Chapter 19 Double Outlet Ventricle
The earliest report that alluded to double outlet right ventricle was published in French in 1703.1 Ninety years elapsed before an English-language publication appeared.2 In 1793, John Abernathy,2 an assistant surgeon at St Bartholomew’s Hospital in London, described “partial transposition” of the great arteries, and in 1898, Karl von Vierordt3 called double outlet right ventricle partial transposition to signify that the aorta was transposed but the pulmonary trunk was normally aligned. It was not until 1957 that Witham4 introduced double outlet right ventricle as a diagnostic term for a partial transposition complex. Witham’s term is now preferred to the synonymous right ventricular origin of both great arteries. Double outlet left ventricle is the rarest of the ventriculoarterial malalignments (see subsequent section).5,6
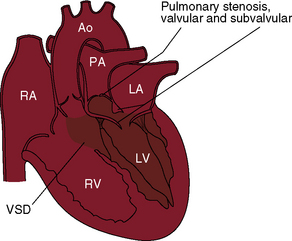
The question of how best to define double outlet right ventricle has been much debated, and the debate continues without a consensus.7,8 It has been argued that the malformation is virtually unclassifiable because of its excessively complex and diverse anatomy. Should double outlet right ventricle be defined as a connection between the great arterial trunks and the right ventricular mass, or as a malformation in which the leaflets of both great arterial valves are supported by right ventricular infundibular musculature?7 How much overriding of one or the other arterial valves is acceptable? In this chapter, double outlet right ventricle is defined pragmatically as a malformation in which the greater part of the circumference of both arterial valves is supported within a morphologic right ventricle in hearts with two distinct ventricular chambers and concordant atrioventricular connections (Table 19-1).7 The aorta and main pulmonary artery are separated by an outlet septum housed exclusively in the right ventricle. A conus resides beneath each of the two great arterial valves (double conuses), although either conus may be attenuated. The position of the outlet septum establishes two types of infundibular relationships: namely, anterior/posterior and side-by-side. In the more common anterior/posterior relationship, the aorta arises from the posterior infundibulum, and the ventricular septal defect is subaortic. In the less common side-by-side relationship, the pulmonary trunk arises from the medial infundibulum, and the ventricular septal defect is subpulmonary.
Table 19-1 Double Outlet Right Ventricle: Clinical Classification
| The More Common Types |
| The Less Common Types |
Connections of the great arteries to the ventricles
The aorta is either to the right of and anterior to the pulmonary trunk or side-by-side with the aorta to the right.9 Each great artery arises above a conus that prevents fibrous continuity with atrioventricular valve tissue, but conal attenuation occasionally permits fibrous continuity.9–11 When each great artery is equipped with a separate conus, and both great arteries arise exclusively from the right ventricle, the term double outlet right ventricle is not disputed. However, the appropriate terminology for hearts with a biventricular great arterial valve is unresolved.
Double outlet right ventricle with a subaortic ventricular septal defect, pulmonary stenosis, and an aortic override greater than 50% resembles Fallot’s tetralogy (Box 19-1; see Chapter 18). Double outlet right ventricle with a subpulmonary ventricular septal defect and a posterior non–border-forming biventricular pulmonary trunk resembles complete transposition of the great arteries (see Box 19-1; see Chapter 27). Double outlet right ventricle, Fallot’s tetralogy, and complete transposition have been proposed to represent a spectrum of anomalies resulting from embryonic arrest of the normal rotation of the junction of the outflow tract and the great arteries. In 1949, Taussig and Bing12 published a case of complete transposition of the aorta and levoposition of the pulmonary artery, which arose chiefly from the right ventricle (see subsequent section).
Box 19-1 Double Outlet Right Ventricle: Major Clinical Patterns
Relationship of the ventricular septal defect to the great arteries
A ventricular septal defect is usually subaortic or subpulmonary and provides the left ventricle with its only outlet (Figure 19-1). Less often, the defect is committed to both great arteries, or the ventricular septum is intact (see Table 19-1).9,13,14 Rarely, the defect is in muscular or inlet septum and is therefore not committed to either great artery.15,16
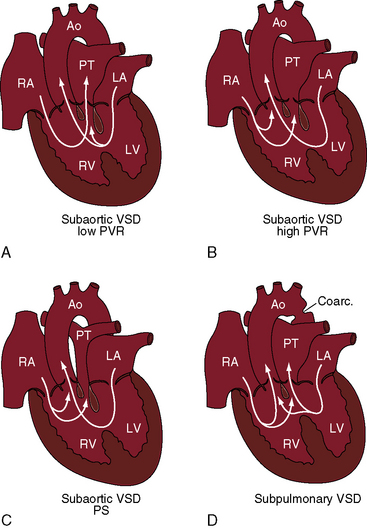
Figure 19-1 Illustrations of four major clinical patterns of double outlet right ventricle (see Table 19-1 and Box 19-1). A, Subaortic ventricular septal defect (VSD), low pulmonary vascular resistance, no pulmonary stenosis. B, Subaortic ventricular septal defect, high pulmonary vascular resistance. C, Subaortic ventricular septal defect with pulmonary stenosis. D, Subpulmonary ventricular septal defect. (Ao = aorta; RA = right atrium; PT = pulmonary trunk; LA = left atrium; LV = left ventricle; RV = right ventricle.)
The location of the ventricular septal defect is the major determinant of intracardiac streaming. When the defect is committed to the aorta, blood flow is preferentially channeled into the aorta (see Figure 19-1A). Streaming is secondarily influenced by pulmonary vas cular resistance and pulmonary stenosis. In the Taussig-Bing anomaly (see previous), which constitutes less than 10% of cases of double outlet right ventricle, the aorta arises completely from the right ventricle, a nonrestrictive ventricular septal defect is subpulmonary, and the pulmonary trunk is biventricular, although principally in the right ventricle.12 Left ventricular blood preferentially enters the pulmonary artery through the subpulmonary ventricular septal defect (Figures 19-1D and 19-2).10–12,17
Obstruction to ventricular outflow
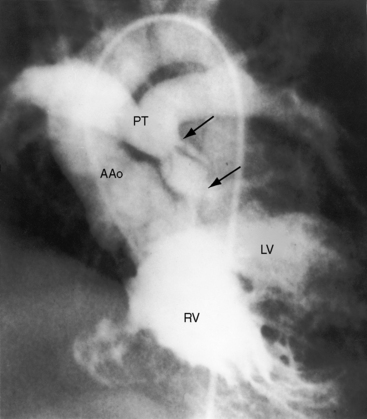
Pulmonary stenosis occurs in 40% to 70% of cases of double outlet right ventricle with subaortic ventricular septal defect (see Figure 19-1C)10,18 and is represented by an underdeveloped subpulmonary conus or by a stenotic bicuspid pulmonary valve.9 A subpulmonary ventricular septal is rarely accompanied by pulmonary stenosis.19 When there is pulmonary artesia (Figure 19-3), the right ventricle has a single functional outlet—the aorta—so double outlet then refers to ventriculoarterial alignment.
A nonrestrictive ventricular septal defect is physiologically advantageous because it provides the left ventricle with an unobstructed exit. An inherently restrictive subaortic ventricular septal defect or a spontaneous decrease in size is a form of subaortic stenosis.20–22 The decrease in size may culminate in complete closure,13,22 or rarely, the ventricular septum is congenitally intact and the left ventricle is hypoplastic.13,23 Aortic stenosis can also be caused by an underdeveloped subaortic infundibulum, which occurs in about 50% of cases with a subaortic ventricular septal defect.
Nearly a third of patients with straddling atrioventricular valves (biventricular insertion of chordae tendineae) have a double outlet right ventricle (see Figure 19-10 and Video 9-1).24 The straddle involves the right-sided or left-sided portion of the atrioventricular valve.24 Overriding refers to biventricular commitment of the atrioventricular annulus, which is not a feature of double outlet right ventricle.
The atrioventricular node is normally located, and the conduction system penetrates the right side of the central fibrous body (see section Electrocardiogram).25 The location of the atrioventricular bundle is related to the ventricular septal defect as in isolated ventricular septal defect (see Chapter 17).
The classification of double outlet right ventricle in Table 19-1 is based on anatomic faults that are principally responsible for the physiologic derangements and clinical expressions of a subaortic or subpulmonary ventricular septal defect and from the absence or presence of pulmonary stenosis or pulmonary vascular disease.
The physiologic consequences of double outlet right ventricle with a subaortic ventricular septal defect and no pulmonary stenosis resemble an isolated nonrestrictive perimembranous ventricular septal defect (see Box 19-1; see Chapter 17). Because the defect is committed to the aorta, left ventricular blood preferentially enters the aorta, and low pulmonary vascular resistance permits a substantial portion of left ventricular blood to stream into the pulmonary circulation and permits right ventricular blood to stream almost exclusively into the pulmonary trunk (see Figure 19-1A). Pulmonary blood flow is increased, and aortic oxygen saturation is virtually normal. As pulmonary vascular resistance rises, right ventricular blood is diverted into the aorta and left ventricular blood is diverted away from the pulmonary trunk (see Figure 19-1B). Pulmonary blood flow declines, and aortic oxygen saturation declines in parallel.
When pulmonary stenosis occurs with double outlet right ventricle, the ventricular septal defect is almost always subaortic. Pulmonary stenosis may initially be absent or mild and then develop and progress. Pulmonary stenosis diverts right ventricular and left ventricular blood away from the pulmonary artery and into the aorta (see Figure 19-1C), so pulmonary blood flow and aortic oxygen saturation fall. The more severe the pulmonary stenosis, the more blood from right and left ventricles enters the aorta; and in the presence of pulmonary atresia, all blood from both ventricles enters the aorta (see Figure 19-3).26 Double outlet right ventricle with a nonrestrictive subaortic ventricular septal defect and severe pulmonary stenosis or atresia physiologically resembles Fallot’s tetralogy (see Box 19-1; see Chapter 18).10,26
When the ventricular septal defect is subpulmonary, left ventricular blood preferentially enters the pulmonary trunk, and right ventricular blood preferentially enters the aorta (see Figures 19-1D and 19-2), so pulmonary arterial oxygen saturation exceeds aortic oxygen saturation. When pulmonary vascular resistance is low, pulmonary blood flow is increased, systemic arterial oxygen saturation is high, cyanosis is relatively mild, and the left ventricle is volume overloaded as in complete transposition of the great arteries (see Chapter 27). A rise in pulmonary vascular resistance diverts right ventricular blood from the pulmonary artery into the aorta. Pulmonary blood flow declines, systemic arterial oxygen saturation falls, and left ventricular volume overload is curtailed (see Figure 19-1D).
Double outlet right ventricle with subaortic ventricular septal defect
The clinical manifestations closely resemble isolated nonrestrictive perimembranous ventricular septal defect (see Figure 19-1A and B and Box 19-1). Male:female ratio is estimated at 1.7:1.27 A large kindred included a second cousin with double outlet right ventricle, a first cousin with complete transposition of the great arteries, and two siblings with truncus arteriosus.28
History
The murmur of ventricular septal defect dates from birth because flow from the left ventricle through the defect is obligatory and does not await the neonatal fall in pulmonary vascular resistance. However, the intensity of the murmur increases as neonatal pulmonary resistance falls and as increased left ventricular stroke volume is ejected through the ventricular septal defect into both great arteries. Increased pulmonary blood flow results in volume overload of the left ventricle, congestive heart failure, and poor growth and development. Cyanosis is mild or absent because left ventricular blood preferentially enters the aorta and right ventricular blood preferentially enters the pulmonary artery (see Figure 19-1A). A rise in pulmonary vascular resistance curtails pulmonary blood flow and relieves the left ventricular of volume overload. Patients occasionally reach young adulthood (Figure 19-4A); one patient underwent intracardiac repair at age 53 years (Figure 19-4B), and one survived to age 65 years.29
Physical Appearance
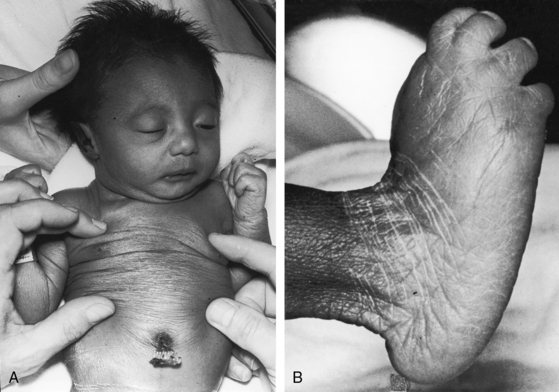
Transient neonatal cyanosis coincides with transient elevation in neonatal pulmonary vascular resistance. The subsequent fall in pulmonary vascular resistance is accompanied by an increase in pulmonary blood flow, volume overload of the left ventricle, and the catabolic appearance of congestive heart failure. A subsequent rise in pulmonary resistance diverts left ventricular blood from the pulmonary trunk and diverts right ventricular blood into the aorta (see Figure 19-1B)—Eisenmenger’s syndrome—with the appearance of cyanosis and clubbing (see Box 19-1). Double outlet right ventricle is occasionally associated with trisomy 18 and the distinctive overlapping fingers (clinodactyly), rocker-bottom feet, and lax skin (Figure 19-5).30–32
Jugular Venous Pulse
When increased pulmonary blood flow results in biventricular failure, the jugular venous A wave, V wave, and mean pressure are elevated. The height and waveform of the jugular pulse normalize when pulmonary vascular resistance increases. The right ventricle then ejects at systemic resistance with little extra help from the right atrium, as in Eisenmenger’s syndrome (see Chapter 17).
Precordial Movement and Palpation
A hyperactive precordium with Harrison’s grooves result from chronic dyspnea. A right ventricular impulse is accompanied by the impulse of a dilated hypertensive pulmonary trunk and a palpable pulmonary valve closure sound. A thrill generated by the ventricular septal defect is maximal in the third and fourth intercostal spaces at the left sternal border (see section Auscultation).
Auscultation
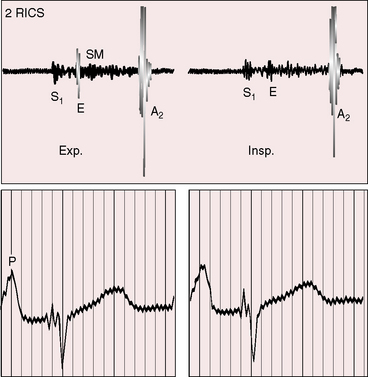
The first heart sound is soft because the PR interval tends to be prolonged (see section Electrocardiogram).33Low pulmonary vascular resistance results in a holosystolic murmur of ventricular septal defect that is maximal in the third and fourth intercostal spaces at the left sternal border. The pulmonary component of the second heart sound is loud because the hypertensive dilated pulmonary trunk is anterior. The aortic component of the second sound is prominent because the aortic valve lies side by side and to the right of the pulmonary trunk rather than posterior. Inspiratory splitting is preserved as long as pulmonary vascular resistance is less than systemic. Increased flow across the mitral valve generates an apical mid-diastolic murmur.
Elevated pulmonary vascular resistance reduces pulmonary blood flow and left ventricular volume overload (see Figure 19-1B). The murmur of ventricular septal defect becomes decrescendo and is softer (Figure 19-6) but does not disappear because flow from left ventricle into aorta is obligatory. Except for the soft decrescendo murmur, the auscultatory signs are analogous to Eisenmenger’s syndrome with an isolated nonrestrictive ventricular septal defect: namely, a pulmonary ejection sound, a loud single second heart sound, and a Graham Steell murmur of pulmonary hypertensive pulmonary regurgitation (see Chapter 17).
Electrocardiogram
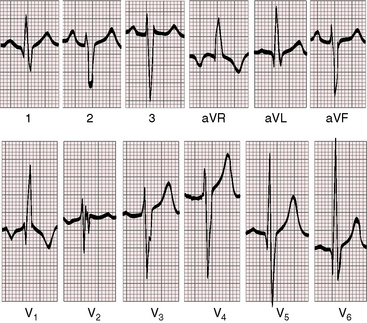
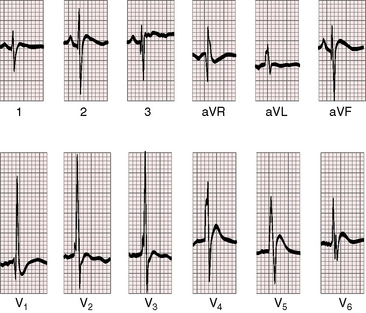
PR interval prolongation is common (Figure 19-7; see previous) because of the unusually long course of the common atrioventricular bundle.33 When pulmonary blood flow is increased, peaked right atrial P waves are associated with bifid broad left atrial P waves (Figure 19-8). Left axis deviation with counterclockwise depolarization is an important feature of double outlet right ventricle with a subaortic ventricular septal defect, no pulmonary stenosis, and increased pulmonary blood flow (see Figure 19-7).25,33 The mechanism of left axis deviation is unknown but cannot be related to an abnormality of the conduction system, which is structurally the same when pulmonary vascular resistance is elevated and there is right axis deviation (see Figure 19-8) and when double outlet right ventricle exists with pulmonary stenosis and right axis deviation.25 The QRS duration is normal, but right ventricular conduction defects sometimes occur, including right bundle branch block.33 Right ventricular hypertrophy is obligatory and is manifested by tall R waves in leads V1 and aVR with deep S waves in left precordial leads (see Figures 19-7 and 19-8). Left ventricular volume overload is indicated by large RS complexes in midprecordial leads and by tall R waves in left precordial leads (see Figure 19-7). Elevated pulmonary vascular resistance is associated with right axis deviation and pure right ventricular hypertrophy (see Figure 19-8).
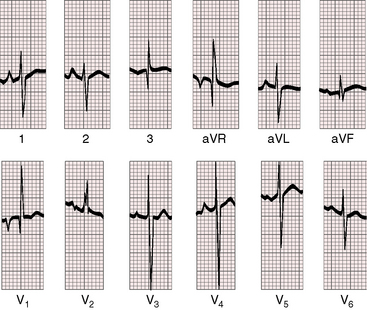
Figure 19-8 Electrocardiogram from a 6-week-old male with trisomy 18 (see Figure 19-5) and double outlet right ventricle, subaortic ventricular septal defect, and moderately elevated pulmonary vascular resistance. Peaked right atrial P waves are present in leads 1 and 2, and a broad bifid left atrial P wave is present in lead V2. Right axis deviation is moderate, and depolarization is clockwise. The q wave in lead V1 indicates right atrial enlargement. Right ventricular hypertrophy is manifested by tall R waves in leads V1 and aVR and by deep S waves in left precordial leads.
X-Ray
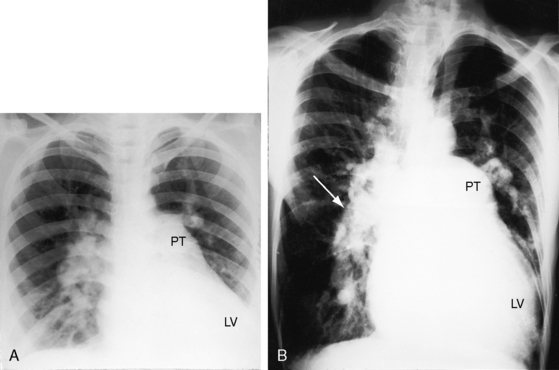
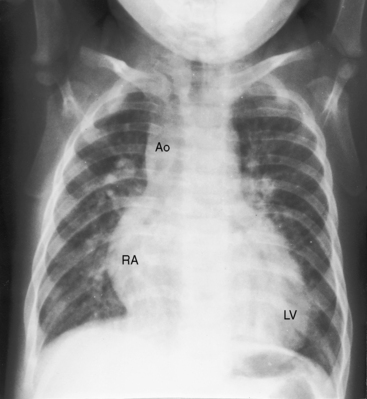
With double outlet right ventricle, nonrestrictive subaortic ventricular septal defect, and low pulmonary vascular resistance, the x-ray resembles isolated nonrestrictive perimembranous ventricular septal defect with increased pulmonary blood flow (Figures 19-4 and 19-9A).27 Thymus is present even though there is transposition of the aorta (Figure 19-9B), in contrast to complete transposition of both great arteries in which thymus is typically absent (see Chapter 27). The pulmonary trunk is prominent because it carries increased volume at systemic pressure and is not posterior to the aorta (see Figure 19-4). Left atrial and left ventricular enlargement reflect the volume overload of increased pulmonary blood flow (see Figure 19-4). With the advent of congestive heart failure, the right atrium and right ventricle dilate conspicuously.
The lung fields are oligemic when left ventricular volume overload is curtailed by elevated pulmonary vascular resistance, either before the neonatal fall (see Figure 19-9B) or after the development of pulmonary vascular disease. The right ventricle copes with systemic resistance without enlarging significantly. The x-ray is indistinguishable from a nonrestrictive ventricular septal defect and Eisenmenger’s syndrome (see Figure 17-9).
Echocardiogram
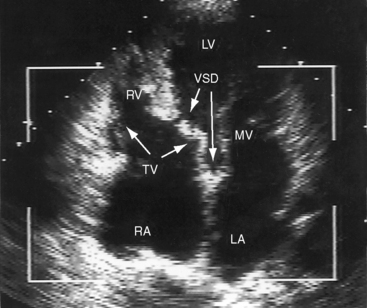
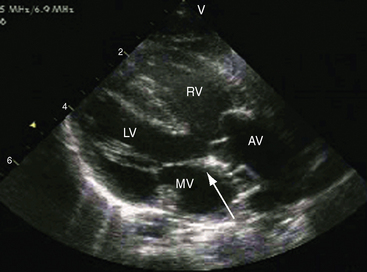
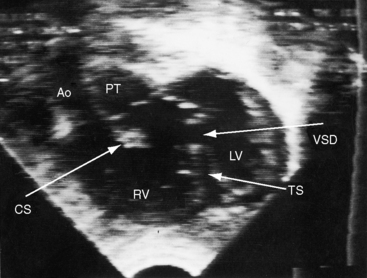
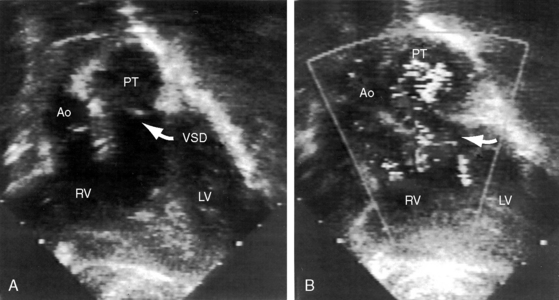
Echocardiography with color flow imaging and Doppler interrogation34 provides diagnostic information on: (1) the right ventricular origin and spatial relationships of the great arteries; (2) the presence and position of the infundibular septum; (3) the size of the ventricular septal defect and its relationship to the aortic and pulmonary valves; (4) mitral/semilunar valve discontinuity (see Figures 19-19 and 19-20)35; and (5) straddling of the atrioventricular valve tensor apparatus (Figure 19-10 and Video 19-1). The aortic root and pulmonary trunk are parallel and are separated by a prominent outlet septum. The aorta arises entirely from the right ventricle, and the pulmonary trunk arises predominantly, if not entirely, from the right ventricle (see Figure 19-19). In the short axis, the great arteries appear as double circles, with the aorta to the right of the pulmonary trunk as in complete transposition of the great arteries (see Chapter 27). The pulmonary trunk is identified by its bifurcation, and the aorta is identified by its brachiocephalic branches. Bilateral subarterial conuses separate the aortic and pulmonary valves from the atrioventricular valves. The course of the outlet septum establishes the alignment of the ventricular septal defect with the aortic or the pulmonary valve. If the outlet septum curves leftward toward the ventriculo/infundibular fold, the ventricular septal defect is subaortic. If the outlet septum is straight and parallel to the trabecular septum, the ventricular septal defect is subpulmonary. The echocardiogram can identify a single coronary artery, which is estimated to occur in 11% of cases of double outlet right ventricle.36 Fetal echocardiography can be used to determine most of the essential anatomic features.37
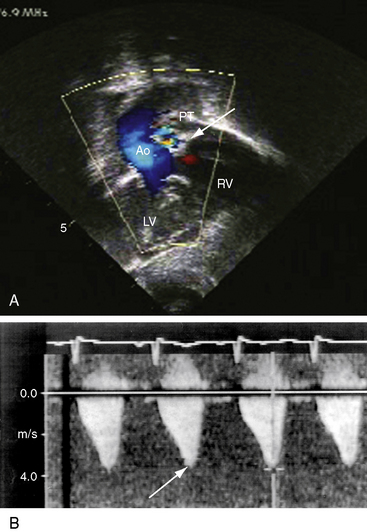
Figure 19-20 A, Black and white print of a color flow image from the 2-month-old male with double outlet right ventricle, subaortic ventricular septal defect, and pulmonary stenosis (see Figure 19-19 for two-dimensional echocardiogram). Both great arteries arise from the right ventricle (RV). Blood flow through the ventricular septal defect (VSD) selectively enters the aorta (Ao). The high-velocity jet of pulmonary stenosis (PS) enters the pulmonary trunk (PT). (RV/LV = right and left ventricles.) B, Continuous wave Doppler scan records a peak velocity of 4 msec for a peak instantaneous gradient of 64 mm Hg from right ventricle to pulmonary trunk (Videos 19-3A and 19-3B).
Double outlet right ventricle with subaortic ventricular septal defect and pulmonary stenosis
More than 50% of patients with double outlet right ventricle and a subaortic ventricular septal defect have pulmonary stenosis (see Box 19-1 and Figure 19-1C). The clinical manifestations closely resemble Fallot’s tetralogy (see Chapter 18).
History and Physical Appearance
Pulmonary stenosis, which varies from mild to severe to atresia, can be present at birth or delayed with a progressive increase in severity (Figure 19-11). Cyanotic patients squat as in Fallot’s tetralogy. The clinical course and longevity are better than in double outlet right ventricle without pulmonary stenosis because pulmonary blood flow is more effectively regulated (see previous).10,38
Jugular Venous Pulse and Arterial Pulse
The A wave is normal because the right ventricle ejects at but not above systemic vascular resistance and does not need an increase in right atrial contractile force, analogous to Fallot’s tetralogy (see Chapter 18). The arterial pulse is normal because biventricular ejection into the aorta maintains a normal stroke volume.
Precordial Movement and Palpation
The right ventricular impulse is analogous to the gentle impulse of a normal neonatal heart and is assigned to the fourth and fifth left intercostal spaces and subxyphoid area because the stenosis is subpulmonary (see Figure 19-11). A systolic thrill is maximal in the third left intercostal space for the same reason. A left ventricular impulse is not palpable in cyanotic patients because pulmonary blood flow is reduced, so the left ventricle is underfilled.
Auscultation
Acyanotic patients with mild pulmonary stenosis have a holosystolic murmur of ventricular septal defect at the lower left sternal border and a midsystolic murmur of pulmonary stenosis in the second and third left intercostal spaces (Figure 19-12A, C). The pulmonary component of the second heart sound is appropriately delayed (see Figure 19-12A). When pulmonary blood flow is increased, an apical third heart sound introduces a mid-diastolic murmur across the mitral valve (Figure 19-12B).
Patients with severe pulmonary stenosis or atresia have auscultatory signs indistinguishable from cyanotic Fallot’s tetralogy (see Chapter 18). The duration of the pulmonary stenotic murmur varies inversely with the severity of stenosis. Pulmonary atresia is accompanied by an aortic ejection sound, a soft midsystolic murmur into the dilated aorta, and a loud single second heart sound of aortic valve closure (Figure 19-13). Exceptionally, there is a long decrescendo systolic murmur at the left sternal border because of obligatory flow from left ventricle into aorta through a restrictive ventricular septal defect that constitutes a zone of subaortic stenosis. A left ventricular fourth heart sound can then be heard because the left ventricular hypertrophy of subaortic stenosis is associated with an increased force of left atrial contraction. Audibility of the fourth heart sound is enhanced by prolongation of the PR interval. An early diastolic murmur of aortic regurgitation is occasionally present in double outlet right ventricle with pulmonary stenosis as in Fallot’s tetralogy.17,38
Electrocardiogram
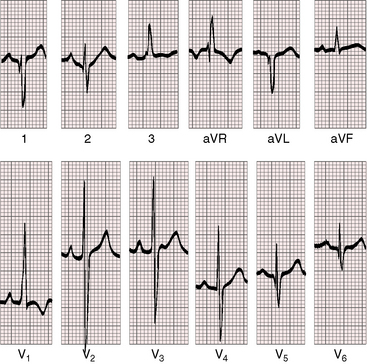
Because the right ventricle is systemic, peaked right atrial P waves occur even with mild pulmonary stenosis and may be accompanied by left atrial P waves because pulmonary blood flow is increased (Figure 19-14). With severe pulmonary stenosis, the P waves are either normal or peaked and low amplitude (Figure 19-15) but are occasionally peaked and tall (Figure 19-16). The PR interval is likely to be prolonged (see Figures 19-14, 19-15, and 19-16).
When pulmonary stenosis is mild, the electrocardiogram retains the left axis deviation and counterclockwise depolarization that occur in the absence of pulmonary stenosis (see Figure 19-14). As stenosis increases, the QRS axis becomes vertical or rightward. It is here that distinctive albeit subtle features arouse suspicion of double outlet right ventricle with pulmonary stenosis.33 The distinctive feature is persistence of counterclockwise initial forces that generate q waves in leads 1 and aVL even when the axis is vertical or rightward (see Figure 19-15). Q waves in leads 1 and aVL are rare in neonates and young children39 and are virtually unknown in Fallot’s tetralogy (see Chapter 18). The terminal forces are deep and prolonged with a tendency for broad slurred S waves in leads 1, aVL, and V5-6 and a broad R wave in lead aVR (see Figures 19-14 and 19-15).33 With pulmonary atresia, the electrocardiogram is similar if not indistinguishable from Fallot’s tetralogy with pulmonary atresia (see Figure 19-16). A restrictive subaortic ventricular septal defect is functionally subaortic stenosis, which can cause left ventricular hypertrophy.
X-Ray
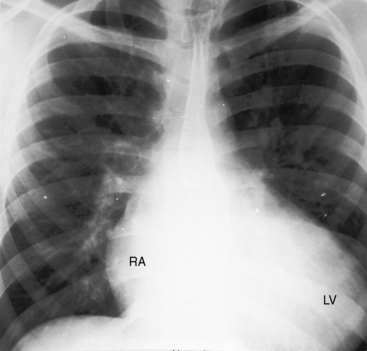
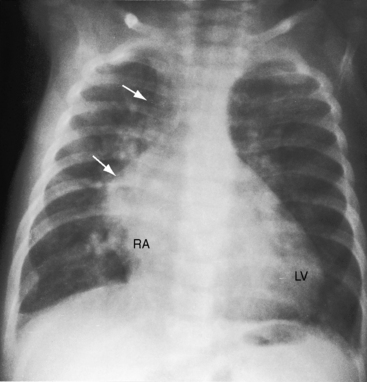
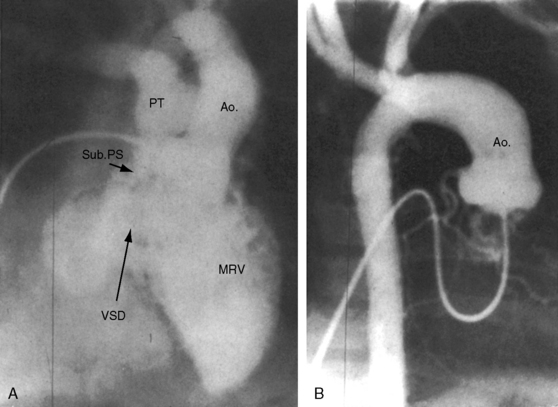
The pulmonary trunk is not dilated because the stenosis is subpulmonary (Figures 19-10 and 19-17). When the stenosis is mild, pulmonary vascularity is increased and the left ventricle is dilated (see Figure 19-17). When pulmonary stenosis is severe, pulmonary vascularity is reduced, the heart size is normal, and the apex is convex (Figure 19-18A).27 In the presence of pulmonary atresia, the ascending aorta is enlarged, the main pulmonary artery segment is concave, and the apex is boot-shaped (Figures 19-3A, B and 19-18B).
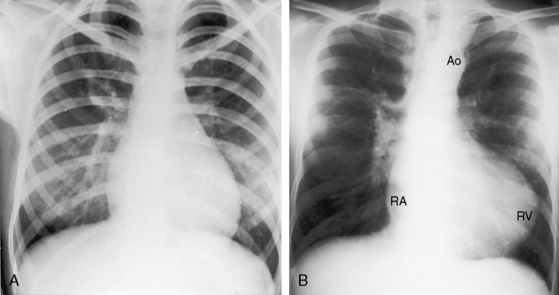
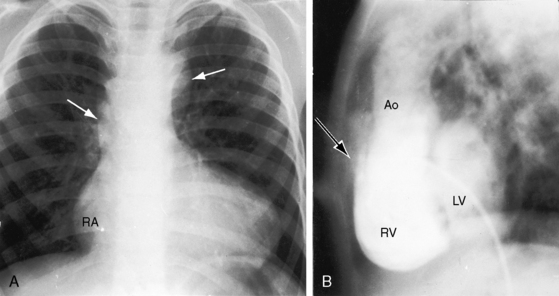
Figure 19-18 A, X-ray from a 13-year-old girl with double outlet right ventricle, subaortic ventricular septal defect, and severe subpulmonary stenosis. Pulmonary vascularity is normal. The heart size is normal with a slightly rounded apex. B, X-ray from a 29-year-old man with double outlet right ventricle, a subaortic ventricular septal defect, and pulmonary atresia (compare with Figure 19-3A). The lung fields have the lacy appearance of systemic-to-pulmonary artery collaterals. The pulmonary trunk is conspicuously absent, but the right and left branches are well-formed. The aortic knuckle (Ao) is prominent and continues as a left descending aorta. The boot-shaped apex is occupied by the right ventricle (RV). The right atrium (RA) is moderately convex.
Echocardiogram
Echocardiography with color flow imaging and Doppler interrogation establishes the subaortic location of the ventricular septal defect and the presence, type, and degree of pulmonary stenosis (Figures 19-19 and 19-20 and Videos 19-2, 19-3A, and 19-3B). Both great arteries are imaged above the right ventricle, and subpulmonary stenosis with a nondilated pulmonary trunk is identified (see Figure 19-19). Continuous wave Doppler scan establishes the pulmonary stenotic gradient (see Figure 19-20B). In the short axis, the great arteries appear as double circles, and a bicuspid stenotic pulmonary valve is occasionally identified at the origin of the pulmonary trunk to the left of the aorta.
Double outlet right ventricle with subpulmonary ventricular septal defect: the taussig-bing anomaly
Clinical manifestations of the Taussig-Bing anomaly resemble complete transposition of the great arteries with a nonrestrictive ventricular septal defect (see Box 19-1; see Chapter 27). About 50% of these patients have congenital malformations of the aortic arch, including coarctation, isthmic hypoplasia, interruption, and patent ductus arteriosus, in addition to subaortic stenosis.17,40,41
History
Cyanosis dates from birth or early infancy because flow from right ventricle into aorta is obligatory (see Figure 19-1D).12,17,42 As neonatal pulmonary vascular resistance falls, left ventricular blood preferentially enters the pulmonary trunk across the subpulmonary ventricular septal defect (see Figure 19-2). Systemic arterial oxygen saturation is relatively high but at the price of increased pulmonary blood flow, left ventricular volume overload, and congestive heart failure.10,17 The clinical course is especially poor when coarctation of the aorta elevates systemic systolic pressure, which augments already abundant pulmonary blood flow by diverting still more right ventricular blood into the pulmonary trunk (see Figure 19-1D).10,41 A rise in pulmonary vascular resistance occasionally regulates pulmonary blood flow and ameliorates congestive heart failure. Cyanosis increases, but longevity improves; and an occasional patient reaches the second, third, or fourth decade (Figure 19-21).42
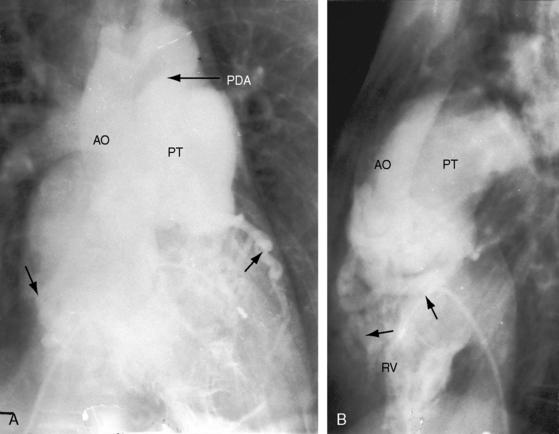
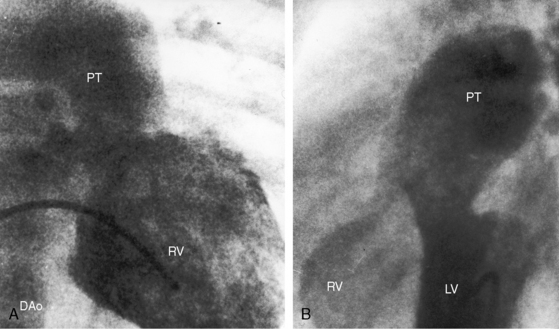
Figure 19-21 Right ventriculograms (RV) from a 40-year-old woman with the Taussig-Bing anomaly characterized by double outlet right ventricle, a subpulmonary ventricular septal defect, a biventricular pulmonary trunk, and pulmonary vascular disease A, The ascending (AO) and the pulmonary trunk (PT) are side by side with the aorta to the right. A large patent ductus arteriosus (PDA) was distal to an aortic coarctation. B, Lateral projection shows the aorta (AO) anterior to the pulmonary trunk (PT). Two unmarked arrows point to dilated ectatic coronary arteries (see Chapter 32). (RV = right ventricle.)
Physical Appearance
Infants with increased pulmonary blood flow have the catabolic effects of congestive heart failure with poor growth and development. When a moderate elevation in pulmonary vascular resistance curtails pulmonary blood flow, congestive heart failure is relieved and growth and development improve. Suprasystemic pulmonary vascular resistance results in reversed differential cyanosis, a distinctive physical appearance that is manifest because ductal flow is right to left (Figure 19-22).17 The toes are less cyanotic and less clubbed than the fingers because oxygenated blood from the left ventricle flows through the subpulmonary ventricular septal defect into the pulmonary trunk and through the patent ductus into the descending aorta, whereas unoxygenated blood from the right ventricle flows into the aorta and to the upper extremities. Trisomy 18 is another distinctive physical appearance with either a subpulmonary or a subaortic ventricular septal defect (see Figure 19-5).30–32
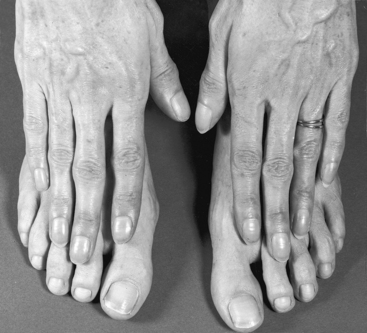
Figure 19-22 Hands and feet of the 40-year-old woman with the Taussig-Bing anomaly referred to in Figure 19-21. There is reversed differential cyanosis, with fingers more cyanotic than toes because unoxygenated blood from the right ventricle entered the ascending aorta and the bracheocephalic arteries while oxygenated blood from the left ventricle entered the pulmonary trunk through the subpulmonary ventricular septal defect and preferentially flowed through the patent ductus arteriosus into the descending thoracic aorta. Compare with Figure 20-12, which illustrates the differential cyanosis that characterizes a reversed shunt through an isolated patent ductus arteriosus.
Arterial Pulse
Upper and lower extremity arterial pulses are diagnostically important because of the frequency of coarctation of the aorta.10,17 However, the femoral pulses are preserved when a nonrestrictive patent ductus is distal to the coarctation (see Figure 19-21).
Jugular Venous Pulse
In the presence of biventricular failure, the right atrial A wave, V wave, and mean pressure are elevated. Increased pulmonary vascular resistance curtails pulmonary blood flow and alleviates congestive heart failure, so the jugular venous pulse normalizes (Figure 19-23, second panel).
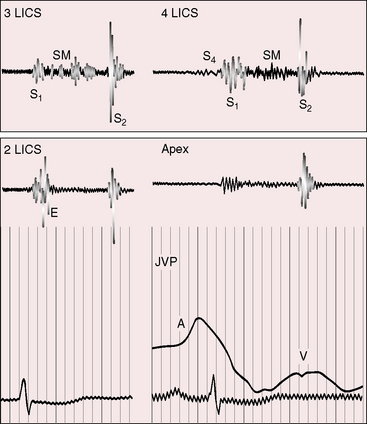
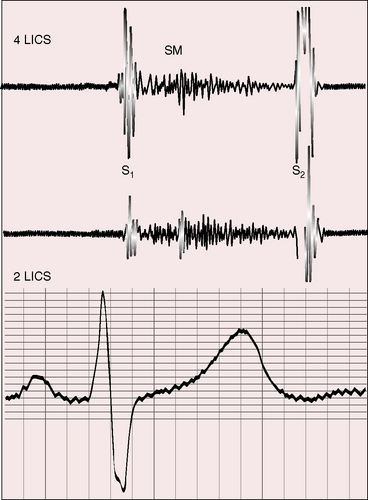
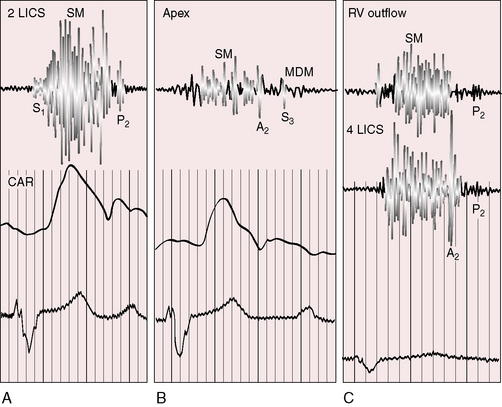
Figure 19-23 Phonocardiograms from the 40-year-old woman with the Taussig-Bing anomaly referred to in Figures 19-21 and 19-22. A soft systolic murmur (SM) is maximal in the third left intercostal space (3 LICS) and ends before a loud single second heart sound (S2). The pulmonary ejection sound (E) originated in a dilated hypertensive pulmonary trunk and was recorded in the second left intercostal space (2 LICS). A soft fourth heart sound (S4) was present in the fourth left intercostal space (4 LICS). The jugular venous pulse (JVP) shows a small dominant A wave.
Precordial Movement and Palpation
An obigatory right ventricular impulse becomes more conspicuous in the presence of biventricular failure. The dilated hypertensive pulmonary trunk (see Figures 19-2 and 19-21) and the loud pulmonary second heart sound can be palpated in the second left intercostal space. Volume overload of the left ventricle is responsible for a left ventricular impulse. A thrill is located as high as the second left intercostal space because the ventricular septal defect is subpulmonary. As pulmonary vascular resistance rises, pulmonary blood flow falls, left ventricular volume overload decreases, the left ventricular impulse may disappear, the right ventricular impulse decreases, and the ventricular septal defect thrill vanishes.
Auscultation
The systolic murmur that originates from a ventricular septal defect can be located as high as the second left intercostal space because the left ventricle ejects directly into the pulmonary trunk through the subpulmonary ventricular septal defect (see Figure 19-1D).17,42 The pulmonary component of the second heart sound is loud, and splitting is preserved when pulmonary resistance is lower than systemic. An apical mid-diastolic murmur signifies increased pulmonary blood flow with increased flow across the mitral valve. As pulmonary resistance rises, pulmonary blood flow and left ventricular volume overload decline, the murmur through the subpulmonary ventricular septal defect is attenuated (see Figure 19-23), and the mitral flow murmur disappears. A pulmonary ejection sound, a soft short mid-systolic murmur, and a Graham Steell murmur originate in the dilated hypertensive pulmonary trunk. The second heart sound is loud and single because of synchronous closure of aortic and pulmonary valves.
Electrocardiogram
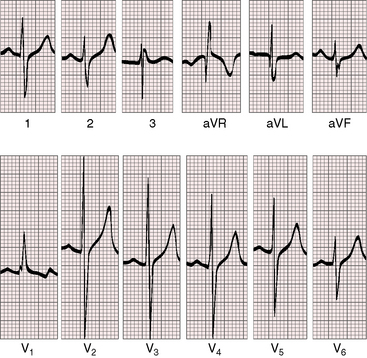
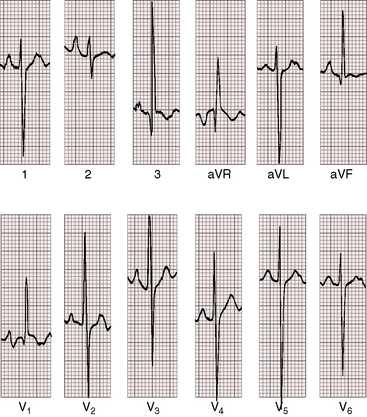
PR interval prolongation is less frequent than with double outlet right ventricle and a subaortic ventricular septal defect (Figure 19-24). Biatrial P wave abnormalities reflect the combination of volume overload of the left atrium and enlargement of the right atrial caused by right ventricular failure. The QRS axis is vertical or rightward with clockwise depolarization (see Figure 19-24), resembling the QRS axis of complete transposition of the great arteries with a nonrestrictive ventricular septal defect (see Chapter 27). Right ventricular hypertrophy is reflected in tall R waves in lead V1 and aVR and deep S waves in left precordial leads (see Figure 19-24). Volume overload of left ventricle is represented by well-developed R waves in leads V5-6 (see Figure 19-24). Elevated pulmonary vascular resistance curtails pulmonary blood flow and reduces volume overload of the left ventricle, but right ventricular pressure overload is unchanged. Accordingly, tall peaked right atrial P waves persist with residual evidence of a left atrial abnormality and with biventricular hypertrophy in mid precordial leads (Figure 19-25).
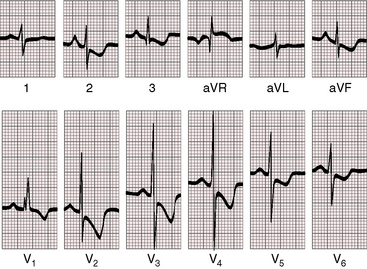
Figure 19-25 Electrocardiogram from the 40-year-old woman with the Taussig-Bing anomaly referred to in Figures 19-21 and 19-22. The P wave in lead 2 is tall and peaked (right atrial abnormality) and broad (left atrial abnormality). The PR interval is 180 msec. The QRS axis is plus 90 degrees. Right ventricular hypertrophy is indicated by the rR pattern in lead V1, the R wave in lead aVR, the deep S waves in left precordial leads, and inverted T waves in right and midprecordial leads. Biventricular hypertrophy from a well-developed left ventricle is implied by the large RS complexes in midprecordial leads.
X-Ray
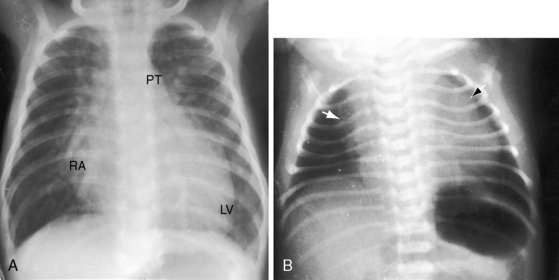
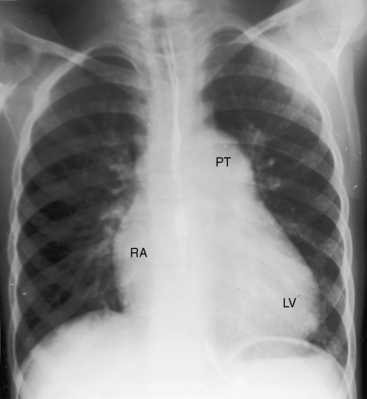
An increase in pulmonary arterial and pulmonary venous vascularity results from low pulmonary vascular resistance and congestive heart failure (Figure 19-26). The left atrium and left ventricle are enlarged because of volume overload, and the right atrium and right ventricle are enlarged because of congestive heart failure (Figure 19-26 and 19-27). The dilated pulmonary trunk projects prominently to the left when the great arteries are side-by-side. The x-ray then resembles a nonrestrictive perimembranous ventricular septal defect (Figure 19-28). When the dilated pulmonary trunk is posterior and therefore not border-forming, the x-ray resembles complete transposition of the great arteries (see Figure 19-26),12,42 except for the presence of a thymus (see Figure 19-27). With the advent of pulmonary vascular disease, pulmonary arterial blood flow decreases, pulmonary venous vascularity disappears, and volume overload of the left ventricle is curtailed, but dilation of the pulmonary trunk persists, so the x-ray resembles a nonrestrictive ventricular septal defect with Eisenmenger’s syndrome (Figures 19-28 and 19-29).
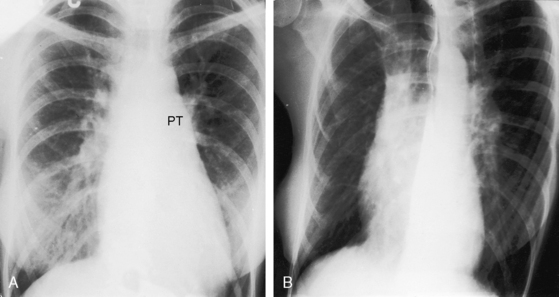
Figure 19-29 X-rays from the 40-year-old woman with the Taussig-Bing anomaly referred to in Figures 19-21 and 19-22. A, Pulmonary soft tissue densities are enhanced by breast tissue, but pulmonary vascularity is otherwise normal. The cardiac silhouette is normal except for the mildly convex dilated posterior pulmonary trunk (PT). B, A left anterior oblique projection confirms the normal size of the right atrium and both ventricles.
Echocardiogram
The following points are relevant in addition to the features described in the section on double outlet right ventricle with subaortic ventricular septal defect: (1) the pulmonary trunk overrides a subpulmonary ventricular septal defect (Figure 19-30 and 19-31); (2) pulmonary stenosis is absent; and (3) subaortic stenosis, coarctation of the aorta, and patent ductus arteriosus often coexist.
Double outlet left ventricle
In biventricular hearts, origin of both great arteries from the morphologic left ventricle is among the rarest of ventriculoarterial malalignments.5,43–45 The malformation was described in the early 19th century, rediscovered in 1967, and defined clinically and at necropsy in 1970.46 Morphogenesis has been assigned to misalignment of the septal anlagen of the embryonic conus and the conal ridges.9,47 Double outlet left ventricle is the converse of double outlet right ventricle because both great arteries arise entirely or predominantly from the morphologic left ventricle (Figure 19-32).47 The only exit for the right ventricle is a subaortic or subpulmonary ventricular septal defect. Rarely, the ventricular septum is intact.48
In hearts with two well-formed noninverted ventricles, this rare malformation is associated with a subaortic ventricular septal defect that tends to occur with pulmonary stenosis (see Figure 19-32).49 Less commonly, the ventricular septal defect is subpulmonary, and the outflow obstruction is aortic stenosis.45
Double outlet left ventricle with subaortic ventricular septal defect and pulmonary stenosis (see Figure 19-32) resembles cyanotic Fallot’s tetralogy, including a right aortic arch, hypoxic spells, and a pulmonary stenotic murmur whose length varies inversely with the severity of obstruction. The electrocardiogram shows right ventricular hypertrophy. The x-ray shows little or no increase in cardiac size and normal or reduced pulmonary vascularity.45 The echocardiogram with Doppler interrogation identifies two noninverted ventricles with left ventricular origin of both great arteries and establishes the location of the ventricular septal defect and the presence and degree of pulmonary stenosis.20,49 Double outlet left ventricle is distinguished from origin of both great arteries from an inverted morphologic right ventricle (Figure 19-33).50
Double outlet left ventricle with subaortic ventricular septal defect and no pulmonary stenosis resembles complete transposition of the great arteries with a subaortic ventricular septal defect and a posterior aorta. The two circulations run in parallel, with blood from the left ventricle recirculating within the pulmonary circulation and blood from the right ventricle recirculating within the systemic circulation (see Chapter 27). Cyanosis varies inversely with pulmonary arterial blood flow. When pulmonary vascular resistance is low, cyanosis exists with increased pulmonary blood flow as in complete transposition of the great arteries.
Double outlet left ventricle with a nonrestrictive subpulmonary ventricular septal defect and no pulmonary stenosis resembles isolated nonrestrictive ventricular septal defect.45 As neonatal pulmonary vascular resistance falls, left ventricular blood is increasingly diverted into the pulmonary artery. Right ventricular blood preferentially flows into the pulmonary artery because the ventricular septal defect is subpulmonary. Pulmonary blood flow increases, the electrocardiogram shows biventricular hypertrophy, cyanosis is minimal, and congestive heart failure results in poor growth and development.45 Echocardiography identifies two noninverted ventricles, establishes left ventricular origin of both great arteries, and identifies the subpulmonary ventricular septal defect.45
1 Van Praagh S., Davidoff A., Chin A., Shiels F., Reynolds J., Van Praagh R. Double-outlet right ventricle: anatomic types and developmental implications based on a study of 101 autopsied cases. Coeur. 1982;13:389-439.
2 Abernathy J. Surgical and physiological essays. Part II. London: James Evans; 1793.
3 Vierordt H. Die angeborenen Herzkrankheiten. Spez Pathol Ther. 15, 1898.
4 Witham A.C. Double outlet right ventricle; a partial transposition complex. Am Heart J. 1957;53:928-939.
5 Anderson R., Galbraith R., Gibson R., Miller G. Double outlet left ventricle. Br Heart J. 1974;36:554-558.
6 Bharati S., Lev M., Stewart R., Mcallister H.A.Jr, Kirklin J.W. The morphologic spectrum of double outlet left ventricle and its surgical significance. Circulation. 1978;58:558-565.
7 Anderson R.H., Mccarthy K., Cook A.C. Continuing medical education. Double outlet right ventricle. Cardiol Young. 2001;11:329-344.
8 Anderson R.H. Double outlet right ventricle. Eur J Cardiothorac Surg. 2002;22:853.
9 Sridaromont S., Feldt R.H., Ritter D.G., Davis G.D., Edwards J.E. Double outlet right ventricle: hemodynamic and anatomic correlations. Am J Cardiol. 1976;38:85-94.
10 Sondheimer H.M., Freedom R.M., Olley P.M. Double outlet right ventricle: clinical spectrum and prognosis. Am J Cardiol. 1977;39:709-714.
11 Van Praagh R. What is the Taussig-Bing malformation? Circulation. 1968;38:445-449.
12 Taussig H.B., Bing R.J. Complete transposition of the aorta and a levoposition of the pulmonary artery; clinical, physiological, and pathological findings. Am Heart J. 1949;37:551-559.
13 Macmahon H.E., Lipa M. Double-outlet right ventricle with intact interventricular septum. Circulation. 1964;30:745-748.
14 Cheung Y.F., Yung T.C., Leung M.P. Left ventriculo-coronary communications in a double-outlet right ventricle with an intact ventricular septum. Int J Cardiol. 2000;74:227-229.
15 Beekman R.P., Bartelings M.M., Hazekamp M.G., Gittenberger-De Groot A.C., Ottenkamp J. The morphologic nature of noncommitted ventricular septal defects in specimens with double-outlet right ventricle. J Thorac Cardiovasc Surg. 2002;124:984-990.
16 Caffarena J.M., Gomez-Ullate J.M. DORV with non-committed VSD and Taussig-Bing hearts. Controversial anatomic entities. Eur J Cardio-Thoracic Surg. 2003;23:136-137.
17 Wedemeyer A.L., Lucas R.V.Jr, Castaneda A.R. Taussig-Bing malformation, coarctation of the aorta, and reversed patent ductus arteriosus. Operative correction in an infant. Circulation. 1970;42:1021-1027.
18 Bashore T.M. Adult congenital heart disease: right ventricular outflow tract lesions. Circulation. 2007;115:1933-1947.
19 Michaelsson M., Tuvemo T. Double outlet right ventricle with spontaneously developing pulmonary outflow obstruction. Br Heart J. 1974;36:937-940.
20 Lavoie R., Sestier F., Gilbert G., Chameides L., Van Praagh R., Grondin P. Double outlet right ventricle with left ventricular outflow tract obstruction due to small ventricular septal defect. Am Heart J. 1971;82:290-299.
21 Mason D.T., Morrow A.G., Elkins R.C., Friedman W.F. Origin of both great vessels from the right ventricle associated with severe obstruction to left ventricular outflow. Am J Cardiol. 1969;24:118-124.
22 Rao P.S., Sissman N.J. Spontaneous closure of physiologically advantageous ventricular septal defects. Circulation. 1971;43:83-90.
23 Davachi F., Moller J.H., Edwards J.E. Origin of both great vessels from right ventricle with intact ventricular septum. Am Heart J. 1968;75:790-794.
24 Rice M.J., Seward J.B., Edwards W.D., et al. Straddling atrioventricular valve: two-dimensional echocardiographic diagnosis, classification and surgical implications. Am J Cardiol. 1985;55:505-513.
25 Titus J.L., Neufeld H., Edwards J.E. The atrioventricular conduction system in hearts with both great vessels originating from the right ventricle. Am Heart J. 1964;67:588-592.
26 Rogoff J.H., Anthony W. Double-outlet right ventricle with pulmonary valve atresia. Report on a patient surviving to age 25. Am Heart J. 1966;72:259-264.
27 Guo D.W., Lin M.L., Gu Z.Q., Cheng T.O. Double-outlet right ventricle. A clinical-roentgenologic-pathologic study of 28 consecutive patients. Chest. 1984;85:526-532.
28 Rein A.J., Dollberg S., Gale R. Genetics of conotruncal malformations: review of the literature and report of a consanguineous kindred with various conotruncal malformations. Am J Med Genet. 1990;36:353-355.
29 Dickinson C., Walker S., Wilmshurst P. Double outlet right ventricle with unprotected pulmonary vasculature presenting in a woman of 65. Heart. 1996;76:187.
30 Butler L.J., Snodgrass G.J., Sinclair L., France N.E., Russell A. E (16–18) trisomy syndrome: analysis of 13 cases. Arch Dis Child. 1965;40:600-611.
31 Rogers T.R., Hagstrom J.W., Engle M.A. Origin of both great vessels from the right ventricle associated with the trisomy-18 syndrome. Circulation. 1965;32:802-807.
32 Rohde R.A., Hodgman J.E., Cleland R.S. Multiple congenital anomalies in the E1-Trisomy (Group 16-18) Syndrome. Pediatrics. 1964;33:258-270.
33 Krongrad E., Ritter D.G., Weidman W.H., Dushane J.W. Hemodynamic and anatomic correlation of electrocardiogram in double-outlet right ventricle. Circulation. 1972;46:995-1004.
34 Saleeb S.F., Juraszek A., Geva T. Anatomic, imaging, and clinical characteristics of double-inlet, double-outlet right ventricle. Am J Cardiol. 2010;105:542-549.
35 Child J. Transthoracic and transesophageal echocardiographic imaging: anatomic and hemodynamic assessment. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: WB Saunders Company, 2009.
36 Ewing S., Silverman N.H. Echocardiographic diagnosis of single coronary artery in double-outlet right ventricle. Am J Cardiol. 1996;77:535-539.
37 Gedikbasi A., Oztarhan K., Gul A., Sargin A., Ceylan Y. Diagnosis and prognosis in double-outlet right ventricle. Am J Perinatol. 2008;25:427-434.
38 Khattri H.N., Misra K.P., Dutta B.N. Double outlet right ventricle with long survival. Br Heart J. 1968;30:569-570.
39 Robinson B.W., Anisman P.C., Sandhu S., Sokoloski M., Eshaghpour E. Significance of a Q wave in lead I in the newborn. Am J Cardiol. 1999;84:615-617. A619
40 Khoury G.H., Gilbert E.F. Taussig-Bing malformation with coarctation of the aorta. Angiology. 1970;21:143-150.
41 Parr G.V., Waldhausen J.A., Bharati S., Lev M., Fripp R., Whitman V. Coarctation in Taussig-Bing malformation of the heart. Surgical significance. J Thorac Cardiovasc Surg. 1983;86:280-287.
42 Beuren A. Differential diagnosis of the Taussig-Bing heart from complete transposition of the great vessels with a posteriorly overriding pulmonary artery. Circulation. 1960;21:1071-1087.
43 Dadourian B.J., Perloff J.K., Drinkwater D.C., Child J.S., Mulder D.G. Double outlet left ventricle—long survival after surgical correction. Ann Thorac Surg. 1991;51:159-160.
44 Kerr A.R., Barcia A., Bargeron L.M.Jr, Kirklin J.W. Double-outlet left ventricle with ventricular septal defect and pulmonary stenosis: report of surgical repair. Am Heart J. 1971;81:688-693.
45 Marino B., Bevilacqua M. Double-outlet left ventricle: two-dimensional echocardiographic diagnosis. Am Heart J. 1992;123:1075-1077.
46 Paul M.H., Muster A.J., Sinha S.N., Cole R.B., Van Praagh R. Double-outlet left ventricle with an intact ventricular septum. Clinical and autopsy diagnosis and developmental implications. Circulation. 1970;41:129-139.
47 Manner J., Seidl W., Steding G. Embryological observations on the formal pathogenesis of double-outlet left ventricle with a right-ventricular infundibulum. Thorac Cardiovasc Surg. 1997;45:172-177.
48 Beitzke A., Suppan C. Double outlet left ventricle with intact ventricular septum. Int J Cardiol. 1984;5:175-183.
49 Bengur A.R., Snider A.R., Peters J., Merida-Asmus L. Two-dimensional echocardiographic features of double outlet left ventricle. J Am Soc Echocardiogr. 1990;3:320-325.
50 Battistessa S., Soto B. Double outlet right ventricle with discordant atrioventricular connexion: an angiographic analysis of 19 cases. Int J Cardiol. 1990;27:253-263. discussion 265–257