Chapter 57 Dorsal and Lateral Thoracic and Lumbar Fusion
When surgical treatment of degenerative disorders is required, following neurologic decompression, fusion may be indicated in cases associated with spondylolisthesis or significant scoliosis. One well-known study,1 for example, demonstrated better clinical and radiographic outcomes following decompression and noninstrumented fusion, compared to decompression alone in the treatment of degenerative spondylolisthesis. Spine fusion may also be useful in cases in which significant iatrogenic instability has been created by wide decompression or by resection of significant lesions. Lumbar spine fusion as a treatment of degenerative disc disease alone, however, is generally not indicated.
The addition of segmental instrumentation has been shown to increase fusion rates and improve clinical outcomes.2,3 While internal fixation may be associated with complications, particularly in the setting of osteoporosis, it should also be considered for patients who are thought to be candidates for spine fusion.
Historical Background
Albee4 and Hibbs5 were the first surgeons to independently describe the technique of arthrodesis. They created greenstick fractures of spinous processes in patients with Pott disease. Albee used tibial autograft to augment the fusion. Mackenzie-Forbes6 and Hibbs7 introduced the technique of laminar decortications extending the fusion further laterally. Hibbs also described the concept of facet joint fusion in a series of his scoliosis patients.7 The technique was further modified by Howorth,8 McBride,9 and Moe.10
The subsequent introduction of intertransverse process fusion by Mathieu and Demirleau provided an alternative technique to dorsal fusion that proved beneficial for patients in whom laminectomy was contemplated.11 Adkins12 implemented this technique in spondylolisthesis patients. Watkins13 described a paramedian approach to gain access to transverse processes lateral to paraspinous musculature, thus sparing the spinous processes and interspinous ligaments. Wiltse et al.14 devised a muscle-splitting approach between the longissimus and multifidus muscles to gain access to the intertransverse process region. The introduction of spinal instrumentation further improved the fusion results.
Biology of Bone Grafting and Spine Fusion
Bone healing following fracture involves a regenerative process, usually leading to complete restoration of the structural integrity of the affected bone. The physiology of spine fusion is similar to the physiology involved in fracture healing. The process of fusion begins by hematoma formation at the surgical site. The rich vascular arcade around the dorsal elements delivers oxygen to the fusion site and also leads to an influx of various inflammatory cells. The healing process begins within hours after surgery. The macrophages infiltrating the hematoma remove the necrotic debris at the fusion site. During this process, the macrophages secrete a number of inflammatory cytokines that stimulate migration of other inflammatory cells into the fusion area.15 Platelets within the hematoma secrete platelet-derived growth factor, which is chemotactic to fibroblasts and other mesenchymal cells in the area.16 The inflammatory markers secreted by platelets and other inflammatory cells promote proliferation and differentiation of primitive mesenchymal cells into cells of osteoid or chondroid lineage.17 Bone morphogenetic protein plays an active role in bone synthesis.18–20
The second phase of fusion involves a repair process.21 Inflammatory markers secreted during the repair process promote ingrowth of new blood vessels. Formation of cartilaginous matrix by chondroblasts as well as osteoid formation by osteoblasts leads to new bone formation. Mineralization of the matrix leads to primary or woven bone formation. Compression and distraction forces22 at the fusion site may provide a stimulus for remodeling21 and consolidation of the fusion mass. Incomplete ossification may lead to fibrous nonunion or pseudarthrosis.
Graft Selection for Dorsal and Lateral Thoracolumbar Fusions
The ideal graft contains an ample number of viable osteoprogenitor cells. Cortical and cancellous autografts and allografts are the most commonly used graft23–26 materials for thoracic and lumbar fusions. Autogenous cancellous grafts are considered more likely to promote fusion than are cortical bone grafts because they are more rapidly vascularized and are more osteoinductive.19,20,27,28 However, cortical autografts are used for their structural integrity in ventral fusion procedures of the thoracic and lumbar spine. Autograft is limited in quantity, however, and is associated with significant morbidity from the autologous bone harvest.27,29–32
Allografts may provide an osteoconductive matrix necessary for fusion. They have limited osteoinductive properties, however, and are inferior to autogenous grafts. Allografts may evoke significant host immune response.33,34 Allografts are considered useful in patients with a limited bone supply or when a large quantity of graft material is needed.35 Adequate fusion rates can be achieved with autograft or allograft when supplemented with segmental instrumentation in young patients with thoracolumbar scoliosis.23–26 Thoracolumbar spine fusion rates are much higher, however, when autograft is used in comparison to allograft alone. A higher pseudarthrosis rate has been demonstrated with the use of allograft as compared to autograft for intertransverse and interlaminar fusion in the lumbar spine.36–39
Surgical Technique
Paraspinal (Wiltse) Approach to the Lumbar Spine
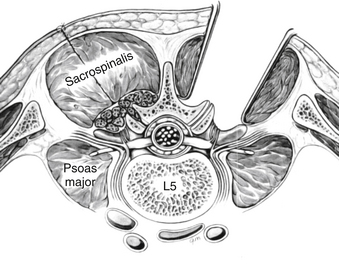
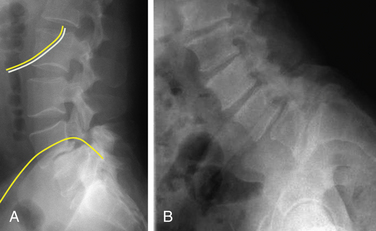
In the paraspinal (Wiltse) approach,14 two paramedian incisions are made approximately 5 cm from the midline just medial to the posterior superior iliac spine. The incision can be planned from the preoperative MRI images by measuring the distance of the plane of separation between the multifidus and longissimus muscles and the midline (Fig. 57-1). The dissection is advanced to the deep dorsal fascia. The fascial incisions are curved medially at their caudal ends to provide adequate muscular retraction. The dissection is further advanced through muscle fascia. The interval between the longissimus and the multifidus muscles can be separated by blunt finger dissection. The dissection should be directed medially to reach the transverse processes and lateral surface of the facet joints. Self-retaining retractors are placed deep to the fascia, and the transverse processes are exposed subperiosteally.
Preparation of Fusion Bed and Bone Grafting
Preparation of Interlaminar and Intertransverse Region
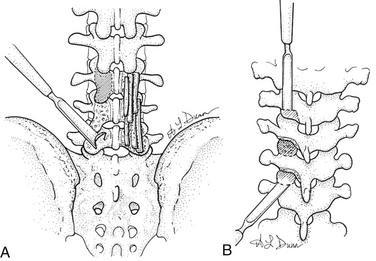
The dorsal surface of the lamina, lateral surface of the facet joint, mamillary body, and lateral pars should be cleared of soft tissues. The exposed area is decorticated by using either a rongeur or a high-speed bur. Graft material is then placed over the decorticated bony elements (Fig. 57-2A).
Facet Joint Fusion
In the thoracic spine, the inferior articular process can be excised by using an osteotome or a Capner gouge to expose the facet joint and cartilaginous surface of the superior articular process.40 The cartilage is then removed to expose bleeding cancellous surface. The bone graft can then be placed on this exposed surface (Fig. 57-2B).
In the lumbar spine, the facet joints can be exposed by excising the facet capsule. Once exposed, the cartilage from the joint can be excised by using a narrow rongeur or a high-speed bur. The exposed bleeding joint surface can then be packed with cancellous bone chips to facilitate fusion across the joint (see Fig. 57-2A).
Complications and Avoidance
Hemorrhage
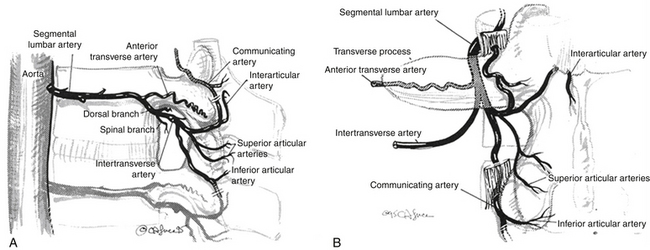
Perforation of terminal branches of lumbar and thoracic segmental arteries during the exposure of the facet joints, lateral pars interarticularis, and transverse processes can lead to significant blood loss. These arteries are found deep to the paraspinal muscles at the superior and inferior aspects of the facet joints (rostral and caudal articular arteries) and on the dorsal surface of the transverse process (communicating artery) (Fig. 57-3). Bleeding from these vessels can be reduced by identification and cauterization prior to dissection. Venous bleeding in the lumbar spine can be avoided by careful positioning of the patient to avoid increased intra-abdominal pressure. Autologous blood donation and the use of cell savers should be considered in patients in whom excessive intraoperative blood loss is anticipated. Intraoperative venous bleeding can be greatly decreased by reducing pressure on the abdomen. This decreases pressure on the vena cava, thus reducing the pressure in the epidural and paravertebral venous plexus.41
Pressure and Traction Injuries
Pseudarthrosis
Successful fusion is defined as the presence of continuous bridging trabeculae of bone between spinal segments. A successful fusion inherently requires the absence of motion between the segments and may provide relief of symptoms caused by mechanical instability. Failure of fusion at the surgical site at or after 1 year from index surgery indicates a pseudarthrosis and needs further investigation into etiology and treatment. Pseudarthrosis is one of the leading causes for revision lumbar spine surgery.43
A variety of factors are thought to be responsible for pseudarthrosis, including metabolic abnormalities, smoking, infection, and persistent motion at the fusion site. Smoking has been shown to be associated with increase in nonunion rates from 8% in nonsmokers to 40% in smokers.44 Persistent motion across the fusion segments is thought to be associated with pseudarthrosis. The incidence of pseudarthrosis increases with increase in the number of levels fused owing to the presence of a longer area that needs to be bridged by the fusion process as well as the presence of more motion across the fusion area.45,46 Spinal instrumentation has been shown to increase the fusion rate by limiting the motion across the fusion segments.3,46–48 A prospective randomized study by Fischgrund et al. showed significantly improved fusion rates at the end of 2 years when lumbar decompression and dorsolateral fusion were combined with instrumentation as compared to noninstrumented decompression and dorsolateral fusion.49
Use of flexion/extension radiographs for diagnosis of pseudarthrosis is controversial and is affected by high interobserver variability.50 Thin-cut CT scans are considered more accurate than are plain radiographs in determining the integrity of the fusion. The presence of metallic artifacts in instrumented fusion, however, decreases the sensitivity of CT scan as the diagnostic modality of choice. Thin-slice CT scan combined with a high index of clinical suspicion may be considered a most reliable diagnostic option. However, exploration of the fusion mass is considered the most specific and sensitive test for diagnosis of pseudarthrosis.
Failure of fusion may present with persistence of back pain, progression of deformity in scoliosis surgery, or recurrence of symptoms in spondylolisthesis. Pseudarthrosis is associated with worse clinical outcomes.51 The treatment of pseudarthrosis should begin with identification of biologic and mechanical factors that contribute to poor bone healing after spine fusion. Correction of endocrine and nutritional factors may assist in achieving solid fusions. Addition of nonrigid mechanical fixation has also been shown to lead to higher fusion rates. The incidence of pseudarthrosis is higher at the thoracolumbar junction as well as the lumbosacral junction. Surgical treatment includes repair of pseudarthrosis by exposure of the fusion area, removal of instrumentations, thorough decortication, and bone grafting with large quantity of autogenous iliac crest bone graft. Instrumentation is then reapplied with compressive forces across the fusion area. RhBMP-252 and osteogenic protein-1 (OP1) have been found to be useful in increasing the fusion rates in lumbar spine fusion surgeries.
Infection
The rate of infections following spine surgery procedures has been shown to correlate with the duration of the procedure, associated patient comorbidities, and the use of instrumentation. The rate of infection is 2% to 4% in noninstrumented spine fusion and 6% to 11% in instrumented spine fusion.53 Staphylococcus aureus is the most common organism responsible for postoperative spinal infections. Postoperative infections may result from direct inoculation or through hematogenous spread of infection from a remote source of infection. Statistically significant preoperative risk factors that are associated with increased risk of infection include age more than 60 years, smoking, diabetes, previous surgical infection, increased body mass index, and alcohol abuse. Intraoperative factors that are associated with increased risk of infection include staged procedure, operative time more than 5 hours, and fusions involving 7 to 13 levels.54
Attempts to reduce preoperative and intraoperative risk factors may decrease the risk of infection and improve patient outcomes following spine fusion surgery. Surgeon familiarity with decortication, bone harvesting, and instrumentation techniques may reduce the operative time and decrease the risk of bacterial colonization. Preoperative antibiotics55 and repeat administration of antibiotics every 4 hours for long-duration surgeries, adequate intraoperative wound irrigation, intermittent repositioning of self-retaining retractors, and limiting the use of monopolar cautery may also decrease the incidence of postoperative infections. The risk of a hematogenous spread of infection can be decreased by recognition and treatment of remote sources of infection. Early patient mobilization and the use of incentive spirometery may help to decrease the risk of pulmonary infections. Careful sterile technique is essential during administration of the Foley catheter.
Surgical treatment of postoperative wound infections should include debridement of infected and necrotic tissues and obtaining sufficient cultures. Exposure of the fusion mass and the hardware with removal of infected unincorporated bone graft material followed by thorough irrigation with a pulsatile lavage system should be carried out. Bone grafting material can then be replaced. Postoperative wound infection can usually be treated without removal of hardware.56
Minimally Invasive Transpsoas (Lateral) Interbody Fusion
The ALIF procedure is typically performed through a transperitoneal or ventral retroperitoneal approach. These open approaches require retraction of the peritoneum and its contents. Postoperative ileus is a common condition following an ALIF procedure. Manipulation of the major retroperitoneal vascular structures may also be required. This can be associated with increased risk of vascular injury.57 While the open ventral approach provides excellent and reliable exposure of L5-S1, exposure of the L4-5 disc requires control of the recurrent iliolumbar vein and segmental vessels to achieve safe retraction of the iliac vessels. Ventral exposure can also be complicated by injury to the sympathetic chain leading to retrograde ejaculation.58 The direct ventral approach can also be more challenging in obese patients and as such may be associated with increased risk of complications in this group of patients.
The lateral transpsoas approach to the lumbar spine was developed as an alternative to the traditional ALIF. The goal was to achieve the interbody biomechanical and biologic benefits of the open ALIF procedure but with reduced complications and reduced invasiveness.59 The transpsoas (lateral) interbody fusion offers certain biomechanical and biologic advantages of the traditional ALIF approach but does not require vascular or visceral retraction.
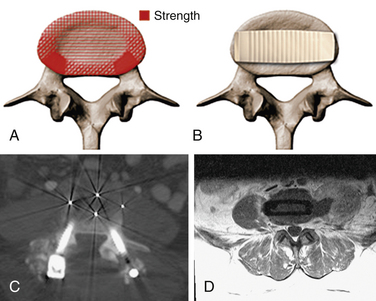
The lateral transpsoas approach requires a small flank incision and placement of a laterally based retractor. This provides access to the lateral aspect of the disc through the psoas muscle. After bilateral anular release and complete discectomy, interbody grafts or devices can be introduced through this minimally invasive approach. This provides a large fusion surface in the compressive environment of the anterior columns. Since the laterally placed interbody graft or device bears weight bilaterally over the apophyseal ring, it provides excellent interbody mechanical support to maintain restoration of interbody height and resist subsidence60 (Fig. 57-4).
Indications and Clinical Applications61
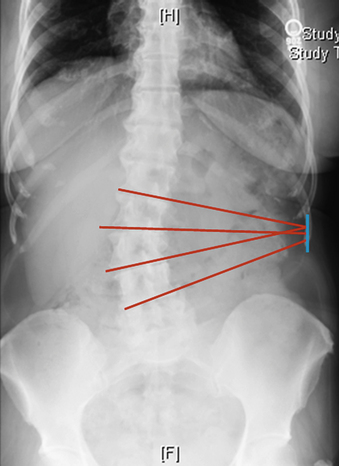
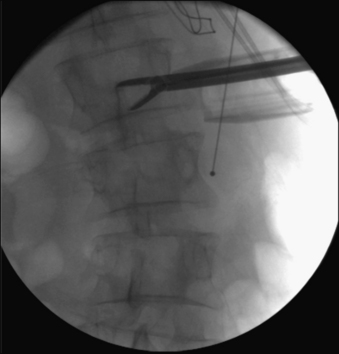
As currently developed, the transpsoas lateral interbody fusion procedure can be applied from the thoracolumbar junction to L5 but is most frequently used in the midlumbar segments. However, thoracic procedures including discectomy and fusion as well as corpectomy have been performed. More typically, this minimally invasive approach is used for interbody lumbar fusion in the setting of adult degenerative disorders, including scoliosis. With coronal plane deformities, the approach is usually performed on the concave side of the curve to provide access to multiple disc spaces through a single flank skin incision (Fig. 57-5). After ipsiplateral and contralateral release of the anulus and Sharpey fibers from the end plates, placement of large interbody spacers can achieve gentle correction of the scoliosis. In patients with degenerative spondylolisthesis, placement of an interbody spacer through a transpsoas technique may help in the restoration of segmental lordosis as well as to increase the foraminal height. Similar results can be obtained in patients with low-grade isthmic spondylolisthesis. The transpsoas technique may be an adjunct to the dorsal treatment of pseudarthrosis and may also be applied to the treatment of patients with adjacent segment disease. We have also used this technique to perform open biopsies and curettage of spinal tumors that were not amenable to CT-guided biopsy (Fig. 57-6). Application of the transpsoas fusion procedure, like any fusion procedure, for the treatment of degenerative disc disease without instability is controversial and is unlikely to be well indicated.
Currently, laterally placed interbody implants should typically not be considered adequate as stand-alone ventral fusion devices. Dorsally and laterally based instrumentation is recommended as an adjunct. Dorsal instrumentation may be placed in a minimally invasive fashion, particularly if a direct neurologic decompression is not required after interbody correction. Dorsal instrumentation may also be placed by traditional open techniques, with or without direct decompression. Laterally based instrumentation can be carried out by using a laterally based plate and screw construct or vertebral body screws passed through the interbody device.
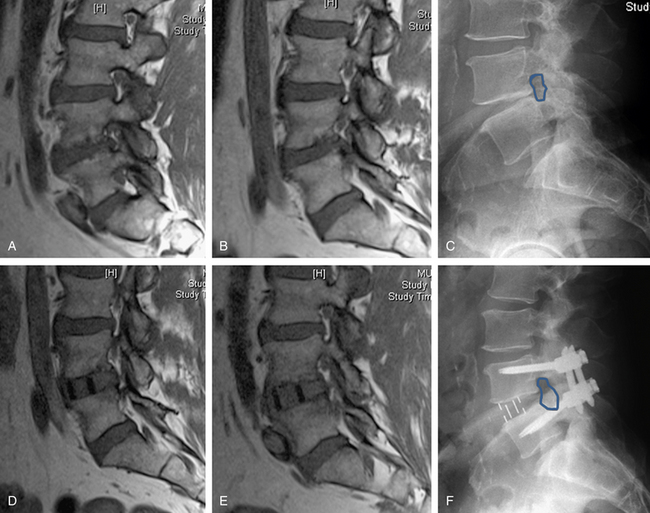
We have used this lateral transpsoas interbody fusion technique most frequently in degenerative deformity or adjacent segment degeneration cases that require direct decompression. It should be noted, however, that in many cases, indirect neurologic decompression can be achieved by restoring the normal interbody anatomic relationship. For example, we have treated isthmic spondylolisthesis in this manner (Fig. 57-7).
Surgical Technique of the Lateral Transpsoas Procedure61
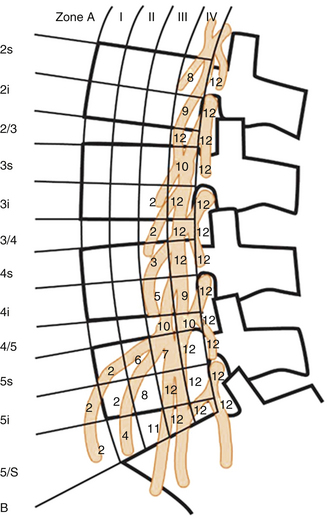
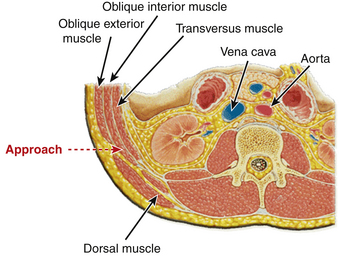
A familiarity with the anatomy of the psoas muscle is useful to guide surgical technique. The psoas muscle originates from the vertebral body, transverse processes, and intervertebral discs from T12 to L4 and covers the vertebral column laterally. The psoas muscle is larger in the lower lumbar region than in the upper lumbar region. A lateral approach through the psoas provides spinal access dorsal to the great vessels and between the segmental vessels, thus minimizing the blood loss and risk of injury to the great vessels. The lumbar plexus traverses the psoas, however, and there is a potential for injury during the approach. Moro et al.62 in their cadaveric study analyzed the distribution of the lumbar plexus within the psoas muscle (Fig. 57-8). The ventral half of the psoas was considered to be the safest to traverse, and the risk was found to increase rostral to caudal. Surgeons should use neuromonitoring while traversing the psoas at the L4-5 level. Care also should be taken to avoid injury to the genitofemoral nerve. The genitofemoral nerve is formed from the branches of the L1 and L2 nerve roots. It traverses the psoas muscle from dorsal to ventral, typically surfacing on the psoas between L3 and L4 and coursing along the ventral surface of the psoas thereafter.62 The nerve ultimately supplies the medial thigh.
Preoperative planning can be very helpful. Posteroanterior and lateral radiographs are assessed to determine whether the lateral approach is applicable to the patient’s anatomy. The iliac crest prevents direct lateral access to L5-S1 and can also interfere with direct lateral access to L4-5 in some patients. This pelvic anatomy is more commonly seen in male patients and can be assessed on radiographs. The presence of long 11th and 12th ribs may also interfere with the approach to the upper lumbar levels. Traversing between ribs or resecting a rib can facilitate the approach (Fig. 57-9).
The determination of a left- versus right-sided approach can also be made on the basis of imaging. Radiographs are used to guide the approach in treating scoliosis. An approach from the convex side of the curve provides easier entry to the disc space because the convex side of the interspace provides more space between the lateral aspect of the end plates in comparison to the concave side. For this reason, we will often approach from the convex side of the deformity when treating at a single level. When treating L4-5 in the setting of coronal malalignment, typically only one side can be used because the iliac crest will obscure the opposite side. This frequently dictates the side of the approach when treating scoliosis. In treating multilevel coronal plane deformities, however, an approach from the concave side may provide access to multiple disc spaces through the same small flank incision (see Fig. 57-5).
Cross-sectional imaging such as MR or CT can be helpful in determining a right- or left-sided approach. Axial images are used to examine the psoas muscle as well as other retroperitoneal structures in determining whether there is a left or right preference. For example, a psoas that is relatively large and relatively ventral may be associated with an increased risk of encountering the nerves of the lumbosacral plexus as compared to a psoas that is slender and relatively dorsal. Additionally, we make it routine practice to identify the path of the ureter from kidney to bladder on MRI, if possible, to help prevent injury. In certain deformities, the path of the ureter has guided the approach.
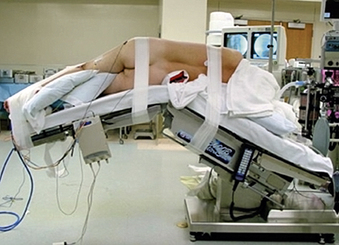
Proper patient positioning speeds the procedure and improves its safety. After intubation and after placement of the neuromonitoring leads, the patient is placed in the lateral decubitus position on an articulating operating table. The pelvis is then secured with 3-inch cloth tape to the operating table just below the table articulation. The hips and knees are flexed to relax the iliopsoas muscle on the operative side. The upper thigh and leg are also secured to the table with 3-inch cloth tape (Fig. 57-10). Bony prominences and upper extremities are well padded, and an axillary role is placed and secured in safe position.
The operating table is then maximally flexed to open the space between the 12th rib and the iliac crest. Biplanar fluoroscopy is used to assess the orientation of the operative vertebrae. The operating table is then moved (in rotation and Trendelenburg planes) so that the vertebral end plate is oriented orthogonally to the walls and floor of the operating room (Figs. 57-11A and B). To achieve this, the cross-table C-arm is positioned so that the beam is parallel to the wall and perpendicular to the floor of the room, and the vertebrae are moved to create a true PA view of the vertebrae. Subsequent placement of the C-arm with its beam parallel to the wall will provide lateral images of the vertebrae. Positioning of the vertebrae in this manner allows the surgeon to approach and traverse the disc in a direct lateral trajectory simply by proceeding straight down. This may reduce the risk of traversing the disc obliquely and inadvertently entering the spinal canal or ventral vascular structures. The skin is then marked directly lateral to the operative disc(s). A transverse incision is used for one- or two-level procedures, and a longitudinal incision is used for three or more levels.
After appropriate prepping and draping, the skin is incised at the marked level (Fig. 57-12). The subcutaneous tissue is dissected to expose the lateral fibers of the external oblique muscle. These are gently split in the line of muscle fibers. Gentle manual retraction in this interval under direct visualization reveals internal oblique muscle fibers 90 degrees to the external oblique muscle. Splitting the fibers of internal oblique muscles reveals transverse running fibers that are separated. The separated fibers are retracted by using hand-held McBurney retractors to provide direct visualization of the transversalis fascia, which is opened to reveal retroperitoneal fat. Blunt finger dissection is then performed so that the retroperitoneal fat is moved ventrally. The quadratus lumborum muscle can be felt by dorsal palpation. Further ventral palpation along the quadratus leads to the tip of the transverse processes of lumbar vertebrae as well the (ventrally) overlying psoas muscle. The dorsolateral and ventral aspects of the psoas muscle belly are determined.
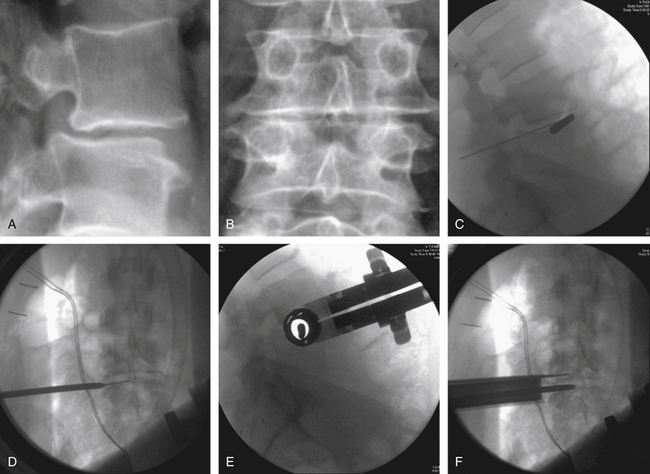
Narrow Wylie renal vein retractors are then placed to allow direct visualization of the lateral surface of the psoas muscle. Using direct visualization may reduce the risk of injury to abdominal structures, including peritoneal contents, as compared to approaches relying on radiographic guidance alone. The surface of the psoas muscle is inspected for identification of the genitofemoral nerve and the ureter. A triggered EMG stimulator probe is then placed upon the lateral aspect of the psoas muscle at the desired surgical level. This is done under direct visualization with confirmation by biplanar fluoroscopy. Stimulation is then applied to assess for potential underlying or adjacent neurologic structures. Biplanar fluoroscopy is used to confirm the optimal position of entry at the lateral surface of the disc and at the junction of the ventral one third and middle one third of the vertebral body (Figs. 57-11C to F).
A guidewire or probe is then placed into the disc. Its position is confirmed by biplanar fluoroscopy. Serial dilation is then performed, and retractor length is determined. The retractor is assembled and passed over the outermost dilator. After removal of the dilators from within the retractor, the lateral aspect of the disc space is inspected. A triggered EMG stimulator is used to stimulate within and around the periphery of the retractor. Subsequently, osseous fixation of the retractor can be performed with certain retractor systems. This is placed adjacent to the end plate, care being taken to not traverse the segmental vessel. The retractor is fixed to the table with a rigid articulating arm (see Fig. 57-11F).
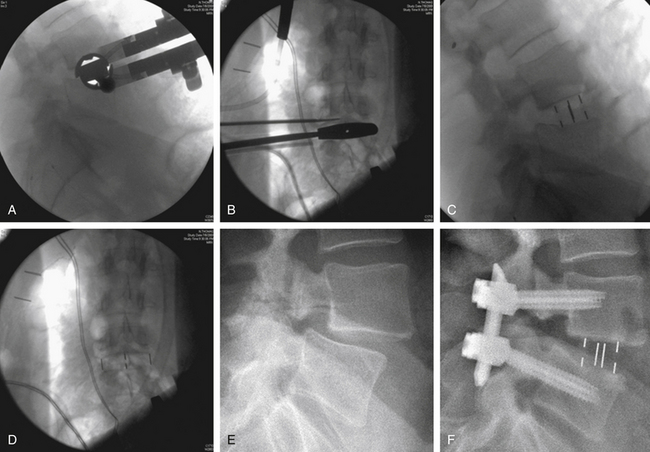
Trial implants of sequentially increasing size are passed to select the proper implant size (Figs. 57-13A and B). The appropriate size spacer is selected and packed with bone graft or bone graft substitute. The implant is advanced between the vertebral bodies under direct biplanar fluoroscopy to ensure proper placement of the implant (Figs. 57-13C to F). Free-running EMG and the more sensitive intermittent tcMEP provide monitoring of the integrity of the local nerve roots throughout the procedure.
Outcomes
The use of the lateral lumbar interbody fusion technique for lumbar interbody fusion is associated with shorter operative times, minimal blood loss, early postoperative patient mobilization, and shorter hospital stays. Diaz et al., in their series of 18 patients, had a mean operative time of 125 minutes, and intraoperative blood loss of 50 mL. The average hospital stay was 2 days.63 The technique led to significant improvement of visual analogue scale and Oswestry Disability Index scores at the end of 1 year. Bergey et al., in their series of 21 patients, observed an average operative time of 149 minutes, average blood loss of 150 mL, and average postoperative hospital stay of 4.1 days.64 Immediate clinical improvement was seen in 84% of patients. The average visual analogue scale score decreased by 5.9 at the end of 6 months, and an excellent clinical outcome was seen in 60% of patients at 6 months. No cases of implant migration or pseudarthrosis were observed in any of the patients.
Complications
The most common complications seen with the lateral interbody fusion technique are transient groin and thigh paraesthesias. Recent papers describing the lateral interbody fusion technique have reported the varying rate of such complications to be bewteen 0.7% and 62.7%.64–67 In a large clinical series by Rogers et al., postoperative neurologic deficit was reported to be 0.7% (4/600 patients).65 Transient thigh paraesthesia and numbness were seen in 30% (6/21) of patients in a clinical series by Bergey et al.64 Improvement from the paraesthesia was seen in 4 weeks in four of these six patients. Uncommon complications such as transient psoas hematoma and transient iliopsoas weakness on the side of surgical approach are also possible. In another clinical series, Wang et al. found postoperative thigh numbness, pain, weakness, and dysaesthesia lateralized to the side of the approach in 30.4% (7/23) of patients.66 The symptoms resolved during the postoperative period in all but one patient, who needed admission to the rehabilitation unit to facilitate recovery. In another clinical series, Cummock et al. reported the incidence of postoperative neurologic deficit to be 62.7%.67 Fifty percent of these patients had their symptoms resolved in 3 months, and 90% within the first postoperative year. Considering the high incidence of postoperative ipsilateral thigh symptoms, we advise discussing this possibility with patients prior to surgery.
Buttermann G.R., Glazer P.A., Bradford D.S. The use of bone allografts in the spine. Clin Orthop Relat Res. 1996;324:75-85.
Cummock M.D., Vanni S., Levi A.D., et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011;15(1):11-18.
Fischgrund J.S., Mackay M., Herkowitz H.N., et al. 1997 Volvo Award winner in clinical studies: degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22(24):2807-2812.
Jorgenson S.S., Lowe T.G., France J., Sabin J. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient: a minimum of 1-year follow-up in 144 patients. Spine (Phila Pa 1976). 1994;19(18):2048-2053.
Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29(7):726-733. discussion 733–734
Moe J.H. A critical analysis of methods of fusion for scoliosis: an evaluation in two hundred and sixty-six patients. J Bone Joint Surg [Am]. 1958;40(3):529-554.
Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443.
Rogers W.B., Gerber E.J., Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011;36(1):26-32.
Simpson A.K., Chambliss H., White A.P. Lateral lumbar transpsoas interbody fusion. Tech Orthop. 2011;26(3):156-165.
Wang M.Y., Mummaneni P.V. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28(3):E9.
1. Herkowitz H.N., Kurz L.T. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am]. 1991;73(6):802-808.
2. Vaccaro A.R., Garfin S.R. Internal fixation (pedicle screw fixation) for fusions of the lumbar spine. Spine (Phila Pa 1976). 1995;20(Suppl 24):S157-S165.
3. Yuan H.A., Garfin S.R., Dickman C.A., Mardjetko S.M. A historical cohort study of pedicle screw fixation in thoracic, lumbar, and sacral spinal fusions. Spine (Phila Pa 1976). 1994;19(Suppl 20):S2279-S2296.
4. Albee F.H. Transplantation of portions of the tibia into spine for Pott’s disease. JAMA. 1911;57:885-886.
5. Hibbs R.A. An operation for progressive spinal deformities. NY J Med. 1911;93:1013-1016.
6. MacKenzie-Forbes A. Technique of an operation for spinal fusion as practised in Montreal. J Orthop Surg. 1920;2B:509-514.
7. Hibbs R.A. A report of fifty-nine cases of scoliosis treated by the fusion operation. J Bone Joint Surg [Am]. 1924;6:3-37.
8. Howorth M.B. Evolution of spinal fusion. Ann Surg. 1943;117(2):278-289.
9. McBride E.D. A mortised transfacet bone block for lumbosacral fusion. J Bone Joint Surg [Am]. 1949;31(2):385-393.
10. Moe J.H. A critical analysis of methods of fusion for scoliosis: an evaluation in two hundred and sixty-six patients. J Bone Joint Surg [Am]. 1958;40(3):524-529.
11. Mathieu P., Demirleau P. Traitement chirurgical du spondylolisthesis douloureaux. Rev Orthop. 1936;23:352-363.
12. Adkins E.W. Lumbo-sacral arthrodesis after laminectomy. J Bone Joint Surg [Br]. 1955;37(2):208-223.
13. Watkins M.B. Posterolateral fusion of the lumbar and lumbosacral spine. J Bone Joint Surg [Am]. 1953;35(4):1014-1018.
14. Wiltse L.L., Bateman J.G., Hutchinson R.H., Nelson W.E. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg [Am]. 1968;50(5):919-926.
15. Rifas L., Shen V., Mitchell K., Peck W.A. Macrophage-derived growth factor for osteoblast-like cells and chondrocytes. Proc Natl Acad Sci U S A. 1984;81(14):4558-4562.
16. Scher C.D., Shepard R.C., Antoniades H.N., Stiles C.D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979;560(2):217-241.
17. Cook S.D., Rueger D.C. Osteogenic protein-1: biology and applications. Clin Orthop Relat Res. 1996;324:29-38.
18. Reddi A.H., Wientroub S., Muthukumaran N. Biologic principles of bone induction. Orthop Clin North Am. 1987;18(2):207-212.
19. Urist M.R. Bone: formation by autoinduction. Science. 1965;150(698):893-899.
20. Urist M.R., Hay P.H., Dubuc F., Buring K. Osteogenetic competence. Clin Orthop Relat Res. 1969;64:194-220.
21. Simmons D.J. Fracture healing perspectives. Clin Orthop Relat Res. 1985;200:100-113.
22. Kushner A. Evaluation of Wolff’s law in bone formation. J Bone Joint Surg [Am]. 1940;22:589-596.
23. Aurori B.F., Weierman R.J., Lowell H.A., et al. Pseudarthrosis after spinal fusion for scoliosis: a comparison of autogeneic and allogeneic bone grafts. Clin Orthop Relat Res. 1985;199:153-158.
24. Bridwell K.H., O’Brien M.F., Lenke L.G., et al. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis: does it work? Spine (Phila Pa 1976). 1994;19(23):2658-2666.
25. Dodd C.A., Fergusson C.M., Freedman L., et al. Allograft versus autograft bone in scoliosis surgery. J Bone Joint Surg [Br]. 1988;70(3):431-434.
26. Fabry G. Allograft versus autograft bone in idiopathic scoliosis surgery: a multivariate statistical analysis. J Pediatr Orthop. 1991;11(4):465-468.
27. Stringa G. Studies of the vascularisation of bone grafts. J Bone Joint Surg [Br]. 1957;39(2):395-420.
28. Abbot L.C., Shcottstaedt E.R., Saunders J.B., Bost F.C. The evaluation of cortical and cancellous bone as grafting material: a clinical and experimental study. J Bone Joint Surg [Br]. 1947;29:381-414.
29. Laurie S.W., Kaban L.B., Mulliken J.B., Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73(6):933-938.
30. Kurz L.T., Garfin S.R., Booth R.E.Jr. Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine (Phila Pa 1976). 1989;14(12):1324-1331.
31. Younger E.M., Chapman M.W. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3(3):192-195.
32. Summers B.N., Eisenstein S.M. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg [Br]. 1989;71(4):677-680.
33. Schaffer J.W., Field G.A., Goldberg V.M., Davy D.T. Fate of vascularized and nonvascularized autografts. Clin Orthop Relat Res. 1985;197:32-43.
34. Heiple K.G., Chase S.W., Herndon C.H. A comparative study of the healing process following different types of bone transplantation. J Bone Joint Surg [Am]. 1963;45:1593-1616.
35. Buttermann G.R., Glazer P.A., Bradford D.S. The use of bone allografts in the spine. Clin Orthop Relat Res. 1996;324:75-85.
36. An H.S., Lynch K., Toth J. Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord. 1995;8(2):131-135.
37. Dawson E.G., Lotysch M.3rd, Urist M.R. Intertransverse process lumbar arthrodesis with autogenous bone graft. Clin Orthop Relat Res. 1981;154:90-96.
38. Jorgenson S.S., Lowe T.G., France J., Sabin J. A prospective analysis of autograft versus allograft in posterolateral lumbar fusion in the same patient: a minimum of 1-year follow-up in 144 patients. Spine (Phila Pa 1976). 1994;19(18):2048-2053.
39. Nugent J.P., Dawson E.G. Intertransverse process lumbar arthrodesis with allogenic fresh-frozen bone graft. Clin Orthop Relat Res. 1991;287:107-111.
40. Bernardi R., Mueller W., editors. Thoracic Fusion Techniques Using Bone. New York: McGraw -Hill, 1996.
41. Batson O.V. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138-149.
42. Tan S.B., Kozak J.A., Dickson J.H., Nalty T.J. Effect of operative position on sagittal alignment of the lumbar spine. Spine (Phila Pa 1976). 1994;19(3):314-318.
43. Martin B.I., Mirza S.K., Comstock B.A., et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976). 2007;32(3):382-387.
44. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;11(9):942-943.
45. DePalma A.F., Rothman R.H. The nature of pseudarthrosis. Clin Orthop Relat Res. 1968;59:113-118.
46. Schwab F.J., Nazarian D.G., Mahmud F., Michelsen C.B. Effects of spinal instrumentation on fusion of the lumbosacral spine. Spine (Phila Pa 1976). 1995;20(18):2023-2028.
47. Wood G.W.2nd, Boyd R.J., Carothers T.A., et al. The effect of pedicle screw/plate fixation on lumbar/lumbosacral autogenous bone graft fusions in patients with degenerative disc disease. Spine (Phila Pa 1976). 1995;20(7):819-830.
48. Zdeblick T.A. A prospective, randomized study of lumbar fusion: preliminary results. Spine (Phila Pa 1976). 1993;18(8):983-991.
49. Fischgrund J.S., Mackay M., Herkowitz H.N., et al. 1997 Volvo Award winner in clinical studies: degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22(24):2807-2812.
50. Cakir B., Richter M., Kafer W., et al. Evaluation of lumbar spine motion with dynamic X-ray—a reliability analysis. Spine (Phila Pa 1976). 2006;31(11):1258-1264.
51. Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29(7):726-733. discussion 733–734
52. Dimar J.R., Glassman S.D., Burkus K.J., et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976). 2006;31(22):2534-2539. discussion 2540
53. Massie J.B., Heller J.G., Abitbol J.J., et al. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99-108.
54. Fang A., Hu S.S., Endres N., et al. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976). 2005;30(12):1460-1465.
55. Horwitz N.H., Curtin J.A. Prophylactic antibiotics and wound infections following laminectomy for lumbar disc herniation. J Neurosurg. 1975;43(6):727-731.
56. Levi A.D., Dickman C.A., Sonntag V.K. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86(6):975-980.
57. Fantini G.A., Pappou I.P., Girardi F.P., et al. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976). 2007;32(24):2751-2758.
58. Christensen F.B., Bunger C.E. Retrograde ejaculation after retroperitoneal lower lumbar interbody fusion. Int Orthop. 1997;21(3):176-180.
59. Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443.
60. Tan J.S., Bailey C.S., Dvorak M.F., et al. Interbody device shape and size are important to strengthen the vertebra-implant interface. Spine (Phila Pa 1976). 2005;30(6):638-644.
61. Simpson A.K., Chambliss H., White A.P. Lateral lumbar transpsoas interbody fusion. Tech Orthop. 2011;26(3):156-165.
62. Moro T., Kikuchi S., Konno S., Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976). 2003;28(5):423-428. discussion 427–428
63. Diaz R, Phillips FM, Pimenta L: Minimally-invasive fusion (XLIF®) in the treatment of symptomatic degenerative lumbar scoliosis. Paper 47: Proceedings of the North American Spine Society, 20th Annual Meeting, Philadelphia, 2005.
64. Bergey D.L., Villavicencio A.T., Goldstein T., Regan J.J. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976). 2004;29(15):1681-1688.
65. Rogers W.B., Gerber E.J., Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011;36(1):26-32.
66. Wang M.Y., Mummaneni P.V. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28(3):E9.
67. Cummock M.D., Vanni S., Levi A.D., et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011;15(1):11-18.