CHAPTER 30 Dorsal Capsuloplasty in Volar Subluxation of the Distal Radius
Instability of the distal radioulnar joint (DRUJ) may be responsible for reduced hand and wrist function from loss of strength and chronic pain on the ulnar side of the wrist. As Bowers noted in 1991, this condition was believed to be rare but became more and more diagnosed and therefore the subject of much discussion and investigation.1 At this time, Bowers’ observation is still of current interest.
The ulna is the stable unit of the forearm, supporting the radius and the hand and allowing controlled movement of the radius along its axis and the DRUJ. The bony anatomy of the DRUJ, however, adds very little to stability. A variety of other structures are responsible for stability of the DRUJ. It is currently assumed that these comprise the pronator quadratus, ulnocarpal ligament, extensor carpi ulnaris subsheath, dorsal radioulnar ligament, volar radioulnar ligament, interosseous membrane, the bony anatomy (sigmoid notch), and the DRUJ capsule.2–10
The soft tissues, together with normal articular cartilage surfaces of the lower end of the ulna and the medial articular facet of the distal radius, ensure a smooth and complete forearm in pronation/supination.1,11 This specific movement is provided not only by rotation from the radius about the ulna but can also only be executed when there is a variable degree of dorsopalmar translation.12,13
Garcia-Elias described in his article on soft tissue anatomy and relationships about the distal ulna that the radius in supination rotates and translates dorsally about the stable ulna, whereas in pronation it rotates and translates palmarly.14 As a result, there is a great variance in joint surface contact of the DRUJ between the sigmoid notch of the distal radius and the articulating surface of the ulnar head. In the neutral forearm position the surface contact is maximal (about 60%). In all other forearm positions there is joint surface contact until it is lost almost completely (less than 10%).15
A stable DRUJ allows rotation, axial motion, as well as translational movement in the dorsopalmar plane. All these movements are based on the osseous contours around the joint, supported by a complex of surrounding soft tissues, which Bowers1 calls the major retaining ligaments. The triangular fibrocartilage complex (TFCC) is considered the major retaining ligament of the DRUJ, with two ligament subsets, the dorsal and volar marginal ligaments (DML-TFC, VML-TFC). These thickenings on the dorsal and volar margins of the central part of the TFCC on the triangular fibrocartilage proper are also referred to as the dorsal and volar radioulnar ligaments.16 There is experimental evidence6,17,18 suggesting that both palmar and dorsal distal radioulnar ligaments need to be intact to maintain complete stability of the joint throughout the whole range of forearm rotation. The relative contribution of each ligament in stabilizing the DRUJ during pronation/supination remains, however, contradictory.6,7,13,17,19
Most of the current treatment options regarding instability of the DRUJ refer to reconstruction of the TFCC. A great variety of techniques have been published. Adams and Berger have classified these techniques into three categories20: (1) a direct radioulnar tether that is extrinsic to the joint,21 (2) an indirect radioulnar link through an ulnocarpal sling or tenodesis,22 and (3) reconstruction of the distal radioulnar ligaments.16,23
In their article, Adams and Berger describe their own technique for reconstruction of both dorsal and palmar radioulnar ligaments, because they believe that gross instability of the DRUJ requires disruption of both ligaments. Reconstruction of both ligaments would therefore be the optimal surgical treatment for post-traumatic, chronic DRUJ instability. For this purpose they use a tendon graft (usually the palmaris longus), which is passed through drill holes in the distal radius and ulna.20
The Authors’ Experience
Patient Selection
The course of 20 consecutive patient treatments during the period from 2003 until 2005 was analyzed retrospectively. Charts were reviewed for preoperative history and physical findings. Fifteen of the patients were female,14 and 5 were men. Of this group, 4 patients were excluded. One patient could not be traced for follow-up, 1 patient developed a pain syndrome (although on the radial side of the wrist), and 2 patients were excluded because of the severity of their TFCC degeneration, which became apparent during operation. Nevertheless we attempted a reefing of the DML, which later proved insufficient. In these 2 patients an ulnar shortening was performed in a second operation.
Surgical Technique
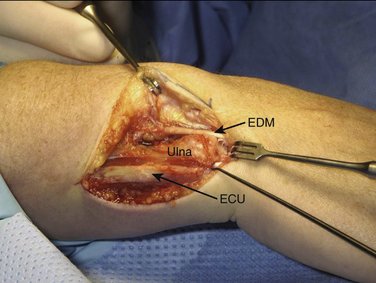
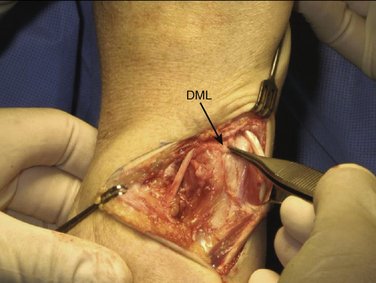
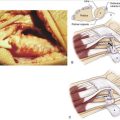
All our patients were operated on as outpatients under regional (axillary block) or general anesthesia with a pneumatic tourniquet around the upper arm to operate under bloodless conditions. The arm is laid on a hand table with the elbow extended and the forearm in pronation. To expose the ulnar head and DRUJ, we make an L-shaped (hockey stick) skin incision of about 5 cm along the sixth extensor sheet area from proximal to distal, curving 5 mm distal to the ulnar styloid to the dorsal aspect of the wrist, and ending just radial to the fifth extensor sheet. To prevent the formation of a painful neuroma it is important to protect the sensory branches of the ulnar nerve, running from volar to dorsal especially in the distal part of the incision. We free the skin from the extensor retinaculum along the fifth and sixth tendon sheets and open them in the same manner as the skin, with a curved incision. The floor of the sixth extensor sheath, however, is preserved. To expose the DRUJ capsule, the extensor carpi ulnaris (ECU) and extensor digiti quinti (EDQ) tendons are retracted radially and ulnarward, respectively (Fig. 30-1).
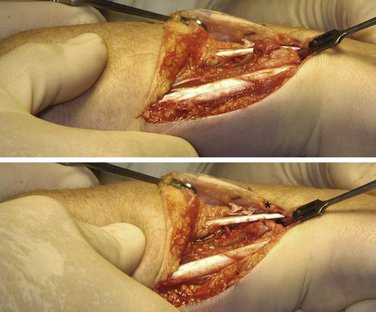
With the wrist still in pronation, the ulnar head can be depressed in a volar direction, that is, correcting the DRUJ instability. In this manner, soft tissue wrinkling is observed around the distal and dorsal aspect of the ulnar head, just proximal to the DML of the TFCC (Fig. 30-2).
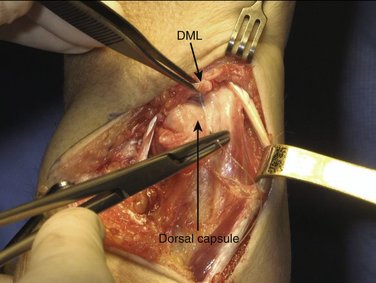
This wrinkling surplus of capsular tissue, which is surprisingly strong, is considered the elongated or attenuated DML. A few millimeters proximal to the DML an incision is made to create a small flap, which is freed from the distal ulnar head but is left connected to the DML. By lifting this flap, the dorsal aspect of the ulnar head and DRUJ are exposed and can be inspected for cartilage condition (Fig. 30-3). A Mitchell trimmer can be inserted and moved between the TFCC and the ulnar head to feel if there are any adhesions that can be released. We then make an incision just distal to the radioulnar ligament to open the ulnocarpal joint for inspection of the triquetrum, the lunate, and the lunotriquetral ligament, as well as the TFCC. In case of TFCC lesions or signs of ulnocarpal abutment, a wafer procedure may be performed simultaneously.
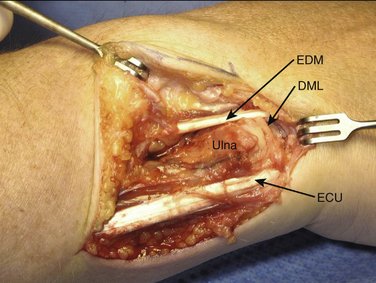
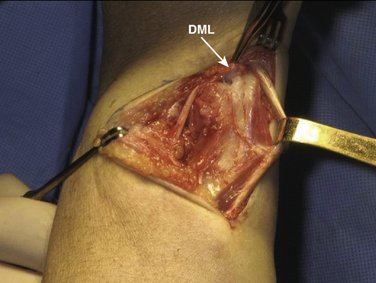
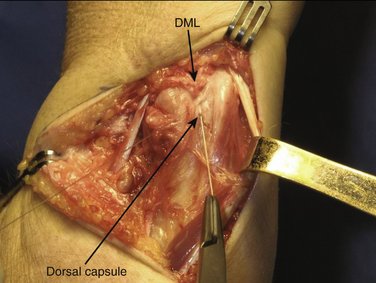
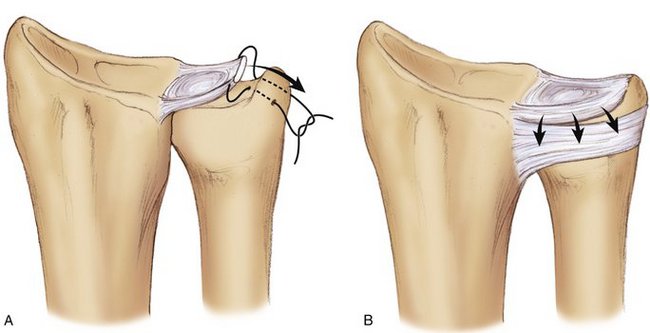
We then start with the final part of the operation—reefing of the DML. After flexing the patient’s elbow, the assistant holds the patient’s wrist in a neutral position with the forearm in a 0-degree position with the thumb pointing toward the patient’s nose. For better visibility and comfort, the surgeon will move to the “lower side” of the arm table. In Figure 30-4, note that the DML is now clearly demonstrated. With the forearm in this neutral position, the ulnar head is only slightly compressed volarly and the DML is brought over the dorsal capsule (Fig. 30-5). We then attach the dorsal portion of the DML with just slight tension to the periosteum of the ulnar head with usually no more than four separate PDS 4-0 stitches (Fig. 30-6). The site of fixation is thus more proximal to the point of original insertion of the DML, but it should never be too tight, in order to prevent a permanent reduction of supination (Fig. 30-7). Transfixion wires through the DRUJ are not used. It is important to maintain the wrist in a neutral position, until the final plaster is applied. The tourniquet is released, and hemostasis is obtained. The fifth and sixth extensor sheaths are closed with resorbable sutures, and the skin is closed with a subcuticular running suture. The site of the incision is covered with a nonadhesive gauze pad, fluffy dressings are applied around the hand and wrist, and a long forearm plaster splint is applied with the elbow flexed and the forearm in neutral position.
Rehabilitation
After 2 weeks the splint is replaced by a circular upper arm cast, for another 4 to 5 weeks. The duration of immobilization is 6 to 7 weeks, depending on the quality of the capsular (DML) tissue.
Results
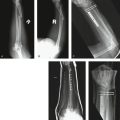
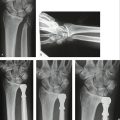
We observed no intraoperative or postoperative complications, except transient paresthesias on the ulnar side of the hand, which disappeared within a few weeks. The wound healing was uneventful (Fig. 30-8).
In all of the 16 patients, reefing of the DML resulted in a clinically stable DRUJ, although minor differences of translational movement between both wrists could be observed in all patients. To obtain objective values for the functional outcome, we measured the total range of motion on the operated wrist compared with the contralateral side (91%), grip strength (78%), and pain according to the visual analog scale.3 When extrapolating these figures to the Mayo Modified Wrist Score combining motion, pain, grip strength, and functional status, the postoperative result score was 85 (excellent: 90-100 points; good: 80-90; fair: 70-80; poor: less than 65). Mean flexion of the operated side versus the contralateral side was 81 versus 86 degrees; extension: 67 versus 76 degrees; pronation: 85 versus 91 degrees; supination: 76 versus 87 degrees; radial deviation: 23 versus 29 degrees; and ulnar deviation: 27 versus 34 degrees.
Discussion
Bowers defines instability as an abnormal path of articular contact occurring during or at the end of the arc of motion attempted. This abnormal path or end point is permitted by alterations in joint surface or by deficiencies in the ligaments that retain the surfaces of the joint or both. DRUJ stability relies mainly on the strength of the supporting soft tissues.1,15 The DML is one of these reinforcing structures, which we use for the dorsal capsuloplasty of the distal ulna.
The advantage of this technique is that it is technically easy and there is limited dissection, which may be helpful in reducing the chance of postoperative limitation of joint motion due to fibrosis. The basic mechanical aim is correcting the volar subluxation of the distal radius. Reefing the DML promotes lifting of the radius along the vertical axis of the sigmoid notch in a dorsal direction and thereby restoring the normal relationship between the radius and ulna in the DRUJ (Fig. 30-9). The vector used for repair is exclusively volar-dorsal and not ulnoradial, which differentiates this from a repair of a peripheral lesion of the TFCC. Another advantage is the use of vascularized, vital tissue instead of a free tendon graft. These grafts may lengthen in time as a result of structure loss of the collagen fibers, with subsequent recurrence of instability. Because the reefing is confined exclusively to the area of the DML, and is done under no tension, the risk of fibrosis and secondary impairment of motion is limited.
Arthroscopy remains our main diagnostic tool in patient selection. It is considered an investigational tool with a high sensitivity and specificity, especially in the diagnosis of lesions of the TFCC.24–26 We believe that clearly visualizing the quality of the tissues along with the ability to test the mechanical quality of the TFCC tissue is of greatest importance in the surgical decision-making. A focal increase in the laxity of the dorsal TFCC is highly suggestive of this type of DRUJ instability.
Previous studies have indicated that the TFCC must rupture for a DRUJ dislocation to occur.6,12,17 In our selected patients we could not detect a rupture of the TFCC during arthroscopy or operation. We believe the instability is caused by attenuation of the fibrous extension of the DML. Wong and coworkers have described a technique for treatment of a specific type of DRUJ instability that they find also on the dorsal side.27 It consists of a duplication capsulorrhaphy of the dorsal capsular structures of the DRUJ in a transverse way. Their series is small (only six patients, but with fairly good results). We use essentially the same fibrous tissues as Wong and coworkers, but we consider the capsule an integral part of the DML, with no relation to the floor of the fifth and sixth extensor sheets. Wong and coworkers’ reefing technique is in a transverse direction, whereas we consider a reefing (or plication) from distal to proximal to be more sound biomechanically in the treatment of a volar-dorsal DRUJ instability. As in our series, no significant TFCC injury could be identified even though typically radioulnar joint laxity usually indicates disruption of soft tissue structures of the DRUJ. Laxity or attenuation of the dorsal extension of the DML seems to explain the volar subluxation of the distal radius and not a lesion of the TFCC itself, which remains inconsistent with earlier reports.10
When during surgery unexpected TFCC lesions or signs of ulnocarpal abutment became visible, we have simultaneously performed a wafer procedure28 in four patients. Some authors prefer a shaft osteotomy for a partial ulnar head resection because the latter may result in scarring between the graft and the exposed bony surface.20 With our technique this sequela is less probable, because we add no other tissue. Indeed in these four cases, which were otherwise not included in our study, no functional loss was observed in relation to scar formation.
An alternative for open surgical repair is arthroscopic suturing of tears of the TFCC, which is now commonly performed. Surgeons in favor of this technique claim a lower risk of neurovascular damage, reduced postoperative pain, and faster rehabilitation. These lesions are mainly ulnar- and radial-sided lesions.29–34 Arthroscopic reefing of the dorsal extension of the DML seems, for the present, not feasible.
Our results seem promising. Comparison with results of other series is difficult because they concern reconstructions of the peripheral tears of the TFCC. The only study on this specific type of instability27 includes only six patients but describes favorable results comparable with ours.
1. Bowers WH. Instability of the distal radioulnar articulation. Hand Clin.. 1991;7:311-327.
2. Kauer TMC, Landsmeer JMF. Functional anatomy of the wrist. In: Tubiana R, editor. The Hand.. Philadelphia: WB Saunders, 1981.
3. Ishii S, Palmer AK, Werner FW, et al. An anatomic study of the ligamentous structure of the triangular fibrocartilage complex. J Hand Surg [Am].. 1998;23:977-985.
4. Johnson RK, Shrewsbury MM. The pronator quadratus in motion and in stabilization of the radius and ulna at the distal radioulnar joint. J Hand Surg.. 1976;1:205-209.
5. Spinner M, Kaplan EB. Extensor carpi ulnaris: its relationship to the stability of the distal radio-ulnar joint. Clin Orthop Relat Res.. 1970;68:124-129.
6. Kihara H, Short WH, Werner FW, et al. The stabilizing mechanism of the distal radioulnar joint during pronation and supination. J Hand Surg [Am].. 1995;20:930-936.
7. Schuind F, An KN, Berglund L, et al. The distal radioulnar ligaments: a biomechanical study. J Hand Surg [Am].. 1991;16:1106-1114.
8. Ward LD, Ambrose CG, Masson MV, Levaro F. The role of the distal radioulnar ligaments, interosseous membrane and joint capsule in distal radioulnar joint stability. J Hand Surg [Am].. 2000;25:341-351.
9. Tolat AR, Stanley JK, Trail IA. A cadaveric study of the anatomy and stability of the distal radioulnar joint in the coronal and transverse planes. J Hand Surg [Br].. 1996;21:587-594.
10. Watanabe H, Berger RA, Berglund LJ, et al. Contribution of the interosseous membrane to distal radioulnar joint constraint. J Hand Surg [Am].. 2005;30:1164-1171.
11. af Ekenstam FW, Hagert CG. Anatomical studies on the geometry and stability of the distal radioulnar joint. Scand J Plast Reconstr Surg.. 1985;19:17.
12. Palmer AK, Werner FW. Biomechanics of the distal radioulnar joint. Clin Orthop Relat Res.. 1984;187:26-35.
13. Hagert CG. The distal radioulnar joint. Hand Clin.. 1987;3:41.
14. Garcia-Elias M. Soft-tissue anatomy and relationships about the distal ulna. Hand Clin.. 1998;14:165-176.
15. af Ekenstam FW. Osseous anatomy and articular relationships about the distal ulna. Hand Clin.. 1998;14:161-164.
16. Scheker LR, Belliappa PP, Acosta R, German DS. Reconstruction of the dorsal ligament of the triangular fibrocartilage complex: a biomechanical study. J Hand Surg [Br].. 1994;19:310-318.
17. Stuart PR, Berger RA, Lindscheid RL, An KN. The dorso-palmar stability of the distal radioulnar joint. J Hand Surg [Am].. 2000;25:689-699.
18. Pirela-Cruz MA, Goll SR, Klug M, Windler D. Stress computed tomography analysis of the distal radioulnar joint: a diagnostic tool for determining translational motion. J Hand Surg [Am].. 1991;16:75-81.
19. Adams BD, Holley KA. Strains in the articular disk of the triangular fibrocartilage complex: a biomechanical study. J Hand Surg [Am].. 1993;18:919-925.
20. Adams BD, Berger RA. An anatomic reconstruction of the distal radioulnar ligaments for posttraumatic distal radioulnar joint instability. J Hand Surg [Am].. 2002;27:243-251.
21. Fulkerson JP, Watson HK. Congenital anterior subluxation of the distal ulna: a case report. Clin Orthop Relat Res.. 1978;131:179-182.
22. Breen TF, Jupiter JB. Extensor carpi ulnaris and flexor carpi ulnaris of the unstable distal ulna. J Hand Surg [Am].. 1989;14:612-617.
23. Bowers WH. The distal radioulnar joint. In: Green DP, Hotchkiss RN, Peterson WC, editors. Green’s Operative Hand Surgery. 4th ed. Philadelphia: Churchill Livingstone; 1999:986-1014.
24. Cooney W. Evaluation of chronic wrist pain by arthrography, arthroscopy and arthrotomy. J Hand Surg [Am].. 1993;18:815-822.
25. Johnstone D, Thorogood S, Smith W, Scott T. A comparison of magnetic resonance imaging and arthroscopy in the investigation of chronic wrist pain. J Hand Surg [Br].. 1997;22:714-718.
26. Pederzini L, Luchetti R, Soragni O, et al. Evaluation of the triangular fibrocartilage complex tears by arthroscopy, arthrography and magnetic resonance imaging. Arthroscopy.. 1992;6:191-197.
27. Wong KH, Yip TH, Wu WC. Distal radioulnar joint distal instability treated with dorsal capsular reconstruction. Hand Surg.. 2004;9:55-61.
28. Feldon P, Terrono AL, Belsky MR. Wafer distal ulna resection for triangular fibrocartilage tears and/or ulna impaction syndrome. J Hand Surg [Am].. 1992;17:731-773.
29. Corso SJ, Savoie FH, Geissler WB, et al. Arthroscopic repair of peripheral avulsion of the triangular fibrocartilage complex of the wrist: a multicenter study. Arthroscopy.. 1997;13:78-84.
30. Haugstvedt JR, Husby T. Results of repair of peripheral tears in the triangular complex using an arthroscopic suture technique. Scand J Plast Reconstr Surg Hand Surg.. 1999;33:439-447.
31. Chou CH, Lee TS. Peripheral tears of the triangular fibrocartilage complex: results of primary repair. Int Orthop.. 2001;25:392-395.
32. Trumble T, Gilbert M, Vedder N. Isolated tears of the triangular fibrocartilage: management by early arthroscopic repair. J Hand Surg [Am].. 1997;22:57-65.
33. Cober SR, Trumble TE. Arthroscopic repair of triangular fibrocartilage complex. Orthop Clin North Am. 2001;30:279-294.
34. Miwa H, Hashizume H, Fujiwara K, et al. Arthroscopic surgery for traumatic triangular fibrocartilage complex injury. J Orthop Sci.. 2004;9:354-359.