Chapter 3 Dose-Response Modifiers in Radiation Therapy
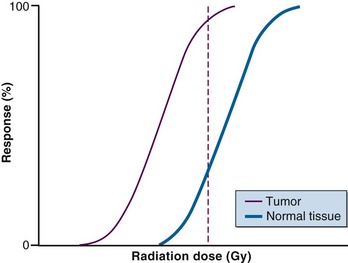
When cancer patients undergo radiation therapy, there is a clear dose-response relationship between the dose delivered and the response of the tumor to the radiation (Fig. 3-1). Unfortunately, damage to normal tissue also increases as the radiation dose increases, and this complication limits the total radiation dose that can be given. Substantial effort has been made to modify these dose-response relationships and increase the separation between tumor tissue and normal tissue dose-response curves. The approach has been to either selectively increase the radiation damage to tumor tissue without affecting normal tissues, or protect normal tissues without protecting tumor tissue to the same extent.
The Hypoxia Problem
The Importance of Oxygen
In 1909, Gottwald Schwarz,1 in a simple but elegant experiment, demonstrated that the radiation response of skin was markedly decreased if the blood flow in the irradiated area was reduced by compression. Although he did not acknowledge that the phenomenon was the result of a lack of oxygen, his study was probably the first radiobiologically oriented clinical study implicating the importance of environmental parameters in the outcome of radiotherapy. This finding was used to introduce the concept of kompressionsanämie, by which the skin was made anemic, thereby allowing a higher dose to be given to deeply situated tumors. Following the work of Schwarz, Müller,2 in 1910, reported that tissues in which the blood flow was stimulated by diathermia showed a more prominent response to radiation. This early study not only demonstrated the importance of the oxygen supply in radiotherapy but was also the first clinical approach showing how resistance could be overcome by using hyperthermia. Subsequently, sporadic clinical and experimental observations indicated the importance of a sufficient blood supply in securing an adequate radiation response. These observations led Gray and colleagues3 in the early 1950s to postulate that oxygen deficiency or hypoxia was a major source of radiation resistance.
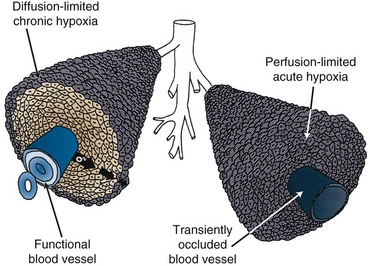
The first clinical indication that hypoxia existed in tumors was made around the same time by Thomlinson and Gray4 when, from histologic observations in carcinoma of the bronchus, they reported seeing viable tumor regions surrounded by a vascular stroma from which the tumor cells obtained their nutrients and oxygen. As the tumors grew, the viable regions expanded and areas of necrosis appeared at the center. The thickness of the resulting shell of viable tissue was 100 to 180 µm, which was within the same range as the calculated diffusion distance for oxygen in respiring tissues. It was thereby suggestive that as oxygen diffused from the stroma, it was consumed by the cells and, although cells beyond the diffusion distance were unable to survive, the cells immediately bordering the necrotic area might be viable but hypoxic. In 1968, Tannock5 described an inverted version of the Thomlinson and Gray picture, with functional blood vessels surrounded by cords of viable tumor cells outside of which areas of necrosis were seen. This “corded” structure, illustrated in Figure 3-2, is the more typical picture found in most solid tumors. It arises because the tumor blood vessels, which are derived from the normal tissue vessels by angiogenesis, are inadequate to meet the needs of the rapidly growing tumor cells. This hypoxia is commonly called chronic hypoxia. It was also suggested that hypoxia in tumors could be acute in nature. Chaplin and colleagues6 confirmed the existence of acutely hypoxic cells in tumors and demonstrated that these cells resulted from transient stoppages in tumor blood flow (see Fig. 3-2). To date, these temporary cessations in blood flow have been observed in mouse and rat tumors as well as in human tumor xenografts, with 4% to 8% of the total functional vessels involved,7 although the exact causes of these stoppages are not known. The current use of “chronic” and “acute” to explain hypoxia in tumors is probably an oversimplification of the real situation. Chronic hypoxia generally refers to prolonged and reduced oxygen concentrations that influence radiation response, but there is evidence that oxygen concentrations that are higher but still below normal physiologic levels are often found. Furthermore, reduced perfusion can be either partial or total, and although cells in the former condition would be oxygen deprived, cells in the latter condition would be starved of oxygen and nutrients and, therefore, their survival and response to therapy would be expected to be different.
Evidence for Hypoxia in Tumors
In experimental tumors it is not only relatively easy to identify hypoxia but one can also quantitatively estimate the percentage of cells that are hypoxic. Three major techniques are routinely used,8 including the paired survival curve, the clamped tumor growth delay, and the clamped tumor control assays. Each involves comparing the response of tumors irradiated under normal air breathing conditions with that of tumors artificially made hypoxic by clamping. Using these procedures, hypoxia has been directly identified in most animal solid tumors, with the values ranging from less than 1% to well over 50% of the total viable cell population.8 Unfortunately, none of these procedures can be applied in clinical situations. One must, therefore, rely on indirect techniques.
Tumor hypoxia has previously been estimated clinically by various methods,9,10 including measurement of tumor vascularization by using such endpoints as intercapillary distance, vascular density, and the distance from tumor cells to the nearest blood vessel; measurement of tumor metabolic activity by using biochemical, high-performance liquid chromatography, bioluminescent, and magnetic resonance techniques; and estimation of the degree of DNA damage by using the Comet assay. The more popular techniques currently under investigation include measurement of the binding of exogenous markers identified by immunohistochemical techniques from histologic sections (e.g., pimonidazole, EF5) or by noninvasive positron emission tomography (PET) (e.g., fluorine-18 [18F]-labeled misonidazole, FAZA), single-photon emission computed tomography (SPECT) (e.g., iodine-123 [123I]-labeled azomycin arabinoside), or magnetic resonance spectroscopy (e.g., SR4554). Also, the up-regulation of endogenous proteins either immunohistochemically (e.g., carbonic anhydrase IX, GLUT-1, HIF-1) or from blood samples (e.g., osteopontin) and the measurement of partial pressure of oxygen (PO2) distributions with polarographic electrodes can be used. The latter method has become by far the most popular. The results of an international multicenter study in head and neck cancer patients are illustrated in Figure 3-3. In this study, the tumor PO2 was measured before radiation therapy and was found to correlate with the overall survival rate in that patients with a lower tumor oxygenation status did significantly worse.11
Probably the best evidence for the existence of hypoxia in human tumors comes from the large number of clinical trials in which hypoxic modification has shown some benefit.12 The latter situation constitutes a circular argument: if hypoxic modification shows an improvement, then hypoxic clonogenic cells must have been present in tumors. It is, however, likely that even tumors with the same histologic makeup and of the same type have substantial heterogeneity with respect to the extent of hypoxia. It must be admitted that today, almost a century after the first clinical description, the importance of hypoxia and its influence on the outcome of radiotherapy is still the subject of substantial debate. However, we will now discuss in detail how the different hypoxic modifiers have been used to modify the radiation dose response of tumors.
Overcoming Tumor Hypoxia
Breathing High-Oxygen-Content Gas
Early experimental studies reported that breathing either oxygen or carbogen (95% O2 + 5% CO2) could substantially enhance the response of murine tumors to radiation and that the best effect was generally seen when the gases were inhaled under hyperbaric (typically 3 atmospheres [atm]) rather than normobaric conditions.13 This is not surprising because hyperbaric conditions would be expected to saturate the blood with more oxygen. However, more recent studies have indicated that the radiosensitization produced by normobaric oxygen or carbogen are quite substantial14; because it is quicker and easier to breathe gas under normobaric conditions, the use of cumbersome, expensive, complex hyperbaric chambers is probably not necessary.
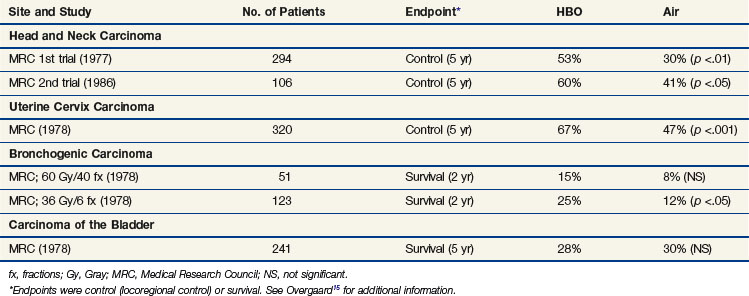
Clinically, the use of high-oxygen-content gas breathing, specifically under hyperbaric conditions, was introduced relatively early.15 Most trials were fairly small and suffered from the applications of unconventional fractionation schemes, but it appeared that the effect of hyperbaric oxygen was superior to that of radiotherapy given in air, especially when few and large fractions were applied. In the large, multicenter clinical trials conducted by the Medical Research Council (Table 3-1), the results from both uterine cervix and advanced head and neck tumors showed a significant benefit in local tumor control and subsequent survival rates. The same findings were not observed in bladder cancer nor were they seen in a number of smaller studies. In retrospect, the use of hyperbaric oxygen was stopped somewhat prematurely. This was partly the result of the introduction of hypoxic radiosensitizers and partly because of problems with patient compliance; it has been claimed that hyperbaric treatment caused significant suffering, but the discomfort associated with such a treatment must be considered minor compared with the often life-threatening complications associated with chemotherapy, which is used with less restrictive indications.
The use of high-oxygen-content gas breathing under normobaric conditions to radiosensitize human tumors has also been tried clinically, but it failed to show any dramatic improvement.16 In the most recent study this may have been the result of size limitation, but in earlier studies it may have been caused by the failure to achieve the optimal preirradiation gas breathing time. Experimental studies have shown that the amount of time is critical for the enhancement of radiation damage and that it can vary from tumor to tumor.17
Hypoxic Cell Radiosensitizers
An alternative approach to the hypoxia problem is the use of chemical agents that mimic oxygen and preferentially sensitize the resistant population to radiation. The advantage of these drugs over oxygen is that they are not rapidly metabolized by the tumor cells through which they diffuse and thus the drugs can penetrate further than oxygen and so reach all the tumor cells. In the early 1960s, researchers found that the efficiency of radiosensitization was directly related to electron affinity,18 and that finding ultimately led to in vitro studies demonstrating preferential radiosensitization of hypoxic cells by highly electron-affinic nitroaromatic compounds.19 Several of these compounds were later shown to be effective at enhancing radiation damage in tumors in vivo,20 and as a result they underwent clinical testing.
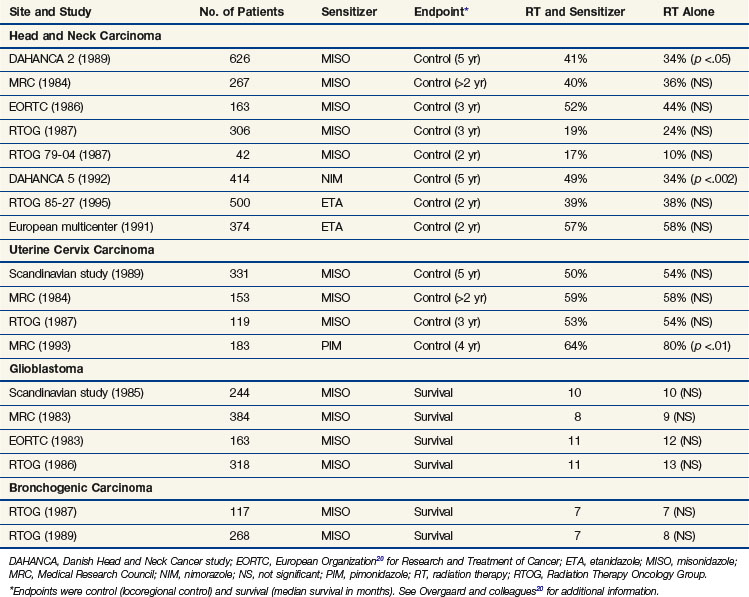
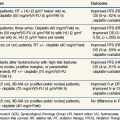
The drugs reaching clinical evaluation include metronidazole, misonidazole, benznidazole, desmethylmisonidazole, etanidazole, pimonidazole, nimorazole, ornidazole, sanazole, and doranidazole. Initial clinical studies were with metronidazole in brain tumors and were followed, in the latter part of the 1970s, by a boom in clinical trials exploring the potential of misonidazole as a radiosensitizer.20 The results from the multicenter randomized trials are summarizsed in Table 3-2. Most of the trials with misonidazole were unable to generate any significant improvement in radiation response, although a benefit was seen in some trials, especially the second Danish Head and Neck Cancer study (DAHANCA 2), which found a highly significant improvement in the stratification subgroup of pharynx tumors but not in the prognostically better glottic carcinomas. The overall impression of the “misonidazole era” was a prolongation of the inconclusive experience from the hyperbaric oxygen trials, namely that the problems related to hypoxia had not been ruled out definitively. Therefore, the search for more efficient or less toxic hypoxic sensitizers continues. Furthermore, the experience from the misonidazole trials has been taken into account to select a more homogeneous tumor population in which hypoxia is more likely to be present.
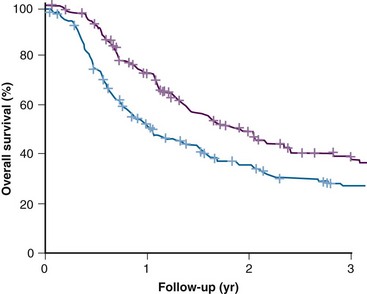
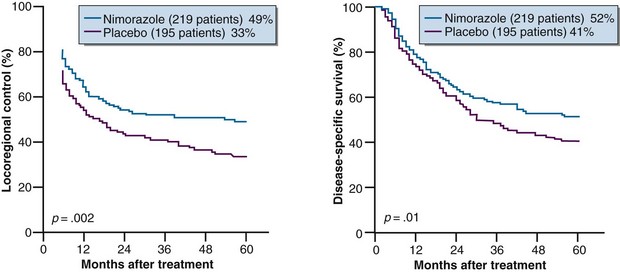
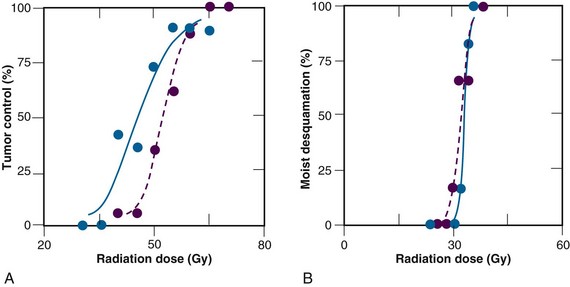
Results from subsequent randomized trials with other nitroaromatic compounds have been conflicting. The European pimonidazole trial in uterine cervical cancer was very disappointing, whereas the two other multicenter trials in head and neck cancer, using etanidazole, showed no benefit. On the other hand, studies with the low-toxicity drug nimorazole given to patients with supraglottic and pharyngeal carcinomas (DAHANCA 5) showed a highly significant benefit in terms of improved locoregional tumor control and disease-free survival rates21 (Fig. 3-4), thereby confirming the result of the DAHANCA 2 study. A more recent International Atomic Energy Agency (IAEA) trial with the 3-nitrotriazole compound sanazole (AK 2123), in uterine cervical cancer, also demonstrated a significant improvement in both local tumor control and overall survival rates.22
Dose Modification Based on Hemoglobin
One of the major factors influencing the delivery of oxygen to tumors is the concentration of hemoglobin; that is, low hemoglobin concentration in general has a negative impact on tumor radiation response. In a review of 51 studies involving 17,272 patients, the prognostic relationship between hemoglobin concentration and local tumor control was analyzed; of these studies, 39 (14,482 patients) showed a correlation and only 12 (2790 patients) did not.23 However, the relationship between hemoglobin concentration and tumor oxygenation status is not clear, because a large (357 patients) international multicenter study in head and neck cancer failed to show a correlation between these parameters.11
The potential benefit of increasing hemoglobin by blood transfusion before radiotherapy has been investigated in a number of studies, with the first clinical investigation in advanced squamous cell carcinoma of the uterine cervix.24 Transfusion to patients with a low hemoglobin level resulted in an increased oxygen tension within the tumor, as measured directly using oxygen electrodes. The same study was the first to show that transfusion to a hemoglobin level of 11 g/dL or higher was able to significantly improve survival rates. A Canadian retrospective study of 605 cervical cancer patients showed that the negative influence of low hemoglobin on prognosis could be overcome by transfusion. However, these observations have not been supported by data from controlled randomized trials. The two available prospective phase III trials, both from the DAHANCA study group, have failed to demonstrate any benefit of transfusion in head and neck cancer patients with low hemoglobin levels.21
Increasing the hemoglobin concentration by stimulation with the hormone erythropoietin (EPO) has also been investigated. Several preclinical studies have shown that the induction of anemia in animals could be corrected by serial injection with erythropoietin and that this treatment also overcame the anemia-induced radiation resistance.25 The concept of using erythropoietin to correct for anemia has also been tested in several clinical trials. However, although low hemoglobin can be effectively and safely improved by erythropoietin,26 studies in patients undergoing treatment for head and neck cancer failed to show any benefit,26 so additional studies are clearly required.
Other “hemoglobin-related” methods for improving tumor oxygenation have been investigated.9 These include using artificial blood substances such as perfluorocarbons, which are small particles capable of carrying more oxygen than hemoglobin, or manipulating the oxygen unloading capacity of blood by modifying the oxyhemoglobin dissociation curve. This can be achieved either by increasing the red blood cell 2,3-DPG content—2,3-DPG being one of the most important allosteric factors controlling the hemoglobin-oxygen dissociation curve—or by using antilipidemic drugs. Although each of these approaches has been shown to improve the oxygenation status of experimental tumors and/or enhance radiation damage, none of them has yet reached controlled clinical testing. Therefore their potential usefulness in the clinical setting is uncertain.
Dealing with the Problem of Fluctuating (Acute) Hypoxia
Although several of the procedures used to combat radiation resistance caused by hypoxic cells have met with some success, the results are far from satisfactory. A possible explanation is that most of these procedures used clinically seem to operate primarily against diffusion-limited chronic hypoxia and have little or no influence on fluctuating hypoxia, which is caused by transient variations in tumor blood flow.6,9
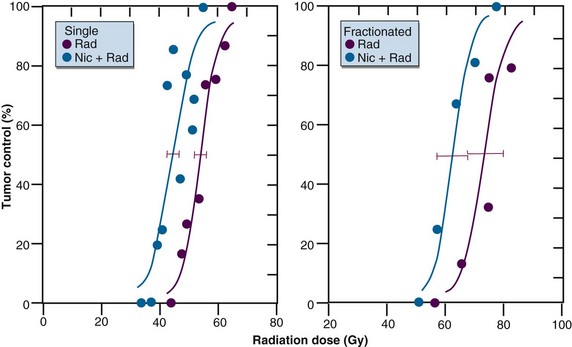
Experimental studies have clearly demonstrated that the vitamin B3 analog nicotinamide can enhance radiation damage in a variety of murine tumor models using both single and fractionated treatments6 (Fig. 3-5). The enhancement of radiation damage appears to depend on the tumor type, drug dose, and time of irradiation after drug administration. The drug can also enhance radiation damage in certain normal tissues, but generally the effects are less pronounced than that seen in tumors. Nicotinamide seems to prevent or reduce the transient fluctuations in tumor blood flow that normally lead to the development of acute hypoxia. This finding led to the suggestion that the optimal approach would be to combine nicotinamide with treatments that specifically overcome chronic hypoxia. This was subsequently demonstrated with hyperthermia, perfluorochemical emulsions, and carbogen inhalation.6 Combining nicotinamide with carbogen is now being tested in a number of European clinical studies, and preliminary clinical results already suggest that such an approach can improve radiation response.27
Bioreductive Drugs
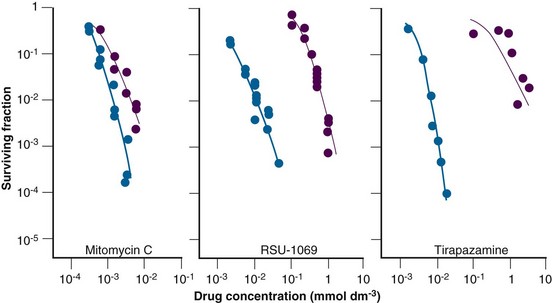
The early preclinical studies with electron-affinic radiosensitizers showed that these agents, which were relatively nontoxic to cells under normal oxygenated conditions, became more toxic under hypoxic conditions. This led to the development of various bioreductive drugs that preferentially killed the radiation-resistant hypoxic tumor cell population. Basically, these drugs can be divided into three major groups, as illustrated in Figure 3-6. They are the quinones, nitroaromatics, and N-oxides.28
The prototype bioreductive drug is mitomycin C (MMC), which is activated by bioreduction to form products that cross-link DNA. It has been used for many years in patients as a chemoradiosensitizer (discussed elsewhere in this book). Several randomized clinical trials in patients with squamous cell carcinoma of the head and neck have now been undertaken, specifically using mitomycin C to counteract the effects of hypoxia. Initial studies reported an improvement in local tumor control without any enhancement of radiation reactions in normal tissues. However, two other trials showed no major influence on response or survival rates. This absence of any improved response is perhaps not surprising, because the drug was given only once or twice during the radiation schedule and the results showed little differential between aerobic and hypoxic cell killing (see Fig. 3-6). Porfiromycin and EO9 were developed as attempts at finding more efficient quinones.28 Although EO9 has gone through only preliminary phase I and II testing, porfiromycin was included in a prospective randomized trial in combination with radiation therapy in head and neck cancer, but it was found to be no better than mitomycin C.28
Misonidazole’s proven preferential toxicity toward hypoxic cells spurred efforts to find other nitroimidazole radiosensitizers that were effective as hypoxic cell cytotoxins. To that end RSU 1069 was developed (see Fig. 3-6). This compound has the classic 2-nitroimidazole radiosensitizing properties, but an aziridine ring at the terminal end of the chain gives the molecule substantial potency as a hypoxic cell cytotoxin. Although RSU 1069 showed substantial activity in hypoxic cells in vitro and in tumors in vivo, large animal studies indicated dose-limiting gastrointestinal toxicity. A less toxic prodrug was then developed, RB 6145, which in vivo is reduced to RSU 1069. Although this drug showed potent antitumor activity in experimental systems, additional large animal toxicity studies revealed the potential of this agent to cause blindness in dogs, and therefore further development was halted. However, other nitroaromatic compounds are under development, including NLCQ-1, CB1954, SN-23862, and PR-104.28
One of the more promising group of bioreductives is the organic nitroxides, of which the benzotriazene di-N-oxide, tirapazamine, is the lead compound28 (see Fig. 3-6). The parent moiety shows limited toxicity toward aerobic cells, but after reduction under hypoxic conditions a product forms that is highly toxic to cells in vitro and can substantially enhance radiation damage in tumors in vivo. Most clinical studies have involved combining tirapazamine with chemotherapy, although there have been a few trials of radiation alone or in combination with chemotherapy.28 The results from the phase II trials generally showed promise, but in the few randomized trials that have been completed the results have been somewhat disappointing. However, a possible benefit of tirapazamine might be achieved if patients with hypoxic tumors could be selected by positron emission tomography (PET) imaging before treatment. Other N-oxides currently under development include chlorambucil N-oxide and AQ4N (banoxantrone), the latter being combined with radiation in a number of phase II trials.28
Vascular Targeting Agents
Angiogenesis Inhibitors
The tumor’s vascular supply plays a critical role in determining the tumor microenvironmental factors that influence radiotherapy. The vasculature develops from the normal tissue vessels via angiogenesis, which is a highly complex process triggered by the release of specific growth factors from the tumor cells.29 These growth factors initiate a series of physical steps, including local degradation of the basement membrane surrounding capillaries, invasion of the surrounding stroma by the endothelial cells in the direction of the angiogenic stimulus, proliferation of the endothelial cells, and, finally, organization of the endothelial cells into three-dimensional structures that connect with other similar structures to form the new blood vessel network. The importance of this process makes it an attractive target for therapy, and numerous approaches for inhibiting the various steps in the angiogenic process have been tested in preclinical models.30 Many of these therapies have now moved into clinical evaluation and, of these, the anti–vascular endothelial growth factor (anti-VEGF) antibody bevacizumab has been shown to improve outcome in a number of chemotherapy-based trials. Preclinical studies using rodent and human tumor xenografts show that certain angiogenesis inhibitors can be effectively combined with radiation to improve tumor response30 (Table 3-3) and, as a result, a limited number of clinical studies have been initiated combining certain angiogenesis inhibitors with radiation therapy.
TABLE 3-3 Vascular Targeting Agents Combined with Radiation*
| Angiogenesis Inhibitors | Vascular Disrupting Agents |
|---|---|
* See Horsman and Siemann30 for additional information.
The consensus opinion is that the improvement in radiation response found in preclinical studies is the consequence of normalization of the tumor vasculature, resulting in a decrease in tumor hypoxia. Although there are certainly preclinical studies showing improved tumor oxygenation status with such treatment, there are just as many studies showing no change and even a decrease in tumor oxygenation.30 These findings not only make it unclear as to the role of vessel normalization in influencing the combination of angiogenesis inhibitors with radiation, they also indicate that timing and sequencing of the two modalities may be critical for an optimal benefit.
Vascular Disrupting Agents
An alternative approach for targeting tumor vasculature involves using agents that can damage the already established tumor vessels.30 This is not a new concept; it was first demonstrated with the tubulin-binding agent colchicine back in the 1940s. Since then, a number of vascular disrupting agents (VDAs) have proved capable of preferentially damaging tumor vessels, leading to a reduction in tumor perfusion, which results in an increase in tumor ischemia and necrosis, subsequently producing an inhibitory effect on tumor growth.30 The VDAs include physical treatments (e.g., hyperthermia, photodynamic therapy, and even radiation), chemotherapeutic agents (e.g., tumor necrosis factor, vinca alkaloids, arsenic trioxides), and small molecule agents (e.g., flavenoid derivatives such as 5,6-dimethylxanthenone-4-acetic acid; tubulin-binding drugs such as combretastatin A-4 phosphate).
As with the angiogenesis inhibitors, several of the VDAs have been combined with radiation (Table 3-3), and significant improvements in response have been seen in preclinical models. This is illustrated in Figure 3-7, using a C3H mammary carcinoma grown in CDF1 mice. The radiation dose needed to control 50% of treated animals (±95% confidence interval) following single-dose radiation treatment alone was 53 Gy (51 to 56 Gy). This was significantly reduced (Chi-squared test; p <.05) to 46 Gy (42 to 49 Gy) when, in mice, tumor irradiation was followed in 30 minutes by a single intraperitoneal injection of a large but nontoxic dose of combretastatin A-4 phosphate (CA4P), the leading vascular disrupting agent in clinical evaluation. On its own—in this tumor model—such a drug dose will only slow the growth of the tumors by about 2 days. The enhancement of tumor radiation damage is known to be time and schedule dependent, with the greatest effect seen when the drug is given within a few hours after irradiation.30 It is also tumor specific, with no enhancement of radiation response seen in normal tissues. This has been shown for CA4P in early-responding normal skin (see Fig. 3-7) or in late-responding bladder and lung. These differences between the tumor tissue and normal tissue results are entirely consistent with the drug’s ability to induce damage in tumor tissue but not normal tissue vessels.30
Radiation Protectors
Sulfhydryl-Containing Compounds
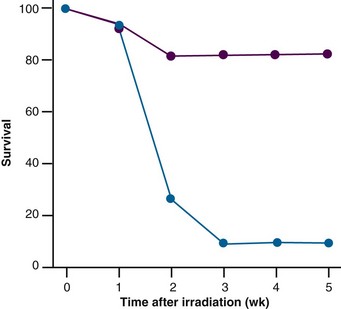
More than 50 years ago researchers realized that certain amino acids, glutathione, and ascorbic acid were able to modulate radiation-induced inactivation of biologic material. Based on those observations, Patt and colleagues31 investigated the effect of treating mice with the thiol-containing amino acid cysteine. They found that administering this compound to mice before whole-body irradiation resulted in a remarkable increase in animal survival rates (Fig. 3-8). In contrast, no effect was observed when cysteine was given after irradiation. During the Cold War, this finding led to a large research program at the Walter Reed Army Institute of Research, aimed at developing a drug that could protect soldiers from nuclear weapon radiation. Numerous sulfhydryl-containing substances with substantial radioprotective properties were detected, but only WR-2771 (amifostine) was found to exhibit acceptable toxicity. The idea of using amifostine in oncology arose when preclinical studies performed mainly in the 1970s and 1980s suggested a selective protection of normal tissue from damage induced not only by irradiation but also by chemotherapy.32 Despite these findings, the interest in amifostine over the years has waxed and waned, although it was boosted by commercialization of the drug, U.S. Food and Drug Administration (FDA) approval, and the establishment of authoritative guidelines. The lack of interest was probably related to the fact that there is no “fail-safe” modification of traditional radiotherapy. Many normal tissues have dose-limiting properties; therefore evaluating the therapeutic benefit of a radioprotector requires either that the protector have absolute normal tissue selectivity (and thus fewer complications with an unchanged rate of tumor control for a given radiation dose) or, if selectivity is uncertain, that an increase in radiation dose may be needed to maintain the same rate of tumor control. However, this scenario requires that the protective effect on tumor tissues is predictable and exceeded by the protection offered to all relevant normal tissues. In addition, the “perfect” radioprotector must have an acceptable toxicity profile and must be easy to handle if generalized clinical use is to be expected with routine fractionated radiotherapy.33
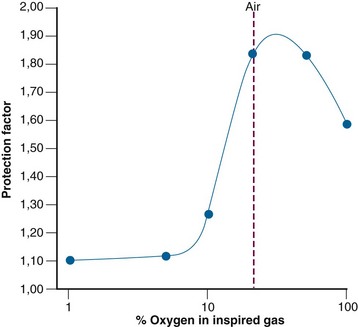
It is not entirely clear how amifostine induces radioprotection. The drug must first undergo dephosphorylation to its active metabolite WR-1065, which is further metabolized to the disulfide WR-33278; the latter metabolite may also afford some protection, although to a lesser extent.34 Several mechanisms are involved in radioprotection, depending on the quality of the radiation. Protection against sparsely ionizing radiation, such as that of x rays, is mainly obtained by the scavenging of free radicals.35,36 Because WR-1065 and WR-33278 react with free radicals in competition with oxygen, the protection obtained by scavenging is highly influenced by oxygen tension (Fig. 3-9). Here the protection is maximal at intermediate levels of oxygen (20% to 50% oxygen in the inspired air). At higher oxygen tensions, WR-1065 is counterbalanced by excess oxygen and the protection is gradually lost. The degree of protection is also diminished at low oxygen tensions where scavenging of free radicals is no longer important, because the lack of oxygen by itself provides radioprotection. Additional and complex mechanisms are undoubtedly involved. Some of these may involve hypoxia created locally by direct interaction of thiols with oxygen, chemical repair by thiol donation of hydrogen, or decreased accessibility of radiolytic attack sites by induction of DNA packaging.33
Preclinical studies have shown that many tissues can be protected from radiation damage by amifostine (Table 3-4). However, the protection observed in different normal tissues is unfortunately very heterogeneous. Some normal tissues—such as central nervous system tissue, which is often dose limiting in radiotherapy—are not protected, because amifostine probably does not cross the blood-brain barrier. In other normal tissues, such as salivary gland tissue and hematopoietic system tissue, amifostine affords significant radioprotection. These variations are probably explained by tissue variations in oxygen concentration, dephosphorylation activity, and distribution of amifostine and its metabolites.34,36 To make things even more complicated, tumor protection has been impossible to rule out by preclinical experiments. In addition, large single doses of irradiation have often been used and relevant comparison with tumor effects has been absent and difficult to translate into a clinically meaningful context.33
TABLE 3-4 Protection Factors Achieved by Amifostine in Different Normal Tissues and Tumors
| Tissue | Protection Factor |
|---|---|
| Salivary gland | 2.3-3.3 |
| Bone marrow | 1.8-3.0 |
| Jejunum | 1.5-2.1 |
| Skin | 1.4-2.1 |
| Testis | 1.5-1.6 |
| Kidney | 1.3-1.5 |
| Bladder | 1.3-1.5 |
| Lung | 1.2-1.4 |
| Heart | >1.0 |
| Tumor | 1.0-2.8 |
On the clinical side, there have been far too many publications of phase I and II studies with a limited number of patients and a few underpowered randomized studies.37 In addition, chemotherapy has often been applied together with radiotherapy, making it difficult to evaluate the results (Table 3-5). Despite the long list of preclinical normal tissue studies with a proven effect of amifostine, disappointingly few studies have been confirmed in the clinical setting. Amelioration of acute radiation toxicity has been observed in studies that often employed various types of treatment intensification such as concomitant chemotherapy or accelerated radiotherapy. However, definite confirmation regarding late morbidity such as fibrosis has not been obtained. A pivotal trial by Brizel and colleagues38 showed that amifostine significantly protected against radiation-induced xerostomia with no apparent loss of tumor control in head and neck cancers. Unfortunately, the study was not powered as an equivalence trial, so the researchers could not rule out the possibility of amifostine-induced tumor protection. In addition, postoperative radiotherapy was used in two-thirds of the included patients, diluting potential protection in the one-third treated with definitive radiotherapy. Finally, the study required that at least 75% of both parotid glands be irradiated. Modern three-dimensional target definition and dose delivery, for instance by intensity-modulated radiotherapy, often makes sparing of significant parts of the parotids possible, thereby making the attempt for pharmacologic protection more or less redundant.
Modifiers of the Oxygen Supply
Because hypoxia reduces radiation sensitivity, decreasing the availability of oxygen to tissues may be a way to achieve radiation protection. One way to accomplish this might be to modify hemoglobin-oxygen affinity. The most widely studied agents in this context are the substituted benzaldehyde BW12C and its derivatives,39 which preferentially bind to oxyhemoglobin and so increase the affinity of the hemoglobin for oxygen.40 Consequently, this decreases the amount of oxygen available to the tissues. Although this has been shown to provide radioprotection for some normal tissues,39 there is limited evidence that in certain normal tissues BW12C can actually increase perfusion,41 which should increase oxygen delivery. In addition, there is clear evidence from experimental studies that BW12C will also provide significant radioprotection for tumors.39
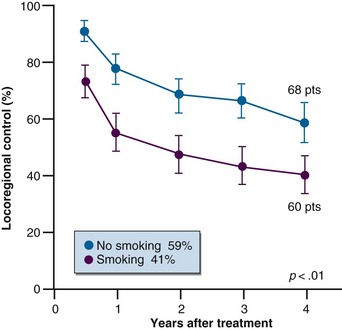
Carbon monoxide reduces oxygen transport to tissues by binding to hemoglobin, thereby decreasing the amount available for oxygen transport, as well as causing a leftward shift of the hemoglobin-oxygen dissociation curve. Therefore any oxygen that binds to the hemoglobin does so more strongly.42 This approach to increase hypoxia and reduce radiation response has been well documented in experimental systems.43 Unfortunately, this effect was demonstrated in tumors and not in normal tissues. The effect of carbon monoxide in patients has also been observed, as shown in Figure 3-10, which illustrates that patients with head and neck carcinomas who were smokers (and therefore had higher carboxyhemoglobin levels) had a poorer response to radiation therapy than nonsmokers.
Because most of the oxygen-supply modifiers already discussed also provide radioprotection for tumors as well as normal tissues, their use in this context must be considered limited. However, this may not be true for pentoxifylline. This drug alters red blood cell deformability and inhibits platelet aggregation and fibrinolytic activity.44 As a result, red blood cells are better able to transverse narrowed arterioles and capillaries. When given before irradiation, pentoxifylline enhances radiation damage in tumors,45 presumably because of an improvement in oxygen delivery, but it has no effect on the response of normal tissues.45 However, when administered on a daily basis after irradiation, pentoxifylline had no effect on early skin reactions in mice but did significantly reduce the incidence of late reactions.46
Other Radioprotectors
Various cytokines are reported to be capable of providing radioprotection to certain normal tissues, although the radioprotective role is somewhat controversial.47,48 Injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to rescue tissue in mice, dogs, and even monkeys from radiation injury. Radioprotection has also been seen with interleukin-1 (IL-1), tumor necrosis factor–alpha (TNF-α), and transforming growth factor–beta (TGF-β). However, the effects of IL-1 and TNF-α were time dependent; although survival of intestinal crypt cells was increased when either agent was administered 20 hours before irradiation, radiosensitization was observed with only a 4-hour interval. Moreover, the production of IL-1, TNF-α, and TGF-β has been reported to be elevated after normal tissue irradiation, and this may be one of the important steps in the development of fibrosis. Radioprotection has also been seen in lung and bone tissue after treatment with basic fibroblast growth factor; but more significantly, such treatment had absolutely no effect on the radiation response of at least one murine tumor model.
summary in relation to Cancer Stem Cells
A major focus of current cancer research is the role of cancer stem cells in tumorigenesis and therapy. These cells make up only 1% to 25% of the total viable tumor cell population,49 but they are the cells that must be eliminated to obtain local tumor control with radiation therapy.50 Therefore, substantial effort is being applied to identify these cells and specifically target them. Recent evidence suggests a possible link between hypoxia and cancer stem cells.50 Hypoxia may affect cancer stem cell generation and maintenance through the up-regulation of hypoxia-induced factors.49,50 Preclinical studies have shown an inverse correlation between hypoxia and local tumor control after irradiation,50 indicating that hypoxia may actually protect the cancer stem cells from the lethal effects of radiation. Using treatments that eliminate hypoxia should, therefore, be an effective method for enhancing the radiosensitivity of cancer stem cells. This fact alone should argue for substantially more effort being made to get hypoxia-modifying treatments established clinically.
1 Schwarz G. über Desensibiliserung gegen Röntgen-und Radiumstrahlen. Munchener Medizinische Wochenschrift. 1909;24:1-2.
2 Müller C. Eine neue Behandlungsmethode bösartiger Geschwülste. Munchener Medizinische Wochenschrift. 1910;28:1490-1493.
3 Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638-648.
4 Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539-549.
5 Tannock IF. The relationship between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22:258-273.
6 Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor. Radiobiological effects. Cancer Res. 1987;47:597-601.
7 Horsman MR. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. Acta Oncol. 1995;34:571-587.
8 Moulder JE, Rockwell S. Hypoxic fractions of solid tumour. Int J Radiat Oncol Biol Phys. 1984;10:695-712.
9 Horsman MR. Hypoxia in tumours: Its relevance, identification and modification. In: Beck-Bornholdt HP, editor. Current topics in clinical radiobiology of tumours. Berlin: Springer-Verlag; 1993:99-112.
10 Horsman MR. Measurement of tumour oxygenation. Int J Radiat Oncol Biol Phys. 1998;42:701-704.
11 Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18-24.
12 Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6:10-21.
13 Suit HD, Marshall N, Woerner D. Oxygen, oxygen plus carbon dioxide, and radiation therapy of a mouse mammary carcinoma. Cancer. 1972;30:1154-1158.
14 Rojas A. Radiosensitization with normobaric oxygen and carbogen. Radiother Oncol. 1991;20(Suppl. 1):65-70.
15 Overgaard J. Sensitization of hypoxic tumour cells—clinical experience. Int J Radiat Biol. 1989;56:801-811.
16 Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with carbogen breathing for squamous cell carcinoma of the head and neck. Cancer. 2005;104:332-337.
17 Chaplin DJ, Horsman MR, Siemann DW. Further evaluation of nicotinamide and carbogen as a strategy to reoxygenate hypoxic cells in vivo. Importance of nicotinamide dose and pre-irradiation breathing time. Br J Cancer. 1993;68:269-273.
18 Adams GE, Cooke MS. Electron-affinic sensitization. I. A structural basis for chemical radiosensitizers in bacteria. Int J Radiat Biol. 1969;15:457-471.
19 Asquith JC, Watts ME, Patel K, et al. Electron-affinic sensitization V. Radiosensitization of hypoxic bacteria and mammalian cells in vitro by some nitroimidazoles and nitropyrazoles. Radiat Res. 1974;60:108-118.
20 Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol Res. 1994;6:509-518.
21 Overgaard J, Sand Hansen H, Overgaard M, et al. A randomised double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish head and neck cancer study (DAHANCA) protocol 5-85. Radiother Oncol. 1998;46:135-146.
22 Dobrowsky W, Huigol NG, Jayatilake RS, et al. Ak-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer. Results of an IAEA multicentre randomized trial. Radiother Oncol. 2007;82:24-29.
23 Grau C, Overgaard J. Significance of haemoglobin concentration for treatment outcome. In: Molls M, Vaupel P, editors. Medical radiology: blood perfusion and microenvironment of human tumours. Heidelberg: Springer-Verlag; 1998:101-112.
24 Thomas GM. Raising hemoglobin. An opportunity for increasing survival? Oncology. 2002;63:19-28.
25 Stuben G, Pottgen C, Knuhmann K, et al. Erythropoietin restores the anemia-induced reduction in radiosensitivity of experimental human tumors in nude mice. Int J Radiat Oncol Biol Phys. 2003;55:1358-1362.
26 Hoskin PJ, Robinson M, Slevin N, et al. Effect of epoetin alfa on survival and cancer treatment-related anemia and fatigue in patients receiving radical radiotherapy with curative intent for head and neck cancer. J Clin Oncol. 2009;27:5751-5756.
27 Hoskin PJ, Rojas A, Saunders MI. Accelerated radiotherapy, carbogen, and nicotinamide (ARCON) in the treatment of advanced bladder cancer. Mature results of a phase II nonrandomized study. Int J Radiother Oncol Biol Phys. 2009;73:1425-1431.
28 McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs. From concept to clinic. Clin Oncol. 2007;19:427-442.
29 Fidler IJ, Langley RR, Kerbel RS, Ellis LM. Angiogenesis. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer principles and practice of oncology. ed 7. Philadelphia: Lippincott Williams & Wilkins; 2005:129-137.
30 Horsman MR, Siemann DW. Pathophysiological effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520-11539.
31 Patt HM, Tyree EB, Straub RL, Smith DE. Cysteine protection against X irradiation. Science. 1949;110:213-214.
32 Yuhas JM. Protective drugs in cancer therapy. Optimal clinical testing and future directions. Int J Radiat Oncol Biol Phys. 1982;8:513-517.
33 Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000;57:113-118.
34 Savoye C, Swenberg C, Hugot S, et al. Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int J Radiat Biol. 1997;71:193-202.
35 Denekamp J, Michael BD, Rojas A, Stewart FA. Radioprotection of mouse skin by WR-2721. The critical influence of oxygen tension. Int J Radiat Oncol Biol Phys. 1982;8:531-534.
36 Travis EL. The oxygen dependence of protection by aminothiols. Implications for normal tissues and solid tumors. Int J Radiat Oncol Biol Phys. 1984;10:1495-1501.
37 Winczura P, Jassem J. Combined treatment with cytoprotective agents and radiotherapy. Cancer Treat Rev. 2010;36(3):268-275.
38 Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339-3345.
39 Adams GE, Barnes DW, du-Boulay C, et al. Induction of hypoxia in normal and malignant tissues by changing the oxygen affinity of hemoglobin—implications for therapy. Int J Radiat Oncol Biol Phys. 1986;12:1299-1302.
40 Keidan AJ, Franklin IM, White RD. Effect of BW12C on oxygen affinity of haemoglobin in sickle cell disease. Lancet. 1986;1:831-834.
41 Honess DJ, Hu DE, Bleehen NM. BW12C. Effects on tumour hypoxia, tumour radiosensitivity and relative tumour and normal tissue perfusion in C3H mice. Br J Cancer. 1991;64:715-722.
42 Roughton FJW, Darling RC. The effect of carbon monoxide on the oxyhemoglobin dissociation curve. Am J Phys. 1944;141:17-31.
43 Siemann DW, Hill RP, Bush RA. The importance of the pre-irradiation breathing times of oxygen and carbogen (5% CO2; 95% O2) on the in vivo radiation response of a murine sarcoma. Int J Radiat Oncol Biol Phys. 1977;2:903-911.
44 Ward A, Clissold SP. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50-97.
45 Lee I, Kim JH, Levitt SH, Song CW. Increases in tumor response by pentoxifylline alone or in combination with nicotinamide. Int J Radiat Oncol Biol Phys. 1992;22:425-429.
46 Dion MW, Hussey DH, Osborne JW. The effect of pentoxifylline on early and late radiation injury following fractionated irradiation in C3H mice. Int J Radiat Oncol Biol Phys. 1989;17:101-107.
47 Hendry JH. Biological response modifiers and normal tissue injury after irradiation. Semin Radiat Oncol. 1994;4:123-132.
48 Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99-109.
49 Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106-111.
50 Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Revs Cancer. 2008;8:545-554.