Chapter 164 Dorsal Dynamic Spine Stabilization
An estimated 1 million spine procedures are performed each year in the United States.1,2 The primary strategies used in these procedures include decompression of neural elements or stabilization using rigid fusion techniques. Spine stabilization surgeries are performed to correct or prevent deformity, diminish pathologic motion, compensate for iatrogenic destabilization, or eliminate pain generators. The introduction of rigid instrumentation to these procedures dramatically improved the rate and success of fusion.3,4 The use of rigid instrumentation provides an optimal environment for intervertebral or posterolateral fusion to occur by immobilizing two or more spinal segments. In addition, spinal instrumentation has allowed for more aggressive decompression and direct reduction or stabilization of spondylolisthesis.
The initial outcomes of fusion surgeries dramatically improved with rigid instrumentation, and long-term consequences have since been recognized.4 Patients undergoing arthrodesis are exposed to numerous long-term morbidities, including pseudarthrosis, fixed sagittal alignment that cannot adapt to postural change, and adjacent-level degeneration. The risk to adjacent levels is the most concerning of these because the degenerative changes are noted to be significantly accelerated compared with the natural history.5,6 Rigid titanium or stainless steel constructs have a supraphysiologic degree of stiffness that far exceeds normal bone or a noninstrumented fusion. This stiffness at the instrumented levels has a direct relationship to the stress load on the adjacent disc and facet joints.4,7 Over time, this additional stress can result in segment hypermobility, osteophyte formation, facet hypertrophy, disc herniation, and stenosis.4
Although the reported findings of radiographically evident adjacent-level disease are widely variable in the literature, the incidence of symptomatic adjacent-level disease is higher in patients who have undergone an instrumented fusion (12.2–18.5%) than in patients who have undergone a noninstrumented fusion (5.2–5.6%) in the lumbosacral spine.4,8,9 Other contributors to adjacent-level disease include muscular and ligamentous disruption, bone removal, and the underlying disease process; however, the supraphysiologic biomechanical stress created by rigid fixation seems to be the most influential factor.3–57 Many patients subsequently need further surgery that may require an extension of their fusion.10
An alternative goal of dynamic stabilization is to restore the function of segmental mobility by replicating the behavior and biomechanics of a healthy spinal segment. Intervertebral motion is maintained or restored, while restricting the extremes of movement or dampening the kinetic energy of motion.2 Dynamic stabilization systems may help spine pain syndromes by restricting movement to a range in which only normal loading may occur, preventing the spinal segment from reaching any position where abnormal loading generates pain.11 In addition, a dynamic stabilizing device must withstand physiologic static and dynamic loads in any plane.10
Advantages of Dynamic Stabilization
The advantages associated with using dynamic constructs are primarily anticipated over the long term. Implantation of most dorsal dynamic stabilizing devices has similar immediate postoperative effects to a rigid construct. Both approaches have the capacity to restore lumbar lordosis, sacral tilt, and foraminal diameter in the immediate postoperative period, showing that dynamic devices have the potential for equally good short-term results regarding sagittal spine alignment and decompression of neural elements.12 The only short-term advantage lies in the elimination of morbidities associated with harvesting autograft when the dynamic device is replacing a potential fusion construct.13
Pseudarthrosis is a significant factor associated with poor outcomes after surgical fusions. The incidence rate for pseudarthrosis varies widely depending on the location, surgical technique, and number of levels fused. A meta-analysis on pseudarthrosis after lumbar arthrodesis reported a rate of 14%.14 Most reported incidence rates of pseudarthrosis after circumferential fusion are less than 10%.13 Anterior cervical discectomy and fusion has a low rate of pseudarthrosis (<10%), although this is increased if multiple levels are involved. Regardless of this variability, pseudarthrosis is a clinically significant adverse outcome after spine fusion. Dynamic devices used in place of fusion constructs eliminate the potential adverse outcome of pseudarthrosis.
Because the prevalence of adjacent-level disease is so variable in the literature, it is difficult to make generalizations regarding its occurrence after fusion surgeries. The level, location, and length of the fusion all appear to have an impact on the likelihood of developing future degeneration at adjacent spinal levels. The reported incidence of the prevalence of adjacent-level disease during long-term follow-up after rigid spine fusion ranges from 32% to 36% in the lumbar spine.13 Regardless of the exact etiology, a significant percentage of patients are affected by this problem after a successful spine fusion.
Several retrospective studies evaluating various posterior dynamic devices have shown lower rates of reoperation for and prevalence of adjacent-level disease. The theoretical reduction of this problem has yet to be definitively proven in the literature, however, and long-term follow-up of randomized controlled trials is necessary to determine absolutely the advantage of dynamic technology and the appropriate patient population for its application.10,15,16
Indications
The indications for dorsal dynamic stabilization vary depending on the device being considered and are likely to expand as this new technology evolves. The longest-standing indication has been for augmentation of interbody fusion. The typical adjunct to interbody fusion is rigid fixation with pedicle screws and rods. The supraphysiologic rigidity of these constructs creates stress shielding of the interbody graft and likely contributes to a certain portion of resulting pseudarthroses.17 A dorsal dynamic stabilization device allows for an increase in anterior load sharing that augments fusion, while limiting any extremes of motion that could result in graft displacement.2
Dynamic devices may also be used in an iatrogenically destabilized spine to provide controlled motion. Decompressive surgeries that involve laminectomy and disruption of the facet joint are used to treat lumbar stenosis and lateral recess stenosis. If a significant amount of encroachment of the superior facet is involved, the decompression required may lead to iatrogenic destabilization. Typical management options for such a situation include primary fusion of the spine at the affected levels or observation for the development of sagittal plane imbalance or deformity.2 Dorsal dynamic stabilization provides another option after a potentially destabilizing procedure and could allow for controlled mobility at the treated spinal levels, while avoiding the need for arthrodesis.18
Patients with osteoporosis or osteopenia may benefit from dorsal dynamic stabilization. These patients are especially prone to construct failure when rigid stabilization devices are used because the bone-metal interface creates significant bony destruction. Using less rigid fixation may be ideal in these patients and reduce the incidence of construct failure.2
A controversial and poorly understood indication for the use of dorsal dynamic stabilization involves its use to protect and restore degenerated facet joints and intervertebral discs. Rather than attempting to remove or destroy the pain generators in the disc or facet joints via fusion, the placement of a dorsal dynamic device may shield the disc and facet joints from destructive motion, allowing for a reduction in inflammatory processes and permitting self-repair mechanisms to operate.2 This application may become a more valid indication as understanding of the pathophysiology of back pain improves.
Finally, complete circumferential reconstruction of the motion segment may be possible with combined technologies. The presence of facet disease is a contraindication for total disc arthroplasty because this procedure alone is thought to accelerate facet degeneration. A dorsal dynamic stabilization device may be used in conjunction with a total disc replacement, allowing for reconstruction of all mobile joints in a spinal segment.2
Fundamental Concepts
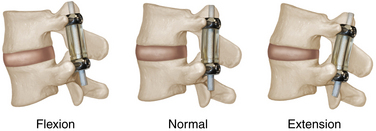
The cervical spine facet joints are oriented in a plane that is midway between a coronal and an axial plane. They resist extension and anterior translation.19,20 The facet joints are loaded by anterior shear forces during extension in the cervical spine. Facet joints in the lumbar spine are oriented in a plane that is midway between the sagittal and coronal planes with a slight anterior incline. Lumbar facet joints resist extension and bear large compressive loads while in extension. During flexion or axial compression, lumbar facets are unloaded.20 Anterior shear forces load the lumbar facet joints, whereas posterior shear forces unload them. The prevalence of substantial and repetitive physiologic loads on the lumbar facets is clinically relevant given their source as major pain generators.19 The posterior supraspinous and intraspinous ligaments resist posterior translation and bear tensile loads.
The biomechanics of dynamic posterior stabilization constructs vary among devices. Typically, the pain-generating tissue is left in situ, while the device restricts certain types of motion and alters load transfer.19 Engineering of posterior dynamic stabilization devices must be done with the kinematics of a functional spinal unit in mind. The posterior and anterior elements of a motion segment move in harmony with each other, and a significant disruption of one could result in excessive loads being placed on the other. Dynamic implants should be constructed with the capacity to maintain range of motion similar to a healthy spinal segment. Excessive motion could lead to degeneration that may include facet arthrosis or hypertrophy, ligamentum flavum hypertrophy, or disc degeneration. Inadequate motion prevents the goals of the dynamic device from being met.19
Some applications of dynamism apply the phenomenon of Wolff’s law to promote bone healing. Wolff’s law states that bone remodels and becomes stronger under increased loads to resist those loads. The reverse is also true, and decreasing the load on a bone results in its weakening. When applied to spine fusions, this theory states that transmitting forces to an intervertebral graft and avoiding stress shielding increase the rate and success of arthrodesis.18 Rigid posterior instrumentation may unload an intervertebral graft, resulting in fusion failure. However, a dynamic device loads the graft when used as a posterior tension band supplement, increasing the likelihood of fusion.
Avoiding adjacent-level disease is one of the driving goals behind the development of dynamic devices. The biomechanics behind this adverse effect have been studied in cadaver and animal models.19,21,22 Fusion of a spinal segment increases the stress on the anulus and end plates of adjacent levels and increases intradiscal pressures. Restricted motion at the fused segment also leads to higher mobility at adjacent segments. In flexion and extension, the loss of mobility across a rigidly fused segment is compensated predominantly in the first rostral adjacent segment.4 In a dynamic stabilization, the compensation is distributed across the first and second rostral segments and in the caudal adjacent segment.23 Additionally, the fixed sagittal alignment prevents accommodation of regional alignment changes that occur with different postural positions. If a segment is fused with suboptimal sagittal alignment, degeneration of adjacent levels may be accelerated further.19
Dorsal Dynamic Devices
A wide variety of dorsal dynamic stabilization devices are in various stages of development and clinical investigation and use. Any implant that is not rigid and allows some motion provides some degree of dynamism. An absorbable implant could be considered dynamic because it permits delayed spine movement after its structural integrity is lost.24 Absorbable implants in the posterior spine, in particular, the lumbosacral spine, are rare, and the focus of this classification is on deformable implants. Deformable implants may permit either angular deformation or axial deformation. The generalized objectives considered when designing these devices include avoidance of fatigue failure, maintenance of normal spine resting posture without excessive kyphosis or lordosis, prevention of abnormal load distributions, and easy salvage in case of construct failure.
Dorsal Dynamic Neutral Fixation
Available dorsal dynamic neutral fixation devices allow for limited movement to occur between the screw and the plate. The most common mechanism is a screw head that can pivot in a concave bed within the plate, allowing a “rocking” motion. Although this type of permissive motion may minimize the chance of failure at the screw-plate interface, the cyclic loading at the screw-bone interface causes degradation, and screws have the potential to pull out under flexion or axial loads. The weak point with this type of fixation is the screw-bone interface. In contrast, in rigid techniques, the weakest point is the screw-plate or screw-rod interface.25 Considering this difference, a dynamic neutral plating technique in a patient with osteoporotic bone would lead to a high likelihood of construct failure given the poor screw pull-out resistance and the dynamic nature of the construct.25 A dorsal dynamic neutral construct should always be coupled with sufficient axial load resistance to be stable in flexion. If either the intrinsic axial load-resisting abilities are insufficient or interbody support is not present, excessive flexion can lead to construct failure and potential spinal canal compromise.
Dorsal Dynamic Compressive Fixation
Different forms of dynamic spinal instrumentation allow for varying degrees of intersegmental movement. Although some degree of compressive motion augments bone healing according to Wolff’s law, excessive intersegmental movement suppresses bony fusion. This concept can be applied to spine surgery in the form of dorsal compressive fixation. A dynamic dorsal tension band that supplements a ventral interbody fusion by providing compressive forces enhances bone healing by encouraging subsidence. When applying a dorsal dynamic compression device for axial loading, the presence of either a solid ventral interbody or intact spinal elements that would not allow excessive flexion is required. Given the substantial axial loads in the thoracic and lumbar portions of the spine, this concept has primarily been used for cervical fusions.24
Posterior Interspinous Devices
The purpose of this category of dorsal dynamic fixation device is to create neural decompression with only a minimal amount of tissue resection. The superficial location of these devices allows for implantation with minimal dissection and can be performed without general anesthesia if necessary. The decompression is indirect, and laminectomy is typically not performed, avoiding the risk of a cerebrospinal fluid leak or epidural scarring. The indication for placement of a posterior interspinous device is neurogenic claudication and pain associated with facet joint disease. The spine is kept in flexed position, allowing the device to distract the canal and vertebral foramen.2 Extension is limited, unloading the facet joints and theoretically relieving any associated pain. Because of this limit in spine extension, however, there is some concern for the development of kyphotic deformity.
Wallis System
The Wallis system (Abbott Spine, Austin, TX), introduced in 1986, was the first interspinous device to be used clinically. The first version of the device consisted of a titanium spacer inserted between spinous processes and held in place by an artificial Dacron ligament wrapped around adjacent processes. The second-generation device changed the material to polyetheretherketone (PEEK), a polymer with an elastic modulus closer to bone, making the construct less rigid. Initial clinical studies showed the safety and efficacy of this device, and long-term follow-up in patients who had the Wallis device placed after initial plans to undergo decompression and fusion found that arthrodesis was avoided in 80% of patients.26 A prospective controlled trial showed a lower incidence of adjacent-segment disease and need for reintervention when this device was placed at the spinal level rostral to a short fusion segment.27
X-Stop Device
The X-Stop device (Medtronic, Minneapolis, MN) was approved for use by the U.S. Food and Drug Administration (FDA) in 2005. It consists of an oval titanium spacer that fits between two adjacent spinous processes (Fig. 164-1). It is placed through a small midline incision after subperiosteal elevation of paraspinal muscles. The supraspinous ligament is left intact. Dilators expand a space between the spinous processes until the ligament is taut, and a sized X-Stop device is inserted. There is no rigid attachment; the implant is kept in place by the lamina, supraspinous ligament, caudal and rostral spinous processes, and device wings laterally.28 Clinical studies have established the efficacy of the device for the treatment of neurogenic claudication secondary to lumbar stenosis and have shown improved outcomes compared with conservative management.29 At 2-year follow-up of 175 patients selected for X-Stop implantation, only 4.6% of patients needed further surgical treatment.30
DIAM System
The DIAM spine stabilization system (Medtronic) consists of a soft interspinous spacer created by a silicone core surrounded by polyethylene coating. In contrast to the X-Stop device, the interspinous ligament is removed before placement of the DIAM system directly above the ligamentum flavum. It is secured to the spinous processes. The goal of this implant is to reduce intradiscal pressure, retighten posterior elements, and reduce rotatory dislocation via posterior shock absorption of the implant; this theoretically protects the entire motion segment from excessive loads and slows the degenerative process.31 The DIAM device has been used in Europe to treat foraminal stenosis and disc protrusions. Studies have shown efficacy and satisfaction among patients.2,32 A prospective study following 68 patients who had the DIAM device placed after decompressive surgery found that none of the patients had recurrence of disease requiring further intervention, and all patients showed clinical improvement.33 Controlled studies with this device have not been published yet.
Pedicle Screw and Rod-Based Dynamic Devices
Pedicle screw and rod-based dynamic devices are designed to function by maintaining normal motion within a physiologic range, unloading degenerated discs and facet joints, and stabilizing the abnormal segment. The resultant decrease in load potentially alleviates any pain coming from degenerating joints. Another goal of these devices is the prevention of adjacent-segment disease. This goal can be approached either by placing a dynamic system as an entire construct or by using a dynamic device to extend a rigid construct across adjacent levels. When used as an adjunct to intervertebral fusion, these devices may provide an optimal environment by load sharing to the intervertebral implant and allowing micromovements across end plates. Another indication for their use is for stabilization after iatrogenic destabilizing procedures, such as wide laminectomies and facetectomies. Many of these implants have been approved for use with fusion, and many require further evidence of their efficacy as stand-alone constructs.
Graf System
The Graf ligamentoplasty system (Surgicraft, Redditch, UK) has a relatively long history of clinical application compared with other dynamic devices. Titanium pedicle screws are connected by prosthetic ligaments made of braided polyester bands. These bands are connected under a compressive force to hold the spinal segment in lordosis. The objective of this construct is to maintain a fixed lordosis of the lumbar spine, compress the posterior anulus to prevent anular tears, and splint the motion segment, allowing repair to damaged joints.34 The posterior anulus acts as a fulcrum between the tensile forces of the construct and the compressive forces across the disc space. Load is transferred from the anterior disc to the posterior anulus and facet joints, limiting pathologic motion.
The device is indicated for the treatment of flexion instability, but it does not correct spondylolisthesis or scoliotic deformity. Over time, the bands relax, allowing for the return of some kyphotic motion. Although initially developed as a method of treating back pain from disc degeneration, this device has also been used to stabilize spondylolistheses after posterior decompression. The success of this procedure depends on appropriate patient selection, and its indication is restricted to patients with less than 25% of a vertebral slip, minimal disc space narrowing, and coronal facet tropism.34 Long-term results have shown Graf ligamentoplasty to be an effective treatment option in appropriately selected patients. Concerns with this device include the development of iatrogenic lateral recess stenosis, overloading of the posterior anulus and facet joints, and some reports of high revision rates.2,19,35,36
Dynesys System
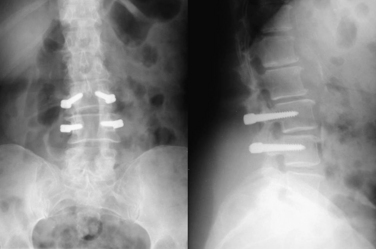
The Dynesys system (Zimmer Spine, Minneapolis, MN) consists of standard pedicle screws attached by polyester cords that pass through compression-resistant polycarbonate urethane spacers. This device has been in use since the early 1990s.2 The Dynesys system is used to treat dynamic instability in the early stages of degeneration, degenerative disease causing low back pain, or iatrogenic instability after decompression.10 This device realigns and stabilizes a spinal segment into its physiologic position, while neutralizing excessive loads. The dynamic relationship between the polyester cords and the spacers limits segmental mobility to a physiologic level and neutralizes bending, torsional, and shear forces.37 The polyester cords resist flexion, whereas the spacers resist compressive forces and prevent excessive lordosis. The combination of these construct components limits the loading of the disc (Fig. 164-2).
Clinical studies have shown the efficacy of this device and shown improved outcome over conservative treatment. The short-term results are comparable to conventional rigid fixation.38 Concerns with this system have focused on the high reported rate of construct failure (17% to 19%).38 Excessive compressive loads on the spacers produce bending moments on the pedicle screws that can lead to breakage or loosening. Another concern is the potential for the compressive sleeves to become kyphogenic under excessive distraction. These sleeves also increase the rigidity of the construct and limit its ability to maintain physiologic motion.19 Whether or not the Dynesys device decreases the development of adjacent-level disease remains to be seen. One study comparing range of motion after instrumented fusion and posterior dynamic stabilization using Dynesys at L4-5 analyzed preoperative and postoperative flexion-extension radiographs. Global and L4-5 segmental range of motion was reduced in the fusion group and unchanged in the Dynesys group. However, no significant changes in mobility at the rostral or caudal adjacent levels were seen in either group (Fig. 164-3).39
Semirigid Rods
Several indications exist for the use of semirigid rods. When attempting to treat spondylolisthesis, recurrent disc herniation, or degenerative disc disease with a solid osseous arthro-desis, semirigid rods may be used to create less potential stress on adjacent spinal segments. Another indication involves patients with a prior instrumented fusion. If symptomatic adjacent-level disease develops, an extension of the instrumentation to the newly affected level can be undertaken with semirigid rods to prevent a subsequent recurrence. Semirigid rods can be used to replace the entire construct length or as an adjunct extension over the adjacent level. A third indication for this type of construct involves patients with spondylolisthesis and stenosis. An osseous arthrodesis may be avoided in these cases if semirigid rods are placed after a facet-sparing laminectomy; this maintains the posterior tension band and limits any progression of the pathologic process.4
Dorsal rods made of the PEEK polymer (Medtronic) have semirigid characteristics. This polymer has a modulus of elasticity between cortical and cancellous bone.4 The rods allow some motion but resist any marked degree of flexion, extension, axial loading, or lateral rotation. Their use reduces stress and hypermobility at adjacent levels compared with rigid titanium constructs. PEEK rods can also reduce stress at the bone-screw interface, reducing the risk of construct failure.4 Although these rods may address the causative factors of adjacent-segment disease, no studies establishing their long-term clinical outcomes have been reported.
The AccuFlex rods (Globus Medical, Audubon, PA) have helical cuts that allow for a limited range of motion, while providing a posterior tension band that unloads the intervertebral disc.18 This system has been approved by the FDA for use in conjunction with an interbody graft, and studies have shown fusion rates and outcomes similar to rigid fixation. Further studies are needed to evaluate the impact on adjacent-level disease and its potential use as a stand-alone dynamic construct. Other semirigid dorsal rods are in development; however, limited clinical data are available on these devices.2
Total Facet Replacement Systems
The restoration of functional facet joints is a relatively new focus for dorsal dynamic stabilization devices. The facet joints can be potential pain generators whether associated disc degeneration is present or absent.2 A primary indication for total facet arthroplasty is pain originating from degenerative facet joints. Another indication focuses on reconstructing an iatrogenically destabilized spine. Combining aggressive decompressive surgeries with total facet arthroplasty may reduce the likelihood of iatrogenic destabilization and the need for arthrodesis. A final application for this technology is for its use in combination with total disc replacement. Disease of the facet joints is currently a contraindication for total disc arthroplasty, but the combination of these technologies may allow for a circumferential motion segment reconstruction and a broader application of both devices (Fig. 164-4).2
Stabilimax NZ Device
The Stabilimax NZ device (Applied Spine Technologies, Wellesley, MA) is designed to support a degenerating spinal segment while preserving normal mobility. This system potentially reduces pain associated with diseased joints and reduces adjacent-level disease. Removal of bony elements is not required to place this construct. Clinical trials are under way to establish its efficacy (Fig. 164-5).
TFAS Device
The TFAS device (Archus Orthopedics, Redmond, WA) can be used to restore motion after resection of diseased facet joints. It is typically used to treat patients with moderate to severe spinal stenosis. Biomechanical studies have verified restoration of physiologic mobility with movement of a sphere along the curved plate of the device. Clinical trials are under way to demonstrate effectiveness and safety compared with instrumented fusion for the treatment of lumbar stenosis after decompression and facetectomy.2,40
TOPS Device
The TOPS device (Impliant, Princeton, NJ) was designed to stabilize a motion segment after laminectomy and medial facetectomy. It shares axial loading with the intervertebral disc and controls lateral, axial, and lordotic mobility. Pedicle screws anchor the device, and an interlocking polyurethane core connects rostral and caudal titanium plates to create a construct that allows controlled physiologic motion. Clinical trials are under way with this device.
Disadvantages and Complications
Pedicle screw fixation was initially designed to stabilize a spinal segment while the fusion completed and assumed the load-bearing function. In the setting of a dynamic implant, pedicle screws may be expected to anchor a device permanently. The extended cyclic loading of the pedicle screws can lead to loosening or fatigue fracture.13 One study found a 10% incidence of radiographically evident pedicle screw loosening after placement of a dynamic device.41 Loosening usually occurred within 6 months after implantation and was unlikely to occur after 1 year.
A motion segment that has been successfully fused is typically stable over the long term. The absence of motion tends to prevent ligamentous or facet hypertrophy, disc herniation, or the recurrence of neural element compression. Preserving mobility at a spinal segment means preserving the possibility of future degeneration, a problem typically avoided with solid fusion. Degenerative changes can occur at the intervertebral disc, the facet joints, or the ligamentum flavum. The disc may herniate or develop pain generators, the facet joints may become arthritic or hypertrophic, and the ligamentum flavum may hypertrophy and contribute to stenosis. The prevalence of degeneration at dynamically stabilized spinal levels has not been established in the literature and may be difficult to distinguish from errors in patient selection.13
Conclusion
The biomechanical applications, appropriate indications, and long-term efficacy of dorsal dynamic stabilization are still being elucidated. Regardless, this technology is likely to be an important tool in the future management of degenerative spine disease. Developing dynamic constructs that are able to relieve pain, restore physiologic mobility, and endure repetitive loads is a tremendous challenge. Optimizing these devices requires a detailed understanding of the pathophysiology of spinal pain syndromes, spinal anatomy, motion segment kinematics, and applied loads, along with sound engineering principles and materials science.19
Ekman P., Moller H., Shalabi A., et al. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175-1186.
Huang R.C., Girardi F.P., Moe R.L., et al. Advantages and disadvantages of nonfusion technology in spine surgery. Orthop Clin North Am. 2005;36:263-269.
Kanayama M., Togawa D., Hashimoto T., et al. Motion-preserving surgery can prevent early breakdown of adjacent segments: comparison of posterior dynamic stabilization with spinal fusion. J Spinal Disord Tech. 2009;22:463-467.
Khoueir P., Kim K.A., Wang M.Y. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
Park P., Garton H.J., Gala V.C., et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938-1944.
1. Cassidy J.D., Cote P., Carroll L.J., et al. Incidence and course of low back pain episodes in the general population. Spine (Phila Pa 1976). 2005;30:2817-2823.
2. Khoueir P., Kim K.A., Wang M.Y. Classification of posterior dynamic stabilization devices. Neurosurg Focus. 2007;22:E3.
3. Bono C.M., Lee C.K. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine (Phila Pa 1976). 2004;29:455-463.
4. Highsmith J.M., Tumialan L.M., Rodts G.E. Flexible rods and the case for dynamic stabilization. Neurosurg Focus. 2007;22:E11.
5. Ekman P., Moller H., Shalabi A., et al. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175-1186.
6. Lee C.S., Hwang C.J., Lee S.W., et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637-1643.
7. Akamaru T., Kawahara N., Tim Yoon S., et al. Adjacent segment motion after a simulated lumbar fusion in different sagittal alignments: a biomechanical analysis. Spine (Phila Pa 1976). 2003;28:1560-1566.
8. Park P., Garton H.J., Gala V.C., et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976). 2004;29:1938-1944.
9. Rahm M.D., Hall B.B. Adjacent-segment degeneration after lumbar fusion with instrumentation: a retrospective study. J Spinal Disord. 1996;9:392-400.
10. Schwarzenbach O., Berlemann U., Stoll T.M., et al. Posterior dynamic stabilization systems: DYNESYS. Orthop Clin North Am. 2005;36:363-372.
11. Mulholland R.C., Sengupta D.K. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11(Suppl 2):S198-S205.
12. Korovessis P., Papazisis Z., Lambiris E. The role of rigid vs. dynamic instrumentation for stabilization of the degenerative lumbosacral spine. Stud Health Technol Inform. 2002;91:457-461.
13. Huang R.C., Girardi F.P., Moe R.L., et al. Advantages and disadvantages of nonfusion technology in spine surgery. Orthop Clin North Am. 2005;36:263-269.
14. Turner J.A., Erske M., Herron L., et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-911.
15. Kanayama M., Hashimoto T., Shigenobu K., et al. Adjacent-segment morbidity after Graf ligamentoplasty compared with posterolateral lumbar fusion. J Neurosurg. 2001;95:5-10.
16. Kanayama M., Togawa D., Hashimoto T., et al. Motion-preserving surgery can prevent early breakdown of adjacent segments: comparison of posterior dynamic stabilization with spinal fusion. J Spinal Disord Tech. 2009;22:463-467.
17. Heggeness M.H., Esses S.I. Classification of pseudoarthroses of the lumbar spine. Spine (Phila Pa 1976). 1991;16(Suppl 8):S449-S454.
18. Mandigo C.E., Sampath P., Kaiser M. Posterior dynamic stabilization of the lumbar spine: pedicle-based stabilization with the AccuFlex rod system. Neurosurg Focus. 2007;22:E9.
19. Huang R.C., Wright T.M., Panjabi M.M., et al. Biomechanics of nonfusion implants. Orthop Clin North Am.. 2005;36:271-280.
20. Sharma M., Langrana N.A., Rodrigueq J. Role of ligaments and facets in lumbar spinal stability. Spine (Phila Pa 1976). 1995;20:887-900.
21. Eck J.C., Humphreys S.C., Lim T.H., et al. Biomechanical study on the effect of cervical spine fusion on adjacent level intradiscal pressure and segmental motion. Spine (Phila Pa 1976). 2002;27:2431-2434.
22. Phillips F.M., Reuben J., Wetzel F.T. Intervertebral disc degeneration adjacent to a lumbar fusion: an experimental rabbit model. J Bone Joint Surg [Br]. 2002;84:289-294.
23. Delank K.S., Gercek E., Kuhn S., et al. How does spinal canal decompression and dorsal stabilization affect segmental mobility? A biomechanical study. Arch Orthop Trauma Surg. 2010;130:285-292.
24. Benzel E.C. Qualitative attributes of spinal implants. In Benzel EC, editor:. In: Biomechanics of spine stabilization. Rolling Meadows, IL: AANS; 2001:171-188.
25. Benzel E.C. Subsidence and dynamic spine stabilization. In Benzel EC, editor:. In: Biomechanics of spine stabilization. Rolling Meadows, IL: AANS; 2001:431-452.
26. Senegas J., Vital J.M., Pointillart V., et al. Clinical evaluation of a lumbar interspinous dynamic stabilization device (the Wallis system) with a 13-year mean follow-up. Neurosurg Rev. 2009;32:335-341.
27. Korovessis P., Repantis T., Zacharatos S., et al. Does Wallis implant reduce adjacent segment degeneration above lumbosacral instrumented fusion? Eur Spine J. 2009;18:830-840.
28. Anderson P.A., Tribus C.B., Kitchel S.H. Treatment of neurogenic claudication by interspinous decompression: application of the X-STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463-471.
29. Zucherman J.F., Hsu K.Y., Hartjen C.A., et al. A multicenter, prospective, randomized trial evaluating the X-STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine (Phila Pa 1976). 2005;30:1351-1358.
30. Kuchta J., Sobottke R., Eysel P., et al. Two-year results of interspinous spacer (X-stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar stenosis. Eur Spin J. 2009;18:823-829.
31. Minns R.J., Walsh W.K. Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine (Phila Pa 1976). 1997;22:1819-1827.
32. Christie S.D., Song J.K., Fessler R.G. Dynamic interspinous process technology. Spine (Phila Pa 1976). 2005;30(Suppl 16):S73-S78.
33. Hrabalek L., Machac J., Vaverka M. The DIAM system stabilisation system to treat degenerative disease of the lumbosacral spine. Acta Chir Orthop Traumatol Cech. 2009;76:417-423.
34. Kanayama M., Hashimoto T., Shigenobu K. Rationale, biomechanics, and surgical indications for Graf ligamentoplasty. Orthop Clin North Am. 2005;36:373-377.
35. Kanayama M., Hashimoto T., Shigenobu K., et al. Non-fusion surgery for degenerative spondylolisthesis using artificial ligament stabilization: surgical indication and clinical results. Spine (Phila Pa 1976). 2005;30:588-592.
36. Rigby M.C., Selmon G.P., Foy M.A., et al. Graf ligament stabilisation: mid-to long-term follow-up. Eur Spine J. 2001;10:234-236.
37. Schnake K.J., Schaeren S., Jeanneret B. Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine (Phila Pa 1976). 2006;31:442-449.
38. Grob D., Benini A., Jung A., et al. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine (Phila Pa 1976). 2005;30:324-331.
39. Cakir B., Carazzo C., Schmidt R., et al. Adjacent segment mobility after rigid and semirigid instrumentation of the lumbar spine. Spine (Phila Pa 1976). 2009;34:1287-1291.
40. Phillips F.M., Tzermiadianos M.N., Voronov L.I., et al. Effect of the total facet arthroplasty system after complete laminectomy-facetectomy on the biomechanics of implanted and adjacent segments. Spine J. 2009;9:96-102.
41. Stoll T.M., Dubois G., Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl 2:S170-S178.