Chapter 130 Dorsal Root Entry Zone Lesions
Ablation of the dorsal root entry zone (DREZ) of the spinal cord, also known as the DREZ procedure, is primarily used for a select group of medical refractory pain syndromes.1 The procedure typically involves thermocoagulation using a fine probe at the entry zone of the dorsal spinal roots under microscopic guidance, after performing a laminectomy and dural opening. DREZ ablation was first performed in France in 1972 by Sindou et al. as a treatment for pain due to Pancoast’s syndrome and cancer-related pain and was later described by Nashold and Ostdahl for other applications.2–5 In the 35 years since the clinical introduction of DREZ ablation, the procedure has been successfully performed for treatment of refractory pain of varying etiologies including brachial plexus avulsion (BPA), spinal cord injury (SCI), postamputation pain, and radiation-induced plexopathy. The mechanistic rational for targeting the DREZ for surgical pain treatment is due to the role of this region in the integration, modulation, and transmission of pain sensory impulses.6
Anatomy
The DREZ is the region of the spinal cord that contains the dorsolateral fasciculus of Lissauer and Rexed laminae I to V (Fig. 130-1). The anatomy of the DREZ in both the cervical spine and the lumbosacral outflow region has been studied in detail by Sindou and others.3,7–11 The dorsal nerve roots enter the dorsolateral spinal cord as a linear arrangement of small rootlets.12 The division into rootlets occurs approximately 1 cm before entering the dorsolateral spinal cord, and the rootlets may travel in a subpial plane for 1 mm before entering the cord.3 Microanatomic studies have indicated that the cervical dorsal nerves divide into an average of 7.7 rootlets prior to entering the lateral sulcus, with fewer rootlets in the upper cervical spine.8,9 The length of the rootlets generally increases in the lower cervical spine.13 Within the rootlets, small fibers, including the nociceptive afferents, are laterally located, while the larger fibers from somatic receptors enter a medial location. A portion of the lateral small fibers proceed to enter Lissauer’s tract prior to terminating in laminae I and II of the dorsal horn. Lissauer’s tract modulates synaptic transmission and is composed of a medial part with a large percentage of unmyelinated fibers from the lateral rootlets and lateral portion that contains propriospinal fibers.14–16
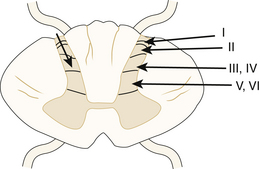
FIGURE 130-1 Cross-sectional diagram of the spinal cord demonstrating the locations of the Rexed laminae within the dorsal gray matter of the cord: posteromarginal nucleus (I), substantia gelatinosa (2), nucleus proprius (III and IV), and the Rexed laminae (V and VI). The arrowhead on the left marks the depth of the DREZ lesion as discussed by Sindou.3 This is typically 3 mm in depth in the region of the brachial plexus.
Figure 130-2 presents a diagram of the DREZ in the cervical region that illustrates the three characteristic geometric measures of the exiting rootlets, including (for a given cervical segment) the angle of exit of the superiormost and inferiormost rootlets with respect to the cord and the vertical length of the DREZ along the cord. Alleyene et al. measured these quantities from the C3 to T1 segments in a set of 10 adult cadavers (128 cervical roots).7 The average angle with respect to the cord for the inferior rootlets ranged from 116 degrees (C5) to 156 degrees (T1), while the superior angle ranged from 132 degrees (C6) to 166 degrees (T1). Similarly, Xiang et al. explored the cervical DREZ region in a series of 20 adult cadavers (200 dorsal cervical roots, C5-T1).9 The average inferior angle the inferior rootlets made with the axis of the spinal cord (180 degrees, the angle of Alleyne et al.7) varied gradually from 65.6 ± 3.6 degrees at C5 to 19.8 ± 2.9 degrees at T1.9 In addition, these authors measured the distances from the posterior median sulcus to the posterior lateral sulcus (the anatomic sulcus at which the root fibers enter the cord). This quantity varied from 3.54 ± 0.12 mm at C5 to 2.23 ± 0.28 mm at the T1 level. The average distance of the DREZ from the median sulcus is 2.95 mm, with the distance decreasing in the lower cervical spine.8,9
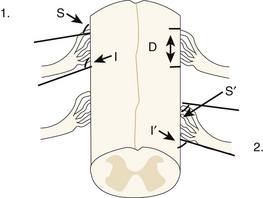
FIGURE 130-2 Box 1: Definitions of the superior (S) and inferior (I) rootlet angles, and the length of the dorsal root entry zone (D) as defined by Alleyne7. Box 2: Definitions of the superior (S’) and inferior (I’) angles as defined by Xiang9.
In general, thoracic region DREZ procedures are rarely performed, for example, only when there is an SCI resulting in segmental pain. However, DREZ procedures in the conus region are more frequently performed, specifically for avulsion of lumbar and sacral roots or trauma to the region.5,17
The caudal termination of the spinal cord is called the conus medullaris. This conical structure occurs caudal to the sites of lumbar and sacral nerve root emergence and is located near the L1/L2 vertebrae in adults.12 The angle of the lumbar nerve roots with respect to the thecal sac decreases from L1 to S1. In an analysis of magnetic resonance images of healthy volunteers, the average nerve root angle decreased from 40.9 degrees at L1 to 17.1 degrees at S1.11 The size of the ventral and dorsal sacral roots has been shown to decrease in size from S1 to S5, with an average S1 nerve root diameter of 1.70 mm (ventral) and 2.39 mm (dorsal) decreasing to 0.17 mm (ventral) and 0.40 mm (dorsal) by S5.10
Indications
DREZ ablation has been applied as a treatment for pain from several etiologies, including neoplasm, trauma, and infection. The application of the DREZ procedure for treatment of pain following BPA has been the most widely described application and is associated with the best results.18–23 Avulsion or deafferentation pain, which occurs following BPA injuries, is commonly described by patients as a constant crushing-type sensation, with can be episodic and involve pain in the hand or bursts of pain traveling down the arm.24 The severity of this pain can be disabling and resistant to most medical therapies. Sensation is also abnormal in the affected limb, and these patients report phantom-like sensations, possibly related to somatosensory reorganization after deafferentation.24 Deafferentation-related changes in higher central nervous system structures such as the cuneate nucleus or the ventral caudal nucleus of the thalamus can take decades to evolve. These changes represent the slow deafferentation process that occurs with second- and third-order relay neurons after an injury and imply slow plasticity changes in higher brain structures.25
Htut et al. found that the intensity of the patient’s ongoing chronic pain rating after BPA was correlated with the number of roots avulsed when measured at a mean of 4 years after injury.24 In a cohort of 76 patients, 56% of patients with BPA injuries also described referred sensations mapping to an unaffected dermatome at some point after their injury. The intensity of these sensations declined over time in the majority. It has been hypothesized that the pain after BPA occurs secondary to abnormal network activity in the deafferented dorsal horn; however, there is some evidence that neighboring injured roots might also play a role as pain mediators.21,26,27 Bertelli and Ghizoni studied 10 patients with chronic neuropathic pain after brachial plexus injuries and selectively blocked nonavulsed roots under computed tomography guidance.27 The patients all exhibited decreases in pain ratings in this small, uncontrolled study, but inflammatory-driven central sensitization from neighboring roots might have future implications for the extent of DREZ lesions.
Guenot et al. performed single-unit analysis of the spinal dorsal horn in patients with various types of neuropathic pain in the context of the DREZ procedure.28 They studied three categories of patients, including patients with peripheral nerve injury, true deafferentation injury from BPA, and disabling spasticity. They found that the coefficient of variation in firing rates was highest for the patients with BPA for cells recorded in the dorsal horn. In addition, BPA patients and spasticity patients had similar patterns of firing behavior, whereas the peripheral nerve injury group demonstrated the most “nonrandom” forms of firing discharges for these cells, manifested in a serial dependency of the interspike intervals.28
Animal models for BPA have also been developed to probe the possible molecular causes of the subsequent pain syndrome. Quintão et al. studied BPA in mice and examined the effects of blocking tumor necrosis factor A with a selective antibody.29 This study demonstrated abolishment of the hypernociception that occurs after injury through intravenous injections of the blocking antibody, thereby pointing toward a possible pathway that could be blocked as a therapy.29 However, in general, neuropathic pain syndromes developing after BPA have been difficult to treat medically, and even newer, novel treatments fail to produce drastic reductions in pain.30
Similar to avulsion-type injuries, the pain following spinal cord or cauda equina injury is a common phenomenon, with a large percentage of patients reporting severe pain.31,32 An estimated 65% of patients with SCI experience pain and 50% of patients with SCI have a level of pain severe enough to interfere with rehabilitation.33,34 Two types of pain following SCI have been described: nociceptive and neuropathic. Nociceptive pain is thought to be the result of activation of peripheral nociceptors due to ongoing tissue damage and is generally considered to be responsive to nonsteroidal anti-inflammatory drugs and other treatments such as physical therapy.35 In contrast, neuropathic pain is the result of an abnormally functioning nervous system.35 Neuropathic pain can develop below, as well as above, the level of the lesion and is thought to be related to spinothalamic tract dysfunction.36,37
The mechanism of neuropathic pain following SCI has been proposed to involve neuronal hyperactivity and hyperexcitability.35 In addition, there appear to be differences in the deafferentation process that SCI patients demonstrate with or without the development of neuropathic pain. In patients with neuropathic pain below the spinal cord lesion, the electroencephalogram peak frequency is significantly lower than that in patients without neuropathic pain.37 Interestingly, in patients suffering from neuropathic pain after SCI, there also appears to be a significant reorganization of primary somatosensory cortex (S1) compared to the arrangement in SCI patients without an associated below-the-injury pain syndrome.38 Wrigley et al. compared the receptive fields for little finger, thumb, and lip brushing derived during functional magnetic resonance imaging for 20 subjects after SCI (10 with and 10 without neuropathic pain), along with 21 controls without SCI. The SCI patients had suffered complete thoracic level injuries that had occurred 2 to 37 years prior to the study. The receptive field for little finger sensation was statistically different in the neuropathic pain patients, having migrated toward the leg representation.38 This effect was correlated with the intensity of pain the subjects experienced.
Hulsebosch et al. hypothesized that the types of neuropathic pain arising after SCIs have distinct mechanisms of production. Neuropathic pain “below the level” may have its origin in an inflammatory cascade after the injury involving the release of glutamate, chemokines, and the activation of microglial cells in the dorsal horn, eventually leading to neighboring neuronal hyperexcitability.39 It has been further proposed that the chronic neuropathic pain developing “at the level” of the injury is likely due to persistent activation of several intracellular kinase pathways, including mitogen-activated protein kinase, leading to persistent activation of the cyclic adenosine monophosphate response element–binding transcription factor. This transcription factor leads to products that phosphorylate N-methyl-d-aspartic acid–activated channels, tipping the neurons toward a hyperexcitable state.39 Finally, “above the level” pain conditions may involve a peripheral sensitization mechanism. The below-the-level type of pain does respond to the DREZ procedure, as outlined later; however, future therapies for this condition and its associated central sensitization might include viral-mediated gene transfer or stem cell transplantation as their basis.40
In an effort to find alternatives to pharmacologic treatments that are accompanied by systemic side effects, DREZ ablation has been evaluated in the setting of pain resulting from SCI.41–45 It has been reported by Sindou and colleagues that DREZ ablation is only effective in segmental pain corresponding to the level of the spinal cord lesion or adjacent pathologic processes such as gliosis or cavitation.3,43 Diffuse, bilateral, and primarily sacral pain levels are less likely to respond.46
There are other less commonly reported indications for the DREZ operation. DREZ ablation has been reported for the treatment of postherpetic neuralgia, occipital neuralgia, phantom limb pain, and radiation-induced plexopathy.23,42,47–50 Amputation of a limb or BPA can also result in a chronic form of phantom limb pain.51–54 The range of sensations associated with the phantom limb syndrome include the sensation of shortening of the limb, numbness, itchiness, temperature differences, cramping, and sometimes even lengthening of the phantom representation.54,55 The central neurodegenerative phenomena associated with phantom pain evolve over the course of several years to even decades and are related to the gradual progression of axonal withdrawal in the thalamus and cortex.25,33 Some small series have demonstrated a gradual reduction in pain intensity over time.33 A few authors have reported series of patients with phantom limb pain treated with the DREZ procedure.50,55 Interestingly, both Saris et al.50 and Zheng et al.55 describe greater efficacy for the procedure in patients who also have BPA. In 6 patients out of the 14 reported by Zheng et al., there were also alterations in the phantom limb sensations themselves, which disappeared in 2 patients in the postoperative period.55
Finally, the finding of decreased muscle tone and abolished stretch reflex in patients undergoing DREZ ablation for treatment of pain conditions prompted that application of DREZ ablation for spasticity treatment.17,56 Results reported by Sindou and Jeanmonod on lower extremity spasticity have indicated that DREZ ablation improved spasms and abnormal postures and may improve motor performance in some patients.17 In addition, medication-resistant upper limb spasticity in the setting of hemiplegia has been treated with DREZ ablation.56
Surgical Technique
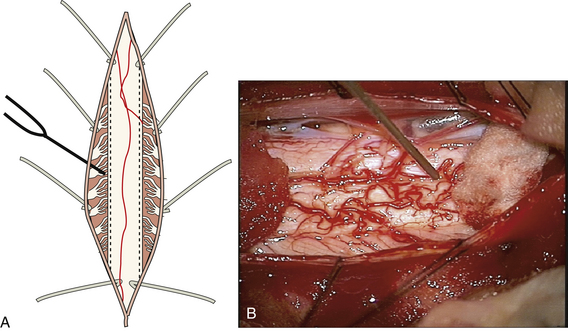
Several surgical approaches and techniques have been described for DREZ ablation. The first technique, developed in the 1970s by Sindou and colleagues, is often referred to as the microsurgical DREZotomy.2,3 This technique was designed to selectively destruct excitatory pain fibers, including the unmyelinated and small myelinated pain fibers in the ventrolateral DREZ and the medial portions of Lissauer’s tract.57 For upper limb procedures, the patient is placed in the prone position with three-pin head fixation. To allow for exposure of the rootlets of the upper limbs (C5-T1), a cervical hemilaminectomy is performed for unilateral procedures while a complete laminectomy is performed for bilateral procedures. The dura is opened, and the dorsal rootlets are exposed. Root identification is performed with bipolar stimulation, with a 2.5-Hz frequency and an increasing voltage from 1 to 6 V. If the dorsal rootlets are still present, an incision to a depth of 2 mm is made at the junction of the rootlets and the dorsolateral funiculus. Given that the rootlets are often totally avulsed and the DREZ is often scarred and difficult to expose, we have found it useful to expose both sides of the cord and allow identification of the root entry zone on the noninjured side for comparison. Exposing both rostrally and caudally to have normal rootlets present also helps in identification of the entry zone. Finally, stimulation of the dorsal columns with recording of a somatosensory evoked potential can be used to identify the midline and aid in defining anatomy in a scarred cord. Microcoagulation is then performed at a 35-degree angle to a depth of 3 mm.3,57
Other techniques have been described that utilized different methods to produce the DREZ lesion. The use of radiofrequency-generated heat lesions was initially described by Nashold and Ostdahl and has been used by several groups.4,19 This technique uses a cordotomy needle placed in the posterolateral sulcus (Fig. 130-3). The lesion produced is visually monitored by the surgeon until a slight contraction of the tissue is evident (10-20 seconds). An alternative approach is to heat the electrode to 75°C for 15 seconds. Multiple lesions are placed at 1- to 2-mm intervals covering the area of avulsion. For maximal results, it is important to continue the DREZ lesions into an area of partial rootlet injury contiguous with the avulsion. The creation of DREZ lesions with a carbon dioxide laser was introduced by Levy et al. as a method to improve the precision of lesion production compared to radiofrequency ablation.58 Ultrasound-mediated DREZ ablation has also been performed; however, far fewer data have been reported in the literature.18,59
Little data have compared the efficacy of the alternative ablation techniques. In one of the few comparative studies, a direct comparison of radiofrequency lesions to laser-produced lesions was performed, and the laser-created lesions were found to be more precise and uniform.60 In general, although there are differences in size and location of the lesions, the use of radiofrequency ablation, laser ablation, or ultrasound ablation results in destruction of the entire DREZ and dorsal horn structures.3 Other techniques, such as computer assistance and guidance by evoked potentials, have been proposed to improve the accuracy of the DREZ procedure.45,61,62 For example, Jeanmonod et al. and Guenot et al. described techniques that allow for intraoperative electrophysiologic unit recordings of the DREZ that may assist localization and allow for analysis of pathologic states.63,64 These recordings were achieved using a novel microelectrode that is implanted intraoperatively and capable of floating freely to allow for stable unit recording.
Outcomes
Reported series of patients benefiting from this procedure have grown over time. They primarily involved patients with BPA and SCI and occasionally involved patients with postherpetic neuralgia, arachnoiditis, pain secondary to spinal cysts, and cauda equina lesions.1 No randomized trials comparing DREZ ablation to alternative therapies have been reported in the literature. As such, all efficacy data have been derived primarily from single-institution case series with no control populations. Cetas et al. reviewed all published case series on the use of the DREZ procedure; the reported indications included pain secondary to BPA injuries, SCI, postherpetic neuralgia, arachnoiditis, spinal cysts, cauda equina lesions, and other forms of deafferentation, denervation, and mixed intractable pain syndromes.1 For these various syndromes, across the series, pain relief rates of more than 50% were obtained in most patients. Pain relief after SCI and BPA tends to be the highest postlesioning, with 54% to 86% reporting at least 50% pain relief.1
The success rates from several case series with long-term follow-up ranging over several decades have been reported for use of the procedure to reduce pain resulting from BPA injury. An early review of the results at the Johns Hopkins Hospital with radiofrequency heat lesions revealed a mean pain relief rate of 85%, with an average follow-up of 28 months.19 Likewise, the results from 124 patients undergoing ultrasonic DREZ ablation revealed good results in 87% of patients, with an average follow-up of 47.5 months.18 A more recent analysis of 55 patients treated with microsurgical DREZ ablation indicated that 94.6% of patients had excellent pain relief on hospital discharge, with 65.9% showing persistent relief on long-term follow-up.21 Pain relief was greater in patients with paroxysmal pain alone compared to that in patients with continuous background pain. The overall literature range for the percentage of pain relief over a several-month follow-up for DREZ performed after BPA is 42.8% to 94.6%, with some evidence for a slow loss in efficacy after 1 year.1
The results of DREZ ablation have also been evaluated in the treatment of pain resulting from SCI. Several groups have described improved results for patients with segmental pain corresponding to the level of the SCI compared to those with diffuse pain in the areas below the level of injury.41–43 In a series of 44 patients who underwent microsurgical DREZ for segmental SCI pain, good results were obtained in 73% and 68% at 3 months and 1 year, respectively.43 In addition, it has been observed that better results are obtained with incomplete SCIs.41,43,44
Less is known regarding the outcomes following DREZ ablation for the treatment of other pain syndromes, such as postherpetic neuralgia and postamputation phantom pain. These reports have included very small case series with variable follow-up times. For the treatment of postherpetic neuralgia, an early report of 17 patients indicated that 10 had good results, with improvement seen primarily in the superficial burning pain.23 A more recent report of 10 patients indicated that persistent pain relief was present in only 20% on long-term follow-up.42 Likewise, postamputation pain consisting of phantom limb pain has been treated with DREZ ablation, although there are a limited number of reports in the literature on outcomes.3,48,65 Patients with phantom limb pain have been described to respond to DREZ ablation, with good long-term relief in 36%.48 However, it has been reported that pain in the amputation stump does not respond to DREZ ablation.3 Finally, the use of DREZ ablation to treat radiation-induced plexopathy has been reported.49,66 In a recent series of 10 patients with radiation-induced plexopathy unresponsive to medical therapies, 8 patients reported full pain relief at the end of follow-up.49
Complications
Postoperative complications have been described by several groups following DREZ ablation. These complications can be divided into general complications following spinal surgery and neurologic complications as a result of DREZ ablation. A case series of 55 patients by Sindou et al. reported a postoperative cerebrospinal fluid (CSF) leak in 1 patient and bacterial meningitis in 2 patients.21 Likewise, a series of 28 patients treated with DREZ for postamputation pain reported a CSF leak in 1 patient and a postoperative epidural hematoma in 1 patient.48 Neurologic complications as a result of DREZ ablation have included decreased sensation or dysesthesias ipsilateral to the DREZ ablation, permanent motor disturbances, and sphincter dysfunction. Rates of immediate postoperative changes in the neurologic exam have been reported to be as high as 50% to 60%, although it is felt that the majority of these may resolve over time.22,67 Sindou et al. reported a 5.4% rate of permanent disabling neurologic complications in a series of 55 patients, and Thomas and Jones reported persistent significant neurologic changes in 12%.21,22
Alleyne C.H.Jr., Cawley C.M., Barrow D.L., Bonner G.D. Microsurgical anatomy of the dorsal cervical nerve roots and the cervical dorsal root ganglion/ventral root complexes. Surg Neurol. Sep 1998;50(3):213-218.
Bertelli J.A., Ghizoni M.F. Pain after avulsion injuries and complete palsy of the brachial plexus: the possible role of nonavulsed roots in pain generation. Neurosurgery. May 2008;62(5):1104-1113. discussion 1113–1104
Campbell J.N., Solomon C.T., James C.S. The Hopkins experience with lesions of the dorsal horn (Nashold’s operation) for pain from avulsion of the brachial plexus. Appl Neurophysiol. 1988;51(2-5):170-174.
Cetas J.S., Saedi T., Burchiel K.J. Destructive procedures for the treatment of nonmalignant pain: a structured literature review. J Neurosurg. Sep 2008;109(3):389-404.
Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. Jul 2002;1(3):182-189.
Guenot M., Bullier J., Rospars J.P., et al. Single-unit analysis of the spinal dorsal horn in patients with neuropathic pain. J Clin Neurophysiol. Apr 2003;20(2):143-150.
Jeanmonod D., Sindou M., Magnin M., Boudet M. Intra-operative unit recordings in the human dorsal horn with a simplified floating microelectrode. Electroencephalogr Clin Neurophysiol. May 1989;72(5):450-454.
Nashold B.S.Jr., Ostdahl R.H. Dorsal root entry zone lesions for pain relief. J Neurosurg. Jul 1979;51(1):59-69.
Rath S.A., Braun V., Soliman N., et al. Results of DREZ coagulations for pain related to plexus lesions, spinal cord injuries and postherpetic neuralgia. Acta Neurochir (Wien). 1996;138(4):364-369.
Sampson J.H., Cashman R.E., Nashold B.S.Jr., Friedman A.H. Dorsal root entry zone lesions for intractable pain after trauma to the conus medullaris and cauda equina. J Neurosurg. Jan 1995;82(1):28-34.
Sindou M.. Microsurgical DREZotomy, Schmidek H., Roberts D. Schmidek and Sweet’s Operative Neurosurgical Techniques: Indications, Methods, and Results, 5th ed., Saunders, 2005. :2445-2459
Sindou M., Jeanmonod D. Microsurgical DREZ-otomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. May 1989;24(5):655-670.
Sindou M., Blondet E., Emery E., Mertens P. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. Jun 2005;102(6):1018-1028.
Sindou M., Fischer G., Goutelle A., Mansuy L. Selective surgery of posterior nerve roots. First results of surgery for pain. Neurochirurgie. Sep-Oct 1974;20(5):391-408.
Sindou M., Mertens P., Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: long-term results in a series of 44 patients. Pain. May 2001;92(1-2):159-171.
Tanaka N., Fujimoto Y., An H.S., et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976). Feb 1 2000;25(3):286-291.
Werhagen L., Budh C.N., Hultling C., Molander C. Neuropathic pain after traumatic spinal cord injury: relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. Dec 2004;42(12):665-673.
Xiang J.P., Liu X.L., Xu Y.B., et al. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28(1):17-20.
1. Cetas J.S., Saedi T., Burchiel K.J. Destructive procedures for the treatment of nonmalignant pain: a structured literature review. J Neurosurg. Sep 2008;109(3):389-404.
2. Sindou M., Fischer G., Goutelle A., Mansuy L. Selective surgery of posterior nerve roots. First results of surgery for pain. Neurochirurgie. Sep-Oct 1974;20(5):391-408.
3. Sindou M.. Microsurgical DREZotomy, Schmidek H., Roberts D. Schmidek and Sweet’s Operative Neurosurgical Techniques: Indications, Methods, and Results, 5th ed., Saunders, 2005. :2445-2459
4. Nashold B.S.Jr., Ostdahl R.H. Dorsal root entry zone lesions for pain relief. J Neurosurg. Jul 1979;51(1):59-69.
5. Moossy J.J., Nashold B.S.Jr. Dorsal root entry zone lesions for conus medullaris root avulsions. Appl Neurophysiol. 1988;51(2-5):198-205.
6. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. Nov 19 1965;150(699):971-979.
7. Alleyne C.H.Jr., Cawley C.M., Barrow D.L., Bonner G.D. Microsurgical anatomy of the dorsal cervical nerve roots and the cervical dorsal root ganglion/ventral root complexes. Surg Neurol. Sep 1998;50(3):213-218.
8. Karatas A., Caglar S., Savas A., et al. Microsurgical anatomy of the dorsal cervical rootlets and dorsal root entry zones. Acta Neurochir (Wien). Feb 2005;147(2):195-199. discussion 199
9. Xiang J.P., Liu X.L., Xu Y.B., et al. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28(1):17-20.
10. Hauck E.F., Wittkowski W., Bothe H.W. Intradural microanatomy of the nerve roots S1-S5 at their origin from the conus medullaris. J Neurosurg Spine. Aug 2008;9(2):207-212.
11. Hasegawa T., Mikawa Y., Watanabe R., An H.S. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine (Phila Pa 1976). May 1 1996;21(9):1005-1009.
12. Carpenter M.B., Sutin J. Human neuroanatomy, 8th ed., Baltimore: Williams & Wilkins, 1983.
13. Tanaka N., Fujimoto Y., An H.S., et al. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976). Feb 1 2000;25(3):286-291.
14. Earle K.M. The tract of Lissauer and its possible relation to the pain pathway. J Comp Neurol. Feb 1952;96(1):93-111.
15. Chung K., Coggeshall R.E. Primary afferent axons in the tract of Lissauer in the cat. J Comp Neurol. Aug 1 1979;186(3):451-463.
16. Rand R.W. Further observations on Lissauer tractolysis. Neurochirurgia (Stuttg). Oct 1960;3(151):68.
17. Sindou M., Jeanmonod D. Microsurgical DREZ-otomy for the treatment of spasticity and pain in the lower limbs. Neurosurgery. May 1989;24(5):655-670.
18. Dreval O.N. Ultrasonic DREZ operations for treatment of pain due to brachial plexus avulsion. Acta Neurochir (Wien). 1993;122(1-2):76-81.
19. Campbell J.N., Solomon C.T., James C.S. The Hopkins experience with lesions of the dorsal horn (Nashold’s operation) for pain from avulsion of the brachial plexus. Appl Neurophysiol. 1988;51(2-5):170-174.
20. Thomas D.G., Kitchen N.D. Long-term follow up of dorsal root entry zone lesions in brachial plexus avulsion. J Neurol Neurosurg Psychiatry. Jun 1994;57(6):737-738.
21. Sindou M., Blondet E., Emery E., Mertens P. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. Jun 2005;102(6):1018-1028.
22. Thomas D.G., Jones S.J. Dorsal root entry zone lesions (Nashold’s procedure) in brachial plexus avulsion. Neurosurgery. Dec 1984;15(6):966-968.
23. Friedman A.H., Nashold B.S.Jr. Dorsal root entry zone lesions for the treatment of postherpetic neuralgia. Neurosurgery. Dec 1984;15(6):969-970.
24. Htut M., Misra P., Anand P., et al. Pain phenomena and sensory recovery following brachial plexus avulsion injury and surgical repairs. J Hand Surg Br.. Dec 2006;31(6):596-605.
25. Graziano A., Jones E.G. Early withdrawal of axons from higher centers in response to peripheral somatosensory denervation. J Neurosci. Mar 25 2009;29(12):3738-3748.
26. Djouhri L., Koutsikou S., Fang X., et al. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. Jan 25 2006;26(4):1281-1292.
27. Bertelli J.A., Ghizoni M.F. Pain after avulsion injuries and complete palsy of the brachial plexus: the possible role of nonavulsed roots in pain generation. Neurosurgery. May 2008;62(5):1104-1113. discussion 1113–1104
28. Guenot M., Bullier J., Rospars J.P., et al. Single-unit analysis of the spinal dorsal horn in patients with neuropathic pain. J Clin Neurophysiol. Apr 2003;20(2):143-150.
29. Quintão N.L., Balz D., Santos A.R., et al. Long-lasting neuropathic pain induced by brachial plexus injury in mice: role triggered by the pro-inflammatory cytokine, tumour necrosis factor alpha. Neuropharmacology. Apr 2006;50(5):614-620.
30. Berman J.S., Symonds C., Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. Dec 2004;112(3):299-306.
31. Werhagen L., Budh C.N., Hultling C., Molander C. Neuropathic pain after traumatic spinal cord injury: relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. Dec 2004;42(12):665-673.
32. Siddall P.J., Taylor D.A., McClelland J.M., et al. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. May 1999;81(1-2):187-197.
33. Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. Jun 2003;103(3):249-257.
34. Siddall P.J., Loeser J.D. Pain following spinal cord injury. Spinal Cord. Feb 2001;39(2):63-73.
35. Eide P.K. Pathophysiological mechanisms of central neuropathic pain after spinal cord injury. Spinal Cord. Sep 1998;36(9):601-612.
36. Finnerup N.B., Sorensen L., Biering-Sorensen F., et al. Segmental hypersensitivity and spinothalamic function in spinal cord injury pain. Exp Neurol. Sep 2007;207(1):139-149.
37. Wydenkeller S., Maurizio S., Dietz V., Halder P. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. Eur J Neurosci. Jul 2009;30(1):91-99.
38. Wrigley P.J., Press S.R., Gustin S.M., et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. Jan 2009;141(1-2):52-59.
39. Hulsebosch C.E., Hains B.C., Crown E.D., Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. Apr 2009;60(1):202-213.
40. Eaton M.J. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma. Mar-Apr 2006;23(3-4):549-559.
41. Spaic M., Petkovic S., Tadic R., Minic L. DREZ surgery on conus medullaris (after failed implantation of vascular omental graft) for treating chronic pain due to spine (gunshot) injuries. Acta Neurochir (Wien). 1999;141(12):1309-1312.
42. Rath S.A., Braun V., Soliman N., et al. Results of DREZ coagulations for pain related to plexus lesions, spinal cord injuries and postherpetic neuralgia. Acta Neurochir (Wien). 1996;138(4):364-369.
43. Sindou M., Mertens P., Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: long-term results in a series of 44 patients. Pain. May 2001;92(1-2):159-171.
44. Sampson J.H., Cashman R.E., Nashold B.S.Jr., Friedman A.H. Dorsal root entry zone lesions for intractable pain after trauma to the conus medullaris and cauda equina. J Neurosurg. Jan 1995;82(1):28-34.
45. Falci S., Best L., Bayles R., et al. Dorsal root entry zone microcoagulation for spinal cord injury-related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J Neurosurg. Sep 2002;97(suppl 2):193-200.
46. Friedman A.H., Nashold B.S.Jr. DREZ lesions for relief of pain related to spinal cord injury. J Neurosurg. Oct 1986;65(4):465-469.
47. Dubuisson D. Treatment of occipital neuralgia by partial posterior rhizotomy at C1-3. J Neurosurg. Apr 1995;82(4):581-586.
48. Saris S.C., Iacono R.P., Nashold B.S.Jr. Dorsal root entry zone lesions for post-amputation pain. J Neurosurg. Jan 1985;62(1):72-76.
49. Teixeira M.J., Fonoff E.T., Montenegro M.C. Dorsal root entry zone lesions for treatment of pain-related to radiation-induced plexopathy. Spine (Phila Pa 1976). May 1 2007;32(10):E316-319.
50. Saris S.C., Iacono R.P., Nashold B.S.Jr. Successful treatment of phantom pain with dorsal root entry zone coagulation. Appl Neurophysiol. 1988;51(2-5):188-197.
51. Sherman R.A. Stump and phantom limb pain. Neurol Clin. May 1989;7(2):249-264.
52. Melzack R.. Phantom limb pain. Patol Fiziol Eksp Ter, Jul-Aug 1992;4:52-54
53. Jensen T.S., Krebs B., Nielsen J., Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. Mar 1985;21(3):267-278.
54. Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. Jul 2002;1(3):182-189.
55. Zheng Z., Hu Y., Tao W., et al. Dorsal root entry zone lesions for phantom limb pain with brachial plexus avulsion: a study of pain and phantom limb sensation. Stereotact Funct Neurosurg. 2009;87(4):249-255.
56. Sindou M., Mifsud J.J., Boisson D., Goutelle A. Selective posterior rhizotomy in the dorsal root entry zone for treatment of hyperspasticity and pain in the hemiplegic upper limb. Neurosurgery. May 1986;18(5):587-595.
57. Jeanmonod D., Sindou M. Somatosensory function following dorsal root entry zone lesions in patients with neurogenic pain or spasticity. J Neurosurg. Jun 1991;74(6):916-932.
58. Levy W.J., Nutkiewicz A., Ditmore Q.M., Watts C. Laser-induced dorsal root entry zone lesions for pain control. Report of three cases. J Neurosurg. Nov 1983;59(5):884-886.
59. Kandel E.I., Ogleznev K., Dreval O.N.. Destruction of the entry zone of the posterior roots as a method of treating chronic pain in traumatic damage to the brachial plexus. Zh Vopr Neirokhir Im N N Burdenko, Nov-Dec 1987;6:20-27
60. Levy W.J., Gallo C., Watts C. Comparison of laser and radiofrequency dorsal root entry zone lesions in cats. Neurosurgery. Mar 1985;16(3):327-330.
61. Tomas R., Haninec P. Dorsal root entry zone (DREZ) localization using direct spinal cord stimulation can improve results of the DREZ thermocoagulation procedure for intractable pain relief. Pain. Jul 2005;116(1-2):159-163.
62. Edgar R.E., Best L.G., Quail P.A., Obert A.D. Computer-assisted DREZ microcoagulation: posttraumatic spinal deafferentation pain. J Spinal Disord. Feb 1993;6(1):48-56.
63. Jeanmonod D., Sindou M., Magnin M., Boudet M. Intra-operative unit recordings in the human dorsal horn with a simplified floating microelectrode. Electroencephalogr Clin Neurophysiol. May 1989;72(5):450-454.
64. Guenot M., Hupe J.M., Mertens P., et al. Human spinal microelectrode recordings. Stereotact Funct Neurosurg. 1999;72(2-4):246.
65. Rath S.A., Seitz K., Soliman N., et al. DREZ coagulations for deafferentation pain related to spinal and peripheral nerve lesions: indication and results of 79 consecutive procedures. Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):161-167.
66. Zeidman S.M., Rossitch E.J., Nashold B.S.Jr. Dorsal root entry zone lesions in the treatment of pain related to radiation-induced brachial plexopathy. J Spinal Disord. Feb 1993;6(1):44-47.
67. Friedman A.H., Nashold B.S.Jr., Bronec P.R. Dorsal root entry zone lesions for the treatment of brachial plexus avulsion injuries: a follow-up study. Neurosurgery. Feb 1988;22(2):369-373.