as Pneumocystic carinii) pneumonia. They are also effective against methicillin-resistant S. aureus. Sulfamethoxazole is commonly combined with trimethoprim, presumably to improve antimicrobial efficacy. Both are folic acid synthesis inhibitors and have traditionally been contraindicated in pregnancy because of an increased risk of neural tube defects13,14,31 and other congenital abnormalities, such as cleft palate.13,14,31,41,42 A number of observational studies have demonstrated an increased risk of cardiovascular and urinary tract malformations in the offspring of women treated with trimethoprim-sulfamethoxazole in the first trimester.42,48 Sulfonamides are contraindicated near term because of their association with kernicterus.13,14,41,42 They compete with bilirubin for protein-binding sites, leaving large amounts of free bilirubin to diffuse freely into the brain. This results in bilirubin deposition in the infant’s brain, causing kernicterus. To date, however, this complication has not been reported in neonates, presumably because free bilirubin is effectively cleared by the placental circulation.13,14 In contradistinction, kernicterus has occurred in newborns exposed to sulfonamides after birth.13,14 Sulfonamides are excreted into breast milk in low concentrations and are generally tolerated by a healthy neonate.12–14 They should be avoided, however, in ill or premature infants and in infants with hyperbilirubinemia or glucose-6-phosphate dehydrogenase deficiency.12–14
Tetracyclines.: Tetracyclines are effective in a number of infections, including infections with atypical bacteria, Yersinia pestis infection, and anthrax. Both tetracycline and doxycycline readily cross the placenta.13,14,31 Tetracycline, but not doxycycline, has been associated with the development of fatal fatty liver in pregnant women.13,14,31 Tetracycline also chelates calcium, causing abnormalities in bone growth and staining of decidual teeth.13,14,31,41,42 It has also been associated with fetal genitourinary anomalies, inguinal hernias, and limb abnormalities.13,14,31,41,42 Tetracycline should therefore be avoided during pregnancy. Doxycycline, on the other hand, does not bind to calcium and is associated less with stained teeth than is tetracycline.13,14,31 In addition, it does not appear to cause an increase in any type of congenital malformation.13,14,31 Despite these findings, doxycycline is not recommended in pregnancy.13,14,31,42
Because tetracycline binds to breast milk calcium, only a small amount reaches the nursing infant, and it may be used for short periods (<10 days) during breast-feeding.13,14,31 Doxycycline does not bind to breast milk calcium and is present in greater quantities in breast milk.12–14 This could theoretically increase its side effects in the newborn. Its use in nursing infants is best avoided.12–14,31
Vancomycin.: Vancomycin is effective against methicillin-resistant S. aureus and C. difficile. It has not been linked to birth defects in animals or in humans.13,14,31,49 Reports of auditory abnormalities and renal insufficiency in neonates of mothers treated with vancomycin are believed to be false positives because these abnormalities were resolved on retesting.13,14 Vancomycin is excreted into milk but not well absorbed by the gastrointestinal tract.12–14,31 Its effects on the nursing infant have not been studied.12–14,31
Antifungal Medications
Nystatin has a long safety profile during pregnancy and lactation. It is poorly absorbed from skin, mucous membranes, and the gastrointestinal tract, and it is considered the antifungal agent of first choice for treatment of mucocutaneous fungal infections.13,14,31 Clotrimazole, miconazole, and ketoconazole seem to be safe during pregnancy and lactation as they have not been associated with major birth defects.13,14,31 However, a minor increase in the incidence of hypoplastic left ventricle was reported in one case-control study.13,14,31,50 In addition, ketoconazole is teratogenic in rats.13,14,31,50 For these reasons, clotrimazole, miconazole, and ketoconazole are considered second-line treatment of fungal infections in pregnancy.13,14,31,50 Fluconazole is teratogenic in high doses (>400 mg/day) and has been associated with an increased incidence of craniofacial and cardiovascular defects in offspring and multiple abnormalities of the skeleton and cartilage.13,14,31,50 These anomalies were not noted when lower doses were used or with single-dose (150 mg) therapy for vaginal candidiasis.13,14,31,50
Ketoconazole, fluconazole, and itraconazole are excreted into breast milk.12–14 On the basis of the safe use of ketoconazole in neonates and the lack of negative reports, it is considered compatible with breast-feeding.12–14
Antituberculous Agents
Untreated tuberculosis places the mother and fetus at greater risk than does the use of antituberculous medications. Isoniazid, ethambutol, and rifampin cross the placenta,13,14,31 and in a review of antituberculous treatment during pregnancy, no association was found between these medications and major congenital malformations.13,14,51 Rifampin has been associated with hemorrhagic disease of the newborn.13,14,51 Despite this adverse effect, it maintains its first-line quality in the treatment of tuberculosis.13,14,51 All three antituberculous medications are considered compatible with breast-feeding.12,13,51
Antiviral Agents
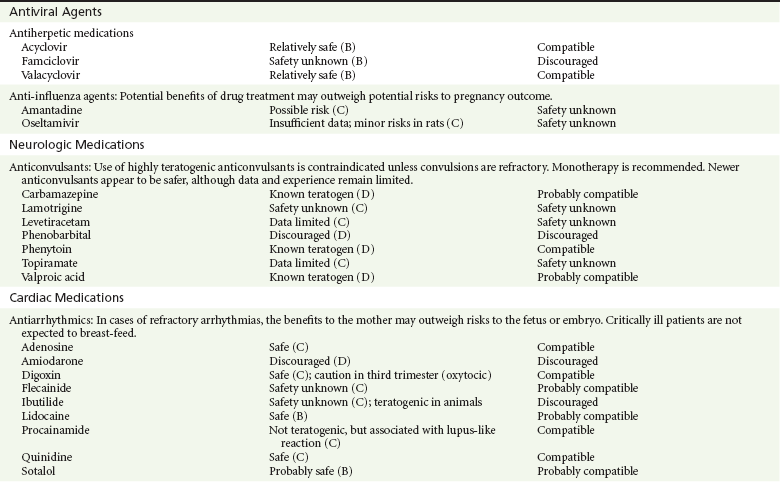
Antiherpetic Drugs.: Acyclovir is a purine analogue commonly used in the treatment of herpesvirus infections. Valacyclovir is rapidly metabolized to L-valine and acyclovir, the active metabolite.13,14 In humans, acyclovir readily crosses the placenta and reaches higher concentrations in fetal circulation than in maternal circulation.13,14 Neither acyclovir nor valacyclovir has been associated with congenital malformations or adverse effects on the offspring.13,14,52 Intravenous acyclovir is the drug of choice for life-threatening maternal herpes simplex virus infections,13,14,53 such as disseminated disease, herpes encephalitis, and varicella pneumonia, which carries a maternal mortality of 44% if it is untreated.13,14,53 For non–life-threatening genital herpes infection in pregnant women, either acyclovir or valacyclovir may be used.13,53,54 Experience with famciclovir is limited and it is therefore not recommended for use in pregnancy.13,14,52 Acyclovir is concentrated in milk, in which levels may be higher than in plasma.12–14 Because there are no reported adverse outcomes in infants of mothers taking acyclovir or in infants treated with acyclovir for disseminated herpes, it is considered safe during breast-feeding.12–14
Anti-Influenza Drugs.: Influenza in pregnancy carries a high risk of morbidity and mortality. During the 2009-2010 H1N1 pandemic, 6% of confirmed H1N1 deaths in the United States occurred in pregnant women.55 Antiviral therapy with either M2 ion channel inhibitors, such as amantadine and rimantadine, or neuraminidase inhibitors, such as oseltamivir and zanamivir, is associated with improved maternal and pregnancy outcomes.13,14,56,57 Whereas amantadine had been linked to various malformations including cardiac defects in humans and is considered teratogenic and embryotoxic in rats,13,14 a cohort study found that neither class of anti-influenza drugs was associated with an increase in malformations or stillbirths.13,57 There was, however, an increase in the incidence of necrotizing colitis in premature newborns exposed to either of these classes of drugs.13,14 Oseltamivir appears safe in lactation.13,14
Anti-HIV Drugs.: Anti-HIV drugs may be indicated immediately after a needle-stick injury or sexual contact with an infected individual. No specific pattern of birth defects has been described with the use of these drugs,13,14 but there are a number of unanswered questions relating to the drugs’ mutagenesis and carcinogenesis and their long-term effects on the liver, heart, and reproductive system.13,14
Animal and human data suggest that didanosine, lamivudine, stavudine, zidovudine, and zalcitabine present a small risk of structural malformations and mitochondrial dysfunction in the developing fetus,13,14 but no specific pattern of birth defects has been described with protease inhibitors, such as ritonavir and nelfinavir.13,14 Despite potential risks, it is thought that the benefit from treatment of HIV infection far outweighs the risk of these drugs, and treatment should not be withheld. In addition, zidovudine has been shown to significantly reduce vertical and perinatal transmission of HIV from the mother to the fetus.13,14,53,58 Because of the risk of postnatal HIV transmission through milk, the Centers for Disease Control and Prevention advises against breast-feeding by HIV-positive mothers.53
Cardiovascular Agents
Atrial and ventricular arrhythmias are common during pregnancy. Most are benign; however, malignant degeneration occasionally occurs.59–62 All unstable tachycardias should be treated with electrical cardioversion and according to advanced cardiac life support guidelines.59–62 Stable patients may be treated medically, but the choice of drugs needs to be modified to protect the patient as well as the fetus from the drug’s harmful effects.59–62
Adenosine.: Adenosine is a naturally occurring compound that is metabolized quickly in the body. It has been used safely throughout pregnancy and is the drug of choice for termination of maternal supraventricular tachycardia.13,14,59–62 Adenosine has also been used safely in termination of incessant tachycardia in the fetus.13,14,59–62 Adenosine is safe in lactation.12–14
Amiodarone.: Amiodarone is a class D agent. It contains large amounts of iodine and has been associated with congenital goiter and transient neonatal hyperthyroidism and hypothyroidism.13,14,59–63 In addition, amiodarone has been linked to many congenital abnormalities, including growth restriction, structural cardiac abnormalities, corneal deposits, and developmental delay.13,14,59–63 It should be used only in refractory cases of supraventricular or ventricular tachycardias in the mother and incessant tachycardias in the fetus.13,14,59–63 Because of its high iodine content, its excretion into milk, and its long elimination half-life, amiodarone should not be used in nursing mothers.12–14,31
Digoxin and Quinidine.: Digoxin and quinidine are considered safe for use during pregnancy and lactation.* Neither has been linked to congenital defects in humans or animals, and they are first-line agents for the treatment of significant maternal dysrhythmias.* They have also been successfully used in fetal tachycardia.* Overdoses of these drugs, on the other hand, have led to premature labor, spontaneous abortions, and, in the case of digoxin, fetal death.13,14 During lactation, digoxin appears compatible with breast-feeding; there is very little information about quinidine’s use in breast-feeding.12–14
Lidocaine.: Lidocaine is a weak base. It rapidly crosses the placenta and becomes ion trapped in the fetus.13,14 There is no evidence of a link between use of lidocaine in the first trimester and any fetal developmental malformations.13,14,31,59–61 However, high doses used near term are associated with neonatal CNS depression, apnea, hypotonia, seizures, and bradycardia.13,14,59–61 Lidocaine is considered compatible with breast-feeding.12–14
Procainamide.: Procainamide has been successfully and safely used in the treatment of stable wide-complex tachydysrhythmias during pregnancy.59–62 It has not been associated with fetal developmental abnormalities and appears well tolerated when it is used for short durations.13,14,59–62 It has been associated with a high incidence of maternal antinuclear antibodies and the occurrence of a lupus-like reaction in humans.13,14,31 During lactation, both procainamide and its metabolite, N-acetylprocainamide, have been found in breast milk,13,14 and although the AAP considers its short-term use compatible with breast-feeding,12 other authorities do not recommend it.13,14
Flecainide.: Flecainide is a class IC antiarrhythmic agent that is structurally related to procainamide.13,14 It has been used safely to terminate maternal and fetal tachycardia,* but it has been associated with fetal hyperbilirubinemia, hepatotoxicity, and loss of fetal heart rate variability.* Flecainide has also been found to be teratogenic in some animal species, resulting in cardiac and musculoskeletal abnormalities.* Flecainide is found in breast milk, but apparently in concentrations that are not toxic to infants.12–14 The AAP considers flecainide compatible with breast-feeding despite limited experience.12,13
Ibutilide.: Ibutilide is a class III antiarrhythmic used to terminate atrial fibrillation and flutter. There are only a few case reports of its successful and safe use during the latter part of pregnancy in humans.* In animals, however, ibutilide was found to be teratogenic and caused cardiac septal defects as well as skeletal dysgenesis in rats,13,14 especially when high doses were given. Ibutilide should be reserved for refractory cases in which the benefits of therapy outweigh any fetal risk.13,14
Sotalol.: Sotalol has been used in pregnant women to successfully and safely treat atrial arrhythmias as well as hypertension.59–62 It has also been successfully used to terminate fetal atrial tachycardias.59–62,64 It does not appear to have teratogenic effects in animals.13,14 Some of the negative effects of sotalol include bradycardia in the newborn, persisting for 24 hours.13,14 Sotalol is concentrated in milk but does not appear to result in bradycardia or hypotension in the nursing infant, and according to the AAP it is compatible with breast-feeding.12–14
Antihypertensives
Hypertension complicates 12% of pregnancies and is most commonly seen in the third trimester.65 It accounts for 16% of maternal deaths in the United States.65 Labetalol is the agent of choice for hypertensive emergencies in pregnancy.66–68
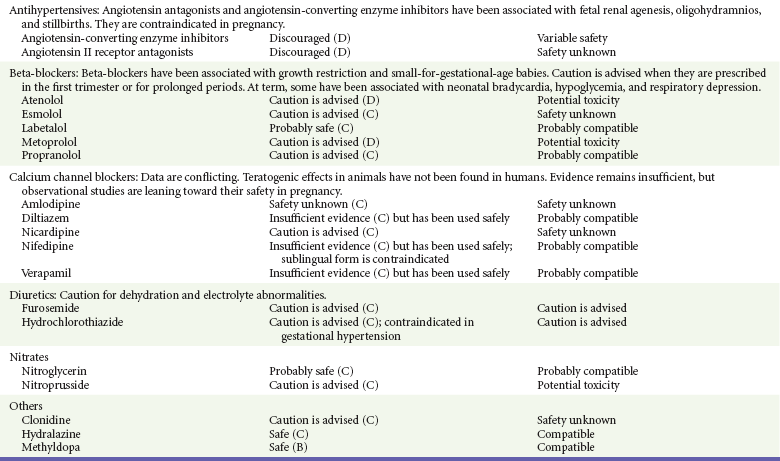
Angiotensin-Converting Enzyme (ACE) Inhibitors.: ACE inhibitors are classified as category D drugs and are contraindicated for use during pregnancy.13,14,69 Furthermore, ACE inhibitors are embryocidal in animals and increase the rate of stillbirths in some animal species.13,14 In humans, the most significant adverse fetal effects occur when they are used in the second and third trimesters.13,14,66–69 These include oligohydramnios, anuria, renal agenesis resulting in death, increased risk of stillbirth, intrauterine growth restriction, fetal skull abnormalities, pulmonary hypoplasia, respiratory distress syndrome, and fetal and neonatal hypotension.13,14,66–69 Captopril and enalapril are considered compatible with breast-feeding.12–14,68
Angiotensin II Receptor Antagonists.: Angiotensin II receptor antagonists should be avoided during pregnancy because their use has been reported to result in fetal abnormalities similar to the abnormalities seen with ACE inhibitors, including renal agenesis, neonatal anuria, oligohydramnios, intrauterine growth restriction, persistent patent ductus arteriosus, abnormal ossification, and death.13,14,66–69 Their safety in lactation is unknown.
Beta-Blockers.: Beta-blockers have become a first-line treatment of hypertension in pregnancy.70,71 They have not been associated with fetal malformations and appear to be safe when they are used for short periods.† Adverse fetal effects include intrauterine growth restriction and a low placental weight.† Beta-blockers lacking intrinsic sympathomimetic activity, such as acebutolol, atenolol, nadolol, and propranolol, are more likely to be associated with these adverse effects.† When beta-blockers are given near term, they have been associated with persistent beta blockade in the newborn.† Nonselective beta-blockers, such as propranolol, also have resulted in neonatal hypoglycemia, respiratory depression, and hyperbilirubinemia in the newborn.* These adverse effects are less common when a cardioselective beta-blocker, such as atenolol or metoprolol, is used.* Esmolol, a short-acting beta-blocker commonly used to terminate maternal supraventricular dysrhythmias and hypertension during surgery, has been associated with fetal bradycardia, neonatal bradycardia and hypotonia, and fetal distress requiring emergent cesarean section.* Beta-blockers have variable effects on the nursing infant, and close monitoring of the infant for adverse effects is recommended.12–14,68
Calcium Channel Blockers.: Calcium channel blockers have been used for the treatment of hypertension and the termination of supraventricular rhythm disturbances during pregnancy.† Intravenous verapamil has also been used to terminate fetal tachycardia,† and intravenous nicardipine has been used for severe preeclampsia.† In addition, some calcium channel blockers, such as nifedipine and diltiazem, have been used as tocolytic agents.13,14,31 In laboratory animals, use of calcium channel blockers in the first trimester was associated with a dose-dependent increase in embryonic mortality and skeletal abnormalities.13,14,31 To date, however, these abnormalities have not been seen in humans, although data remain limited.13,14,66–68 A prospective multicenter observational study of 299 patients exposed to calcium channel blockers in the first trimester did not reveal an increase in congenital malformations compared with controls.71 Some complications of calcium channel blocker use during pregnancy include maternal hypotension, tachycardia, and fetal distress,† especially pronounced when sublingual nifedipine or intravenous nicardipine is used.13,14 The AAP considers these drugs compatible with breast-feeding.12,13,31
Clonidine has been safely used in the treatment of severe hypertension throughout pregnancy, but experience during the first trimester remains limited.13,14,66–68 It does not appear to be teratogenic in laboratory animals and does not increase fetal mortality.13,14 Transient neonatal hypertension has been reported in neonates.4,14,66–68 Its effects on breast-feeding neonates are unknown, but it is considered to be compatible with breast-feeding.12–14,68
Hydralazine is a direct vasodilator that has a rapid onset of action. Its use, however, has been associated with higher rates of maternal hypotension, placental abruption, and neonatal distress compared with labetalol.‡ It is therefore no longer recommended as a first-line agent in the treatment of severe acute hypertension in pregnancy. It may still be used as a second-line agent.66–68,73 Hydralazine is considered compatible with breast-feeding.12–14,68
Methyldopa has been safely used to treat chronic hypertension throughout pregnancy, and most reviews have not linked it to any teratogenic effects on the offspring or adverse effects on the pregnancy.13,14,31,66–68 Many clinicians continue to prescribe it as first-line therapy for hypertension during pregnancy. Methyldopa is compatible with breast-feeding.12–14,68
Diuretics.: Diuretics are no longer used for gestational hypertension or preeclampsia because, in general, affected patients tend to be hypovolemic.13,66–68
Loop diuretics such as furosemide are indicated in the treatment of pulmonary edema due to congestive heart failure. In laboratory animals, furosemide has been linked to renal and skeletal abnormalities when it is used in pregnancy.13,14 These effects have not been seen in humans, but a slightly increased risk of hypospadias has been reported.13,14 Furosemide is secreted into breast milk but is considered compatible with breast-feeding.12–14
Thiazide diuretics have been associated with hypoglycemia and electrolyte abnormalities in neonates when they are given near term and an increase in meconium staining and perinatal mortality.13,14 Moreover, thiazide diuretics may have a direct effect on smooth muscle and inhibit labor.13,14 In general, these agents are excreted in low quantities into breast milk and are considered safe during breast-feeding.12–14
Nitrates.: Nitroglycerin has not been shown to cause fetal harm in animal studies.13,14,31 Limited reports in humans do not show any major effects on the fetus or neonate.13,14,31 Nitroglycerin is rarely used during pregnancy, but it appears to be a safe, effective, rapidly acting and short-acting agent.* It appears to be effective in relieving intrapartum fetal distress related to uterine hyperactivity.76 Nitroprusside for the treatment of hypertensive emergencies in pregnancy has the same advantages and disadvantages as in nonpregnant patients. During prolonged administration of high doses, nitroprusside may result in cyanide toxicity and severe acidosis.13,14,74,75 It readily crosses the placenta, and fetal levels of cyanide can increase as high as twice maternal levels.13,14,74,75 Standard doses do not seem to subject the fetus to major risk of toxicity,13,14 but with the availability of safer alternatives, notably labetalol, nitroprusside is considered a last-resort agent.† No data are available on its use during lactation, but breast-feeding is not expected to continue during critical illness.
Vasopressors
Vasopressors are indicated in shock states only after intravascular volume has been restored. Whereas they are effective in restoration of intravascular resistance and blood pressure, all have the potential to increase uterine vascular resistance, resulting in a proportional decrease in placental blood flow.74,75,77,78 Each of the vasopressors has its indications, its benefits, and its inherent risks. At this time, on the basis of its safety profile, phenylephrine appears to be the vasopressor of choice in the treatment of vascular collapse during pregnancy.74,75,77,78
Dobutamine.: Dobutamine is an inotrope used in the setting of cardiac dysfunction and sepsis.74,75 It has not been associated with any negative reproductive effects in laboratory animals or in humans.13,14
Dopamine.: Dopamine has a dose-dependent effect on dopaminergic, alpha, and beta receptors and has been used in maternal shock, including spinal shock due to spinal anesthesia.13,14,74,75 Low-dose dopamine has been successfully used to improve cardiac and urine output in patients with preeclampsia and oliguria,74,75 but this has not been shown to improve mortality or renal function.79 No significant fetal side effects have been directly linked to its use in animal studies, even though it has been shown to decrease uterine vascular flow.13,14 Its safety during breast-feeding has not been studied, but breast-feeding is not expected to continue during critical illness.12,13
Epinephrine.: Epinephrine is the preferred agent for the treatment of anaphylaxis and is commonly used in status asthmaticus. It has also been used to treat shock from any cause during pregnancy.13,74,75 However, it has been associated with anoxic injury to the fetus, intracranial hemorrhage, and increased incidence of inguinal hernias.13,14,74,75 Safety during breast-feeding has not been studied.12,13
Norepinephrine.: Norepinephrine use during pregnancy has been associated with the occurrence of vascular and skeletal abnormalities in rodents and a significant decrease in placental blood flow and fetal oxygenation.13,14 It has also been associated with increased incidence of cerebral hemorrhage.13,14,74,75 Its safety during breast-feeding has not been studied,13 but breast-feeding is not expected to continue during critical illness.12,13
Ephedrine and Phenylephrine.: Ephedrine and phenylephrine are both effective in the treatment of shock from any cause in pregnancy.74,75,77 Compared with phenylephrine, ephedrine was associated with higher heart rates, more maternal gastric upset, and greater incidence of fetal acidosis.13,14,74,75,77 Ephedrine is no longer the agent of choice in shock during pregnancy as it has been replaced by phenylephrine.77
Endocrine Agents
Diabetes mellitus is associated with a number of congenital malformations involving multiple organ systems.13,14,78 It is also associated with a significant increase in perinatal morbidity.13,14,78 Glycemic control in pregnancy is therefore important and should be accomplished in a controlled manner as hypoglycemia is also associated with adverse pregnancy outcomes.13,14,78
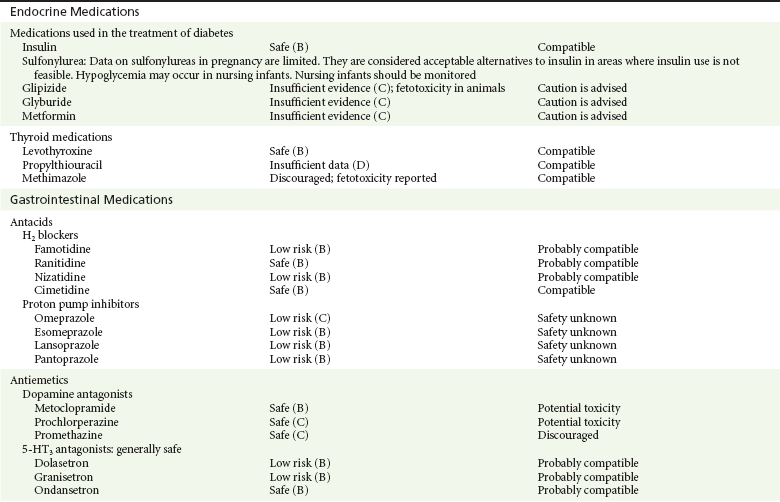
Insulin.: Insulin is a large peptide that does not cross the blood-placenta barrier. It is considered to be the drug of choice in the United States for diabetes mellitus types 1 and 2 in pregnancy.13,78
Sulfonylureas.: Sulfonylureas are regarded as possibly teratogenic and less effective than insulin in the control of gestational diabetes.* They have also been associated with neonatal hypoglycemia with use at term.* In reality, outside the perinatal period, there is little information about their use during pregnancy, and in a randomized study, glyburide proved to be as effective and safe as insulin during pregnancy.80 Glyburide and glipizide are highly protein bound and are not likely to pass into breast milk; nursing infants should nevertheless be monitored.12,13,78
Metformin.: Metformin has been associated with serious adverse effects in adults, including life-threatening metabolic acidosis and hepatotoxicity.13,14,78 It has not been associated with fetal malformations in animals,13,14,31,78 and there were no major birth defects in women taking metformin for polycystic ovary disease in a 2004 report.13,81 In addition, metformin does not appear to result in neonatal hypoglycemia, a complication commonly seen with the use of sulfonylureas at term.13,14,78,81 In the developing world, where the administration of insulin may be problematic, metformin is a good alternative to insulin for type 2 diabetes mellitus when diet alone is not effective.13,81 Whereas metformin is considered compatible with breast-feeding by the AAP,12 it should be used with caution in lactating mothers because of its potential for serious effects in adults and its excretion into milk.13
Thyroid Medications
Maternal hyperthyroidism is associated with an increased risk of spontaneous abortion, preterm labor, placental abruption, and maternal congestive heart failure. Effects of the disease on the offspring include intrauterine growth restriction, intrauterine fetal death, and neonatal goiter.13
Propylthiouracil (PTU).: PTU, the drug of choice during pregnancy, prevents the synthesis of thyroid hormone and prevents the peripheral conversion of T4 to T3.13,31 Complications of treatment include transient goiter and hypothyroidism of the newborn.13,82 There does not appear to be an increase in the risk of congenital defects with PTU.13,14,31,82 Other drugs used to control hyperthyroidism in pregnancy include methimazole and carbimazole, both of which have been associated with abnormal development of the skin, albeit inconsistently.13,14,31,82 PTU is excreted into breast milk in insignificant amounts and is considered compatible with breast-feeding.12,13
Iodides.: Iodides are reserved for thyrotoxic patients. These agents are readily taken up by the fetal thyroid gland, resulting in prolonged fetal hypothyroidism and goiter.13,14
Levothyroxine.: Hypothyroidism in pregnancy may result in an increased risk for spontaneous abortion, intrauterine growth restriction, placental abruption, and fetal demise and has been associated with severe neurologic impairment of the offspring.13,14 Levothyroxine, a synthetic formulation of naturally occurring T4, has not been associated with any negative effects on the offspring and is the treatment of choice for hypothyroidism in pregnant women.13,14,31 It is compatible with breast-feeding.12,13
Gastrointestinal Agents
H2 Receptor Antagonists.: Antacids are commonly prescribed throughout pregnancy. None of the H2 receptor antagonists has been linked to congenital malformation, and they all appear to be safe for the nursing infant.13,14 There is one report in the literature, however, linking in utero gastric suppression to an increased incidence of asthma during childhood.13,14,83
Proton Pump Inhibitors.: Despite the limited size of most studies on proton pump inhibitor use in pregnancy, a meta-analysis found no association with an increased risk for major congenital birth defects, spontaneous abortions, or preterm delivery.84 Proton pump inhibitors, such as esomeprazole, lansoprazole, pantoprazole, and rabeprazole, are accepted for use during pregnancy.13,14 There are reports, however, of an increased incidence of gastrointestinal, hepatic, and thyroid cancers in rats and mice.13,14 There are no human data studying the effect of proton pump inhibitors on nursing infants.12,13
Antiemetic Medications
Phenothiazines.: Phenothiazines, such as metoclopramide, prochlorperazine, and promethazine, are dopamine antagonists commonly used in the treatment of nausea and vomiting during pregnancy. Although there are reports of increased risk of cardiac defects,13,14 these reports did not consider other factors, such as the mother’s health, when the drug was reviewed. The bulk of evidence does not support a link to congenital abnormalities.13,14,85 These agents are excreted in milk. The AAP cautions against their use in nursing mothers as they may cause sedation and other untoward effects.12,13
Neurologic Agents
Anticonvulsants are known teratogens, and 30% of neonates exposed to commonly used anticonvulsants exhibit congenital anomalies.* The risks for birth defects increase with the duration of exposure and with the number of agents used.86,87 Valproate is associated with the most frequent serious adverse effects on the pregnancy and fetus (20.3% incidence of serious adverse outcomes) compared with phenytoin, carbamazepine, and lamotrigine (10.7%, 8.2%, and 1.0%, respectively).13,86,87 Despite the risks, most practitioners believe that it is important to control seizures during pregnancy. Generalized seizures during pregnancy are associated with an increased risk of spontaneous abortion, hypoxic injury to the fetus, and impaired neuropsychological functioning.13,31
Monotherapy is the most appropriate option and is recommended at the lowest effective anticonvulsant dose.13,31,86,87 Dividing of the daily dose to decrease peak plasma levels may be considered. Adjustment of the dosage upward is often required to maintain adequate seizure control.13,31,86,87
Classic Anticonvulsants.: The classic anticonvulsants are considered class D agents and are teratogenic.* Carbamazepine has been linked to an increased risk of craniofacial defects, neural tube defects, and developmental delay.* Phenobarbital has been associated with a slightly increased risk of congenital heart disease and cleft lip or palate.* In addition, its chronic use during pregnancy has been associated with neonatal withdrawal.* Phenytoin use during pregnancy has been associated with the fetal hydantoin syndrome, which affects 5 to 10% of pregnancies.* This syndrome is characterized by varying degrees of ossification abnormalities of the extremities and digits, craniofacial abnormalities including cleft lip and palate, impaired growth, delayed neurologic development, and multiple cardiovascular anomalies.* Valproic acid is associated with multiple facial anomalies, neural tube defects, strabismus, and congenital heart defects.*
Carbamazepine, phenobarbital, and phenytoin have also been associated with hemorrhagic disease of the newborn, presumably because they competitively inhibit placental transport of vitamin K.13,14,31
Carbamazepine, phenytoin, and valproic acid are secreted into breast milk in low quantities and are considered compatible with breast-feeding.11–14 Phenobarbital, on the other hand, has been associated with neonatal sedation and toxicity and is not advised during breast-feeding.11–14
Newer Anticonvulsants.: Newer anticonvulsants, such as lamotrigine, levetiracetam, and topiramate, have been associated with a slightly increased incidence of major birth defects, such as oral clefts, skeletal abnormalities, and hypospadias.13,14,86,87 The incidence of these birth defects increases significantly when these substances are combined with other anticonvulsants, such as valproic acid.13,31,86,87 These findings, however, were not seen in two studies comparing the newer anticonvulsants.88,89 In the lamotrigine pregnancy registry,88 a study conducted by the manufacturer of lamotrigine, and in an observational study from Denmark,89 first-trimester exposure to lamotrigine, oxcarbazepine, topiramate, gabapentin, or levetiracetam was not associated with an increased risk of major birth defects.
These agents also appeared to be well tolerated by the nursing infant, except for topiramate, which caused excess sedation.13,31
Migraine Medications
Ergot Alkaloids.: Ergotamine and dihydroergotamine are commonly used in the treatment of migraines. Whereas neither has been associated with teratogenic effects, they are contraindicated in pregnancy because of their oxytocic effects13,14 and their effects on uterine blood flow.13,14 In a number of animals, these alkaloids have been associated with intrauterine growth restriction, probably because of reductions in uteroplacental blood flow.13,14 They are also contraindicated during breast-feeding because of possible ergot poisoning of the nursing infant manifested by convulsions and gastrointestinal symptoms.12–14
Triptans.: The triptans are selective serotonin agonists that are effective in the treatment of vascular headaches, including migraines and cluster headaches. They have been found to be teratogenic in a number of animal species,13,14 but recent data in humans appear to favor their safety during pregnancy.90 Sumatriptan is excreted into milk, but oral bioavailability appears to be low, and very little reaches the nursing infant.13,14 It is considered compatible with breast-feeding, especially if the milk is discarded for 8 hours after the last dose.12,13
Respiratory Agents
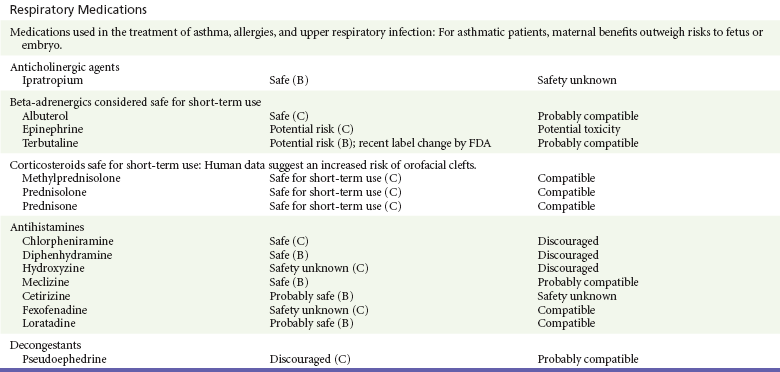
Antihistamines including chlorpheniramine, diphenhydramine, doxylamine, hydroxyzine, and meclizine have been safely used in the treatment of allergic reactions during pregnancy and as antiemetics in the treatment of nausea and vomiting during pregnancy.13,14,31 This has been further confirmed in recent meta-analyses involving more than 200,000 patients.91,92 First-generation antihistamines are not recommended during breast-feeding because they are thought to inhibit lactation.12–14 In addition, serious adverse CNS effects, including seizures, have been reported to develop in neonates receiving antihistamines, especially when they are premature.12,13
The newer generation antihistamines, such as cetirizine and loratadine, also appear safe during pregnancy.13,14,92,93 They may be acceptable alternatives for severe allergies if the first-generation antihistamines are not tolerated.13,14,92,93 These drugs are excreted into human milk, but in the case of loratadine, very small amounts reach the neonate.13,14 The AAP classifies these drugs as compatible with breast-feeding.12,13
Asthma Medications
Pregnant women with asthma are at risk for neonatal death, preterm birth, low-birth-weight infants, preeclampsia, and small-for-gestational-age infants.94 Asthmatic mothers may also have a higher rate of chorioamnionitis, hypertensive disorders of pregnancy, cesarean section, and prolonged hospital stay compared with control mothers.94 Better asthma control has been associated with an improved outcome.94
Beta-adrenergic medications such as albuterol, metaproterenol, and terbutaline are commonly used in the treatment of asthma. Beta-adrenergic agents have also been used during the last trimester to treat premature labor.13,14 Albuterol is the most commonly prescribed and is the treatment of choice in asthma.* It appears safe even when it is used long term.13,14,92,94 None of the beta-adrenergic medications has been linked to fetal or congenital malformations, but some have been associated with significant cardiovascular and metabolic effects, which are transient and generally well tolerated by the fetus.* Transient hyperglycemia followed by insulin secretion may also occur, resulting in neonatal hypoglycemia, especially in diabetic patients.* Terbutaline, when it is used intravenously or orally in pregnant women, may result in significant maternal and fetal arrhythmias.13,14 Maternal pulmonary edema and death have been reported.13,14 The FDA has recommended a label change to add a warning against its use in preterm labor because safer beta2-agonists and tocolytic agents are available.95 Long-acting beta-agonists also appear to be safe during pregnancy.13,14,92,94 Albuterol is compatible with breast-feeding.12–14
Ipratropium has not been found to be teratogenic in numerous animal models,13,14,92,94 but there are few data regarding its safety in human pregnancy.13,14 It is considered compatible with breast-feeding.12,13
Cromolyn sodium has not been associated with any significant risk of birth defects or negative perinatal outcomes.13,14 It is considered compatible with breast-feeding.12,13
Corticosteroids
Corticosteroids are commonly used during pregnancy for the treatment of various disorders, including autoimmune diseases and asthma. Inhaled corticosteroids are the main therapy for the prevention of asthma exacerbations during pregnancy. Oral corticosteroids are the mainstay of therapy for acute exacerbations of asthma. Although they are not considered human teratogens, there may be a slightly increased incidence of orofacial clefts when oral steroids are used during the first trimester.* Furthermore, their use in the third trimester has been linked to an increased incidence of preterm delivery, low birth weight, preeclampsia, and cataracts in the newborn.† Other authors have also raised concerns about the development of congenital adrenal hyperplasia in newborns.13,14 Prednisone is considered safe during breast-feeding.12,13
Data on the use of leukotriene antagonists in pregnancy are limited. One study did not find an association with congenital abnormalities, but there was a slight increase in intrauterine growth restriction. However, these results should be interpreted with caution because of the small sample size of the study.13,14,98 Zileuton is mutagenic in animal studies and should be avoided during pregnancy and lactation.13,14,98
Decongestants
Decongestants with strong vasoconstrictive properties, such as phenylpropanolamine and pseudoephedrine, cause placental vasoconstriction and are not recommended during pregnancy.13,14 There are limited data suggesting that their use in the first trimester may result in an increased incidence of abnormalities typically associated with placental vascular disruption, such as gastroschisis and intestinal atresia.13,14 The risk, however, appears to be low. Pseudoephedrine is excreted in milk in small quantities, limiting the nursing infant’s exposure.13,14 The AAP classifies pseudoephedrine compatible with breast-feeding.12,13
References
1. Schardein, JL. Principles of teratogenesis applicable to drug and chemical exposure. In: Schardein JL, ed. Chemically Induced Birth Defects. New York: Dekker; 2000:1–65.
2. O’Rahilly, RR, Muller, F. Teratology and prenatal diagnosis. In: O’Rahilly RR, Muller F, eds. Human Embryology and Teratology. 3rd ed. New York: John Wiley; 2001:115–133.
3. Shepard, TH. Catalog of Teratogenic Agents, 13th ed. Baltimore: Johns Hopkins University Press; 2010.
4. Fine, JS. Reproductive and perinatal principles. In: Goldfrank LR, et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York: McGraw-Hill; 2011:423–445.
5. Buhimschi, CS, Weiner, CP. Medications in pregnancy and lactation: Part 1. Teratology. Obstet Gynecol. 2009;113:166–188.
6. Mitchell, AA. Systematic identification of drugs that cause birth defects—a new opportunity. N Engl J Med. 2003;349:2556.
7. Doering, PL, Boothby, LA, Cheok, M. Review of pregnancy labeling of prescription drugs: Is the current system adequate to inform of risks? Am J Obstet Gynecol. 2002;187:333.
8. Lo, WY, Friedman, JM. Teratogenicity of recently introduced medications in human pregnancy. Obstet Gynecol. 2002;100:465.
9. Proposed rule: Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Fed Regist. 2008;73:30831.
10. Ostrea, EM, Mantaring, JB, Silvestre, MA. Drugs that affect the fetus and newborn infant via the placenta or breast milk. Pediatr Clin North Am. 2004;51:539–579.
11. Ito, S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–126.
12. American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776.
13. Briggs, GG, et al. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, 9th ed. Baltimore: Lippincott Williams & Wilkins; 2011.
14. REPROTOX: An information system on environmental hazards to reproduction and development. www.REPROTOX.org.
15. Feldkamp, ML. Acetaminophen use in pregnancy and risk of birth defects: Findings from the National Birth Defects Prevention Study. Obstet Gynecol. 2010;115:109–115.
16. Wilkes, JM. Acetaminophen overdose in pregnancy. South Med J. 2005;98:1118–1122.
17. Sørensen, HT. Aspirin in early pregnancy is not associated with an increased risk of congenital anomalies—meta-analysis. Evid Based Obstet Gynecol. 2003;5:103.
18. Kozer, E, et al. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: A meta-analysis. Am J Obstet Gynecol. 2002;187:1523.
19. Askie, LM, et al. Antiplatelet agents for prevention of pre-eclampsia: A meta-analysis of individual patient data. Lancet. 2007;369:1791.
20. Østensen, M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209.
21. Nielsen, GL, Sørensen, HT, Larsen, H, Pedersen, L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: Population-based observational study and case-control study. BMJ. 2001;322:266.
22. Ofori, B, et al. Risk of congenital anomalies in pregnant users of non-steroidal anti-inflammatory drugs: A nested case control study. Birth Defects Res B Dev Reprod Toxicol. 2006;77:268.
23. Koren, G, et al. NSAIDS during third trimester and the risk of prenatal closure of the ductus arteriosus: A meta-analysis. Ann Pharmacother. 2006;40:824–829.
24. Jones, HE, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331.
25. Koren, G, et al. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704.
26. Cheek, TG, Baird, E. Anesthesia for the nonobstetric surgery: Maternal and fetal considerations. Clin Obstet Gynecol. 2009;52:535–545.
27. Kuczkowski, KM. Laparoscopic procedures during pregnancy and the risks of anesthesia: What does an obstetrician need to know? Arch Gynecol Obstet. 2007;276:201–209.
28. Kuczkowski, KM. The safety of anesthetics in pregnant women. Expert Opinion Drug Saf. 2006;5:251–264.
29. Nitsun, M, et al. Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006;79:549–557.
30. Wikner, BN, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: Neonatal outcome and congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16:1203–1210.
31. Buhimschi, CS, Weiner, CP. Medications in pregnancy and lactation: Part 2. Drugs with minimal or unknown human teratogenic effect. Obstet Gynecol. 2009;113(Pt 1):417–432.
32. Chan, KY, Gilbert-Barness, E, Tiller, G. Warfarin embryopathy. Pediatr Pathol Mol Med. 2003;22:277.
33. Santoro, R, et al. Efficacy and safety of the long-term administration of low-molecular-weight heparins in pregnancy. Blood Coagul Fibrinolysis. 2009;20:240–243.
34. Ahearn, GS, et al. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: A case report and review of treatment options. Arch Intern Med. 2002;162:1221–1227.
35. Bailey, B. Are there teratogenic risks associated with antidotes used in the acute management of poisoned pregnant women? Birth Defects Res A Clin Mol Teratol. 2003;67:133–140.
36. Nassar, AH, et al. Pregnancy in patients with beta thalassemia intermedia: Outcome of mothers and newborns. Am J Hematol. 2006;81:499–502.
37. Weizsaecker, K. Lead toxicity during pregnancy. Primary Care Update OB/GYNS. 2003;10:304–309.
38. Albert, M, Lessin, MS, Gilchrist, BS. Methylene blue: A dangerous dye. J Pediatr Surg. 2003;38:1244–1245.
39. Guinsburg, R, Wyckoff, M. Naloxone during neonatal resuscitation: Acknowledging the unknown. Clin Perinatol. 2006;33:121–132.
40. Adhikari, K, Ghosh, A, Alauddin, MD, Moitra, A, Datta, AK. Organophosphate poisoning in pregnancy. J Obstet Gynaecol. 2011;31:290–292.
41. Mylonas, I. Antibiotic chemotherapy during pregnancy and lactation period: Aspects for consideration. Arch Gynecol Obstet. 2011;283:7–18.
42. Crider, CS, et al. Antibacterial medication use in pregnancy and risk of birth defects. Arch Pediatr Adolesc Med. 2009;163:978–985.
43. Czeizel, AE, Rockenbauer, M, Olsen, J, Sørensen, HT. A teratological study of aminoglycoside antibiotic treatment during pregnancy. Scand J Infect Dis. 2000;32:309–313.
44. Bar-Oz, B, et al. The safety of fluoroquinolones: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2009;142:75–78.
45. Bar-Oz, B, et al. Pregnancy outcome after gestational exposure to the new macrolides: A prospective multicenter observational study. Eur J Obstet Gynecol Reprod Biol. 2008;141:31–34.
46. Diav-Citrin, O, Shechtman, S, Gotteiner, T, Arnon, J, Ornoy, A. Pregnancy outcome after gestational exposure to metronidazole : A prospective controlled cohort study. Teratology. 2001;63:186–192.
47. Bruel, H, et al. Hemolytic anemia in a newborn after treatment with nitrofurantoin at the end of pregnancy. Arch Pediatr. 2000;7:745–747.
48. Czeizel, AE, et al. The teratogenic risk of trimethoprim-sulfonamides: A population based case-control study. Reprod Toxicol. 2001;15:637–646.
49. Czeizel, AE, et al. A teratologic study of aminoglycoside antibiotic treatment during pregnancy. Scand J Infect Dis. 2000;32:309–313.
50. Carter, C, et al. Antifungals and the risk of selected birth defects. Am J Obstet Gynecol. 2008;19:191.
51. Czeizel, AE, et al. A population-based case-control study of the safety of oral anti-tuberculosis drug treatment during pregnancy. Int J Tuberc Lung Dis. 2001;5:564–568.
52. Pasternak, B, Hviid, A. Use of acyclovir, valacyclovir and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304:905–906.
53. Workowski, KA, Berman, S, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–112.
54. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. No. 82 June 2007. Management of herpes in pregnancy. Obstet Gynecol. 2007;109:1489–1498.
55. Centers for Disease Control and Prevention, Pregnant women and novel influenza A (H1N1) virus: Considerations for clinicians. June 30, 2009.
56. Greer, LG, et al. Maternal and neonatal outcomes after antepartum treatment of influenza with antiviral medications. Obstet Gynecol. 2010;115:711–716.
57. Tanaka, T, et al. Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnancy and breast feeding women. CMAJ. 2009;181:55–58.
58. Ahmad, N. The vertical transmission of human immunodeficiency virus type 1: Molecular and biological properties of the virus. Crit Rev Clin Lab Sci. 2005;42:1–34.
59. Trappe, HJ. Emergency therapy of maternal and fetal arrhythmias during pregnancy. J Emerg Trauma Shock. 2010;3:153–159.
60. Newstead-Angel, J, Gibson, PS. Cardiac drug use in pregnancy: Safety, effectiveness and obstetric implications. Expert Rev Cardiovasc Ther. 2009;7:1569–1580.
61. Burkart, TA, Conti, JB. Cardiac arrhythmias during pregnancy. Curr Treat Options Cardiovasc Med. 2010;12:457–471.
62. Ghosh, N, et al. The acute treatment of maternal supraventricular tachycardias during pregnancy: A review of the literature. J Obstet Gynaecol Can. 2011;33:17–23.
63. Strasburger, JF, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375–379.
64. Oudjik, MA, et al. Treatment of fetal tachycardia with sotalol: Transplacental pharmacokinetics and pharmacodynamics. J Am Coll Cardiol. 2003;42:765.
65. Chang, J, et al. Pregnancy-related mortality surveillance—United States, 1991-1999. MMWR Surveill Summ. 2003;52:1–8.
66. Duley, L, Henderson-Smart, DJ, Meher, S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. (3):2006.
67. Podymow, T, August, P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–969.
68. Ghanem, FA, Movahed, A. Use of antihypertensive drugs during pregnancy and lactation. Cardiovasc Ther. 2008;26:38–49.
69. Cooper, WO, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451.
70. Magee, LA, et al. Risks and benefits of beta-receptor blockers for pregnancy induced hypertension: Overview of the randomized trials. Eur J Obstet Gynecol Reprod Biol. 2000;88:15.
71. Weber-Schoendorfer, C, et al. The safety of calcium channel blockers during pregnancy: A prospective, multicenter, observational study. Reprod Toxicol. 2008;26:24–30.
72. Khan, K, et al. Safety concerns for the use of calcium channel blockers in pregnancy for the treatment of spontaneous preterm labour and hypertension: A systematic review and meta-regression analysis. J Matern Fetal Neonatal Med. 2010;23:1030–1038.
73. Magee, LA, Cham, C, Waterman, EJ, Ohlsson, A, von Dadelszen, P. Hydralazine for treatment of severe hypertension in pregnancy: Meta-analysis. BMJ. 2003;327:955–960.
74. Miller, MA, Bourjeily, G. Managing the critically ill pregnant patient. www.chestnet.org/accp, Apr 15, 2009.
75. Munnur, U, Bandi, V, Guntupalli, KK. Management principles of the critically ill obstetric patient. Clin Chest Med. 2011;32:53–60.
76. Thurlow, JA, Kinsella, SM. Intrauterine resuscitation: Active management of fetal distress. Int J Obstet Anesthes. 2002;11:105–116.
77. Reidy, J, Douglas, J. Vasopressors in obstetrics. Anesthesiol Clin. 2008;26:75–88.
78. Young, J, Aresh, A. Diabetic medications in pregnancy. Curr Diabetes Rev. 2009;5:252–258.
79. Friedrich, JA, et al. Meta-analysis: Low dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142:510–524.
80. Langer, L, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134.
81. Glueck, CJ, et al. Metformin, pre-eclampsia and pregnancy outcomes in women with polycystic ovary syndrome. Diabet Med. 2004;21:829–836.
82. Diav-Citrin, O, Ornoy, A. Teratogen update: Antithyroid drugs. Methimazole, carbimazole and propylthiouracil. Teratology. 2002;65:38–44.
83. Yen, EH, et al. Acid blocking therapy during pregnancy increases the odds for childhood asthma. J Allergy Clin Immunol. 2008;121:794.
84. Gill, SK, et al. The safety of proton pump inhibitors (PPIs) in pregnancy: A meta-analysis. Am J Gastroenterol. 2009;104:1541–1545.
85. Magee, LA, Mazzotta, P, Koren, G. Evidence-based view of safety and effectiveness of pharmacologic therapy for nausea and vomiting of pregnancy (NVP). Am J Obstet Gynecol. 2002;186:5.
86. Holmes, LB, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132.
87. Meador, KJ, NEAD Study Group. In utero antiepileptic drug exposure: Fetal death and malformations. Neurology. 2006;67:407.
88. The Lamotrigine Pregnancy Registry: Final report. 1 September 1992 through 31 March 2010. Wilmington, NC: GlaxoSmithKline, July 2010.
89. Molgaard-Nielsen, D, Hviid, A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305:1996–2002.
90. Evans, EW, Lorber, KC. Use of 5-HT1 agonists in pregnancy. Ann Pharmacother. 2008;42:543–549.
91. Seto, A, Einarson, T, Koren, G. Pregnancy outcome following first trimester exposure to antihistamines: Meta-analysis. Am J Perinatol. 1997;14:119–123.
92. Kallen, B. Use of antihistamine drugs in early pregnancy and delivery outcome. J Matern Fetal Neonatal Med. 2000;11:146.
93. Tan, KS, Thompson, NC. Asthma in pregnancy. Am J Med. 2000;109:727.
94. Chambers, C. The safety of asthma and allergy medication during pregnancy. Immunol Allergy Clin North Am. 2006;26:13.
95. U.S. Food and Drug Administration. Terbutaline: Label change—warnings against use for treatment of preterm labor. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm243843.htm, 2011.
96. Blais, L, et al. High doses of inhaled corticosteroids during the first trimester of pregnancy and congenital malformations. J Allergy Immunol. 2009;124:1229–1234.
97. Hviid, A, Molgaard-Nielsen, D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796–804.
98. Bakhireva, LN, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007;119:618.
*References 11, 13, 14, 31, 41, 42.
†References 10, 13, 14, 31, 41, 42.
*References 13, 14, 31, 59, 60, 62.
*References 13, 14, 31, 59–62.
†References 13, 14, 66–68, 70.
†References 13, 14, 66–68, 71, 72.
‡References 13, 14, 31, 66–68, 73.
*References 13, 14, 31, 74, 75.
†References 13, 14, 66–68, 74, 75.
*References 13, 14, 31, 78, 80.
*References 13, 14, 31, 86, 87.
*References 13, 14, 31, 86, 87.
*References 13, 14, 31, 92, 94.