Tularemia is a zoonosis caused by Francisella tularensis. Humans of any age, sex, or race are universally susceptible to this systemic infection. Tularemia is primarily a disease of wild animals and persists in contaminated environments, ectoparasites, and animal carriers. Human infection is incidental and usually results from interaction with biting or blood-sucking insects, contact with wild or domestic animals, ingestion of contaminated water or food, or inhalation of infective aerosols. The illness is characterized by various clinical syndromes, the most common of which consists of an ulcerative lesion at the site of inoculation, with regional lymphadenopathy and lymphadenitis. Systemic manifestations, including pneumonia, typhoidal tularemia, meningitis, and fever without localizing findings, pose a greater diagnostic challenge.
ETIOLOGY AND EPIDEMIOLOGY
F. tularensis is a class A bioterrorism agent (Chap. 261e). With rare exceptions, tularemia is the only disease produced by F. tularensis—a small (0.2 μm by 0.2–0.7 μm), gram-negative, pleomorphic, nonmotile, non-spore-forming bacillus. Bipolar staining results in a coccoid appearance. The organism is a thinly encapsulated, nonpiliated strict aerobe that invades host cells. In nature, F. tularensis is a hardy organism that persists for weeks or months in mud, water, and decaying animal carcasses. Dozens of biting and blood-sucking insects, especially ticks and tabanid flies, serve as vectors. Ticks and wild rabbits are the source for most human cases in endemic areas of the southeastern United States. In Utah, Nevada, and California, tabanid flies are the most common vectors. Animal reservoirs include wild rabbits, squirrels, birds, sheep, beavers, muskrats, and domestic dogs and cats. Person-to-person transmission is rare or nonexistent.
The four subspecies of F. tularensis are tularensis, holarctica, novicida, and mediasiatica. The first three of these subspecies are found in North America; in fact, subspecies tularensis has been isolated only in North America, where it accounts for >70% of cases of tularemia and produces more serious human disease than other subspecies (although, with treatment, the associated fatality rate is <2%). The progression of illness depends on the infecting strain’s virulence, the inoculum size, the portal of entry, and the host’s immune status.
Ticks pass F. tularensis to their offspring transovarially. The organism is found in tick feces but not in large quantities in tick salivary glands. In the United States, the disease is carried by Dermacentor andersoni (Rocky Mountain wood tick), Dermacentor variabilis (American dog tick), Dermacentor occidentalis (Pacific Coast dog tick), and Amblyomma americanum (Lone Star tick). F. tularensis is transmitted frequently during blood meals taken by embedded ticks after hours of attachment. It is the taking of a blood meal through a fecally contaminated field that transmits the organism. Transmission by ticks and tabanid flies takes place mainly in the spring and summer. However, continued transmission in the winter by trapped or hunted animals has been documented.
![]() Tularemia is most common in the southeastern United States; Arkansas, Missouri, and Oklahoma account for more than half of all reported cases in this country. Small outbreaks in higher-risk populations (e.g., professional landscapers cutting up brush, mowing, and using a leaf blower) have been reported. Although the irregular distribution of cases of tularemia makes worldwide estimates difficult, increasing numbers of cases have been reported between latitudes 30° and 71°N (the Holarctic region) in the Northern Hemisphere. Cases of tularemia have been reported from Europe, Turkey, Canada, Mexico, and Asia. If the disease is caused by subspecies tularensis, the clinical manifestations are similar to those in the United States. However, in areas where tularemia is due largely to subspecies holarctica, oropharyngeal disease is common. Disease acquisition results from the consumption of water contaminated by live organisms shed by animals (e.g., muskrats, beavers). Subspecies holarctica is known to cause milder disease than other subspecies and responds well to fluoroquinolones, especially ciprofloxacin.
Tularemia is most common in the southeastern United States; Arkansas, Missouri, and Oklahoma account for more than half of all reported cases in this country. Small outbreaks in higher-risk populations (e.g., professional landscapers cutting up brush, mowing, and using a leaf blower) have been reported. Although the irregular distribution of cases of tularemia makes worldwide estimates difficult, increasing numbers of cases have been reported between latitudes 30° and 71°N (the Holarctic region) in the Northern Hemisphere. Cases of tularemia have been reported from Europe, Turkey, Canada, Mexico, and Asia. If the disease is caused by subspecies tularensis, the clinical manifestations are similar to those in the United States. However, in areas where tularemia is due largely to subspecies holarctica, oropharyngeal disease is common. Disease acquisition results from the consumption of water contaminated by live organisms shed by animals (e.g., muskrats, beavers). Subspecies holarctica is known to cause milder disease than other subspecies and responds well to fluoroquinolones, especially ciprofloxacin.
PATHOGENESIS AND PATHOLOGY
The most common portal of entry for human infection is through skin or mucous membranes, either directly—through the bite of ticks, other arthropods, or other animals—or via inapparent abrasions. Inhalation or ingestion of F. tularensis also can result in infection. F. tularensis is extremely infectious: Although >108 organisms are usually required to produce infection via the oral route (oropharyngeal or gastrointestinal tularemia), as few as 10 organisms can result in infection when injected into the skin (ulceroglandular/glandular tularemia) or inhaled (pulmonary tularemia). After inoculation into the skin, the organism multiplies locally; within 2–5 days (range, 1–10 days), it produces an erythematous, tender, or pruritic papule. The papule rapidly enlarges and forms an ulcer with a black base (chancriform lesion). The bacteria spread to regional lymph nodes, producing lymphadenopathy (buboes). All forms can lead to bacteremia with spread to distant organs, including the central nervous system.
Tularemia is characterized by mononuclear cell infiltration with pyogranulomatous pathology. The histopathologic findings can be quite similar to those in tuberculosis, although tularemia develops more rapidly. As a facultatively intracellular bacterium, F. tularensis can parasitize both phagocytic and nonphagocytic host cells and can survive intracellularly for prolonged periods. In the acute phase of infection, the primary organs affected (skin, lymph nodes, liver, and spleen) include areas of focal necrosis, which are initially surrounded by polymorphonuclear leukocytes (PMNs). Subsequently, granulomas form, with epithelioid cells, lymphocytes, and multinucleated giant cells surrounded by areas of necrosis. These areas may resemble caseation necrosis but later coalesce to form abscesses.
Conjunctival inoculation can result in infection of the eye, with regional lymph node enlargement (preauricular lymphadenopathy, Parinaud’s complex). Aerosolization and inhalation or hematogenous spread of organisms can result in pneumonia. In the lung, an inflammatory reaction develops, including foci of alveolar necrosis and cell infiltration (initially polymorphonuclear and later mononuclear) with granulomas. Chest roentgenograms usually reveal bilateral patchy infiltrates rather than large areas of consolidation. Pleural effusions are common and may contain blood. Lymphadenopathy occurs in regions draining infected organs. Therefore, in pulmonary infection, mediastinal adenopathy may be evident, whereas patients with oropharyngeal tularemia develop cervical lymphadenopathy. In gastrointestinal or typhoidal tularemia, mesenteric lymphadenopathy may follow the ingestion of large numbers of organisms. (The term typhoidal tularemia may be used to describe severe bacteremic disease, irrespective of the mode of transmission or portal of entry.) Meningitis has been reported as a primary or secondary manifestation of bacteremia. Patients may also present with fever and no localizing signs.
IMMUNOLOGY
Although a complete and widely accepted understanding of the protective immune response to F. tularensis is lacking, significant advances in the study of natural and protective immunity have been made in recent years and may ultimately result in a vaccine candidate. Complete genomic sequencing and the availability of attenuated F. tularensis strains developed through genetic manipulation are facilitating research that will expand our knowledge in this area.
A number of investigators have studied various models and proposed various hypotheses regarding the induction of protective immunity to F. tularensis. Although further research is needed, synergy between humoral and cell-mediated immune (CMI) responses appears to be critical in inducing effective immune protection. Elucidation of the molecular mechanisms for the organism’s evasion of the host response, pathogen-associated molecular patterns, and effective host immune protection has led to novel vaccination strategies tested in animal models. Antibodies to Fc receptors on antigen-presenting cells have been shown to be protective in animal models of pulmonary tularemia, resulting in both mucosal and CMI responses. This enhanced understanding of mucosal and serum antibodies in combination with a targeted CMI response holds great promise for future vaccine development.
CLINICAL MANIFESTATIONS
Tularemia often starts with a sudden onset of fever, chills, headache, and generalized myalgias and arthralgias (Table 195-1). This onset takes place when the organism penetrates the skin, is ingested, or is inhaled. An incubation period of 2–10 days is followed by the formation of an ulcer at the site of penetration, with local inflammation. The ulcer may persist for several months as organisms are transported via the lymphatics to the regional lymph nodes. These nodes enlarge and may become necrotic and suppurative. If the organism enters the bloodstream, widespread dissemination can result.
|
CLINICAL PRESENTATION OF TULAREMIA |
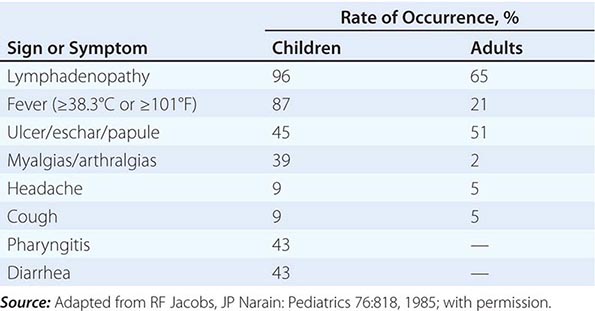
In the United States, most patients with tularemia (75–85%) acquire the infection by inoculation of the skin. In adults, the most common localized form is inguinal/femoral lymphadenopathy; in children, it is cervical lymphadenopathy. About 20% of patients develop a generalized maculopapular rash, which occasionally becomes pustular. Erythema nodosum occurs infrequently. The clinical manifestations of tularemia have been divided into various syndromes, which are listed in Table 195-2.
|
CLINICAL SYNDROMES OF TULAREMIA |
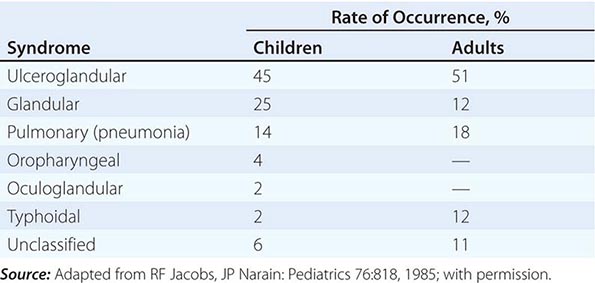
Ulceroglandular/Glandular Tularemia These two forms of tularemia account for ~75–85% of cases. The predominant form in children involves cervical or posterior auricular lymphadenopathy and is usually related to tick bites on the head and neck. In adults, the most common form is inguinal/femoral lymphadenopathy resulting from insect and tick exposures on the lower limbs. In cases related to wild game, the usual portal of entry for F. tularensis is either an injury sustained while skinning or cleaning an animal carcass or a bite (usually on the hand). Epitrochlear lymphadenopathy/lymphadenitis is common in patients with bite-related injuries.
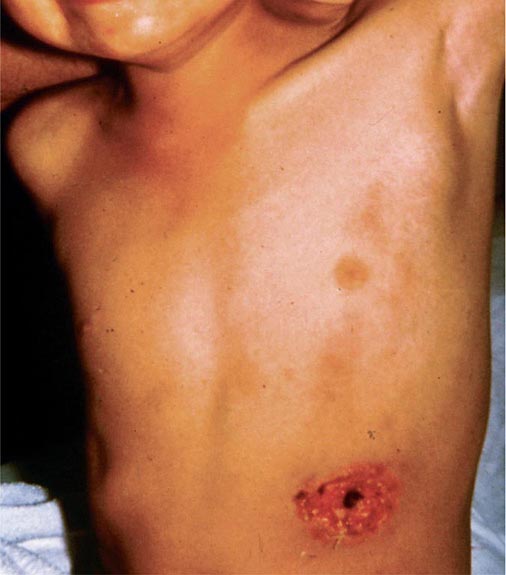
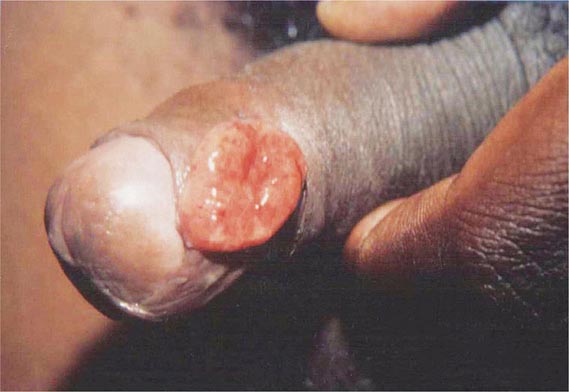
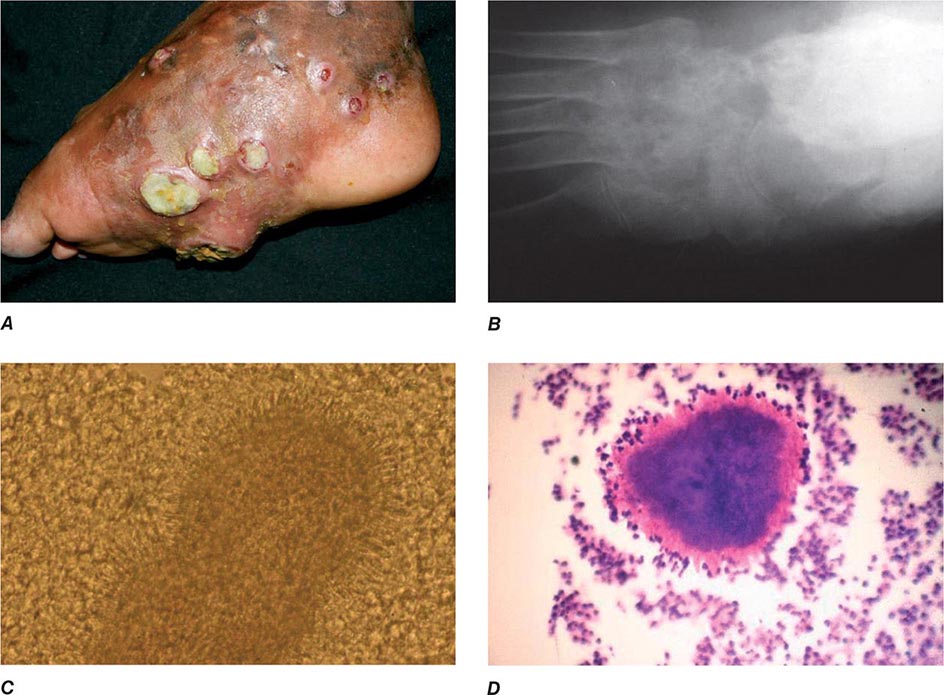
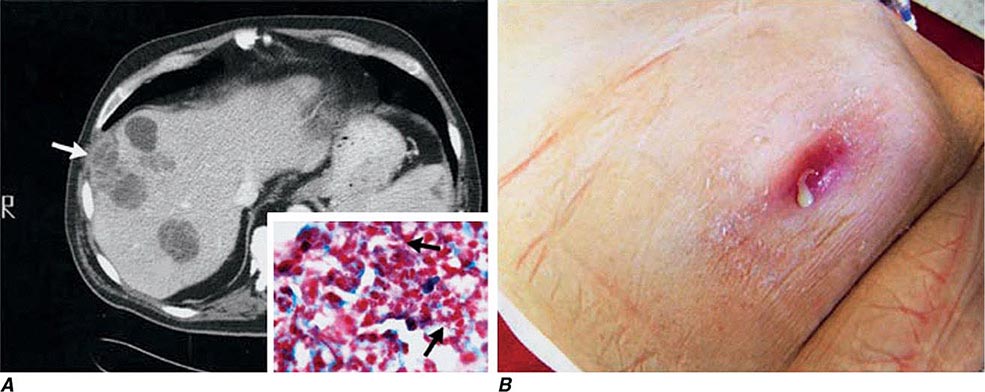
In ulceroglandular tularemia, the ulcer is erythematous, indurated, and nonhealing, with a punched-out appearance that lasts 1–3 weeks. The papule may begin as an erythematous lesion that is tender or pruritic; it evolves over several days into an ulcer with sharply demarcated edges and a yellow exudate. The ulcer gradually develops a black base, and simultaneously the regional lymph nodes become tender and severely enlarged (Fig. 195-1). The affected lymph nodes may become fluctuant and drain spontaneously, but the condition usually resolves with effective treatment. Late suppuration of lymph nodes has been described in up to 25% of patients with ulceroglandular/glandular tularemia. Examination of material taken from these late fluctuant nodes after successful antimicrobial treatment reveals sterile necrotic tissue. In 5–10% of patients, the skin lesion may be inapparent, with lymphadenopathy plus systemic signs and symptoms the only physical findings (glandular tularemia). Conversely, a tick or deerfly bite on the trunk may result in an ulcer without evident lymphadenopathy.
FIGURE 195-1 An 8-year-old boy with inguinal lymphadenitis and associated tick-bite site characteristic of ulceroglandular tularemia.
Oculoglandular Tularemia In ~1% of patients, the portal of entry for F. tularensis is the conjunctiva, which the organism usually reaches through contact with contaminated fingers. The inflamed conjunctiva is painful, with numerous yellowish nodules and pinpoint ulcers. Purulent conjunctivitis with regional lymphadenopathy (preauricular, submandibular, or cervical) is evident. Because of debilitating pain, the patient may seek medical attention before regional lymphadenopathy develops. Painful preauricular lymphadenopathy is unique to tularemia and distinguishes it from tuberculosis, sporotrichosis, and syphilis. Corneal perforation may occur.
Oropharyngeal and Gastrointestinal Tularemia Rarely, tularemia follows ingestion of contaminated undercooked meat, oral inoculation of F. tularensis from the hands in association with the skinning and cleaning of animal carcasses, or consumption of contaminated food or water. Oral inoculation may result in acute, exudative, or membranous pharyngitis associated with cervical lymphadenopathy or in ulcerative intestinal lesions associated with mesenteric lymphadenopathy, diarrhea, abdominal pain, nausea, vomiting, and gastrointestinal bleeding. Infected tonsils become enlarged and develop a yellowish-white pseudomembrane, which can be confused with that of diphtheria. The clinical severity of gastrointestinal tularemia varies from mild, unexplained, persistent diarrhea with no other symptoms to a fulminant, fatal disease. In fatal cases, the extensive intestinal ulceration found at autopsy suggests an enormous inoculum.
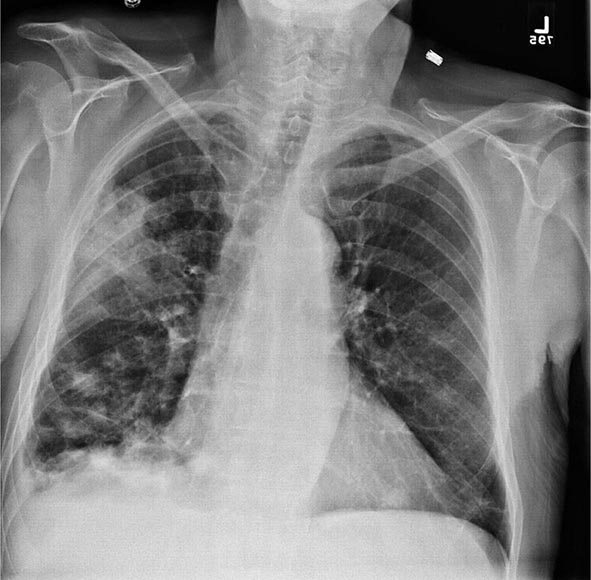
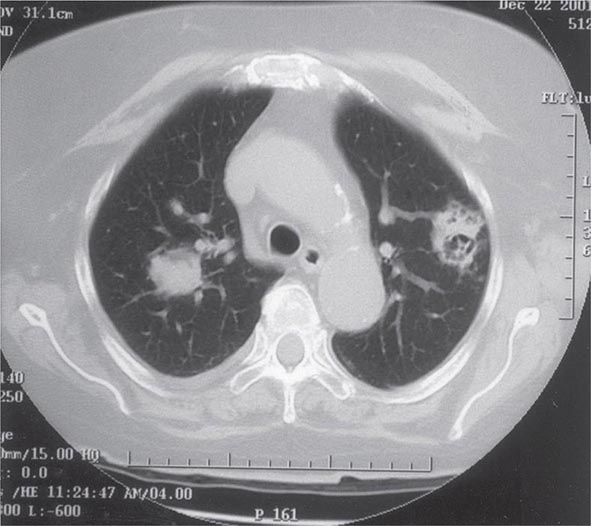
Pulmonary Tularemia Pneumonia due to F. tularensis presents as variable parenchymal infiltrates that are unresponsive to treatment with β-lactam antibiotics. Tularemia must be considered in the differential diagnosis of atypical pneumonia in a patient with a history of travel to an endemic area. The disease can result from inhalation of an infectious aerosol or can spread to the lungs and pleura via bacteremia. Inhalation-related pneumonia has been described in laboratory workers after exposure to contaminated materials and, if untreated, can be associated with a relatively high mortality rate. Exposure to F. tularensis in aerosols from live domestic animals or dead wildlife (including birds) has been reported to cause pneumonia. Hematogenous dissemination to the lungs occurs in 10–15% of cases of ulceroglandular tularemia and in about half of cases of typhoidal tularemia. Previously, tularemia pneumonia was thought to be a disease of older patients, but as many as 10–15% of children with clinical manifestations of tularemia have parenchymal infiltrates detected by chest roentgenography. Patients with pneumonia usually have a nonproductive cough and may have dyspnea or pleuritic chest pain. Roentgenograms of the chest usually reveal bilateral patchy infiltrates (described as ovoid or lobar densities), lobar parenchymal infiltrates, and cavitary lesions. Pleural effusions may have a predominance of mononuclear leukocytes or PMNs and sometimes red blood cells. Empyema may develop. Blood cultures may be positive for F. tularensis.
Typhoidal Tularemia The typhoidal presentation is now considered rare in the United States. The source of infection in typhoidal tularemia is usually associated with pharyngeal and/or gastrointestinal inoculation or bacteremic disease. Fever usually develops without apparent skin lesions or lymphadenopathy. Some patients have cervical and mesenteric lymphadenopathy. In the absence of a history of possible contact with a vector, diagnosis can be extremely difficult. Blood cultures may be positive and patients may present with classic sepsis or septic shock in this acute systemic form of the infection. Typhoidal tularemia is usually associated with a huge inoculum or with a preexisting compromising condition. High continuous fevers, signs of sepsis, and severe headache are common. The patient may be delirious and may develop prostration and shock. If presumptive antibiotic therapy in culture-negative cases does not include an aminoglycoside, the estimated mortality rate is relatively high.
Other Manifestations F. tularensis infection has been associated with meningitis, pericarditis, hepatitis, peritonitis, endocarditis, osteomyelitis, and sepsis and septic shock with rhabdomyolysis and acute renal failure. In cases of tularemia meningitis, a mean white blood cell count of 1788/μL, a predominantly mononuclear cell response (70–100%), a depressed glucose level, an elevated protein concentration, and a negative Gram’s stain are typically found on examination of cerebrospinal fluid.
DIFFERENTIAL DIAGNOSIS
When patients in endemic areas present with fever, chronic ulcerative skin lesions, and large tender lymph nodes (Fig. 195-1), a diagnosis of tularemia should be made presumptively, and confirmatory diagnostic testing and appropriate therapy should be undertaken. When the possibility of tularemia is considered in a nonendemic area, an attempt should be made to identify contact with a potential animal vector. The level of suspicion should be especially high in hunters, trappers, game wardens, professional landscapers, veterinarians, laboratory workers, and individuals exposed to an insect or another animal vector. However, up to 40% of patients with tularemia have no known history of epidemiologic contact with an animal vector.
The characteristic presentation of ulceroglandular tularemia does not pose a diagnostic problem, but a less classic progression of regional lymphadenopathy or glandular tularemia must be differentiated from other diseases (Table 195-3). The skin lesion of tularemia may resemble those seen in various other diseases but is generally accompanied by more impressive regional lymphadenopathy. In children, the differentiation of tularemia from cat-scratch disease is made more difficult by the chronic papulovesicular lesion associated with Bartonella henselae infection (Chap. 197). Oropharyngeal tularemia can resemble and must be differentiated from pharyngitis due to other bacteria or viruses. Pulmonary tularemia may resemble any atypical pneumonia. Typhoidal tularemia and tularemia meningitis may resemble a variety of other infections.
|
TULAREMIA: DIFFERENTIAL DIAGNOSIS, BY CLINICAL DISEASE CATEGORY |
LABORATORY DIAGNOSIS
The diagnosis of tularemia is most frequently confirmed by agglutination testing. Microagglutination and tube agglutination are the techniques most commonly used to detect antibody to F. tularensis. In the standard tube agglutination test, a single titer of ≥1:160 is interpreted as a presumptive positive result. A fourfold increase in titer between paired serum samples collected 2–3 weeks apart is considered diagnostic. False-negative serologic responses are obtained early in infection; up to 30% of patients infected for 3 weeks have sera that test negative. Late in infection, titers into the thousands are common, and titers of 1:20–1:80 may persist for years. Enzyme-linked immunosorbent assays have proved useful for the detection of both antibodies and antigens.
Culture and isolation of F. tularensis are difficult. In one study, the organism was isolated in only 10% of more than 1000 human cases, 84% of which were confirmed by serology. The medium of choice is cysteine-glucose-blood agar. F. tularensis can be isolated directly from infected ulcer scrapings, lymph node biopsy specimens, gastric washings, sputum, and blood cultures. Colonies are blue-gray, round, smooth, and slightly mucoid. On media containing blood, a small zone of α hemolysis usually surrounds the colony. Slide agglutination tests or direct fluorescent antibody tests with commercially available antisera can be applied directly to culture suspensions for identification. Most clinical laboratories will not attempt to culture F. tularensis because of the infectivity of the organism from the culture media and the consequent risk of a laboratory-acquired infection. Although tularemia is not spread from person to person, the organism can be inhaled from culture plates and infect unsuspecting laboratory workers. In most clinical laboratories, biosafety level 2 practices are recommended to handle clinical specimens thought to contain F. tularensis; however, biosafety level 3 conditions are required for procedures that produce aerosols or droplets during manipulation of cultures containing or possibly containing this organism.
A variety of polymerase chain reaction (PCR) methods have been used to detect F. tularensis DNA in many clinical specimens but mostly in ulceroglandular disease. The majority of these methods target the genes encoding outer-membrane proteins (e.g., fopA or tul4). A 16S rDNA sequence identification PCR may be helpful when the patient’s clinical information does not lead the clinician to suspect a diagnosis of tularemia.
|
TREATMENT |
TULAREMIA |
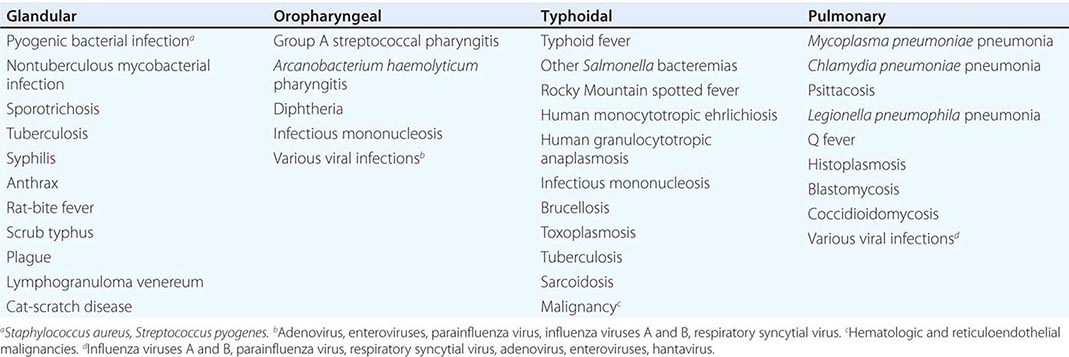
Only aminoglycosides, tetracyclines, chloramphenicol, and rifampin are currently approved by the U.S. Food and Drug Administration for the treatment of tularemia. Gentamicin is considered the drug of choice for both adults and children. The dosage for adults and children is 5 mg/kg daily in two divided doses. Gentamicin therapy is typically continued for 7–10 days; however, in mild to moderate cases of tularemia in which the patient becomes afebrile within the first 48–72 h of gentamicin treatment, a 5- to 7-day course has been successful.
If available, streptomycin given intramuscularly is also effective. The dosage for adults is 2 g/d in two divided doses. For children, the dosage is 30 mg/kg daily in two divided doses (maximal daily dose, 2 g). After a clinical response is demonstrated at 3–5 days, the dosage for children can be reduced to 10–15 mg/kg daily in two divided doses. The total duration of streptomycin therapy in both adults and children is usually 10 days. Unlike streptomycin and gentamicin, tobramycin is ineffective in the treatment of tularemia and should not be used.
Because doxycycline is bacteriostatic against F. tularensis, there is a risk of relapse if the patient is not treated for a long enough period. Therefore, if doxycycline is used, it should be given for at least 14 days. The lack of availability of chloramphenicol limits the utility of this agent as a viable treatment option. Fluoroquinolones—specifically, ciprofloxacin and levofloxacin—have been used with good outcomes to treat infections caused by subspecies holarctica, which is most often found in Europe. The lack of data on the efficacy of these agents against subspecies tularensis limits their use in North America at this time.
F. tularensis cannot be subjected to standardized antimicrobial susceptibility testing because the organism will not grow on the media used. A wide variety of antibiotics, including all β-lactam antibiotics and the cephalosporins, are ineffective for the treatment of tularemia. Several studies indicated that third-generation cephalosporins were active against F. tularensis in vitro, but clinical case reports suggested nearly universal failure of ceftriaxone in pediatric patients with tularemia. Although in vitro data indicate that imipenem may be active, therapy with imipenem, sulfanilamides, and macrolides is not presently recommended because of the lack of relevant clinical data.
Virtually all strains of F. tularensis are susceptible to streptomycin and gentamicin. Hearing screening should be considered before initiation of streptomycin or gentamicin therapy. In successfully treated patients, defervescence usually occurs within 2 days, but skin lesions and lymph nodes may take 1–2 weeks to heal. When therapy is not initiated within the first several days of illness, defervescence may be delayed. Relapses are uncommon with streptomycin or gentamicin therapy. Late lymph-node suppuration, however, occurs in ~40% of children, regardless of the treatment received. These nodes have typically been found to contain sterile necrotic tissue without evidence of active infection. Patients with fluctuant nodes should receive several days of antibiotic therapy before drainage to minimize the risk to hospital personnel.
PROGNOSIS
If tularemia goes untreated, symptoms usually last 1–4 weeks but may continue for months. The mortality rate from severe untreated infection (including all cases of untreated pulmonary and typhoidal tularemia) can be as high as 30%. However, the overall mortality rate for untreated tularemia is <8%. With appropriate treatment, the mortality rate is <1%. Poor outcomes are often associated with long delays in diagnosis and treatment. Lifelong immunity usually follows tularemia.
PREVENTION
The prevention of tularemia is based on avoidance of exposure to biting and blood-sucking insects, especially ticks and deerflies. A wide range of approaches to vaccine development are being evaluated, but no vaccine against tularemia is yet licensed. Prophylaxis of tularemia has not proved effective in patients with embedded ticks or insect bites. However, in patients who are known to have been exposed to large quantities of organisms (e.g., in the laboratory) and who have incubating infection with F. tularensis, early treatment can prevent the development of significant clinical disease.
196 |
Plague and Other Yersinia Infections |
PLAGUE
Plague is a systemic zoonosis caused by Yersinia pestis. It predominantly affects small rodents in rural areas of Africa, Asia, and the Americas and is usually transmitted to humans by an arthropod vector (the flea). Less often, infection follows contact with animal tissues or respiratory droplets. Plague is an acute febrile illness that is treatable with antimicrobial agents, but mortality rates among untreated patients are high. Ancient DNA studies have confirmed that the fourteenth-century “Black Death” in Europe was Y. pestis infection. Patients can present with the bubonic, septicemic, or pneumonic form of the disease. Although there is concern among the general public about epidemic spread of plague by the respiratory route, this is not the usual route of plague transmission, and established infection-control measures for respiratory plague exist. However, the fatalities associated with plague and the capacity for infection via the respiratory tract mean that Y. pestis fits the profile of a potential agent of bioterrorism. Consequently, measures have been taken to restrict access to the organism, including legislation affecting diagnostic and research procedures in some countries (e.g., the United States).
ETIOLOGY
The genus Yersinia comprises gram-negative bacteria of the family Enterobacteriaceae (gamma proteobacteria). Overwhelming taxonomic evidence showing Y. pestis strains as a clonal group within Yersinia pseudotuberculosis suggests recent evolution from the latter organism—an enteric pathogen of mammals that is spread by the fecal-oral route and thus has a phenotype distinctly different from that of Y. pestis. When grown in vivo or at 37°C, Y. pestis forms an amorphous capsule made from a plasmid-specified fimbrial protein, Caf or fraction 1 (F1) antigen, which is an immunodiagnostic marker of infection.
EPIDEMIOLOGY
Human plague generally follows an outbreak in a host rodent population (epizootic). Mass deaths among the rodent primary hosts lead to a search by fleas for new hosts, with consequent incidental infection of other mammals. The precipitating cause for an epizootic may ultimately be related to climate or other environmental factors. The reservoir for Y. pestis causing enzootic plague in natural endemic foci between epizootics (i.e., when the organism may be difficult to detect in rodents or fleas) is a topic of ongoing research and may not be the same in all regions. The enzootic/epizootic pattern may be the result of complex dynamic interactions of host rodents that have different plague susceptibilities and different flea vectors; alternatively, an environmental reservoir may be important.
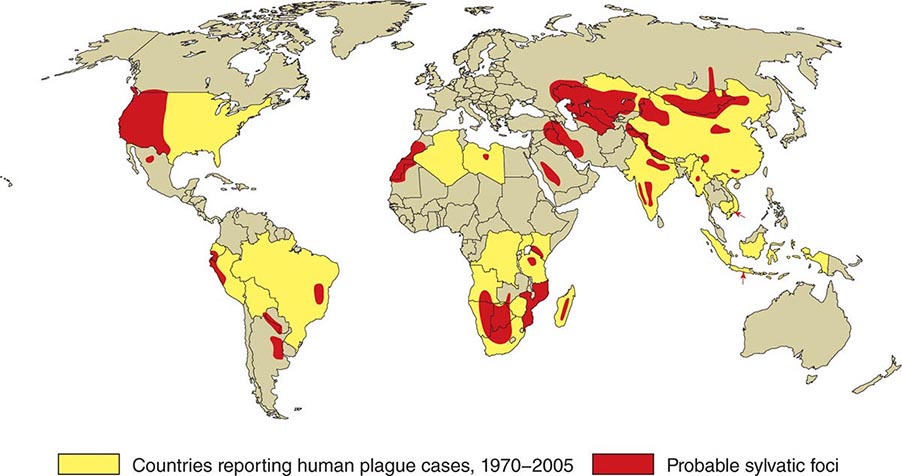
![]() In general, the enzootic areas for plague are lightly populated regions of Africa, Asia, and the Americas (Fig. 196-1). Between 2004 and 2009, 12,503 cases of plague, with a global case-fatality rate of 6.7%, were recorded by the World Health Organization (WHO); these figures were obtained by combining cases notified under the International Health Regulations with data from national surveillance programs and publications. More than 97% of these cases were in Africa; the majority of cases were reported from the Democratic Republic of the Congo and the island of Madagascar. The period covered spans a change in the International Health Regulations from a requirement for nations to notify the WHO of all cases of plague to a requirement to report pneumonic plague or any suspected case of plague in an area not known to be endemic for plague. In the past decade, outbreaks of pneumonic plague have been recorded in the Democratic Republic of the Congo, Uganda, Algeria, Madagascar, China, and Peru.
In general, the enzootic areas for plague are lightly populated regions of Africa, Asia, and the Americas (Fig. 196-1). Between 2004 and 2009, 12,503 cases of plague, with a global case-fatality rate of 6.7%, were recorded by the World Health Organization (WHO); these figures were obtained by combining cases notified under the International Health Regulations with data from national surveillance programs and publications. More than 97% of these cases were in Africa; the majority of cases were reported from the Democratic Republic of the Congo and the island of Madagascar. The period covered spans a change in the International Health Regulations from a requirement for nations to notify the WHO of all cases of plague to a requirement to report pneumonic plague or any suspected case of plague in an area not known to be endemic for plague. In the past decade, outbreaks of pneumonic plague have been recorded in the Democratic Republic of the Congo, Uganda, Algeria, Madagascar, China, and Peru.
FIGURE 196-1 Approximate global distribution of Yersinia pestis. (Compiled from WHO, CDC, and country sources. Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
Plague was introduced into North America via the port of San Francisco in 1900 as part of the Third Pandemic, which spread around the world from Hong Kong. The disease is presently enzootic on the western side of the continent from southwestern Canada to Mexico. Most human cases in the United States occur in two regions: “Four Corners” (the junction point of New Mexico, Arizona, Colorado, and Utah), especially northern New Mexico, northern Arizona, and southern Colorado; and further west in California, southern Oregon, and western Nevada (http://www.cdc.gov/plague/maps/index.html). From 1990 to 2011, 151 cases of plague were reported in the United States, a mean of seven cases per year. Most cases occurred from May to October—the time of year when people are outdoors and rodents and their fleas are most plentiful. The infection is most often acquired by fleabite in peridomestic environments; it can also be acquired through the handling of living or dead small mammals (e.g., rabbits, hares, and prairie dogs) or wild carnivores (e.g., wildcats, coyotes, or mountain lions). Dogs and cats may bring plague-infected fleas into the home, and infected cats may transmit plague directly to humans by the respiratory route. The last recorded case of person-to-person transmission in the United States occurred in 1925.
Plague most often develops in areas with poor sanitary conditions and infestations of rats—in particular, the widely distributed roof rat Rattus rattus and the brown rat Rattus norvegicus (which serves as a laboratory model of plague). Rat control in warehouses and shipping facilities has been recognized as important in preventing the spread of plague since the early twentieth century and features in the current WHO International Health Regulations. Urban rodents acquire infection from wild rodents, and the proximity of the former to humans increases the risk of transmission. The oriental rat flea Xenopsylla cheopis is the most efficient vector for transmission of plague among rats and onward to humans in Asia, Africa, and South America.
Worldwide, bubonic plague is the predominant form reported (80–95% of suspected cases), with mortality rates of 10–20%. The mortality rate is higher (22%) in the small proportion of patients (10–20%) with primary septicemic plague (i.e., systemic Y. pestis sepsis with no bubo; see “Clinical Manifestations,” below) and is highest with primary pulmonary plague; in this, the least common of the main plague presentations, the mortality rate approaches 100% without antimicrobial treatment and is >50% even with such treatment. Rare outbreaks of pharyngeal plague following consumption of raw or undercooked camel or goat meat have been reported.
A total of 81 (76%) of the 107 plague cases reported in the United States from 1990 to 2005 were primary bubonic disease, 19 (18%) were primary septicemic disease, and 5 (5%) were primary pneumonic disease; 2 cases (2%) were not classified. Eleven cases (10%) were fatal.
PATHOGENESIS
![]() As mentioned earlier, genetic evidence suggests that Y. pestis is a clone derived from the enteric pathogen Y. pseudotuberculosis in the recent evolutionary past (9000–40,000 years ago). The change from infection by the fecal-oral route to a two-stage life cycle, with alternate parasitization of arthropod and mammalian hosts, followed the acquisition of two plasmids (pFra and pPst) and the inactivation of remarkably few Y. pseudotuberculosis genes in conjunction with preexisting properties of the Y. pseudotuberculosis ancestor (e.g., the presence of a third plasmid, pYV, and the capacity to cause septicemia). In the arthropod-parasitizing portion of its life cycle, Y. pestis multiplies and forms biofilm-embedded aggregates in the flea midgut after ingestion of a blood meal containing bacteria. In some fleas, biofilm-embedded bacteria eventually fill the proventriculus (a valve connecting the esophagus to the midgut) and block normal blood feeding. Both “blocked” fleas and those containing masses of biofilm-embedded Y. pestis without complete blockage inoculate Y. pestis into each bite site. The ability of Y. pestis to colonize and multiply in the flea requires phospholipase D encoded by the ymt gene on the pFra plasmid, and biofilm synthesis requires the chromosomal hms locus shared with Y. pseudotuberculosis. However, three Y. pseudotuberculosis genes inhibiting biofilm formation or promoting its degradation are inactivated in Y. pestis, together with urease, which causes acute flea gastrointestinal toxicity. Blockage takes days or weeks to come about after initial infection of the flea and is followed by the flea’s death. In addition, many flea vectors (including X. cheopis) are able to transmit plague in an early-phase unblocked state for up to 1 week after feeding, but 10 fleas in this state are required to infect a mammalian host (mass transmission).
As mentioned earlier, genetic evidence suggests that Y. pestis is a clone derived from the enteric pathogen Y. pseudotuberculosis in the recent evolutionary past (9000–40,000 years ago). The change from infection by the fecal-oral route to a two-stage life cycle, with alternate parasitization of arthropod and mammalian hosts, followed the acquisition of two plasmids (pFra and pPst) and the inactivation of remarkably few Y. pseudotuberculosis genes in conjunction with preexisting properties of the Y. pseudotuberculosis ancestor (e.g., the presence of a third plasmid, pYV, and the capacity to cause septicemia). In the arthropod-parasitizing portion of its life cycle, Y. pestis multiplies and forms biofilm-embedded aggregates in the flea midgut after ingestion of a blood meal containing bacteria. In some fleas, biofilm-embedded bacteria eventually fill the proventriculus (a valve connecting the esophagus to the midgut) and block normal blood feeding. Both “blocked” fleas and those containing masses of biofilm-embedded Y. pestis without complete blockage inoculate Y. pestis into each bite site. The ability of Y. pestis to colonize and multiply in the flea requires phospholipase D encoded by the ymt gene on the pFra plasmid, and biofilm synthesis requires the chromosomal hms locus shared with Y. pseudotuberculosis. However, three Y. pseudotuberculosis genes inhibiting biofilm formation or promoting its degradation are inactivated in Y. pestis, together with urease, which causes acute flea gastrointestinal toxicity. Blockage takes days or weeks to come about after initial infection of the flea and is followed by the flea’s death. In addition, many flea vectors (including X. cheopis) are able to transmit plague in an early-phase unblocked state for up to 1 week after feeding, but 10 fleas in this state are required to infect a mammalian host (mass transmission).
Y. pestis disseminates from the site of inoculation in the mammalian host in a process initially dependent on plasminogen activator Pla, which is encoded by the small pPst plasmid. This surface protease activates mammalian plasminogen, degrades complement, and adheres to the extracellular matrix component laminin. Pla is essential for the high-level virulence of Y. pestis in mice by subcutaneous or intradermal injection (laboratory proxies for fleabites) and for the development of primary pneumonic plague. When actual fleabite inoculation is used in mouse models, the fimbrial capsule-forming protein (Ca1 or fraction 1; F1 antigen) encoded on pFra increases the efficiency of transmission, and plasminogen activator is required for the formation of buboes. Because the antiphagocytic systems in Y. pestis are not fully operational at the time of inoculation into the mammalian host, the organism is taken up by macrophages from the inoculation site and transported to regional lymph nodes. After intracellular replication, Y. pestis switches to extracellular replication with full expression of its antiphagocytic systems: the type III secretion machines and their effectors encoded by pYV as well as the F1 capsule. Overproduction of the type III secretion substrate and translocation protein LcrV exerts an anti-inflammatory effect, reducing host immune responses. Likewise, Y. pestis lipopolysaccharide is modified to minimize stimulation of host Toll-like receptor 4, thereby reducing protective host inflammatory responses during peripheral infection and prolonging host survival with high-grade bacteremia—an effect that probably enhances the pathogen’s subsequent transmission by fleabite.
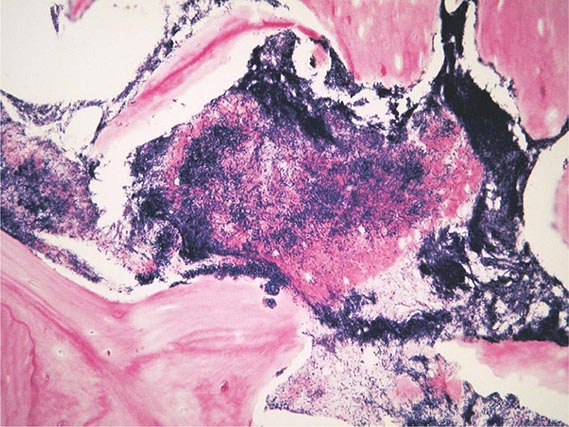
Replication of Y. pestis in a regional lymph node results in the local swelling of the lymph node and periglandular region known as a bubo. On histology, the node is found to be hemorrhagic or necrotic, with thrombosed blood vessels, and the lymphoid cells and normal architecture are replaced by large numbers of bacteria and fibrin. Periglandular tissues are inflamed and also contain large numbers of bacteria in a serosanguineous, gelatinous exudate.
Continued spread through the lymphatic vessels to contiguous lymph nodes produces second-order primary buboes. Infection is initially contained in the infected regional lymph nodes, although transient bacteremia can be detected. As the infection progresses, spread via efferent lymphatics to the thoracic duct produces high-grade bacteremia. Hematogenous spread to the spleen, liver, and secondary buboes follows, with subsequent uncontrolled septicemia, endotoxic shock, and disseminated intravascular coagulation leading to death. In some patients, this septicemic phase occurs without obvious prior bubo development or lung disease (septicemic plague). Hematogenous spread to the lungs results in secondary plague pneumonia, with bacteria initially more prominent in the interstitium than in the air spaces (the reverse being the case in primary plague pneumonia). Hematogenous spread to other organs, including the meninges, can occur.
CLINICAL MANIFESTATIONS
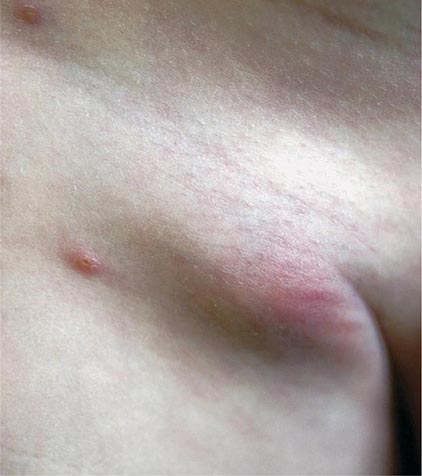
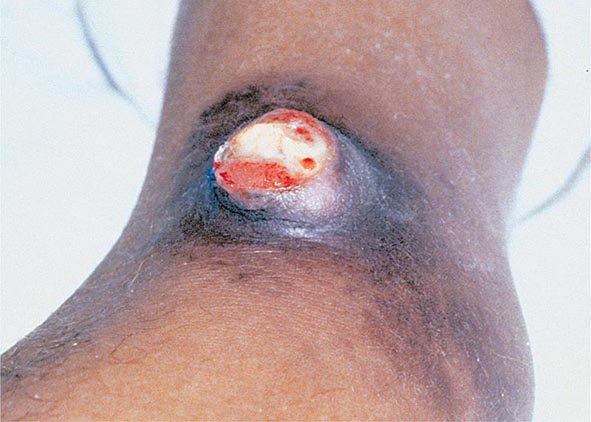
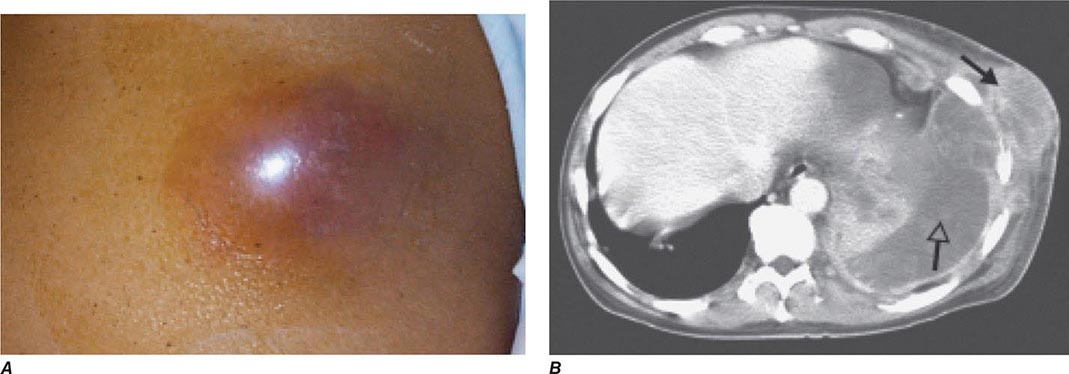
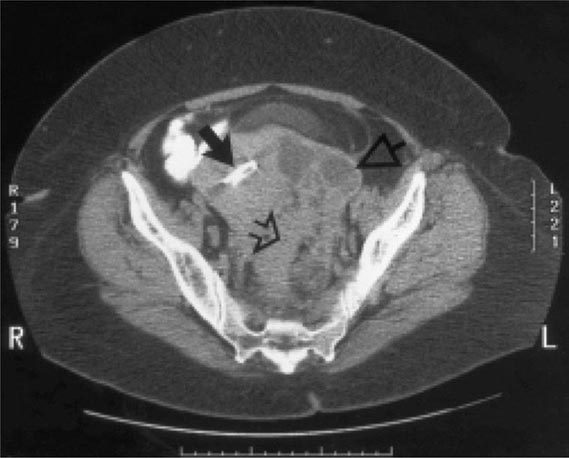
Bubonic Plague After an incubation period of 2–6 days, the onset of bubonic plague is sudden and is characterized by fever (>38°C), malaise, myalgia, dizziness, and increasing pain due to progressive lymphadenitis in the regional lymph nodes near the fleabite or other inoculation site. Lymphadenitis manifests as a tense, tender swelling (bubo) that, when palpated, has a boggy consistency with an underlying hard core. Generally, there is one painful and erythematous bubo with surrounding periganglionic edema. The bubo is most commonly inguinal but can also be crural, axillary (Fig. 196-2), cervical, or submaxillary, depending on the site of the bite. Abdominal pain from intraabdominal node involvement can occur without other visible signs. Children are most likely to present with cervical or axillary buboes.
FIGURE 196-2 Plague patient in the southwestern United States with a left axillary bubo and an unusual plague ulcer and eschar at the site of the infective flea bite. (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
The differential diagnosis includes acute focal lymphadenopathy of other etiologies, such as streptococcal or staphylococcal infection, tularemia, cat-scratch disease, tick typhus, infectious mononucleosis, or lymphatic filariasis. These infections do not progress as rapidly, are not as painful, and are associated with visible cellulitis or ascending lymphangitis—both of which are absent in plague.
Without treatment, Y. pestis dissemination occurs and causes serious illness, including pneumonia (secondary pneumonic plague) and meningitis. Secondary pneumonic plague can be the source of person-to-person transmission of respiratory infection by productive cough (droplet infection), with the consequent development of primary plague pneumonia. Appropriate treatment of bubonic plague results in fever resolution within 2–5 days, but buboes may remain enlarged for >1 week after initial treatment and can become fluctuant.
Primary Septicemic Plague A minority (10–25%) of infections with Y. pestis present as gram-negative septicemia (hypotension, shock) without preceding lymphadenopathy. Septicemic plague occurs in all age groups, but persons older than age 40 years are at elevated risk. Some chronic conditions may predispose to septicemic plague: in 2009 in the United States, a fatal laboratory-acquired infection with an attenuated Y. pestis strain manifested as septicemic plague in a 60-year-old researcher with diabetes mellitus and undiagnosed hemochromatosis. These conditions also carry an increased risk of septicemia with other pathogenic Yersinia species. The term septicemic plague can be confusing since most patients with buboes have detectable bacteremia at some stage, with or without systemic signs of sepsis. In laboratory experiments, however, septicemic disease without histologic changes in lymph nodes is seen in a minority of mice infected via fleabites.
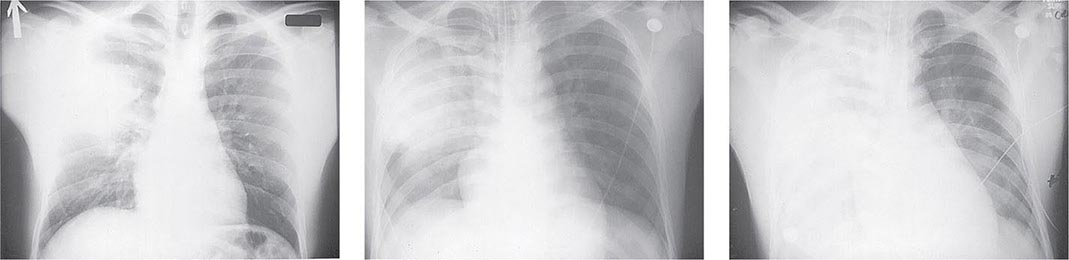
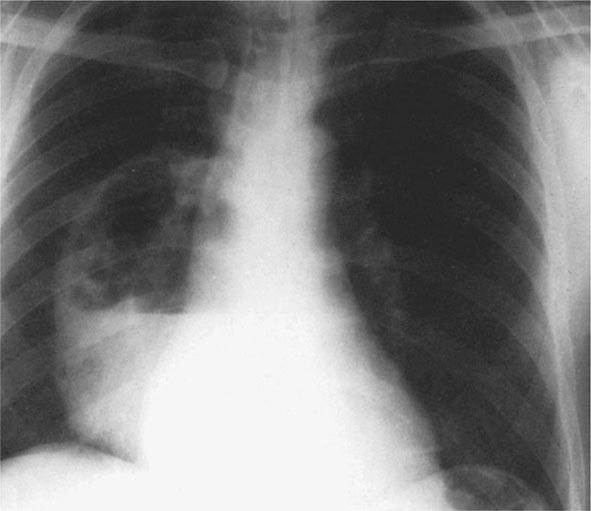
Pneumonic Plague Primary pneumonic plague results from inhalation of infectious bacteria in droplets expelled from another person or an animal with primary or secondary plague pneumonia. This syndrome has a short incubation period, averaging from a few hours to 2–3 days (range, 1–7 days), and is characterized by a sudden onset of fever, headache, myalgia, weakness, nausea, vomiting, and dizziness. Respiratory signs—cough, dyspnea, chest pain, and sputum production with hemoptysis—typically arise after 24 h. Progression of initial segmental pneumonitis to lobar pneumonia and then to bilateral lung involvement may occur (Fig. 196-3). The possible release of aerosolized Y. pestis bacteria in a bioterrorist attack, manifesting as an outbreak of primary pneumonic plague in nonendemic regions or in an urban setting where plague is rarely seen, has been a source of public health concern. Secondary pneumonic plague is a consequence of bacteremia occurring in ~10–15% of patients with bubonic plague. Bilateral alveolar infiltrates are seen on chest x-ray, and diffuse interstitial pneumonitis with scanty sputum production is typical.
FIGURE 196-3 Sequential chest radiographs of a patient with fatal primary plague pneumonia. Left: Upright posteroanterior film taken at admission to the hospital emergency department on the third day of illness, showing segmental consolidation of the right upper lobe. Center: Portable anteroposterior film taken 8 h after admission, showing extension of pneumonia to the right middle and right lower lobes. Right: Portable anteroposterior film taken 13 h after admission (when the patient had clinical adult respiratory distress syndrome), showing diffuse infiltration throughout the right lung and patchy infiltration of the left lower lung. A cavity later developed at the site of the initial right-upper-lobe consolidation. (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed., AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
Meningitis Meningeal plague is uncommon, occurring in ≤6% of plague cases reported in the United States. Presentation with headache and fever typically occurs >1 week after the onset of bubonic or septicemic plague and may be associated with suboptimal antimicrobial therapy (delayed therapy, penicillin administration, or low-dose tetracycline treatment) and cervical or axillary buboes.
Pharyngitis Symptomatic plague pharyngitis can follow the consumption of contaminated meat from an animal dying of plague or contact with persons or animals with pneumonic plague. This condition can resemble tonsillitis, with peritonsillar abscess and cervical lymphadenopathy. Asymptomatic pharyngeal carriage of Y. pestis can also occur in close contacts of patients with pneumonic plague.
LABORATORY DIAGNOSIS
![]() Because of the scarcity of laboratory facilities in regions where human Y. pestis infection is most common, and because of the potential significance of Y. pestis isolation in a nonendemic area or an area from which human plague has been absent for many years, the WHO recommends an initial presumptive diagnosis followed by reference laboratory confirmation (Table 196-1). In the United States, comprehensive national diagnostic facilities for plague have been in place since a federal Laboratory Response Network (LRN; www.bt.cdc.gov/lrn/) was set up in 1999 to detect possible use of biological terrorism agents, including Y. pestis. Routine diagnostic clinical microbiology laboratories that are included in this network as sentinel-level laboratories use joint protocols from the Centers for Disease Control and Prevention (CDC) and the American Society for Microbiology to identify suspected Y. pestis isolates and to refer these specimens to LRN reference laboratories for confirmatory tests (http://www.asm.org/index.php/issues/sentinel-laboratory-guidelines). Y. pestis is designated a “Tier 1 select agent” under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 and subsequent executive orders; the provisions of this act, the Patriot Act of 2001, and related executive orders apply to all U.S. laboratories and individuals working with Y. pestis. Details of the applicable regulations are available from the CDC.
Because of the scarcity of laboratory facilities in regions where human Y. pestis infection is most common, and because of the potential significance of Y. pestis isolation in a nonendemic area or an area from which human plague has been absent for many years, the WHO recommends an initial presumptive diagnosis followed by reference laboratory confirmation (Table 196-1). In the United States, comprehensive national diagnostic facilities for plague have been in place since a federal Laboratory Response Network (LRN; www.bt.cdc.gov/lrn/) was set up in 1999 to detect possible use of biological terrorism agents, including Y. pestis. Routine diagnostic clinical microbiology laboratories that are included in this network as sentinel-level laboratories use joint protocols from the Centers for Disease Control and Prevention (CDC) and the American Society for Microbiology to identify suspected Y. pestis isolates and to refer these specimens to LRN reference laboratories for confirmatory tests (http://www.asm.org/index.php/issues/sentinel-laboratory-guidelines). Y. pestis is designated a “Tier 1 select agent” under the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 and subsequent executive orders; the provisions of this act, the Patriot Act of 2001, and related executive orders apply to all U.S. laboratories and individuals working with Y. pestis. Details of the applicable regulations are available from the CDC.
|
WORLD HEALTH ORGANIZATION CASE DEFINITIONS OF PLAGUE |

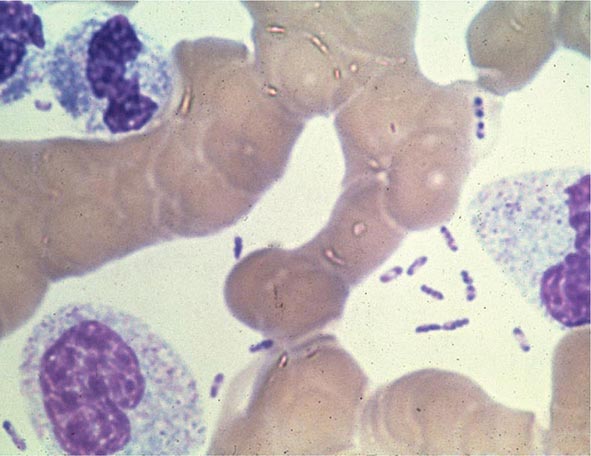
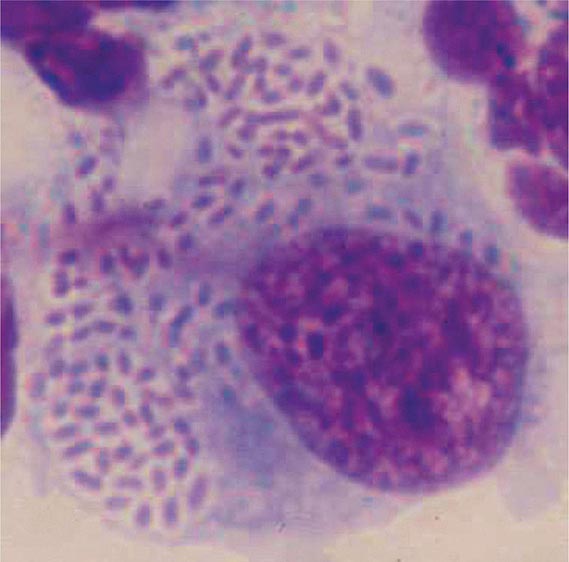
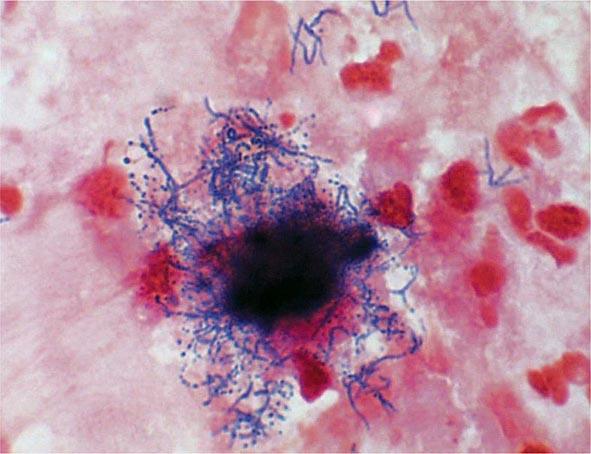
Yersinia species are gram-negative coccobacilli (short rods with rounded ends) 1–3 μm in length and 0.5–0.8 μm in diameter. Y. pestis in particular appears bipolar (with a “closed safety pin” appearance) and pleomorphic when stained with a polychromatic stain (Wayson or Wright-Giemsa; Fig. 196-4). Its lack of motility distinguishes Y. pestis from other Yersinia species, which are motile at 25°C and nonmotile at 37°C. Transport medium (e.g., Cary-Blair medium) preserves the viability of Y. pestis if transport is delayed.
FIGURE 196-4 Peripheral blood smear from a patient with fatal plague septicemia and shock, showing characteristic bipolar-staining Yersinia pestis bacilli (Wright’s stain, oil immersion). (Reprinted with permission from DT Dennis, GL Campbell: Plague and other Yersinia infections, in Harrison’s Principles of Internal Medicine, 17th ed, AS Fauci et al [eds]. New York, McGraw-Hill, Chap. 152, 2008.)
The appropriate specimens for diagnosis of bubonic, pneumonic, and septicemic plague are bubo aspirate, bronchoalveolar lavage fluid or sputum, and blood, respectively. Culture of postmortem organ biopsy samples can also be diagnostic. A bubo aspirate is obtained by injection of 1 mL of sterile normal saline into a bubo under local anesthetic and aspiration of a small amount of (usually blood-stained) fluid. Gram’s staining of these specimens may reveal gram-negative rods, which are shown by Wayson or Wright-Giemsa staining to be bipolar. These bacteria may even be visible in direct blood smears in septicemic plague (Fig. 196-4); this finding indicates very high numbers of circulating bacteria and a poor prognosis.
Y. pestis grows on nutrient agar and other standard laboratory media but forms smaller colonies than do other Enterobacteriaceae. Specimens should be inoculated onto nutrient-rich media such as sheep blood agar (SBA), into nutrient-rich broth such as brain-heart infusion broth, and onto selective agar such as MacConkey or eosin methylene blue (EMB) agar. Yersinia-specific CIN (cefsulodin, triclosan [Irgasan], novobiocin) agar can be useful for culture of contaminated specimens, such as sputum. Blood should be cultured in a standard blood culture system. The optimal growth temperature is <37°C (25–29°C), with pinpoint colonies only on SBA at 24 h. Slower growth occurs at 37°C. Y. pestis is oxidase-negative, catalase-positive, urea-negative, indole-negative, and lactose-negative. Automated biochemical identification systems can misidentify Y. pestis as Y. pseudotuberculosis or other bacterial species.
![]() Reference laboratory tests for definitive identification of isolates include direct immunofluorescence for F1 antigen; specific polymerase chain reaction (PCR) for targets such as F1 antigen, the pesticin gene, and the plasminogen activator gene; and specific bacteriophage lysis. PCR can also be applied to diagnostic specimens, as can direct immunofluorescence for F1 antigen (produced in large amounts by Y. pestis) by slide microscopy. An immunochromatographic test strip for F1 antigen detection by monoclonal antibodies in clinical specimens has been devised in Madagascar. This method is effective for both laboratory and near-patient use and is now widely used in endemic countries. A similar test strip for Pla antigen has recently been developed and could be used to detect wild-type or engineered F1-negative virulent strains. Many other rapid diagnostic kits for possible bioterrorism pathogens, including Y. pestis, have been described in recent years, but none is widely used for primary or reference laboratory identification, and only one (a field real-time PCR for a range of potential bioterrorism agents) is approved by the U.S. Food and Drug Administration (FDA). Detailed phylogeographic DNA sequence data based on culture collections have been accumulated to trace plague evolution, and this system could be adapted in the future to determine real-time clinical plague epidemiology.
Reference laboratory tests for definitive identification of isolates include direct immunofluorescence for F1 antigen; specific polymerase chain reaction (PCR) for targets such as F1 antigen, the pesticin gene, and the plasminogen activator gene; and specific bacteriophage lysis. PCR can also be applied to diagnostic specimens, as can direct immunofluorescence for F1 antigen (produced in large amounts by Y. pestis) by slide microscopy. An immunochromatographic test strip for F1 antigen detection by monoclonal antibodies in clinical specimens has been devised in Madagascar. This method is effective for both laboratory and near-patient use and is now widely used in endemic countries. A similar test strip for Pla antigen has recently been developed and could be used to detect wild-type or engineered F1-negative virulent strains. Many other rapid diagnostic kits for possible bioterrorism pathogens, including Y. pestis, have been described in recent years, but none is widely used for primary or reference laboratory identification, and only one (a field real-time PCR for a range of potential bioterrorism agents) is approved by the U.S. Food and Drug Administration (FDA). Detailed phylogeographic DNA sequence data based on culture collections have been accumulated to trace plague evolution, and this system could be adapted in the future to determine real-time clinical plague epidemiology.
In the absence of other positive laboratory diagnostic tests, a retrospective serologic diagnosis may be made on the basis of rising titers of hemagglutinating antibody to F1 antigen. Enzyme-linked immunosorbent assays (ELISAs) for IgG and IgM antibodies to F1 antigen are also available.
The white blood cell (WBC) count is generally raised (to 10,000–20,000/μL) in plague, with neutrophilic leukocytosis and a left shift (numerous immature neutrophils); in some cases, however, the WBC count is normal or leukopenia develops. WBC counts are occasionally very high, especially in children (>100,000/μL). Levels of fibrinogen degradation products are elevated in a majority of patients, but platelet counts are usually normal or low-normal. However, disseminated intravascular coagulation, with low platelet counts, prolonged prothrombin times, reduced fibrinogen, and elevated fibrinogen degradation product levels, occurs in a significant minority of patients.
|
TREATMENT |
PLAGUE |
Guidelines for the treatment of plague are given in Table 196-2. A 10-day course of antimicrobial therapy is recommended. Streptomycin has historically been the parenteral treatment of choice for plague and is approved for this indication by the FDA. Although not yet approved by the FDA for plague, gentamicin has proven safe and effective in clinical trials in Tanzania and Madagascar and in retrospectively reviewed cases in the United States. In view of streptomycin’s adverse-reaction profile and limited availability, some experts now recommend gentamicin over streptomycin. In 2012, the FDA approved levofloxacin for prophylaxis and treatment of plague (including septicemic and pneumonic disease), making it the first antibiotic approved for a new indication under a regulatory approach based on animal studies alone, known as the Animal Rule. An FDA decision on ciprofloxacin is pending. Levofloxacin is more efficacious than ciprofloxacin for postexposure prophylaxis of inhalational anthrax in animal models and also received FDA approval for this indication (Chap. 261e); thus it is approved for multiagent prophylaxis in possible bioterrorism exposures.
|
GUIDELINES FOR THE TREATMENT OF PLAGUE |
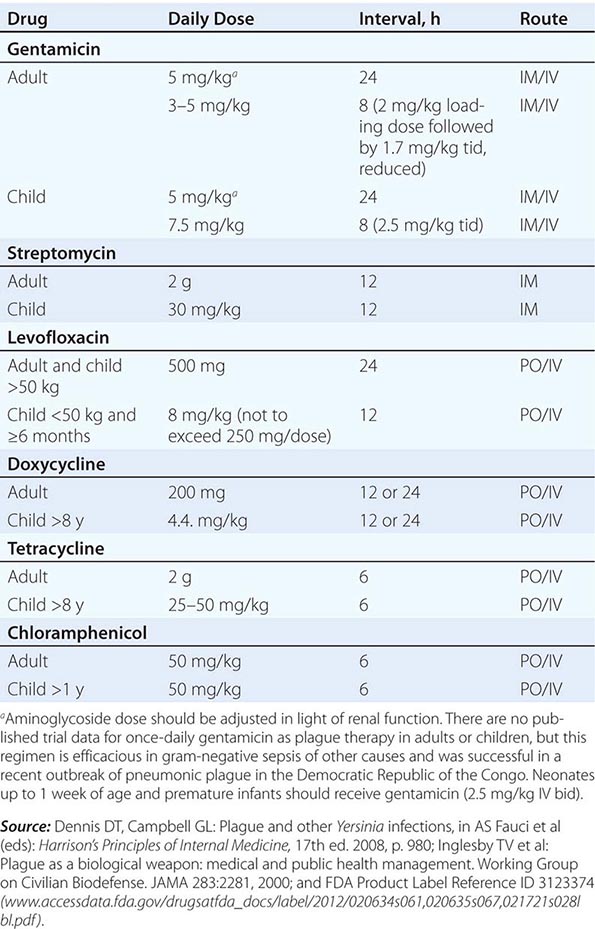
![]() While systemic chloramphenicol therapy is available in the resource-poor countries primarily affected by plague, it is less likely to be available or used in high-income countries because of its adverse effect profile. Tetracyclines are also effective and can be given by mouth but are not recommended for children under the age of 7 years because of tooth discoloration. Doxycycline is the tetracycline of choice; at an oral dosage of 100 mg twice daily, this drug was as effective as IM gentamicin (2.5 mg/kg twice daily) in a trial in Tanzania.
While systemic chloramphenicol therapy is available in the resource-poor countries primarily affected by plague, it is less likely to be available or used in high-income countries because of its adverse effect profile. Tetracyclines are also effective and can be given by mouth but are not recommended for children under the age of 7 years because of tooth discoloration. Doxycycline is the tetracycline of choice; at an oral dosage of 100 mg twice daily, this drug was as effective as IM gentamicin (2.5 mg/kg twice daily) in a trial in Tanzania.
Although Y. pestis is sensitive to β-lactam drugs in vitro and these drugs have been efficacious against plague in some animal models, the response to penicillins has been poor in some clinical cases; thus β-lactams and macrolides are not generally recommended as first-line therapy. Chloramphenicol, alone or in combination, is recommended for some focal complications of plague (e.g., meningitis, endophthalmitis, myocarditis) because of its tissue penetration properties. Fluoroquinolones, effective in vitro and in animal models, are recommended in guidelines for possible bioterrorism-associated pneumonic plague and are increasingly used in therapy, although the only human efficacy data available so far are from a case report. Animal and in vitro studies suggest that fluoroquinolones other than levofloxacin, at doses used in systemic gram-negative sepsis, should be effective as therapy for plague: e.g., ciprofloxacin (400 mg twice daily IV, 500 mg twice daily by mouth), ofloxacin (400 mg twice daily IV or by mouth), or moxifloxacin (400 mg/d IV or by mouth).
PREVENTION
![]() In endemic areas, the control of plague in humans is based on reduction of the likelihood of being bitten by infected fleas or exposed to infected droplets from either humans or animals with plague pneumonia. In the United States, residence and outdoor activity in rural areas of western states where epizootics occur are the main risk factors for infection. To assess potential risks to humans in specific areas, surveillance for Y. pestis infection among animal plague hosts and vectors is carried out regularly as well as in response to observed animal die-offs. Personal protective measures include avoidance of areas where a plague epizootic has been identified and publicized (e.g., by warning signs or closure of campsites). Sick or dead animals should not be handled by the general public. Hunters and zoologists should wear gloves when handling wild-animal carcasses in endemic areas. General measures to avoid rodent fleabite during outdoor activity are appropriate and include the use of insect repellant, insecticide, and protective clothing. General measures to reduce peridomestic and occupational human contact with rodents are advised and include rodent-proofing of buildings and food-waste stores and removal of potential rodent habitats (e.g., woodpiles and junk heaps). Flea control by insecticide treatment of wild rodents is an effective means of minimizing human contact with plague if an epizootic is identified in an area close to human habitation. Any attempt to reduce rodent numbers must be preceded by flea suppression to reduce the migration of infected fleas to human hosts. An oral F1-V subunit vaccine using raccoon poxvirus (RCN) as a vector protects prairie dogs against Y. pestis injections and is being investigated for efficacy in preventing disease in wild animals, thus potentially reducing human exposure.
In endemic areas, the control of plague in humans is based on reduction of the likelihood of being bitten by infected fleas or exposed to infected droplets from either humans or animals with plague pneumonia. In the United States, residence and outdoor activity in rural areas of western states where epizootics occur are the main risk factors for infection. To assess potential risks to humans in specific areas, surveillance for Y. pestis infection among animal plague hosts and vectors is carried out regularly as well as in response to observed animal die-offs. Personal protective measures include avoidance of areas where a plague epizootic has been identified and publicized (e.g., by warning signs or closure of campsites). Sick or dead animals should not be handled by the general public. Hunters and zoologists should wear gloves when handling wild-animal carcasses in endemic areas. General measures to avoid rodent fleabite during outdoor activity are appropriate and include the use of insect repellant, insecticide, and protective clothing. General measures to reduce peridomestic and occupational human contact with rodents are advised and include rodent-proofing of buildings and food-waste stores and removal of potential rodent habitats (e.g., woodpiles and junk heaps). Flea control by insecticide treatment of wild rodents is an effective means of minimizing human contact with plague if an epizootic is identified in an area close to human habitation. Any attempt to reduce rodent numbers must be preceded by flea suppression to reduce the migration of infected fleas to human hosts. An oral F1-V subunit vaccine using raccoon poxvirus (RCN) as a vector protects prairie dogs against Y. pestis injections and is being investigated for efficacy in preventing disease in wild animals, thus potentially reducing human exposure.
Patients in whom pneumonic plague is suspected should be managed in isolation, with droplet precautions observed until pneumonia is excluded or effective antimicrobial therapy has been given for 48 h. Review of the literature published before the advent of antimicrobial agents suggests that the main infective risk is posed by patients in the final stages of disease who are coughing up sputum with plentiful visible blood and/or pus. Cotton and gauze masks were protective in these circumstances. Current surgical masks capable of barrier protection against droplets, including large respiratory particles, are considered protective; a particulate respirator (e.g., N95 or greater) is not required.
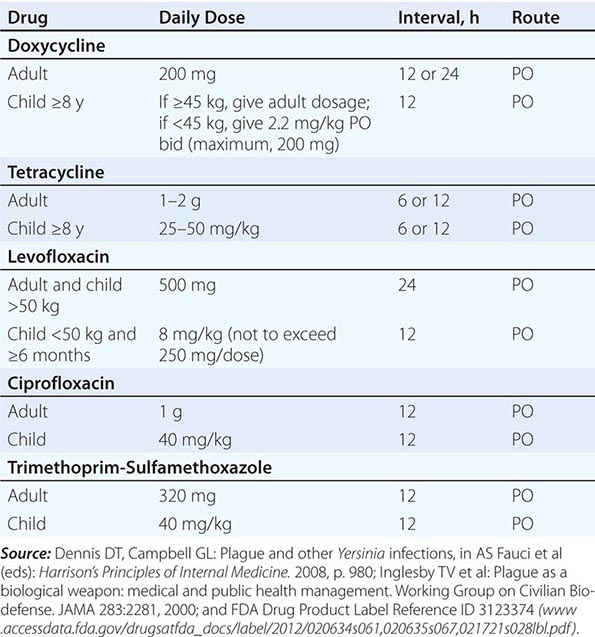
Antimicrobial Prophylaxis Postexposure antimicrobial prophylaxis lasting 7 days is recommended following household, hospital, or other close contact with persons with untreated pneumonic plague. (Close contact is defined as contact with a patient at <2 m.) In animal aerosol-infection studies, levofloxacin and ciprofloxacin are associated with higher survival rates than doxycycline (Table 196-3).
|
GUIDELINES FOR PLAGUE PROPHYLAXIS |
Immunization Studies with candidate plague vaccines in animal models show that neutralizing antibody provides protection against exposure but that cell-mediated immunity is critical for protection and clearance of Y. pestis from the host. A killed whole-cell vaccine used in humans required multiple doses, caused significant local and systemic reactions, and was not protective against pneumonic plague; this vaccine is not currently available in the United States. A live attenuated vaccine based on strain EV76 is still used in countries of the former Soviet Union but has significant side effects. The vaccines closest to licensure are subunit vaccines comprising recombinant F1 (rF1) and various recombinant V (rV) proteins produced in Escherichia coli, which are combined either as a fusion protein or as a mixture, purified, and adsorbed to aluminum hydroxide for injection. This combination protects mice and various nonhuman primates in laboratory models of bubonic and pneumonic plague and has been evaluated in phase 2 clinical trials. Special ethical considerations with controlled clinical studies involving plague in humans make prelicensure field efficacy studies unlikely. In the United States, the FDA is therefore prepared to assess plague vaccines for human use under the Animal Rule, using efficacy data and other results from animal studies as well as antibodies and other correlates of immunity from human vaccine recipients (www.fda.gov/BiologicsBloodVaccines/ScienceResearch/BiologicsResearchAreas/ucm127288.htm). Live attenuated Y. pseudotuberculosis and Salmonella strains expressing Y. pestis–specific antigens have been shown to be protective in laboratory animal models of bubonic and pneumonic plague and could be delivered by the oral route. A wide variety of other delivery mechanisms for Y. pestis antigens are being explored. Antigens other than F1 and V that could be added to subunit vaccines are being investigated. Advances providing impetus for exploration of these antigens are (1) the recovery of F1-negative Y. pestis strains from natural sources and (2) the observation that F1 antigen is not required for virulence in primate models of pneumonic plague.
YERSINIOSIS
Yersiniosis is a zoonotic infection with an enteropathogenic Yersinia species, usually Yersinia enterocolitica or Y. pseudotuberculosis. The usual hosts for these organisms are pigs and other wild and domestic animals; humans are usually infected by the oral route, and outbreaks from contaminated food occur. Yersiniosis is most common in childhood and in colder climates. Patients present with abdominal pain and sometimes with diarrhea (which may not occur in up to 50% of cases). Y. enterocolitica is more closely associated with terminal ileitis and Y. pseudotuberculosis with mesenteric adenitis, but both organisms may cause mesenteric adenitis and symptoms of abdominal pain and tenderness that result in pseudoappendicitis, with the surgical removal of a normal appendix. Diagnosis is based on culture of the organism or convalescent serology. Y. pseudotuberculosis and some rarer strains of Y. enterocolitica are especially likely to cause systemic infection, which is also particularly common among patients with diabetes or iron overload. Systemic sepsis is treatable with antimicrobial agents, but postinfective arthropathy responds poorly to such therapy. Fourteen other Yersinia species are now recognized, but all lack the virulence plasmid pYV common to Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica and are generally considered to be, at most, opportunistic pathogens of humans (Y. aldovae, Y. aleksiciae, Y. bercovieri, Y. entomophaga, Y. frederiksenii, Y. intermedia, Y. kristensenii, Y. massiliensis, Y. mollaretii, Y. nurmii, Y. pekkanenii, Y. rohdei, Y. similis, and Y. ruckeri). Molecular phylogeny shows that Y. enterocolitica is more distantly related to Y. pseudotuberculosis than these other Yersinia species, and the similar virulence plasmid they share has probably been acquired independently by at least one of the two since the species diverged.
EPIDEMIOLOGY
![]() Y. enterocolitica Y. enterocolitica is found worldwide and has been isolated from a wide variety of wild and domestic animals and environmental samples, including samples of food and water. In vitro, Y. enterocolitica is resistant to predation by the protozoon Acanthamoeba castellanii and can survive inside it, suggesting a possible mode of environmental persistence. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Most clinical infections are associated with serogroups O:3, O:9, and O:5,27, with a declining number of O:8 infections in North America. Some O:8 infections, previously confined to North America, have been reported from Europe and Japan in recent years, and serogroup O:8 now causes a high percentage of yersiniosis cases in Poland. Yersiniosis, mostly due to Y. enterocolitica, is the third commonest zoonosis reported in Europe; most reports come from northern Europe, especially Germany and Scandinavia. The incidence is highest among children; children under the age of 4 years are more likely to present with diarrhea than are older children. Abdominal pain with mesenteric adenitis and terminal ileitis is more prominent among older children and adults. Septicemia is more likely in patients with preexisting conditions such as diabetes mellitus, liver disease, any condition involving iron overload (including thalassemia and hemochromatosis), advanced age, malignancy, or HIV/AIDS. As in enteritis of other bacterial etiologies, postinfective complications such as reactive arthritis occur mainly in individuals who are HLA-B27 positive. Erythema nodosum (see Fig. 25e-40) following Yersinia infection is not associated with HLA-B27 and is more common among women than among men.
Y. enterocolitica Y. enterocolitica is found worldwide and has been isolated from a wide variety of wild and domestic animals and environmental samples, including samples of food and water. In vitro, Y. enterocolitica is resistant to predation by the protozoon Acanthamoeba castellanii and can survive inside it, suggesting a possible mode of environmental persistence. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Most clinical infections are associated with serogroups O:3, O:9, and O:5,27, with a declining number of O:8 infections in North America. Some O:8 infections, previously confined to North America, have been reported from Europe and Japan in recent years, and serogroup O:8 now causes a high percentage of yersiniosis cases in Poland. Yersiniosis, mostly due to Y. enterocolitica, is the third commonest zoonosis reported in Europe; most reports come from northern Europe, especially Germany and Scandinavia. The incidence is highest among children; children under the age of 4 years are more likely to present with diarrhea than are older children. Abdominal pain with mesenteric adenitis and terminal ileitis is more prominent among older children and adults. Septicemia is more likely in patients with preexisting conditions such as diabetes mellitus, liver disease, any condition involving iron overload (including thalassemia and hemochromatosis), advanced age, malignancy, or HIV/AIDS. As in enteritis of other bacterial etiologies, postinfective complications such as reactive arthritis occur mainly in individuals who are HLA-B27 positive. Erythema nodosum (see Fig. 25e-40) following Yersinia infection is not associated with HLA-B27 and is more common among women than among men.
Consumption or preparation of raw pork products (such as chitterlings) and some processed pork products is strongly linked with infection because a high percentage of pigs carry pathogenic Y. enterocolitica strains. Outbreaks of Y. enterocolitica infection have been associated with consumption of milk (pasteurized, unpasteurized, and chocolate-flavored) and various foods contaminated with springwater. Person-to-person transmission is suspected in a few cases (e.g., in nosocomial and familial outbreaks) but is much less likely with Y. enterocolitica than with other causes of gastrointestinal infection, such as Salmonella. A multivariate analysis indicates that contact with companion animals is a risk factor for Y. enterocolitica infection among children in Sweden, and low-level colonization of dogs and cats with Y. enterocolitica has been reported. Transfusion-associated septicemia due to Y. enterocolitica, while recognized as a very rare but frequently fatal event for over 30 years, has been difficult to eradicate.
![]() Y. pseudotuberculosis Y. pseudotuberculosis is less frequently reported as a cause of human disease than Y. enterocolitica, and infection with Y. pseudotuberculosis is more likely to present as fever and abdominal pain due to mesenteric lymphadenitis. This organism is associated with wild mammals (rodents, rabbits, and deer), birds, and domestic pigs. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Although outbreaks are generally rare, several have recently occurred in Finland in association with consumption of lettuce and raw carrots.
Y. pseudotuberculosis Y. pseudotuberculosis is less frequently reported as a cause of human disease than Y. enterocolitica, and infection with Y. pseudotuberculosis is more likely to present as fever and abdominal pain due to mesenteric lymphadenitis. This organism is associated with wild mammals (rodents, rabbits, and deer), birds, and domestic pigs. Strains are differentiated by combined biochemical reactions (biovar) and serogroup. Although outbreaks are generally rare, several have recently occurred in Finland in association with consumption of lettuce and raw carrots.
PATHOGENESIS
The usual route of infection is oral. Studies with both Y. enterocolitica and Y. pseudotuberculosis in animal models suggest that initial replication in the small intestine is followed by invasion of Peyer’s patches of the distal ileum via M cells, with onward spread to mesenteric lymph nodes. The liver and spleen can also be involved after oral infection. The characteristic histologic appearance of enteropathogenic yersiniae after invasion of host tissues is as extracellular microabscesses surrounded by an epithelioid granulomatous lesion.
Experiments involving oral infection of mice with tagged Y. enterocolitica show that only a very small proportion of bacteria in the gut invade tissues. Individual bacterial clones from an orally inoculated pool give rise to each microabscess in a Peyer’s patch, and the host restricts the invasion of previously infected Peyer’s patches. A prior model positing progressive bacterial spread from Peyer’s patches and mesenteric lymph nodes to the liver and spleen appears to be inaccurate: spread of individually tagged clones of Y. pseudotuberculosis to the liver and spleen of mice occurs independently of regional lymph node colonization and in mice lacking Peyer’s patches.
![]() Invasion requires the expression of several nonfimbrial adhesins, such as invasin (Inv) and—in Y. pseudotuberculosis—Yersinia adhesin A (YadA). Inv interacts directly with β1 integrins, which are expressed on the apical surfaces of M cells but not enterocytes. YadA of Y. pseudotuberculosis interacts with extracellular matrix proteins such as collagen and fibronectin to facilitate host cell integrin association and invasion. YadA of Y. enterocolitica lacks a crucial N-terminal region and binds collagen and laminin but not fibronectin and does not cause invasion. Inv is chromosomally encoded, whereas YadA is encoded on the virulence plasmid pYV. YadA helps to confer serum resistance by binding host complement regulators such as factor H and C4-binding protein. Another chromosomal gene, ail (attachment and invasion locus), encodes the extracellular protein Ail, which also confers serum resistance by binding these complement regulators.
Invasion requires the expression of several nonfimbrial adhesins, such as invasin (Inv) and—in Y. pseudotuberculosis—Yersinia adhesin A (YadA). Inv interacts directly with β1 integrins, which are expressed on the apical surfaces of M cells but not enterocytes. YadA of Y. pseudotuberculosis interacts with extracellular matrix proteins such as collagen and fibronectin to facilitate host cell integrin association and invasion. YadA of Y. enterocolitica lacks a crucial N-terminal region and binds collagen and laminin but not fibronectin and does not cause invasion. Inv is chromosomally encoded, whereas YadA is encoded on the virulence plasmid pYV. YadA helps to confer serum resistance by binding host complement regulators such as factor H and C4-binding protein. Another chromosomal gene, ail (attachment and invasion locus), encodes the extracellular protein Ail, which also confers serum resistance by binding these complement regulators.
By binding to host cell surfaces, YadA allows targeting of immune effector cells by the pYV plasmid–encoded type III secretion system (injectisome). As a consequence, the host’s innate immune response is altered; toxins (Yersinia outer proteins, or Yops) are injected into host macrophages, neutrophils, and dendritic cells, affecting signal transduction pathways, resulting in reduced phagocytosis and inhibited production of reactive oxygen species by neutrophils, and triggering apoptosis of macrophages. Other factors functional in invasive disease include yersiniabactin (Ybt), a siderophore produced by some strains of Y. pseudotuberculosis and Y. enterocolitica as well as other Enterobacteriaceae. Yersiniabactin allows bacteria to access iron from saturated lactoferrin during infection and reduces the production of reactive oxygen species by innate immune effector cells, thereby decreasing bacterial killing. Y. pseudotuberculosis and Y. pestis make other siderophores in addition to yersiniabactin.
CLINICAL MANIFESTATIONS
Self-limiting diarrhea is the most common reported presentation in infection with pathogenic Y. enterocolitica, especially in children under the age of 4, who form the single largest group in most case series. Blood may be detected in diarrheal stool. Older children and adults are more likely than younger children to present with abdominal pain, which can be localized to the right iliac fossa—a situation that often leads to laparotomy for presumed appendicitis (pseudoappendicitis). Appendectomy is not indicated for Yersinia infection causing pseudoappendicitis. Thickening of the terminal ileum and cecum is seen on endoscopy and ultrasound, with elevated round or oval lesions that may overlie Peyer’s patches. Mesenteric lymph nodes are enlarged. Ulcerations of the mucosa are noted on endoscopy. Gastrointestinal complications include granulomatous appendicitis, a chronic inflammatory condition affecting the appendix that is responsible for ≤2% of cases of appendicitis; Yersinia is involved in a minority of cases. Y. enterocolitica infection can present as acute pharyngitis with or without other gastrointestinal symptoms. Fatal Y. enterocolitica pharyngitis has been recorded. Mycotic aneurysm can follow Y. enterocolitica bacteremia, as can focal infection (abscess) in many other sites and body compartments (liver, spleen, kidney, bone, meninges, endocardium).
![]() In all age groups, Y. pseudotuberculosis infection is more likely to present as abdominal pain and fever than as diarrhea. A superantigenic toxin—Y. pseudotuberculosis mitogen (YPM)—is produced by strains seen in eastern Russia in association with Far Eastern scarlet-like fever, a childhood illness with desquamating rash, arthralgia, and toxic shock. A similar illness is recognized in Japan (Izumi fever) and Korea. Similarities have been noted with Kawasaki disease, the idiopathic acute systematic vasculitis of childhood. There is an epidemiologic link between exposure of populations to superantigen-positive Y. pseudotuberculosis and an elevated incidence of Kawasaki disease.
In all age groups, Y. pseudotuberculosis infection is more likely to present as abdominal pain and fever than as diarrhea. A superantigenic toxin—Y. pseudotuberculosis mitogen (YPM)—is produced by strains seen in eastern Russia in association with Far Eastern scarlet-like fever, a childhood illness with desquamating rash, arthralgia, and toxic shock. A similar illness is recognized in Japan (Izumi fever) and Korea. Similarities have been noted with Kawasaki disease, the idiopathic acute systematic vasculitis of childhood. There is an epidemiologic link between exposure of populations to superantigen-positive Y. pseudotuberculosis and an elevated incidence of Kawasaki disease.
Y. enterocolitica or Y. pseudotuberculosis septicemia presents as a severe illness with fever and leukocytosis, often without localizing features, and is significantly associated with predisposing conditions such as diabetes mellitus, liver disease, and iron overload. Hemochromatosis combines several of these risk factors. Administration of iron chelators like desferrioxamine, which provide iron accessible to Yersinia (and have an inhibitory effect on neutrophil function), may result in Yersinia septicemia in patients with iron overload who presumably have an otherwise mild gastrointestinal infection. HIV/AIDS has been associated with Y. pseudotuberculosis septicemia. The unusual phenomenon of transfusion-associated septicemia is linked to the ability of Y. enterocolitica to multiply at refrigerator temperature (psychrotrophy). Typically, the transfused unit has been stored for >20 days, and it is believed that small numbers of yersiniae from an apparently healthy donor with subclinical bacteremia are amplified to very high numbers by growth inside the bag at ≤4°C, with consequent septic shock after transfusion. A method for preventing this very rare event (i.e., a range of 1 case in 500,000 to 1 case in several million transfused units in countries such as the United States and France) without unacceptable restriction in the blood supply has not yet been devised.
POSTINFECTIVE PHENOMENA
![]() Like other invasive infections of intestinal origin (salmonellosis, shigellosis), reactive arthritis (articular arthritis of multiple joints developing within 2–4 weeks of a preceding infection) results from autoimmune activity initiated by the deposition of bacterial components (not viable bacteria) in joints in combination with the immune response to invading bacteria. The majority of individuals affected by reactive arthritis due to Yersinia are HLA-B27 positive. Myocarditis with electrocardiographic ST-segment abnormalities may occur with Yersinia-associated reactive arthritis. Most Yersinia-associated cases follow Y. enterocolitica infection (presumably because it is more common than infection with other species), but Y. pseudotuberculosis–associated reactive arthritis is also well documented in Finland, where sporadic and outbreak infections with Y. pseudotuberculosis are more common than in other countries. Of infected individuals identified in a recent Y. pseudotuberculosis serotype O:3 outbreak in Finland, 12% developed reactive arthritis affecting the small joints of the hands and feet, knees, ankles, and shoulders and lasting >6 months in most cases. Erythema nodosum (see Fig. 25e-40) occurs after Yersinia infection (more commonly in women) with no evidence of HLA-B27 linkage.
Like other invasive infections of intestinal origin (salmonellosis, shigellosis), reactive arthritis (articular arthritis of multiple joints developing within 2–4 weeks of a preceding infection) results from autoimmune activity initiated by the deposition of bacterial components (not viable bacteria) in joints in combination with the immune response to invading bacteria. The majority of individuals affected by reactive arthritis due to Yersinia are HLA-B27 positive. Myocarditis with electrocardiographic ST-segment abnormalities may occur with Yersinia-associated reactive arthritis. Most Yersinia-associated cases follow Y. enterocolitica infection (presumably because it is more common than infection with other species), but Y. pseudotuberculosis–associated reactive arthritis is also well documented in Finland, where sporadic and outbreak infections with Y. pseudotuberculosis are more common than in other countries. Of infected individuals identified in a recent Y. pseudotuberculosis serotype O:3 outbreak in Finland, 12% developed reactive arthritis affecting the small joints of the hands and feet, knees, ankles, and shoulders and lasting >6 months in most cases. Erythema nodosum (see Fig. 25e-40) occurs after Yersinia infection (more commonly in women) with no evidence of HLA-B27 linkage.
There is a long-standing association between antithyroid and anti-Yersinia antibodies. Antibody evidence of prior Y. enterocolitica infection in Graves’ disease and increased levels of antithyroid antibody in patients with Y. enterocolitica antibodies were first noted in the 1970s. Y. enterocolitica contains a thyroid-stimulating hormone (TSH)–binding site that is recognized by anti-TSH antibodies from Graves’ disease patients. Raised titers of antibodies to Y. enterocolitica whole cells and Yops have been found in some series of Graves’ disease patients but not in others. One Danish study of twins found no evidence of an association between asymptomatic Yersinia infection (as evidenced by anti-Yop antibody titers) and antithyroid antibodies in euthyroid individuals, while another Danish study of twins with and without Graves’ disease found that increased anti-Yop antibody titers were associated with Graves’ disease. It remains unclear whether this cross-reactivity is significant in the etiology of Graves’ disease.
LABORATORY DIAGNOSIS
Standard laboratory culture methods can be used to isolate enteropathogenic Yersinia species from sterile samples, including blood and cerebrospinal fluid. Culture on specific selective media (CIN agar), with or without pre-enrichment in broth or phosphate-buffered saline at either 4°C or 16°C, is the basis of most schema for isolation of yersiniae from stool or other nonsterile samples. Outside known high-incidence areas, specific culture may be carried out by laboratories only upon request. Virulence plasmid–negative strains of Y. enterocolitica can be isolated from cultures of stool from asymptomatic individuals, especially after cold enrichment. These strains usually differ in biotype (typically biovar 1a) from virulence plasmid–possessing strains; although some display apparent pathogenicity in a mouse model, virulence plasmid–negative strains are not commonly accepted as human pathogens. Because of the frequency with which the virulence plasmid is lost on laboratory subculture, combined biochemical identification (with biotyping according to a standard schema) and serologic identification are usually required to interpret the significance of an isolate of Y. enterocolitica from a nonsterile site. Most pathogenic Y. enterocolitica strains currently isolated from humans are of serogroup O:3/biovar 4 or serogroup O:9/biovar 2; this pattern holds even in the United States, where serogroup O:8/biovar 1B strains were previously predominant. Many self-validated multiplex PCR screens for detection of Y. enterocolitica in clinical samples—and rather more for its detection in food—have been described, but none of these assays is widely used outside its originating laboratory. Some CE-marked real-time PCR kits are now available in Europe for the diagnosis of yersiniosis in animals; as molecular diagnosis of enteric infection becomes more routine in human disease, it is likely that Y. enterocolitica will be included in diagnostic multiplex PCR screens of feces. Because of the presence of Ail in biovar 1a strains, this antigen cannot be used alone in diagnostic assays. A standard for PCR detection in food samples is being prepared by the International Organization for Standardization.
Agglutinating or ELISA antibody titers to specific O-antigen types are used in the retrospective diagnosis of both Y. enterocolitica and Y. pseudotuberculosis infections. IgA and IgG antibodies persist in patients with reactive arthritis. Serologic cross-reactions between Y. enterocolitica serogroup O:9 and Brucella are due to the similarity of their lipopolysaccharide structures. Multiple assays are required to cover even the predominant serogroups (Y. enterocolitica O:3, O:5,27, and O:9; Y. pseudotuberculosis O:1a, O:1b, and O:3), and these assays are generally available only in reference laboratories. ELISA and western blot tests for antibodies to Yops, which are expressed by all pathogenic strains of Y. enterocolitica and Y. pseudotuberculosis, are also available; most of the positivity in these assays probably relates to previous infection with Y. enterocolitica.
|
TREATMENT |
YERSINIOSIS |
Most cases of diarrhea caused by enteropathogenic Yersinia are self-limiting. Data from clinical trials do not support antimicrobial treatment for adults or children with Y. enterocolitica diarrhea. Systemic infections with bacteremia or focal infections outside the gastrointestinal tract generally require antimicrobial therapy. Infants <3 months of age with documented Y. enterocolitica infection may require antimicrobial treatment because of the increased likelihood of bacteremia in this age group. Y. enterocolitica strains nearly always express β-lactamases. Because of the relative rarity of systemic Y. enterocolitica infection, there are no clinical trial data to guide antimicrobial choice or to suggest the optimal dose and duration of therapy. On the basis of retrospective case series and in vitro sensitivity data, fluoroquinolone therapy is effective for bacteremia in adults; for example, ciprofloxacin is given at a typical dose of 500 mg twice daily by mouth or 400 mg twice daily IV for at least 2 weeks (longer if positive blood cultures persist). A third-generation cephalosporin is an alternative—e.g., cefotaxime (typical dose, 6–8 g/d in three or four divided doses). In children, third-generation cephalosporins are effective; for example, cefotaxime is given to children ≥1 month of age at a typical dose of 75–100 mg/kg per day in three or four divided doses, with an increase to 150–200 mg/kg per day in severe cases (maximal daily dose, 8–10 g). Amoxicillin and amoxicillin/clavulanate have shown poor efficacy in case series. Trimethoprim-sulfamethoxazole, gentamicin, and imipenem are all active in vitro. Y. pseudotuberculosis strains do not express β-lactamase but are intrinsically resistant to polymyxin. Because human infection with Y. pseudotuberculosis is less common than that with Y. enterocolitica, less case information is available; however, studies in mice suggest that ampicillin is ineffective. Drugs similar to those used against Y. enterocolitica should be used. The best results have been obtained with a quinolone.
Some trials of treatment for reactive arthritis (with a large proportion of cases due to Yersinia) found that 3 months of oral ciprofloxacin therapy did not affect outcome. One trial in which the same therapy was given specifically for Y. enterocolitica–reactive arthritis found that, while outcome indeed was not affected, there was a trend toward faster remission of symptoms in the treated group. Follow-up 4–7 years after initial antibiotic treatment of reactive arthritis (predominantly following Salmonella and Yersinia infections) demonstrated apparent efficacy in the prevention of chronic arthritis in HLA-B27-positive individuals. A trial showing that azithromycin therapy did not affect outcome in reactive arthritis included cases believed to follow yersiniosis, although no breakdown of cases was provided. A Cochrane review evaluating the use of antibiotics for reactive arthritis is in progress.
PREVENTION AND CONTROL
![]() Current control measures are similar to those used against other enteric pathogens like Salmonella and Campylobacter, which colonize the intestine of food animals. The focus is on safe handling and processing of food. No vaccine is effective in preventing intestinal colonization of food animals by enteropathogenic Yersinia. Consumption of food made from raw pork (which is popular in Germany and Belgium) should be discouraged at present because it is not possible to eliminate contamination with the enteropathogenic Yersinia strains found worldwide in pigs. Exposure of infants to raw pig intestine during domestic preparation of chitterlings is inadvisable. Modification of abattoir technique in Scandinavian countries from the 1990s onward included the removal of pig intestines in a closed plastic bag; levels of carcass contamination with Y. enterocolitica were reduced, but such contamination was not eliminated. Experimental pig herds free of pathogenic Y. enterocolitica O:3 (and also of Salmonella, Toxoplasma, and Trichinella) have been established in Norway and may be commercialized in the future because of their enhanced safety. In the food industry, vigilance is required because of the potential for large outbreaks if small numbers of enteropathogenic yersiniae contaminate any ready-to-eat food whose safe preservation is based on refrigeration before consumption.
Current control measures are similar to those used against other enteric pathogens like Salmonella and Campylobacter, which colonize the intestine of food animals. The focus is on safe handling and processing of food. No vaccine is effective in preventing intestinal colonization of food animals by enteropathogenic Yersinia. Consumption of food made from raw pork (which is popular in Germany and Belgium) should be discouraged at present because it is not possible to eliminate contamination with the enteropathogenic Yersinia strains found worldwide in pigs. Exposure of infants to raw pig intestine during domestic preparation of chitterlings is inadvisable. Modification of abattoir technique in Scandinavian countries from the 1990s onward included the removal of pig intestines in a closed plastic bag; levels of carcass contamination with Y. enterocolitica were reduced, but such contamination was not eliminated. Experimental pig herds free of pathogenic Y. enterocolitica O:3 (and also of Salmonella, Toxoplasma, and Trichinella) have been established in Norway and may be commercialized in the future because of their enhanced safety. In the food industry, vigilance is required because of the potential for large outbreaks if small numbers of enteropathogenic yersiniae contaminate any ready-to-eat food whose safe preservation is based on refrigeration before consumption.
The rare phenomenon of contamination of blood for transfusion has proved impossible to eradicate. However, leukodepletion is now practiced in most blood transfusion centers, primarily to prevent nonhemolytic febrile transfusion reactions and alloimmunization against HLA antigens. This measure reduces but does not eliminate the risk of Yersinia blood contamination.
Notification of yersiniosis is now obligatory in some countries.
197 |
Bartonella Infections, Including Cat-Scratch Disease |
Bartonella species are fastidious, facultative intracellular, slow-growing, gram-negative bacteria that cause a broad spectrum of diseases in humans. This genus includes more than 30 distinct species or subspecies, of which at least 16 have been recognized as confirmed or potential human pathogens; Bartonella bacilliformis, Bartonella quintana, and Bartonella henselae are most commonly identified (Table 197-1). Most Bartonella species have successfully adapted to survival in specific domestic or wild mammals. Prolonged intraerythrocytic infection in these animals creates a niche where the bacteria are protected from both innate and adaptive immunity and which serves as a reservoir for human infections. Bartonella characteristically evades the host immune system by modification of its virulence factors (e.g., lipopolysaccharides or flagella) and by attenuation of the immune response. B. bacilliformis and B. quintana, which are not zoonotic, are exceptions. Arthropod vectors are often involved. Isolation and characterization of Bartonella species are difficult and require special techniques. Clinical presentation generally depends on both the infecting Bartonella species and the immune status of the infected individual. Bartonella species are susceptible to many antibiotics in vitro; however, clinical responses to therapy and studies in animal models suggest that the minimal inhibitory concentrations of many antimicrobial agents correlate poorly with the drugs’ in vivo efficacies in patients with Bartonella infections.
|
BARTONELLA SPECIES KNOWN OR SUSPECTED TO BE HUMAN PATHOGENS |
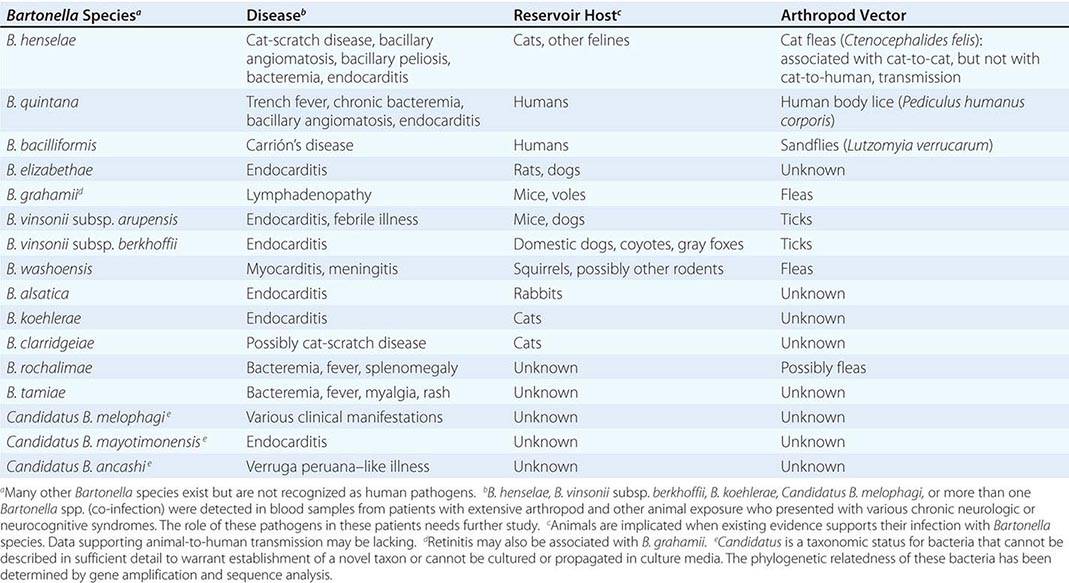
CAT-SCRATCH DISEASE
DEFINITION AND ETIOLOGY
Usually a self-limited illness, cat-scratch disease (CSD) has two general clinical presentations. Typical CSD, the more common, is characterized by subacute regional lymphadenopathy; atypical CSD is the collective designation for numerous extranodal manifestations involving various organs. B. henselae is the principal etiologic agent of CSD. Rare cases have been associated with Afipia felis and other Bartonella species.
EPIDEMIOLOGY
![]() CSD occurs worldwide, favoring warm and humid climates. In temperate climates, incidence peaks during fall and winter; in the tropics, disease occurs year-round. Adults are affected nearly as frequently as children. Intrafamilial clustering is rare, and person-to-person transmission does not occur. Apparently healthy cats constitute the major reservoir of B. henselae, and cat fleas (Ctenocephalides felis) may be responsible for cat-to-cat transmission. CSD usually follows contact with cats (especially kittens), but other animals (e.g., dogs) have been implicated as possible reservoirs in rare instances. In the United States, the estimated disease incidence is ~10 cases per 100,000 population. About 10% of patients are hospitalized.
CSD occurs worldwide, favoring warm and humid climates. In temperate climates, incidence peaks during fall and winter; in the tropics, disease occurs year-round. Adults are affected nearly as frequently as children. Intrafamilial clustering is rare, and person-to-person transmission does not occur. Apparently healthy cats constitute the major reservoir of B. henselae, and cat fleas (Ctenocephalides felis) may be responsible for cat-to-cat transmission. CSD usually follows contact with cats (especially kittens), but other animals (e.g., dogs) have been implicated as possible reservoirs in rare instances. In the United States, the estimated disease incidence is ~10 cases per 100,000 population. About 10% of patients are hospitalized.
PATHOGENESIS
Inoculation of B. henselae, possibly via contaminated flea feces, usually results from a cat scratch or bite. Infection of mucous membranes or conjunctivae via droplets or licking may occur as well. With lymphatic drainage to one or more regional lymph nodes in immunocompetent hosts, a TH1 response can result in necrotizing granulomatous lymphadenitis. Dendritic cells, along with their associated chemokines, play a role in the host inflammatory response and granuloma formation.
CLINICAL MANIFESTATIONS AND PROGNOSIS
Of patients with CSD, 85–90% have typical disease. The primary lesion, a small (0.3- to 1-cm) painless erythematous papule or pustule, develops at the inoculation site (usually the site of a scratch or a bite) within days to 2 weeks in about one-third to two-thirds of patients (Fig. 197–1A, B). Lymphadenopathy develops 1–3 weeks or longer after cat contact. The affected lymph node(s) are enlarged and usually painful, sometimes have overlying erythema, and suppurate in 10–15% of cases (Fig. 197–1C, D, and E). Axillary/epitrochlear nodes are most commonly involved; next in frequency are head/neck nodes and then inguinal/femoral nodes. Approximately 50% of patients have fever, malaise, and anorexia. A smaller proportion experience weight loss and night sweats mimicking the presentation of lymphoma. Fever is usually low-grade but infrequently rises to ≥39°C. Resolution is slow, requiring weeks (for fever, pain, and accompanying signs and symptoms) to months (for node shrinkage).
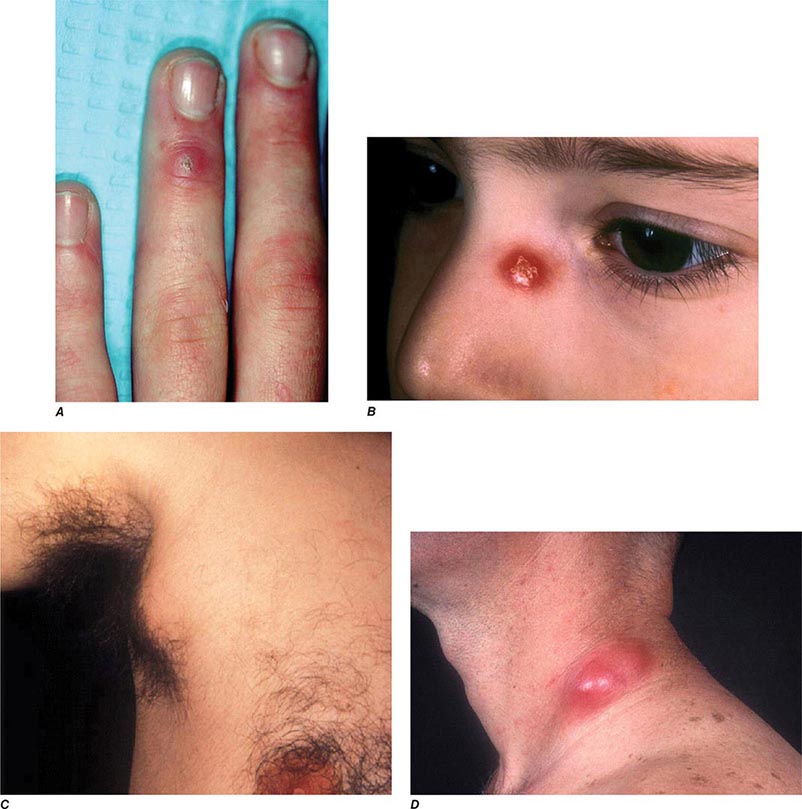
FIGURE 197-1 Manifestations of cat-scratch disease. A. Primary inoculation lesion. Axillary and epitrochlear lymphadenitis appeared 2 weeks later. B. Primary inoculation lesion. Submental lymphadenitis appeared 10 days later. C. Axillary lymphadenopathy of 2 weeks’ duration. The overlying skin appears normal. D. Cervical lymphadenopathy of 6 weeks’ duration. The overlying skin is red. Thick, odorless pus (12 mL) was aspirated. E. Preauricular lymphadenopathy. F. Left-eye neuroretinitis. Note papilledema and stellate macular exudates (“macular star”).
Atypical CSD occurs in 10–15% of patients as extranodal or complicated disease in the absence or presence of lymphadenopathy. Atypical disease includes Parinaud’s oculoglandular syndrome (granulomatous conjunctivitis with ipsilateral preauricular lymphadenitis; Fig. 197–1E), granulomatous hepatitis/splenitis, neuroretinitis (often presenting as unilateral deterioration of vision; Fig. 197–1F), and other ophthalmologic manifestations. In addition, neurologic involvement (encephalopathy, seizures, myelitis, radiculitis, cerebellitis, facial and other cranial or peripheral palsies), fever of unknown origin, debilitating myalgia, arthritis or arthralgia (affecting mostly women >20 years old), osteomyelitis (including multifocal disease), tendinitis, neuralgia, and dermatologic manifestations (including erythema nodosum [see Fig. 25e-40], sometimes accompanying arthropathy) occur. Other manifestations and syndromes (pneumonitis, pleural effusion, idiopathic thrombocytopenic purpura, Henoch-Schönlein purpura, erythema multiforme [see Fig. 25e-25], hypercalcemia, glomerulonephritis, myocarditis) have also been associated with CSD. In elderly patients (>60 years old), lymphadenopathy is more often absent but encephalitis and fever of unknown origin are more common than in younger patients. In immunocompetent individuals, CSD—whether typical or atypical—usually resolves without treatment and without sequelae. Lifelong immunity is the rule.
DIAGNOSIS
Routine laboratory tests usually yield normal or nonspecific results. Histopathology initially shows lymphoid hyperplasia and later demonstrates stellate granulomata with necrosis, coalescing microabscesses, and occasional multinucleated giant cells—findings that, although nonspecific, may narrow the differential diagnosis. Serologic testing (immunofluorescence or enzyme immunoassay) is the most commonly used laboratory diagnostic approach, with variable sensitivity and specificity. Seroconversion may take a few weeks. Other tests are of low sensitivity (culture, Warthin-Starry silver staining), of low specificity (cytology, histopathology), or of limited availability in routine diagnostic laboratories (polymerase chain reaction [PCR], immunohistochemistry). PCR of lymph node tissue, pus, or the primary inoculation lesion is highly sensitive and specific and is particularly useful for definitive and rapid diagnosis in seronegative patients.
|
TREATMENT |
CAT-SCRATCH DISEASE |
(Table 197-2) Treatment regimens are based on only minimal data. Suppurative nodes should be drained by large-bore needle aspiration and not by incision and drainage in order to avoid chronic draining tracts. Immunocompromised patients must always be treated with systemic antimicrobials.
|
ANTIMICROBIAL THERAPY FOR DISEASE CAUSED BY BARTONELLA SPECIES IN ADULTS |
PREVENTION
Avoiding cats (especially kittens) and instituting flea control are options for immunocompromised patients and for patients with valvular heart disease.
TRENCH FEVER AND CHRONIC BACTEREMIA
DEFINITION AND ETIOLOGY
Trench fever, also known as 5-day fever or quintan fever, is a febrile illness caused by B. quintana. It was first described as an epidemic in the trenches of World War I and recently reemerged as chronic bacteremia seen most often in homeless people (also referred to in the latter setting as urban or contemporary trench fever).
EPIDEMIOLOGY
![]() In addition to epidemics during World Wars I and II, sporadic outbreaks of trench fever have been reported in many regions of the world. The human body louse has been identified as the vector and humans as the only known reservoir. After a hiatus of several decades during which trench fever was almost forgotten, small clusters of cases of B. quintana chronic bacteremia were reported sporadically, primarily from the United States and France, in HIV-uninfected homeless people. Alcoholism and louse infestation were identified as risk factors.
In addition to epidemics during World Wars I and II, sporadic outbreaks of trench fever have been reported in many regions of the world. The human body louse has been identified as the vector and humans as the only known reservoir. After a hiatus of several decades during which trench fever was almost forgotten, small clusters of cases of B. quintana chronic bacteremia were reported sporadically, primarily from the United States and France, in HIV-uninfected homeless people. Alcoholism and louse infestation were identified as risk factors.
CLINICAL MANIFESTATIONS
The typical incubation period is 15–25 days (range, 3–38 days). “Classical” trench fever, as described in 1919, ranges from a mild febrile illness to a recurrent or protracted and debilitating disease. Onset may be abrupt or preceded by a prodrome of several days. Fever is often periodic, lasting 4–5 days with 5-day (range, 3- to 8-day) intervals between episodes. Other symptoms and signs include headache, back and limb pain, profuse sweating, shivering, myalgia, arthralgia, splenomegaly, a maculopapular rash in occasional cases, and nuchal rigidity in some cases. Untreated, the disease usually lasts 4–6 weeks. Death is rare. The clinical spectrum of B. quintana bacteremia in homeless people ranges from asymptomatic infection to a febrile illness with headache, severe leg pain, and thrombocytopenia. Endocarditis sometimes develops.
DIAGNOSIS
Definitive diagnosis requires isolation of B. quintana by blood culture. Some patients have positive blood cultures for several weeks. Patients with acute trench fever typically develop significant titers of antibody to Bartonella, whereas those with chronic B. quintana bacteremia may be seronegative. Patients with high titers of IgG antibodies should be evaluated for endocarditis. In epidemics, trench fever should be differentiated from epidemic louse-borne typhus and relapsing fever, which occur under similar conditions and share many features.
|
TREATMENT |
B. QUINTANA BACTEREMIA |
(Table 197–2) In a small, randomized, placebo-controlled trial involving homeless people with B. quintana bacteremia, therapy with gentamicin and doxycycline was superior to administration of placebo in eradicating bacteremia. Treatment of bacteremia is important even in clinically mild cases to prevent endocarditis. Optimal therapy for trench fever without documented bacteremia is uncertain.
BARTONELLA ENDOCARDITIS
DEFINITION AND ETIOLOGY
![]() Coxiella burnetii (Chap. 211) and Bartonella species are the most common pathogens in culture-negative endocarditis (Chap. 155). In France, for example, Bartonella species were identified as the etiologic agents in 28% of 348 cases of culture-negative endocarditis. Prevalence, however, varies by geographic location and epidemiologic setting. In addition to B. quintana and B. henselae (the most common Bartonella species implicated in endocarditis, the former more commonly than the latter), other Bartonella species have reportedly caused rare cases (Table 197-1).
Coxiella burnetii (Chap. 211) and Bartonella species are the most common pathogens in culture-negative endocarditis (Chap. 155). In France, for example, Bartonella species were identified as the etiologic agents in 28% of 348 cases of culture-negative endocarditis. Prevalence, however, varies by geographic location and epidemiologic setting. In addition to B. quintana and B. henselae (the most common Bartonella species implicated in endocarditis, the former more commonly than the latter), other Bartonella species have reportedly caused rare cases (Table 197-1).
EPIDEMIOLOGY
![]() Bartonella endocarditis has been reported worldwide. Most patients are adults; more are male than female. Risk factors associated with B. quintana endocarditis include homelessness, alcoholism, and body louse infestation; however, individuals with no risk factors have had Bartonella endocarditis diagnosed as well. B. henselae endocarditis is associated with exposure to cats. Most cases involve native rather than prosthetic valves; the aortic valve accounts for ~60% of cases. Patients with B. henselae endocarditis usually have preexisting valvulopathy, whereas B. quintana often infects normal valves.
Bartonella endocarditis has been reported worldwide. Most patients are adults; more are male than female. Risk factors associated with B. quintana endocarditis include homelessness, alcoholism, and body louse infestation; however, individuals with no risk factors have had Bartonella endocarditis diagnosed as well. B. henselae endocarditis is associated with exposure to cats. Most cases involve native rather than prosthetic valves; the aortic valve accounts for ~60% of cases. Patients with B. henselae endocarditis usually have preexisting valvulopathy, whereas B. quintana often infects normal valves.
CLINICAL MANIFESTATIONS
Clinical manifestations are usually characteristic of subacute endocarditis of any etiology. However, a substantial number of patients have a prolonged, minimally febrile or even afebrile indolent illness, with mild nonspecific symptoms lasting weeks or months before the diagnosis is made. Initial echocardiography may not show vegetations. Acute, aggressive disease is rare.
DIAGNOSIS
Blood cultures, even with use of special techniques (lysis centrifugation or EDTA-containing tubes), are positive in only ~25% of cases—mostly those caused by B. quintana and only rarely those caused by B. henselae. Prolonged incubation of cultures (up to 6 weeks) is required. Serologic tests—either immunofluorescence or enzyme immunoassay—usually demonstrate high-titer IgG antibodies to Bartonella. Because of cross-antigenicity, serology does not distinguish between B. quintana and B. henselae and may also be low-titer cross-reactive with other pathogens, such as C. burnetii and Chlamydia species. Identification of Bartonella to the species level is usually accomplished by application of PCR-based methods to valve tissue.
|
TREATMENT |
BARTONELLA ENDOCARDITIS |
(Table 197-2) For patients with culture-negative endocarditis suspected to be due to Bartonella species, empirical treatment consists of gentamicin, doxycycline, and ceftriaxone; the major role of ceftriaxone in this regimen is to adequately treat other potential causes of culture-negative endocarditis, including members of the HACEK group (Chap. 183e). Once a diagnosis of Bartonella endocarditis has been established, ceftriaxone is discontinued. Aminoglycosides, the only antibiotics known to be bactericidal against Bartonella, should be included in the regimen for ≥2 weeks. Indications for valvular surgery are the same as in subacute endocarditis due to other pathogens; however, the proportion of patients who undergo surgery (~60%) is high, probably as a consequence of delayed diagnosis.
BACILLARY ANGIOMATOSIS AND PELIOSIS
DEFINITION AND ETIOLOGY
Bacillary angiomatosis (sometimes called bacillary epithelioid angiomatosis or epithelioid angiomatosis) is a disease of severely immunocompromised patients, is caused by B. henselae or B. quintana, and is characterized by neovascular proliferative lesions involving the skin and other organs. Both species cause cutaneous lesions; hepatosplenic lesions are caused only by B. henselae, whereas subcutaneous and lytic bone lesions are more frequently associated with B. quintana. Bacillary peliosis is a closely related angioproliferative disorder caused by B. henselae and involving primarily the liver (peliosis hepatis) but also the spleen and lymph nodes. Bacillary peliosis is characterized by blood-filled cystic structures whose size ranges from microscopic to several millimeters.
EPIDEMIOLOGY
Bacillary angiomatosis and bacillary peliosis occur primarily in HIV-infected persons (Chap. 226) with CD4+ T cell counts <100/μL but also affect other immunosuppressed patients and, in rare instances, immunocompetent patients. The previously reported incidence of ~1 case per 1000 HIV-infected persons is now lower; the decrease is most likely attributable to effective antiretroviral therapy and the routine use of rifabutin and macrolides to prevent Mycobacterium avium complex infection in AIDS patients. Contact with cats or cat fleas increases the risk of B. henselae infection. Risk factors for B. quintana infection are low income, homelessness, and body louse infestation.
CLINICAL MANIFESTATIONS
Bacillary angiomatosis presents most commonly as one or more cutaneous lesions that are not painful and that may be tan, red, or purple in color. Subcutaneous masses or nodules, superficial ulcerated plaques (Fig. 197-2), and verrucous growths are also seen. Nodular forms resemble those seen in fungal or mycobacterial infections. Subcutaneous nodules are often tender. Painful osseous lesions, most often involving long bones, may underlie cutaneous lesions and occasionally develop in their absence. In rare cases, other organs are involved in bacillary angiomatosis. Patients usually have constitutional symptoms, including fever, chills, malaise, headache, anorexia, weight loss, and night sweats. In osseous disease, lytic lesions are generally seen on radiography, and technetium scan shows focal uptake. The differential diagnosis of cutaneous bacillary angiomatosis includes Kaposi’s sarcoma, pyogenic granuloma, subcutaneous tumors, and verruga peruana. In bacillary peliosis, hypodense hepatic areas are usually evident on imaging. In patients with advanced immunodeficiency, B. henselae and B. quintana are important causes of fever of unknown origin. Intermittent bacteremia with positive blood cultures can occur with or without endocarditis.
FIGURE 197-2 Nodular lesion of bacillary angiomatosis with superficial ulceration in an AIDS patient with advanced immunodeficiency. (Reprinted with permission from DH Spach and E Darby: Bartonella Infections, Including Cat-Scratch Disease, in Harrison’s Principles of Internal Medicine, 17th ed, AF Fauci et al [eds]. New York, McGraw-Hill, 2008, p 989.)
PATHOLOGY
Bacillary angiomatosis consists of lobular proliferations of small blood vessels lined by enlarged endothelial cells interspersed with mixed infiltrates of neutrophils and lymphocytes, with predominance of the former. Histologic examination of organs with bacillary peliosis reveals small blood-filled cystic lesions partially lined by endothelial cells that can be several millimeters in size. Peliotic lesions are surrounded by fibromyxoid stroma containing inflammatory cells, dilated capillaries, and clumps of granular material. Warthin-Starry silver staining of bacillary angiomatosis and peliosis lesions reveals clusters of bacilli. Cultures are usually negative.
DIAGNOSIS
Bacillary angiomatosis and bacillary peliosis are diagnosed on histologic grounds. Blood cultures may be positive.
|
TREATMENT |
BACILLARY ANGIOMATOSIS AND PELIOSIS |
(Table 197-2) Prolonged therapy with a macrolide or doxycycline is recommended for both bacillary angiomatosis and bacillary peliosis.
PREVENTION
Control of cat-flea infestation and avoidance of cat scratches (for prevention of B. henselae) and avoidance and treatment of body louse infestation (for prevention of B. quintana) are reasonable strategies for HIV-infected persons. Primary prophylaxis is not recommended, but suppressive therapy with a macrolide or doxycycline is indicated in HIV-infected patients with bacillary angiomatosis or bacillary peliosis until CD4+ T cell counts are >200/μL. Relapse may necessitate lifelong suppressive therapy in individual cases.
CARRIÓN’S DISEASE (OROYA FEVER AND VERRUGA PERUANA)
DEFINITION AND ETIOLOGY
Carrión’s disease is a biphasic disease caused by B. bacilliformis. Oroya fever is the initial, bacteremic, systemic form, and verruga peruana is its late-onset, eruptive manifestation.
EPIDEMIOLOGY AND PREVENTION
![]() Infection is endemic to the geographically restricted Andes valleys of Peru, Ecuador, and Colombia (~500–3200 m above sea level). Sporadic epidemics occur. The disease is transmitted by the phlebotomine sandfly Lutzomyia verrucarum. Humans are the only known reservoir of B. bacilliformis. Sandfly control measures (e.g., insecticides) and personal protection measures (e.g., repellents, screening, bed nets) may decrease the risk of infection.
Infection is endemic to the geographically restricted Andes valleys of Peru, Ecuador, and Colombia (~500–3200 m above sea level). Sporadic epidemics occur. The disease is transmitted by the phlebotomine sandfly Lutzomyia verrucarum. Humans are the only known reservoir of B. bacilliformis. Sandfly control measures (e.g., insecticides) and personal protection measures (e.g., repellents, screening, bed nets) may decrease the risk of infection.
PATHOGENESIS
After inoculation by the sandfly, bacteria invade the blood vessel endothelium and proliferate; the reticuloendothelial system and various organs may also be involved. Upon reentry into blood vessels, B. bacilliformis invades, replicates, and ultimately destroys erythrocytes, with consequent massive hemolysis and sudden, severe anemia. Microvascular thrombosis results in end-organ ischemia. Survivors sometimes develop cutaneous hemangiomatous lesions characterized by various inflammatory cells, endothelial proliferation, and the presence of B. bacilliformis.
CLINICAL MANIFESTATIONS
The incubation period is 3 weeks (range, 2–14 weeks). Oroya fever may present as a nonspecific bacteremic febrile illness without anemia or as an acute, severe hemolytic anemia with hepatomegaly and jaundice of rapid onset leading to vascular collapse and clouded sensorium. Myalgia, arthralgia, lymphadenopathy, and abdominal pain may develop. Temperature is elevated but not extremely so; high fever may suggest intercurrent infection. Subclinical asymptomatic infection also occurs. In verruga peruana, red, hemangioma-like, cutaneous vascular lesions of various sizes appear either weeks to months after systemic illness or with no previous suggestive history. These lesions persist for months up to 1 year. Mucosal and internal lesions may also develop.
DIAGNOSIS AND APPROACH TO THE PATIENT
Systemic illness (with or without anemia) or the development of cutaneous lesions in a person who has been to an endemic area raises the possibility of B. bacilliformis infection. Severe anemia with exuberant reticulocytosis—and sometimes thrombocytopenia—can occur. In systemic illness, Giemsa-stained blood films show typical intraerythrocytic bacilli, and blood and bone marrow cultures are positive. Serologic assays may be helpful. Biopsy may be required to confirm the diagnosis of verruga peruana. Differential diagnosis includes the spectrum of coendemic systemic febrile illnesses (e.g., typhoid fever, malaria, brucellosis) as well as diseases producing cutaneous vascular lesions (e.g., hemangiomata, bacillary angiomatosis, Kaposi’s sarcoma).
|
TREATMENT |
CAREIÓN’S DISEASE |
(Table 197-2) Antibiotic therapy for systemic B. bacilliformis infection usually results in rapid defervescence. Additional antibiotic treatment of intercurrent infection (particularly salmonellosis) is often required. Blood transfusion may be necessary. Treatment of verruga peruana usually is not required, although large lesions or those interfering with function may require excision. Patients with numerous lesions, especially lesions that have been present for only a short period, may respond well to antibiotic therapy.
COMPLICATIONS AND PROGNOSIS
Mortality rates associated with Oroya fever have been reported to be as high as 40% without treatment but are considerably lower (~10%) with treatment. Complications such as bacterial superinfection and neurologic and cardiac manifestations occur frequently. Generalized massive edema (anasarca) and petechiae are associated with poor outcome. Permanent immunity usually develops.
198e |
Donovanosis |
Donovanosis is a chronic, progressive bacterial infection that usually involves the genital region. The condition is generally regarded as a sexually transmitted infection of low infectivity. This infection has been known by many other names, the most common being granuloma inguinale.
ETIOLOGY
The causative organism has been reclassified as Klebsiella granulomatis comb nov on the basis of phylogenetic analysis, although there is ongoing debate about this decision. Some authorities consider the original nomenclature (Calymmatobacterium granulomatis) to be more appropriate in light of analysis of 16S rRNA gene sequences.
Donovanosis was first described in Calcutta in 1882, and the causative organism was recognized by Charles Donovan in Madras in 1905. He identified the characteristic Donovan bodies, measuring 1.5 × 0.7 μm, in macrophages and the stratum malpighii. The organism was not reproducibly cultured until the mid-1990s, when its isolation in peripheral-blood monocytes and human epithelial cell lines was reported.
EPIDEMIOLOGY
![]() Donovanosis has an unusual geographic distribution that includes Papua New Guinea, parts of southern Africa, India, the Caribbean, French Guyana, Brazil, and aboriginal communities in Australia. In Australia, donovanosis has been almost entirely eliminated through a sustained program backed by strong political commitment and resources at the primary health care level. Although few cases are now reported in the United States, donovanosis was once prevalent in this country, with 5000–10,000 cases recorded in 1947. The largest epidemic recorded was in Dutch South Guinea, where 10,000 cases were identified in a population of 15,000 (the Marind-anim people) between 1922 and 1952.
Donovanosis has an unusual geographic distribution that includes Papua New Guinea, parts of southern Africa, India, the Caribbean, French Guyana, Brazil, and aboriginal communities in Australia. In Australia, donovanosis has been almost entirely eliminated through a sustained program backed by strong political commitment and resources at the primary health care level. Although few cases are now reported in the United States, donovanosis was once prevalent in this country, with 5000–10,000 cases recorded in 1947. The largest epidemic recorded was in Dutch South Guinea, where 10,000 cases were identified in a population of 15,000 (the Marind-anim people) between 1922 and 1952.
Donovanosis is associated with poor hygiene and is more common in lower socioeconomic groups than in those who are better off and in men than in women. Infection in sexual partners of index cases occurs to a limited extent. Donovanosis is a risk factor for HIV infection (Chap. 226).
Globally, the incidence of donovanosis has decreased significantly in recent times. This decline probably reflects a greater focus on effective management of genital ulcers because of their role in facilitating HIV transmission.
CLINICAL FEATURES
A lesion starts as a papule or subcutaneous nodule that later ulcerates after trauma. The incubation period is uncertain, but experimental infections in humans indicate a duration of ~50 days. Four types of lesions have been described: (1) the classic ulcerogranulomatous lesion (Fig. 198e-1), a beefy red ulcer that bleeds readily when touched; (2) a hypertrophic or verrucous ulcer with a raised irregular edge; (3) a necrotic, offensive-smelling ulcer causing tissue destruction; and (4) a sclerotic or cicatricial lesion with fibrous and scar tissue.
FIGURE 198e-1 Ulcerogranulomatous penile lesion of donovanosis, with some hypertrophic features.
The genitals are affected in 90% of patients and the inguinal region in 10%. The most common sites of infection are the prepuce, coronal sulcus, frenum, and glans in men and the labia minora and fourchette in women. Cervical lesions may mimic cervical carcinoma. In men, lesions are associated with lack of circumcision. Lymphadenitis is uncommon. Extragenital lesions occur in 6% of cases and may involve the lip, gums, cheek, palate, pharynx, larynx, and chest. Hematogenous spread with involvement of liver and bone has been reported. During pregnancy, lesions tend to develop more quickly and respond more slowly to treatment. Polyarthritis and osteomyelitis are rare complications. In newborn infants, donovanosis may present with ear infection. Cases in children have been attributed to sitting on the laps of infected adults. As the incidence of donovanosis has decreased, the number of unusual case reports has appeared to be increasing.
Complications include neoplastic changes, pseudo-elephantiasis, and stenosis of the urethra, vagina, or anus.
DIAGNOSIS
A clinical diagnosis of donovanosis made by an experienced practitioner on the basis of the lesion’s appearance usually has a high positive predictive value. The diagnosis is confirmed by microscopic identification of Donovan bodies (Fig. 198e-2) in tissue smears. Preparation of a good-quality smear is important. If donovanosis is suspected on clinical grounds, the smear for Donovan bodies should be taken before swab samples are collected to be tested for other causes of genital ulceration so that enough material can be collected from the ulcer. A swab should be rolled firmly over an ulcer previously cleaned with a dry swab to remove debris. Smears can be examined in a clinical setting by direct microscopy with a rapid Giemsa or Wright’s stain. Alternatively, a piece of granulation tissue crushed and spread between two slides can be used. Donovan bodies can be seen in large, mononuclear (Pund) cells as gram-negative intracytoplasmic cysts filled with deeply staining bodies that may have a safety-pin appearance. These cysts eventually rupture and release the infective organisms. Histologic changes include chronic inflammation with infiltration of plasma cells and neutrophils. Epithelial changes include ulceration, microabscesses, and elongation of rete ridges.
FIGURE 198e-2 Pund cell stained by rapid Giemsa (RapiDiff) technique. Numerous Donovan bodies are visible.
A diagnostic polymerase chain reaction (PCR) test was based on the observation that two unique base changes in the phoE gene eliminate Hae111 restriction sites, enabling differentiation of K. granulomatis comb nov from related Klebsiella species. PCR analysis with a colorimetric detection system can now be used in routine diagnostic laboratories. A genital ulcer multiplex PCR that includes K. granulomatis has been developed. Serologic tests are only poorly specific and are not currently used.
The differential diagnosis of donovanosis includes primary syphilitic chancres, secondary syphilis (condylomata lata), chancroid, lymphogranuloma venereum, genital herpes, neoplasm, and amebiasis. Mixed infections are common. Histologic appearances should be distinguished from those of rhinoscleroma, leishmaniasis, and histoplasmosis.
|
TREATMENT |
DONOVANOSIS |
Many patients with donovanosis present quite late with extensive ulceration. They may be embarrassed and have low self-esteem related to their disease. Reassurance that they have a treatable condition is important, as is the need to administer antibiotics and monitor patients for an adequate interval (see below). Epidemiologic treatment of sexual partners and advice about how to improve genital hygiene are recommended.
The recommended drug regimens for donovanosis are shown in Table 198e-1. Gentamicin can be added if the response is slow. Ceftriaxone, chloramphenicol, and norfloxacin are also effective. Patients treated for 14 days should be monitored until lesions have healed completely. Those treated with azithromycin probably do not need such rigorous follow-up.
|
EFFECTIVE ANTIBIOTICS FOR THE TREATMENT OF DONOVANOSIS |
Surgery may be indicated for very advanced lesions.
CONTROL AND PREVENTION
Donovanosis is probably the cause of genital ulceration that is most readily recognizable clinically. Donovanosis is now limited to a few specific locations, and its global eradication is a distinct possibility.
SECTION 7 |
MISCELANEOUS BACTERIAL INFECTIONS |
199 |
Nocardiosis |
Nocardia, a genus of saprophytic aerobic actinomycetes that are common worldwide, resides in soil, contributing to the decay of organic matter. Nearly 100 species have been identified, mostly on the basis of 16S rRNA gene sequences. Nocardiae are relatively inactive in standard biochemical tests, and speciation is difficult without molecular phylogenetic techniques. Historically, the majority of isolates associated with pneumonia and systemic disease were identified as Nocardia asteroides, but the lineage of the type strain was muddled, and it is now clear that human disease is associated with several species. Most clinical laboratories cannot speciate isolates accurately and may identify them simply as N. asteroides or Nocardia species.
Nine species or species complexes are commonly associated with human disease (Table 199-1). Most systemic disease involves Nocardia cyriacigeorgica, Nocardia farcinica, Nocardia pseudobrasiliensis, and species in the Nocardia transvalensis and Nocardia nova complexes. Nocardia brasiliensis is usually associated with disease limited to the skin. Actinomycetoma—an indolent, slowly progressive disease of skin and underlying tissues with nodular swellings and draining sinuses—is often associated with N. brasiliensis, Nocardia otitidiscaviarum, N. transvalensis complex strains, or other actinomycetes.
|
NOCARDIA SPECIES MOST COMMONLY ASSOCIATED WITH HUMAN DISEASE AND THEIR IN VITRO SUSCEPTIBILITY PATTERNS |
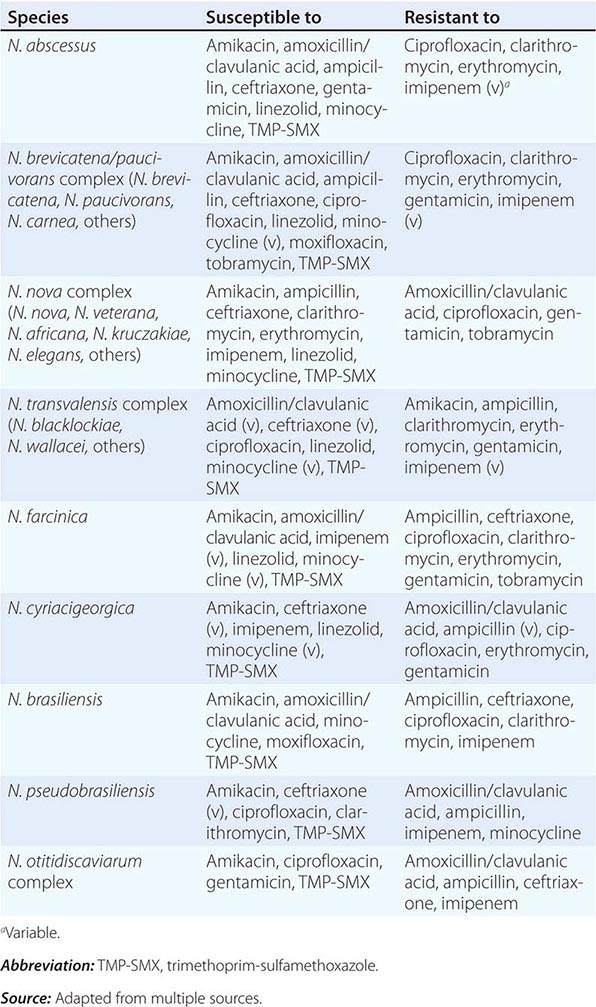
EPIDEMIOLOGY
![]() Nocardiosis occurs worldwide. The annual incidence, estimated on three continents (North America, Europe, and Australia), is ~0.375 cases per 100,000 persons and may be increasing. The disease is more common among adults than among children and more common among males than among females. Nearly all cases are sporadic, but outbreaks have been associated with contamination of the hospital environment, cosmetic procedures, and parenteral illicit drug use. Person-to-person spread is not well documented. There is no known seasonality.
Nocardiosis occurs worldwide. The annual incidence, estimated on three continents (North America, Europe, and Australia), is ~0.375 cases per 100,000 persons and may be increasing. The disease is more common among adults than among children and more common among males than among females. Nearly all cases are sporadic, but outbreaks have been associated with contamination of the hospital environment, cosmetic procedures, and parenteral illicit drug use. Person-to-person spread is not well documented. There is no known seasonality.
The majority of cases of pulmonary or disseminated disease occur in people with a host defense defect. Most have deficient cell-mediated immunity, especially that associated with lymphoma, transplantation, glucocorticoid therapy, or AIDS. The incidence is ~140-fold greater among patients with AIDS and ~340-fold greater among bone marrow transplant recipients than in general populations. In AIDS, nocardiosis usually affects persons with <250 CD4+ T lymphocytes/μL. Nocardiosis has also been associated with pulmonary alveolar proteinosis, tuberculosis and other mycobacterial diseases, chronic granulomatous disease, interleukin 12 deficiency, and treatment with monoclonal antibodies that interfere with tumor necrosis factor. Any child with nocardiosis and no known cause of immunosuppression should undergo tests to determine the adequacy of the phagocytic respiratory burst.
Cases of actinomycetoma occur mainly in tropical and subtropical regions, especially those of Mexico, Central and South America, Africa, and India. The most important risk factor is frequent contact with soil or vegetable matter, especially in laborers.
PATHOLOGY AND PATHOGENESIS
Pneumonia and disseminated disease are both thought to follow inhalation of fragmented bacterial mycelia. The characteristic histologic feature of nocardiosis is an abscess with extensive neutrophil infiltration and prominent necrosis. Granulation tissue usually surrounds the lesions, but extensive fibrosis or encapsulation is uncommon.
Actinomycetoma is characterized by suppurative inflammation with sinus tract formation. Granules—microcolonies composed of dense masses of bacterial filaments extending radially from a central core—are occasionally observed in histologic preparations. The granules are frequently found in discharges from lesions of actinomycetoma but almost never in discharges from lesions in other forms of nocardiosis.
Nocardiae have evolved a number of properties that enable them to survive within phagocytes, including neutralization of oxidants, prevention of phagosome-lysosome fusion, and prevention of phagosome acidification. Neutrophils phagocytose the organisms and limit their growth but do not kill them efficiently. Cell-mediated immunity is important for definitive control and elimination of nocardiae.
CLINICAL MANIFESTATIONS
Respiratory Tract Disease Pneumonia, the most common form of nocardial disease in the respiratory tract, is typically subacute; symptoms have usually been present for days or weeks at presentation. The onset is occasionally more acute in immunosuppressed patients. Cough is prominent and produces small amounts of thick, purulent sputum that is not malodorous. Fever, anorexia, weight loss, and malaise are common; dyspnea, pleuritic pain, and hemoptysis are less common. Remissions and exacerbations over several weeks are frequent. Roentgenographic patterns vary, but some are highly suggestive of nocardial pneumonia. Infiltrates vary in size and are typically dense. Single or multiple nodules are common (Figs. 199-1 and 199-2), sometimes suggesting tumors or metastases. Infiltrates and nodules tend to cavitate (Fig. 199-2). Empyema is present in one-quarter of cases. Co-infection with Nocardia and Mycobacterium tuberculosis has been reported from regions where tuberculosis is common.
FIGURE 199-1 Nocardial pneumonia. A dense infiltrate with a possible cavity and several nodules are apparent in the right lung.
FIGURE 199-2 Nocardial pneumonia. A computed tomography scan shows bilateral nodules, with cavitation in the nodule in the left lung.
Nocardiosis may spread directly from the lungs to adjacent tissues. Pericarditis, mediastinitis, and the superior vena cava syndrome have all been reported. Nocardial laryngitis, tracheitis, bronchitis, and sinusitis are much less common than pneumonia. In the major airways, disease often presents as a nodular or granulomatous mass. Nocardiae are sometimes isolated from respiratory secretions of persons without apparent nocardial disease, usually individuals who have underlying lung or airway abnormalities.
Extrapulmonary Disease In half of all cases of pulmonary nocardiosis, disease appears outside the lungs. In one-fifth of cases of disseminated disease, lung disease is not apparent. The most common site of dissemination is the brain. Other common sites include the skin and supporting structures, kidneys, bone, muscle, and eye, but almost any organ can be involved. Peritonitis has been reported in patients undergoing peritoneal dialysis. Nocardiae have been recovered from blood in a few cases of pneumonia, disseminated disease, or central venous catheter infection. Nocardial endocarditis occurs rarely and can affect either native or prosthetic valves.
The typical manifestation of extrapulmonary dissemination is a subacute abscess. A minority of abscesses outside the lungs or central nervous system (CNS) form fistulas and discharge small amounts of pus. In CNS infections, brain abscesses are usually supratentorial, are often multiloculated, and may be single or multiple (Fig. 199-3). Brain abscesses tend to burrow into the ventricles or extend out into the subarachnoid space. The symptoms and signs are somewhat more indolent than those of other types of bacterial brain abscess. Meningitis is uncommon and is usually due to spread from a nearby brain abscess. Nocardiae are not easily recovered from cerebrospinal fluid (CSF).
FIGURE 199-3 Nocardial abscesses in the right occipital lobe.
Disease Following Transcutaneous Inoculation Disease that follows transcutaneous nocardial inoculation usually takes one of three forms: cellulitis, lymphocutaneous syndrome, or actinomycetoma.
Cellulitis generally begins 1–3 weeks after a recognized breach of the skin, often with soil contamination. Subacute cellulitis, with pain, swelling, erythema, and warmth, develops over days to weeks. The lesions are usually firm and not fluctuant. Disease may progress to involve underlying muscles, tendons, bones, or joints. Dissemination is rare. N. brasiliensis and species in the N. otitidiscaviarum complex are most common in cellulitis cases.
Lymphocutaneous disease usually begins as a pyodermatous nodule at the site of inoculation, with central ulceration and purulent or honey-colored drainage. Subcutaneous nodules often appear along lymphatics that drain the primary lesion. Most cases of nocardial lymphocutaneous syndrome are associated with N. brasiliensis. Similar disease occurs with other pathogens, most notably Sporothrix schenckii (Chap. 243) and Mycobacterium marinum (Chap. 204).
Actinomycetoma usually begins with a nodular swelling, sometimes at a site of local trauma. Lesions (Fig. 199-4A) typically develop on the feet or hands but may involve the posterior part of the neck, the upper back, the head, and other sites. The nodule eventually breaks down, and a fistula appears, typically followed by others. The fistulas tend to come and go, with new ones forming as old ones disappear. The discharge is serous or purulent, may be bloody, and often contains 0.1- to 2-mm white granules consisting of masses of mycelia (Figs. 199-4C and 199-4D). The lesions spread slowly along fascial planes to involve adjacent areas of skin, subcutaneous tissue, and bone. Over months or years, there may be extensive deformation of the affected part. Lesions involving soft tissues are only mildly painful; those affecting bones or joints are more so (Fig. 199-4B). Systemic symptoms are absent or minimal. Infection rarely disseminates from actinomycetoma, and lesions on the hands and feet usually cause only local disability. Lesions on the head, neck, and trunk can invade locally to involve deep organs, with consequent severe disability or death.
FIGURE 199-4 Nocardia brasiliensis mycetoma. A. Draining sinuses and giant white grains with a seropurulent discharge. B. Radiography of the foot showing marked soft tissue enlargement and bony lytic lesions. C. Direct microscopy of grains stained with Lugol’s iodine (×40). D. Periodic acid–Schiff stain of skin biopsy (×40). (Image provided by Roberto Arenas and Mahreen Ameen, St. John’s Institute of Dermatology, Guy’s & St Thomas’ NHS Trust, London, UK. Reprinted with permission from R Arenas, M Ameen: Lancet Infect Dis 10:66, 2010.)
Eye Infections Nocardia species are uncommon causes of subacute keratitis, usually following eye trauma. Nocardial endophthalmitis can develop after eye surgery. In one series, nocardiae accounted for more than half of culture-proved cases of endophthalmitis after cataract surgery. Endophthalmitis can also occur during disseminated disease. Nocardial infection of lachrymal glands has been reported.
DIAGNOSIS
The first step in diagnosis is examination of sputum or pus for crooked, branching, beaded, gram-positive filaments 1 μm wide and up to 50 μm long (Fig. 199-5). Most nocardiae are acid-fast in direct smears if a weak acid is used for decolorization (e.g., in the modified Kinyoun, Ziehl-Neelsen, and Fite-Faraco methods). The organisms often take up silver stains. Recovery from specimens containing a mixed flora can be improved with selective media (colistin–nalidixic acid agar, modified Thayer-Martin agar, or buffered charcoal–yeast extract agar). Nocardiae grow well on most fungal and mycobacterial media, but procedures used for decontamination of specimens for mycobacterial culture can kill nocardiae and thus should not be used when nocardiae are suspected. Nocardiae grow relatively slowly; colonies may take up to 2 weeks to appear and may not develop their characteristic appearance—white, yellow, or orange, with aerial mycelia and delicate, dichotomously branched substrate mycelia—for up to 4 weeks. Several blood culture systems support nocardial growth, although nocardiae may not be detected for up to 2 weeks. The growth of nocardiae is so different from that of more common pathogens that the laboratory should be alerted when nocardiosis is suspected in order to maximize the likelihood of isolation.
FIGURE 199-5 Gram-stained sputum from a patient with nocardial pneumonia. (Image provided by Charles Cartwright and Susan Nelson, Hennepin County Medical Center, Minneapolis, MN.)
In nocardial pneumonia, sputum smears are often negative. Unless the diagnosis can be made in smear-negative cases by sampling lesions in more accessible sites, bronchoscopy or lung aspiration is usually necessary. To evaluate the possibility of dissemination in patients with nocardial pneumonia, a careful history should be obtained and a thorough physical examination performed. Suggestive symptoms or signs should be pursued with further diagnostic tests. Computed tomography (CT) or magnetic resonance imaging (MRI) of the head, with and without contrast material, should be undertaken if signs or symptoms suggest brain involvement. Some authorities recommend brain imaging in all cases of pulmonary or disseminated disease. When clinically indicated, CSF or urine should be concentrated and then cultured. Actinomycetoma, eumycetoma (cases involving fungi; Chap. 243), and botryomycosis (cases involving cocci or bacilli, often Staphylococcus aureus) are difficult to distinguish clinically but are readily distinguished with microbiologic testing or biopsy. Granules should be sought in any discharge. Suspect particles should be washed in saline, examined microscopically, and cultured. Granules in actinomycetoma cases are usually white, pale yellow, pink, or red. Viewed microscopically, they consist of tight masses of fine filaments (0.5–1 μm wide) radiating outward from a central core (Fig. 199-5). Granules from eumycetoma cases are white, yellow, brown, black, or green. Under the microscope, they appear as masses of broader filaments (2–5 μm wide) encased in a matrix. Granules of botryomycosis consist of loose masses of cocci or bacilli. Organisms can also be seen in wound discharge or histologic specimens. The most reliable way to differentiate among the various organisms associated with mycetoma is by culture.
Isolation of nocardiae from sputum or blood occasionally represents colonization, transient infection, or contamination. In typical cases of respiratory tract colonization, Gram-stained specimens are negative and cultures are only intermittently positive. A positive sputum culture in an immunosuppressed patient usually reflects disease. When nocardiae are isolated from sputum of an immunocompetent patient without apparent nocardial disease, the patient should be observed carefully without treatment. A patient with a host-defense defect that increases the risk of nocardiosis should usually receive antimicrobial treatment.
|
TREATMENT |
NOCARDIOSIS |
For mild or moderate cases, therapy with drugs known to be effective against most isolates is usually adequate. For severe cases or cases that do not respond promptly to antimicrobial therapy, isolates should be sent to a laboratory experienced with Nocardia for identification and susceptibility testing. Identification of an isolate to the species level is accomplished with molecular testing, and susceptibility is assessed with a Clinical Laboratory Standards Institute (CLSI)–approved broth dilution test. Nocardial growth is slower than the growth of most clinically important bacteria, and nocardiae tend to clump in suspension so that susceptibility test endpoints are unusual; thus experience is necessary for reliable results. Because nocardiosis is uncommon, data on the relation between susceptibility test results for specific drugs and clinical outcomes in patients treated with these drugs are meager. Careful clinical monitoring is essential, and consultation with clinicians who have experience with nocardiosis is often needed.
Sulfonamides are the drugs of choice (Tables 199-1 and 199-2). The combination of sulfamethoxazole (SMX) and trimethoprim (TMP) is at least equivalent to a sulfonamide alone and may be slightly more effective, but the combination also poses a modestly greater risk of hematologic toxicity. At the outset, 10–20 mg/kg of TMP and 50–100 mg/kg of SMX are given each day in two divided doses. Later, daily doses can be decreased to as little as 5 mg/kg and 25 mg/kg, respectively. In persons with sulfonamide allergies, desensitization usually allows continuation of therapy with these effective and inexpensive drugs.
|
TREATMENT DURATION FOR NOCARDIOSIS |
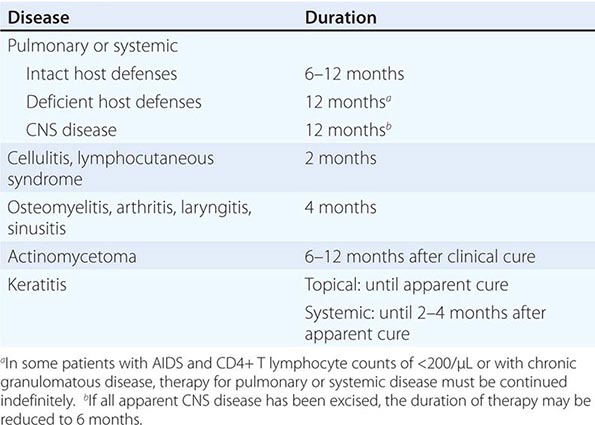
Sulfonamide susceptibility testing is difficult. The CLSI standard methodology includes a technique for TMP-SMX but not for a sulfonamide alone. Reported rates of sulfonamide susceptibility have varied widely, and controversy has ensued about the reliability of sulfonamides for therapy. However, clinical responses to appropriate sulfonamide treatment are nearly always satisfactory. Sulfonamides remain the drugs of choice in nearly all cases.
Clinical experience with other oral drugs is limited. Minocycline (100–200 mg twice a day) is often effective; other tetracyclines are usually less effective. Linezolid is active against all species in vitro and in vivo, but adverse effects are common with long-term use. Tigecycline appears to be active in vitro against some species, but little clinical experience has been reported. Amoxicillin (875 mg) combined with clavulanic acid (125 mg), given twice a day, has been effective but should be avoided in cases involving strains of the N. nova complex, in which clavulanate induces β-lactamase production. Among the quinolones, moxifloxacin and gemifloxacin appear to be most active.
Amikacin, the best-established parenteral drug except in cases involving the N. transvalensis complex, is given in doses of 5–7.5 mg/kg every 12 h or 15 mg/kg every 24 h. Serum drug levels should be monitored during prolonged therapy in patients with diminished renal function and in the elderly. Ceftriaxone and imipenem are usually effective except as indicated in Table 199-1.
Patients with severe disease are initially treated with a combination including TMP-SMX, amikacin, and ceftriaxone or imipenem. Clinical improvement is usually noticeable after 1–2 weeks of therapy but may take longer, especially with CNS disease. After definite clinical improvement, therapy can be continued with a single oral drug, usually TMP-SMX. Some experts use two or more drugs for the entire course of therapy, but whether multiple drugs are better than a single agent is not known, and additional drugs increase the risk of toxicity. In patients with nocardiosis who need immunosuppressive therapy for an underlying disease or prevention of transplant rejection, immunosuppressive therapy should be continued.
Use of SMX and TMP in high-risk populations to prevent Pneumocystis disease or urinary tract infections appears to reduce but not eliminate the risk of nocardiosis. The incidence of nocardiosis is low enough that prophylaxis solely to prevent this disease is not recommended.
Surgical management of nocardial disease is similar to that of other bacterial diseases. Brain abscesses should be aspirated, drained, or excised if the diagnosis is unclear, if an abscess is large and accessible, or if an abscess fails to respond to chemotherapy. Small or inaccessible brain abscesses should be treated medically; clinical improvement should be noticeable within 1–2 weeks. Brain imaging should be repeated to document the resolution of lesions, although abatement on images often lags behind clinical improvement.
Antimicrobial therapy usually suffices for nocardial actinomycetoma. In deep or extensive cases, drainage or excision of heavily involved tissue may facilitate healing, but structure and function should be preserved whenever possible. Keratitis is treated with topical sulfonamide or amikacin drops plus a sulfonamide or an alternative drug given by mouth.
Nocardial infections tend to relapse (particularly in patients with chronic granulomatous disease), and long courses of antimicrobial therapy are necessary (Table 199-2). If disease is unusually extensive or if the response to therapy is slow, the recommendations in Table 199-2 should be exceeded.
With appropriate treatment, the mortality rate for pulmonary or disseminated nocardiosis outside the CNS should be <5%. CNS disease carries a higher mortality rate. Patients should be followed carefully for at least 6 months after therapy has ended.
200 |
Actinomycosis and Whipple’s Disease |
Actinomycosis and Whipple’s disease share characteristics that confound even the skilled diagnostician. Because both diseases are uncommon, the physician’s personal experience with their clinical presentations is limited. The laboratory identification of the etiologic agents from the order Actinomycetales is not routine. Thus they remain a diagnostic challenge. However, both of these chronic infections are curable, usually with medical therapy alone. Therefore, an awareness of the full spectrum of these diseases, prompting clinical suspicion, can expedite their diagnosis and treatment and minimize unnecessary surgical interventions (especially with actinomycosis), morbidity, and mortality risk.
ACTINOMYCOSIS
Actinomycosis is an indolent, slowly progressive infection caused by anaerobic or microaerophilic bacteria, primarily of the genus Actinomyces, that colonize the mouth, colon, and vagina. Mucosal disruption may lead to infection at virtually any site in the body. In vivo growth of actinomycetes usually results in the formation of characteristic clumps called grains or sulfur granules. The clinical presentations of actinomycosis are myriad. Common in the preantibiotic era, actinomycosis has diminished in incidence, as has its timely recognition. Actinomycosis has been called the most misdiagnosed disease, and it has been said that no disease is so often missed by experienced clinicians.
Three “classic” clinical presentations that should prompt consideration of this unique infection are (1) the combination of chronicity, progression across tissue boundaries, and mass-like features (mimicking malignancy, with which it is often confused); (2) the development of a sinus tract, which may spontaneously resolve and recur; and (3) a refractory or relapsing infection after a short course of therapy, since cure of established actinomycosis requires prolonged treatment.
ETIOLOGIC AGENTS
Actinomycosis is most commonly caused by A. israelii, A. naeslundii, A. odontolyticus, A. viscosus, A. meyeri, and A. gerencseriae. Most if not all actinomycotic infections are polymicrobial. Aggregatibacter (Actinobacillus) actinomycetemcomitans, Eikenella corrodens, Enterobacteriaceae, and species of Fusobacterium, Bacteroides, Capnocytophaga, Staphylococcus, and Streptococcus are commonly isolated with actinomycetes in various combinations, depending on the site of infection. Their contribution to the pathogenesis of actinomycosis is uncertain.
Comparative 16S rRNA gene sequencing has led to the identification of an ever-expanding list of Actinomyces species and a reclassification of some species to other genera. At present, 46 species and 2 subspecies have been recognized (www.bacterio.cict.fr/a/actinomyces.html). A. europaeus, A. neuii, A. radingae, A. graevenitzii, A. turicensis, A. cardiffensis, A. houstonensis, A. hongkongensis, A. lingnae, A. massiliensis, A. timonensis, and A. funkei as well as two former Actinomyces species—Arcanobacterium pyogenes and Arcanobacterium bernardiae—are additional causes of human actinomycosis, albeit not always with a “classic” presentation.
EPIDEMIOLOGY
Actinomycosis has no geographic boundaries and occurs throughout life, with a peak incidence in the middle decades. Males have a threefold higher incidence than females, possibly because of poorer dental hygiene and/or more frequent trauma. Improved dental hygiene and the initiation of antimicrobial treatment before actinomycosis fully develops have probably contributed to a decrease in incidence since the advent of antibiotics. Individuals who do not seek or have access to health care, those who have an intrauterine contraceptive device (IUCD) in place for a prolonged period (see “Pelvic Disease,” below), and those who receive bisphosphonate treatment (see “Oral-Cervicofacial Disease,” below) are probably at higher risk.
PATHOGENESIS AND PATHOLOGY
The etiologic agents of actinomycosis are members of the normal oral flora and are often cultured from the bronchi, the gastrointestinal tract, and the female genital tract. The critical step in the development of actinomycosis is disruption of the mucosal barrier. Local infection may ensue. Once established, actinomycosis spreads contiguously in a slow, progressive manner, ignoring tissue planes. Although acute inflammation may initially develop at the infection site, the hallmark of actinomycosis is the characteristic chronic, indolent phase manifested by lesions that usually appear as single or multiple indurations. Central necrosis consisting of neutrophils and sulfur granules develops and is virtually diagnostic. The fibrotic walls of the mass are typically described as “wooden.” The responsible bacterial and/or host factors have not been identified. Over time, sinus tracts to the skin, adjacent organs, or bone may develop. In rare instances, distant hematogenous seeding may occur. As mentioned above, these unique features of actinomycosis mimic malignancy, with which it is often confused.
Foreign bodies appear to facilitate infection. This association most frequently involves IUCDs. Reports have described an association of actinomycosis with HIV infection; transplantation; common variable immunodeficiency; chronic granulomatous disease; treatment with infliximab, glucocorticoids, or bisphosphonates; and radio- or chemotherapy. Ulcerative mucosal infections (e.g., by herpes simplex virus or cytomegalovirus) may facilitate disease development.
CLINICAL MANIFESTATIONS
Oral-Cervicofacial Disease Actinomycosis occurs most frequently at an oral, cervical, or facial site, usually as a soft tissue swelling, abscess, or mass lesion that is often mistaken for a neoplasm. The angle of the jaw is generally involved, but a diagnosis of actinomycosis should be considered with any mass lesion or relapsing infection in the head and neck (Chap. 44). Radiation therapy and especially bisphosphonate treatment have been recognized as contributing to an increasing incidence of actinomycotic infection of the mandible and maxilla (Fig. 200-1). Canaliculitis (also commonly due to Propionibacterium propionicum), otitis, and sinusitis also can develop. Pain, fever, and leukocytosis are variably reported. Contiguous extension to the cranium, cervical spine, or thorax is a potential sequela.
FIGURE 200-1 Bisphosphonate-associated maxillary osteomyelitis due to A. viscosus. A sulfur granule is seen within the bone. (Reprinted with permission from NH Naik, TA Russo: Bisphosphonate related osteonecrosis of the jaw: The role of Actinomyces. Clin Infect Dis 49:1729, 2009. © 2009 University of Chicago Press.)
Thoracic Disease Thoracic actinomycosis, which may be facilitated by foreign material, usually follows an indolent progressive course, with involvement of the pulmonary parenchyma and/or the pleural space. Chest pain, fever, and weight loss are common. A cough, when present, is variably productive. The usual radiographic finding is either a mass lesion or pneumonia. On CT, central areas of low attenuation and ringlike rim enhancement may be seen. Cavitary disease or mediastinal or hilar adenopathy may develop. More than 50% of cases include pleural thickening, effusion, or empyema (Fig. 200-2). Rarely, pulmonary nodules or endobronchial lesions occur. Lesions suggestive of actinomycosis include those that cross fissures or pleura; extend into the mediastinum, contiguous bone, or chest wall; or are associated with a sinus tract. In the absence of these findings, thoracic actinomycosis is usually mistaken for a neoplasm or pneumonia due to more usual causes.
FIGURE 200-2 Thoracic actinomycosis. A. A chest wall mass from extension of pulmonary infection. B. Pulmonary infection is complicated by empyema (open arrow) and extension to the chest wall (closed arrow). (Courtesy of Dr. C. B. Hsiao, Division of Infectious Diseases, Department of Medicine, State University of New York at Buffalo.)
Mediastinal infection is uncommon, usually arising from thoracic extension but rarely from perforation of the esophagus, trauma, or extension of head and neck or abdominal disease. The structures within the mediastinum and the heart can be involved in various combinations; consequently, the possible presentations are diverse. Primary endocarditis (in which A. neuii has been increasingly described) and isolated disease of the breast occur.
Abdominal Disease Abdominal actinomycosis poses a great diagnostic challenge. Months or years usually pass from the inciting event (e.g., appendicitis, diverticulitis, peptic ulcer disease, spillage of gall stones or bile during laparoscopic cholecystectomy, foreign-body perforation, bowel surgery, or ascension from IUCD-associated pelvic disease) to clinical recognition. Because of the flow of peritoneal fluid and/or the direct extension of primary disease, virtually any abdominal organ, region, or space can be involved. The disease usually presents as an abscess, a mass, or a mixed lesion that is often fixed to underlying tissue and mistaken for a tumor. On CT, enhancement is most often heterogeneous and adjacent bowel is thickened. Sinus tracts to the abdominal wall, to the perianal region, or between the bowel and other organs may develop and mimic inflammatory bowel disease (Chap. 351). Recurrent disease or a wound or fistula that fails to heal suggests actinomycosis.
Hepatic infection usually presents as one or more abscesses or masses (Fig. 200-3). Isolated disease presumably develops via hematogenous seeding from cryptic foci. Imaging and percutaneous techniques have resulted in improved diagnosis and treatment.
FIGURE 200-3 Hepatic-splenic actinomycosis. A. Computed tomogram showing multiple hepatic abscesses and a small splenic lesion due to A. israelii. Arrow indicates extension outside the liver. Inset: Gram’s stain of abscess fluid demonstrating beaded filamentous gram-positive rods. B. Subsequent formation of a sinus tract. (Reprinted with permission from Saad M: Actinomyces hepatic abscess with cutaneous fistula. N Engl J Med 353:e16, 2005. © 2005 Massachusetts Medical Society. All rights reserved.)
All levels of the urogenital tract can be infected. Renal disease usually presents as pyelonephritis and/or renal and perinephric abscess. Bladder involvement, usually due to extension of pelvic disease, may result in ureteral obstruction or fistulas to bowel, skin, or uterus. Actinomyces can be detected in urine with appropriate stains and cultures.
Pelvic Disease Actinomycotic involvement of the pelvis occurs most commonly in association with an IUCD. When an IUCD is in place or has recently been removed, pelvic symptoms should prompt consideration of actinomycosis. The risk, although not quantified, appears small. The disease rarely develops when the IUCD has been in place for <1 year, but the risk increases with time. Actinomycosis can also present months after IUCD removal. Symptoms are typically indolent; fever, weight loss, abdominal pain, and abnormal vaginal bleeding or discharge are the most common. The earliest stage of disease—often endometritis—commonly progresses to pelvic masses or a tuboovarian abscess (Fig. 200-4). Unfortunately, because the diagnosis is often delayed, a “frozen pelvis” mimicking malignancy or endometriosis can develop by the time of recognition. Ca125 levels may be elevated, further contributing to misdiagnosis.
FIGURE 200-4 Computed tomogram showing pelvic actinomycosis associated with an intrauterine contraceptive device. The device is encased by endometrial fibrosis (solid arrow); also visible are paraendometrial fibrosis (open triangular arrowhead) and an area of suppuration (open arrow).
Actinomyces-like organisms (ALOs), which are identified in Papanicolaou-stained specimens in (on average) 7% of women using an IUCD, have a low positive predictive value for diagnosis. Nonetheless, although the risk appears small, the consequences of infection are significant. Therefore, until more quantitative data become available, it seems prudent to remove the IUCD in the presence of symptoms that cannot be accounted for, regardless of whether ALOs are detected, and—if advanced disease is excluded—to initiate a 14-day course of empirical treatment for possible early endometritis. The detection of ALOs in the asymptomatic patient warrants education and close follow-up but not removal of the IUCD unless a suitable contraceptive alternative is agreed on.
Central Nervous System Disease Actinomycosis of the central nervous system (CNS) is rare. Single or multiple brain abscesses are most common. An abscess usually appears on CT as a ring-enhancing lesion with a thick wall that may be irregular or nodular. Magnetic resonance perfusion and spectroscopy findings have also been described, as have primary meningitis, epidural or subdural space infection, and cavernous sinus syndrome.
Musculoskeletal and Soft Tissue Infection Actinomycotic infection of bone and joints is usually due to adjacent soft-tissue infection but may be associated with trauma (e.g., fracture of the mandible), injections, surgery, osteoradionecrosis and bisphosphonate osteonecrosis (limited to mandibular and maxillary bones), or hematogenous spread. Because of slow disease progression, new bone formation and bone destruction are seen concomitantly. Infection of an extremity is uncommon and is usually a result of trauma. Skin, subcutaneous tissue, muscle, and bone (with periostitis or acute or chronic osteomyelitis) are involved alone or in various combinations. Cutaneous sinus tracts frequently develop.
Disseminated Disease Hematogenous dissemination of disease from any location rarely results in multiple-organ involvement. A. meyeri is most commonly involved. The lungs and liver are most commonly affected, with the presentation of multiple nodules mimicking disseminated malignancy. The clinical presentation may be surprisingly indolent given the extent of disease.
DIAGNOSIS
The diagnosis of actinomycosis is rarely considered. All too often, actinomycosis is first mentioned by the pathologist after extensive surgery. Since medical therapy alone is frequently sufficient for cure, the challenge for the clinician is to consider the possibility of actinomycosis, to diagnose it in the least invasive fashion, and to avoid unnecessary surgery. The clinical and radiographic presentations that suggest actinomycosis are discussed above. Of note, hypermetabolism has been demonstrated by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in actinomycotic disease. Aspirations and biopsies (with or without CT or ultrasound guidance) are being used successfully to obtain clinical material for diagnosis, although surgery may be required. The diagnosis is most commonly made by microscopic identification of sulfur granules (an in vivo matrix of bacteria, calcium phosphate, and host material) in pus or tissues. Occasionally, these granules are identified grossly from draining sinus tracts or pus. Although sulfur granules are a defining characteristic of actinomycosis, granules also are found in mycetoma (Chaps. 199 and 243) and botryomycosis (a chronic suppurative bacterial infection of soft tissue or, in rare cases, visceral tissue that produces clumps of bacteria resembling granules). These entities can easily be differentiated from actinomycosis with appropriate histopathologic and microbiologic studies. Microbiologic identification of actinomycetes is often precluded by prior antimicrobial therapy or failure to perform appropriate microbiologic cultures. For optimal yield, the avoidance of even a single dose of antibiotics is mandatory. Primary isolation usually requires 5–7 days under anaerobic conditions but may take as long as 2–4 weeks. Although not routinely used, 16S rRNA gene amplification and sequencing have been successfully applied to increase diagnostic sensitivity and specificity. Because actinomycetes are components of the normal oral and genital-tract flora, their identification in the absence of sulfur granules in sputum, bronchial washings, and cervicovaginal secretions is of little significance.
|
TREATMENT |
ACTINOMYCOSIS |
Decisions about treatment are based on the collective clinical experience of the past 65 years. Actinomycosis requires prolonged treatment with high doses of antimicrobial agents; suitable antimicrobial agents and those deemed unreliable are listed in Table 200-1. The need for intensive treatment is presumably due to the drugs’ poor penetration of the thick-walled masses common in this infection and/or the sulfur granules themselves, which may represent a biofilm. Although therapy must be individualized, the IV administration of 18–24 million units of penicillin daily for 2–6 weeks, followed by oral therapy with penicillin or amoxicillin (total duration, 6–12 months), is a reasonable guideline for serious infections and bulky disease. Less extensive disease, particularly that involving the oral-cervicofacial region, may be cured with a shorter course. If therapy is extended beyond the resolution of measurable disease, the risk of relapse—a clinical hallmark of this infection—will be minimized; CT and MRI are generally the most sensitive and objective techniques by which to accomplish this goal. A similar approach is reasonable for immunocompromised patients, although refractory disease has been described in HIV-infected individuals. Although the role played by “companion” microbes in actinomycosis is unclear, many isolates are pathogens in their own right, and a regimen covering these organisms during the initial treatment course is reasonable.
|
APPROPRIATE AND INAPPROPRIATE ANTIBIOTIC THERAPY FOR ACTINOMYCOSISa |
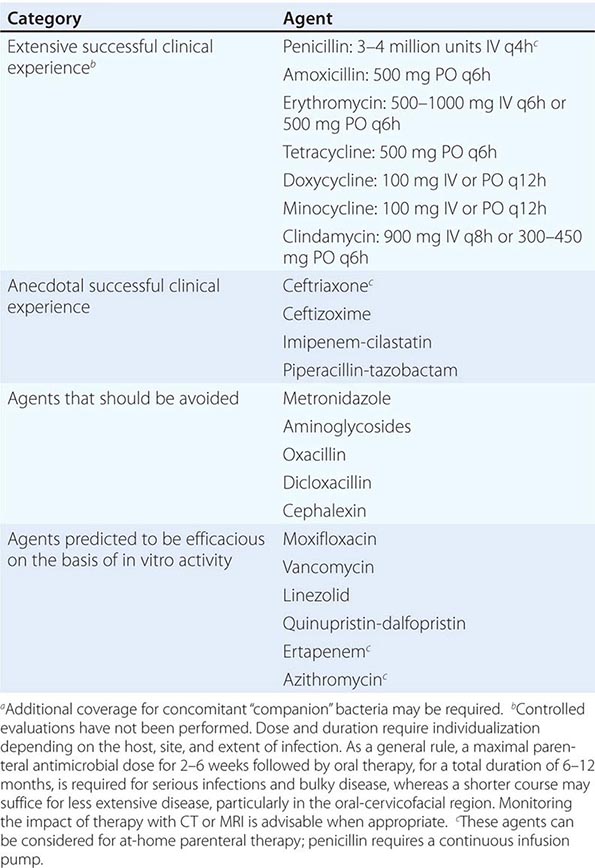
Combined medical-surgical therapy is still advocated in some reports. However, an increasing body of literature now supports an initial attempt at cure with medical therapy alone, even in extensive disease. CT and MRI should be used to monitor the response to therapy. In most cases, either surgery can be avoided or a less extensive procedure can be used. This approach is particularly valuable in sparing critical organs, such as the bladder or the reproductive organs in women of childbearing age. For a well-defined abscess, percutaneous drainage in combination with medical therapy is a reasonable approach. When a critical location is involved (e.g., the epidural space, the CNS), when there is significant hemoptysis, or when suitable medical therapy fails, surgical intervention may be appropriate. In the absence of optimal data, the combination of a prolonged course of antimicrobial therapy and resection—at least of necrotic bone for bisphosphonate-related osteonecrosis of the jaw (BRONJ)—is a reasonable approach.
WHIPPLE’S DISEASE
Whipple’s disease, a chronic multiorgan infection caused by Tropheryma whipplei, was first described in 1907. The long-held belief that Whipple’s disease is an infection was supported by observations on its responsiveness to antimicrobial therapy in the 1950s and the identification of bacilli via electron microscopy in small-bowel biopsy specimens in the 1960s. This hypothesis was finally confirmed by amplification and sequencing of a partial 16S rRNA polymerase chain reaction (PCR)–generated amplicon from duodenal tissue in 1991. The subsequent successful cultivation of T. whipplei enabled whole-genome sequencing and the development of additional diagnostic tests. The development of PCR-based diagnostics has broadened our understanding of both the epidemiology and the clinical syndromes attributable to T. whipplei. Exposure to T. whipplei, which appears to be much more common than has been appreciated, can be followed by asymptomatic carriage, acute disease, or chronic infection. Chronic infection (Whipple’s disease) is a rare development after exposure. “Classic” Whipple’s disease is manifested variably by a combination of arthralgias/arthritis, weight loss, chronic diarrhea, abdominal pain, and fever; less commonly, involvement at sites other than the gastrointestinal tract is documented. Acute infection and chronic organ disease in the absence of intestinal involvement (see “Isolated Infection,” below) are described with increasing frequency. Since untreated Whipple’s disease is often fatal and delayed diagnosis may lead to irreparable organ damage (e.g., in the CNS), knowledge of the clinical scenarios in which Whipple’s should be considered and of an appropriate diagnostic strategy is mandatory.
ETIOLOGIC AGENT
![]() T. whipplei is a weakly staining gram-positive bacillus. Genomic sequence data have revealed that the organism has a small (<1-megabase) chromosome, with many biosynthetic pathways absent or incomplete. This finding is consistent with a host-dependent intracellular pathogen or a pathogen that requires a nutritionally rich extracellular environment. A genotyping scheme based on a variable region has disclosed more than 70 genotypes (GTs) to date. GTs 1 and 3 are most commonly reported, but all GTs appear to be capable of causing similar clinical syndromes.
T. whipplei is a weakly staining gram-positive bacillus. Genomic sequence data have revealed that the organism has a small (<1-megabase) chromosome, with many biosynthetic pathways absent or incomplete. This finding is consistent with a host-dependent intracellular pathogen or a pathogen that requires a nutritionally rich extracellular environment. A genotyping scheme based on a variable region has disclosed more than 70 genotypes (GTs) to date. GTs 1 and 3 are most commonly reported, but all GTs appear to be capable of causing similar clinical syndromes.
EPIDEMIOLOGY
![]() Whipple’s disease is rare but has been increasingly recognized since the advent of PCR-based diagnostic tools. It occurs in all parts of the globe, with an incidence presently estimated at 1 case per 1 million patient-years. Seroprevalence studies indicate that ~50% of Western Europeans and ~75% of Africans from rural Senegal have been exposed to T. whipplei. A predilection for chronic disease has been observed in middle-aged Caucasian men. Males are infected five to eight times more frequently than females. To date, no clear animal or environmental reservoir has been demonstrated. However, the organism has been identified by PCR in sewage water and human feces. Workers with direct exposure to sewage are more likely to be asymptomatically colonized than controls, a pattern suggesting fecal-oral spread. Recent data support oral-oral or fecal-oral spread among family members. Further, the development of acute T. whipplei pneumonia in children raises the possibility of droplet or airborne transmission.
Whipple’s disease is rare but has been increasingly recognized since the advent of PCR-based diagnostic tools. It occurs in all parts of the globe, with an incidence presently estimated at 1 case per 1 million patient-years. Seroprevalence studies indicate that ~50% of Western Europeans and ~75% of Africans from rural Senegal have been exposed to T. whipplei. A predilection for chronic disease has been observed in middle-aged Caucasian men. Males are infected five to eight times more frequently than females. To date, no clear animal or environmental reservoir has been demonstrated. However, the organism has been identified by PCR in sewage water and human feces. Workers with direct exposure to sewage are more likely to be asymptomatically colonized than controls, a pattern suggesting fecal-oral spread. Recent data support oral-oral or fecal-oral spread among family members. Further, the development of acute T. whipplei pneumonia in children raises the possibility of droplet or airborne transmission.
PATHOGENESIS AND PATHOLOGY
Since rates of exposure to T. whipplei appear to be much higher (e.g., ~50% in Western Europe, as stated above) than rates of chronic disease development (0.00001%), it has been hypothesized that chronically infected individuals possess a subtle host-defense abnormality that does not place them at risk for non–T. whipplei infection. The HLA alleles DRB1*13 and DQB1*06 may be associated with an increased risk of infection. Chronic infection results in a general state of immunosuppression characterized by low CD4+ T cell counts, high levels of interleukin 10 production, increased activity of regulatory T cells, alternative activation of macrophages with diminished antimicrobial activity (M2 polarization) and ensuing apoptosis, and blunted development of T. whipplei–specific T cells. Immunosuppressive glucocorticoid treatment or anti–tumor necrosis factor α therapy appears to accelerate progression of disease. Recently, asymptomatic HIV-infected individuals were found to have significantly higher levels of T. whipplei sequence in bronchoalveolar lavage fluid (BALF) than did non-HIV-infected individuals, and these levels decreased with antiretroviral therapy. A weak humoral response, perhaps due to bacterial glycosylation in patients with chronic disease, appears to differentiate persons who clear the bacillus from asymptomatic carriers. In the initiation of chronic infection, the relative importance of the host’s genetic background versus the modulation of the host response by T. whipplei is unknown.
T. whipplei has a tropism for myeloid cells, which it invades and in which it can avoid being killed. Infiltration of infected tissue by large numbers of foamy macrophages is a characteristic finding. In the intestine, villi are flat and wide with dilated lacteals. Involvement of lymphatic or hepatic tissue may manifest as noncaseating granulomas that can mimic sarcoid.
CLINICAL MANIFESTATIONS
Asymptomatic Colonization/Carriage Studies using primarily PCR have detected T. whipplei sequence in stool, saliva, duodenal tissue, and (rarely) blood in the absence of symptoms. Although prevalence rates are still being defined, in Western European countries, detection in saliva (0.2%) is less common than that in stool (1–11%) and appears to occur only with concomitant fecal carriage. The prevalence of fecal carriage is elevated in individuals with exposure to waste water or sewage (12–26%). However, in rural Senegal, 44% of children age 2–10 had T. whipplei detected in fecal samples. The duration of carriage at these sites is still being examined but can be at least 1 year. It is not known how often the carrier state is associated with acute infection, but evolution into chronic disease is uncommon. Bacterial loads are lighter in asymptomatic carriage than in active disease.
Acute Infection T. whipplei has been implicated as a cause of acute gastroenteritis in children. It was also detected via PCR in the blood of 6.4% of febrile patients (primarily children) from two villages in Senegal, often with concomitant cough and rhinorrhea. Further, T. whipplei has been implicated as a cause of acute pneumonia in the United States and France. These data suggest that primary acquisition can result in symptomatic pulmonary or intestinal infection, which may be more common than has been thought, and only rarely results in chronic disease.
Chronic Infection • “CLASSIC” WHIPPLE’S DISEASE So-called classic Whipple’s disease was the initial clinical syndrome recognized, with consequent identification of T. whipplei. This chronic infection is defined by involvement of the duodenum and/or jejunum that develops over years. In most individuals, the initial phase of disease manifests primarily as intermittent, occasionally chronic and destructive migratory oligo- or polyarthralgias/seronegative arthritis. Spondylitis, sacroiliitis, and prosthetic hip infection also have been described. This initial stage is often confused with a variety of rheumatologic disorders and, on average, lasts 6–8 years before gastrointestinal symptoms commence. Treatment of presumed inflammatory arthritis with immunosuppressive agents (e.g., glucocorticoids, tumor necrosis factor α antagonists) can accelerate progression of the disease process. Alternatively, antimicrobial therapy used for another indication may reduce symptoms. In fact, the modulation of symptoms in these settings should prompt consideration of Whipple’s disease. The intestinal symptoms that develop in the majority of cases are characterized by diarrhea with accompanying weight loss and may be associated with fever and abdominal pain. Diagnostic misdirection can be caused by co-infection with Giardia lamblia, which is occasionally identified. Occult gastrointestinal blood loss, hepatosplenomegaly, and ascites are less common. Anemia and hypereosinophilia may be detected. Rheumatoid factor and antinuclear antibody tests are usually negative. The most common finding on abdominal CT is mesenteric and/or retroperitoneal lymphadenopathy. The endoscopic or video capsule observation of pale, yellow, or shaggy mucosa with erythema or ulceration past the first portion of the duodenum suggests Whipple’s disease (Fig. 200-5). In addition to rheumatologic and proximal intestinal disease, neurologic (6–63%), cardiac (17–55%), pulmonary (10–40%), lymphatic (10%), ocular (5–10%), dermal (1–5%), and (in rare instances) other sites are variably involved in classic Whipple’s disease.
FIGURE 200-5 Endoscopic view of the jejunal mucosa demonstrating a thickened, granular mucosa and “white spots” due to dilated lacteals. (Reprinted with permission from J Bureš et al: Whipple’s disease: Our own experience and review of the literature. Gastroenterol Res Pract, 2013. http://dx.doi.org/10.1155/2013/478349.)
Neurologic disease Asymptomatic neurologic involvement in Whipple’s disease has been documented by PCR-based detection in cerebrospinal fluid (CSF). A variety of neurologic manifestations have been reported, the most common of which are cognitive changes progressing to dementia; personality, mood, and sleep-cycle disorders; hypothalamic involvement; and supranuclear ophthalmoplegia. In addition to the latter, neuro-ophthalmologic manifestations of Whipple’s disease include supranuclear gaze palsy, oculomasticatory and oculofacial myorhythmia (highly suggestive of Whipple’s), nystagmus, and retrobulbar neuritis. Focal neurologic presentations (dependent on lesion location), seizures, ataxia, meningitis, encephalitis, hydrocephalus, myelopathy, and distal polyneuropathy also have been described. Neurologic sequelae occur with CNS disease, and the mortality risk is significant.
MRI results may be normal. Identified lesions (solitary or multifocal) are usually T2 and fluid-attenuated inversion recovery (FLAIR) hyperintense and may enhance with gadolinium. Findings are myriad and not diagnostic, but the limbic system is commonly involved. FDG-PET may reveal increased uptake. CSF analysis may be abnormal; leukocytosis (generally lymphocyte-predominant) and an elevated protein concentration are common. A low CSF glucose level has been reported.
Cardiac disease Endocarditis, which is increasingly recognized in Whipple’s disease, presents as culture-negative infection and/or congestive heart failure; hypotension occurs rarely. Embolic events or various arrhythmias may also be noted. Fever is often absent, and Duke clinical criteria are rarely met. Vegetations are identified by echocardiography in 50–75% of cases. All valves, alone or in combination, can be affected; most commonly involved are the aortic and mitral valves. Preexisting valvular disease is found in only a minority of cases, although infection of bioprosthetic valves has been described. Mural, myocardial, or pericardial disease also occurs alone or in combination with valvular involvement. Constrictive pericarditis develops infrequently.
Pulmonary disease Some combination of interstitial disease, nodules, parenchymal infiltrate, and pleural effusion is observed. The clinical significance of T. whipplei dominating sequence reads in BALF from HIV-infected individuals is unresolved.
Lymphatic disease Mesenteric and retroperitoneal lymphadenopathy are common with intestinal disease, and mediastinal adenopathy may be associated with pulmonary infection. Peripheral adenopathy is less common.
Ocular disease (non-neuro-ophthalmologic) Uveitis is the most common form of ocular disease, usually presenting as a change in vision or “floaters.” Anterior (anterior chamber), intermediate (vitreous), and posterior (retina/choroid) uveitis can occur alone or in combination. Postoperative acute or chronic ocular Whipple’s disease has been described in association with local or systemic glucocorticoid use; its detection in this setting raises the possibility that asymptomatic or subclinical disease has been unmasked. Keratitis and crystalline keratopathy also have been reported. Patients may be misdiagnosed with sarcoid or Behçet’s disease prior to the recognition of Whipple’s.
Dermatologic disease Skin hyperpigmentation, particularly in light-exposed areas in the absence of adrenal dysfunction, should be suggestive of Whipple’s disease. A variety of other cutaneous manifestations have been described, including erythematous macular lesions, nonthrombocytopenic purpura, subcutaneous nodules, and hyperkeratosis.
Miscellaneous sites Thyroid, renal, testicular, epididymal, gallbladder, skeletal muscle, and bone marrow involvement have all been described. In fact, almost any organ can be involved in classic Whipple’s disease, with varying frequency, variable combinations, and myriad signs and symptoms. As a result, Whipple’s disease should be considered in the setting of a chronic multisystemic process. Despite its rarity, the combination of rheumatologic and intestinal disease with weight loss, with or without neurologic and cardiac involvement, warrants heightened suspicion.
ISOLATED INFECTION This entity has been defined as infection in the absence of intestinal symptoms, although an occasional small-bowel biopsy may be PCR-positive in this setting. “Isolated infection” is something of a misnomer since multiple nonintestinal sites of T. whipplei infection are not uncommon. Infection at the same nonintestinal sites (single or multiple) that are variably involved in classic Whipple’s disease may also present as “isolated infection.” Endocarditis, neurologic disease, uveitis, rheumatologic manifestations, and pulmonary involvement are most commonly described. Signs and symptoms are similar to those described for T. whipplei infection of these sites in classic Whipple’s disease. With enhanced PCR-based diagnostic capabilities, T. whipplei infection without concomitant intestinal involvement (of which endocarditis is the best example) will probably be diagnosed increasingly often.
REINFECTION/RELAPSING DISEASE/IMMUNE RECONSTITUTION INFLAMMATORY SYNDROME (IRIS) It has been suggested that, if an underlying host immune defect places an individual at risk for chronic infection, then that person may be at risk for reinfection due to occupational exposure or contact with family members who are asymptomatically colonized. One case of apparent relapse that was due to a different genotype supports this contention.
Optimal treatment regimens and durations are still being defined. However, it is clear, especially in the setting of occult or overt CNS disease, that treatment with oral tetracycline or trimethoprim-sulfamethoxazole (TMP-SMX) alone may result in disease relapse.
As in patients treated for HIV or mycobacterial disease, IRIS has been described in patients treated for T. whipplei infection. Prior immunosuppressive therapy increases the likelihood of IRIS, in which inflammation recurs after an initial clinical response to treatment and loss of PCR detection of T. whipplei. Manifestations include fever, arthritis, skin lesions, pleuritis, uveitis, and orbital and periorbital inflammation.
DIAGNOSIS
Considering T. whipplei infection and ensuring that the appropriate tests are performed are the critical steps in making the diagnosis, which otherwise will likely be missed. The clinical presentation will in part dictate which clinical specimens are most likely to enable the diagnosis. In the presence (and perhaps the absence) of gastrointestinal symptoms, postbulbar duodenal biopsies should be performed. As a general rule, diagnostic yield is greater for tissue specimens than for body fluids. Biopsy of normal-appearing skin may detect T. whipplei in the setting of classic Whipple’s disease and serve as a minimally invasive means to establish the diagnosis. It is unclear whether CSF should be obtained in the absence of CNS symptoms, but its collection should be considered: the CNS is the most common site for relapse, and thus the information gained by CSF examination could influence the design of the treatment regimen.
The development and implementation of PCR-based diagnostics have significantly increased the sensitivity and specificity of T. whipplei identification. PCR can be applied to affected tissues (fixed and nonfixed) and various body fluids (e.g., CSF; aqueous or vitreous humor; joint, pericardial, or pleural fluid; BALF; blood; feces). In some clinical scenarios, a generic 16S rRNA bacterial assay combined with amplicon sequencing can be used to detect and identify T. whipplei sequence. Delineation of the T. whipplei genomic sequence has enabled the development and broad availability of more sensitive and specific PCR-based assays. The interpretation of a PCR-based diagnostic approach must take into account limitations such as false-positive results due to sample contamination and false-negative results due to organism load, sample quality, and inadequate DNA extraction.
The diagnosis of classic Whipple’s disease was originally based on histologic findings in intestinal biopsy specimens, and this diagnostic procedure remains important. Infiltration of the lamina propria with macrophages containing inclusions (representing ingested bacteria) that are positive on periodic acid–Schiff (PAS) staining and resistant to diastase is observed. However, PAS is nonspecific, also yielding positive results with mycobacteria (which can be differentiated with Ziehl-Neelsen stain), Rhodococcus equi, Bacillus cereus, Corynebacterium species, and Histoplasma species. T. whipplei can also be detected by silver stain, Brown-Brenn (weakly positive), or acridine orange and is not stained by calcofluor. Staining of other tissues or fluids (e.g., ocular aspirations) for PAS-positive inclusions in macrophages can be performed to support the diagnosis. Electron microscopy can be used to identify the trilaminar cell wall of T. whipplei.
When available, immunohistochemistry has greater specificity and sensitivity than PAS staining and can be performed on archived fixed tissue. T. whipplei has been successfully cultured from blood, CSF, synovial fluid, BALF, valve tissue, duodenal tissue, skeletal muscle, and lymph nodes, but culture is not practical since it takes months to obtain a positive result. Likewise, serology is of limited value for the diagnosis of Whipple’s disease because the prevalence of exposure is much higher than that of chronic disease development and the antibody response to T. whipplei appears to be blunted in the disease state.
Although histologic or cytologic detection of T. whipplei is less specific and sensitive than PCR, a positive result is strongly supportive within the appropriate clinical context and is definitive when combined with a more specific test (e.g., PCR, immunohistochemistry).
|
TREATMENT |
WHIPPLE’S DISEASE |
Data on treatment are emerging, but questions persist regarding the optimal regimen and duration, which may depend on the site of infection (e.g., CNS and heart valve). Appropriate treatment usually results in a rapid and at times remarkable clinical response (e.g., in CNS disease). Maintenance of a durable response has been more challenging.
Rates of relapse, particularly of CNS disease, were unacceptable with oral tetracycline or TMP-SMX monotherapy. Sequence data now indicate that TMP is not active against T. whipplei due to the absence of dihydrofolate reductase, but this drug was used extensively before this fact was known. This information prompted a randomized controlled trial in 40 patients, who received either ceftriaxone (2 g IV q24h) or meropenem (1 g IV q8h) for 2 weeks followed by oral TMP-SMX (160/800 mg) twice a day for 1 year. The efficacy of these regimens was outstanding. The only instance of therapy failure—in a case of asymptomatic CNS infection that was not eradicated by either regimen—was subsequently cured with oral minocycline and chloroquine (250 mg/d after a loading dose). A follow-up trial reported similar efficacy with a regimen of ceftriaxone (2 g IV q24h) for 2 weeks followed by oral TMP-SMX for 3 months. One issue in these trials was that the CNS doses—and perhaps the duration of ceftriaxone and meropenem treatment as well—were not optimal. Further, investigators have speculated that oral regimens with greater CNS penetrance, such as sulfadiazine (2–4 g/d in 3 or 4 divided doses) and/or doxycycline or minocycline (200 mg/d in 2 divided doses) plus hydroxychloroquine (200 mg three times a day, to raise phagosome pH and increase drug activity in vitro), might render the parenteral phase of treatment unnecessary, given that the one failure of therapy for CNS disease was cured with a similar regimen. Another issue is concern about the potential development of resistance to sulfa drugs. Lastly, it is unclear whether oral sulfa- or tetracycline-based regimens will suffice in endocarditis. Until more data become available, it seems prudent—at least in asymptomatic/symptomatic CNS disease or cardiac infection—to administer CNS-optimized doses of IV ceftriaxone (2 g q12h) or meropenem (2 g q8h) for at least 2 weeks followed by oral doxycycline or minocycline plus hydroxychloroquine or chloroquine for at least 1 year, if tolerated. Although data on the use of PCR to guide therapy do not exist, it seems reasonable that continued T. whipplei detection by PCR, especially in the CSF, should dictate at least continuation of therapy and perhaps consideration of an alternative regimen.
The occurrence of a Jarisch-Herxheimer reaction within 24 h of treatment initiation has been described, with rapid resolution. The addition of glucocorticoids may be beneficial in the management of clearly documented IRIS.
Data on certain site-specific treatment issues are even more limited. Anecdotal reports describe successful treatment of uveitis with oral TMP-SMX with or without rifampin, whereas treatment with tetracycline alone has resulted in relapse. Although a role for adjunctive intraocular therapy has been reported, the data are unclear on this point. Surgery may be needed in the setting of endocarditis with significant valve dysfunction; however, timely recognition can result in cure with medical management alone. Although data on the treatment of foreign body–associated infection are virtually nonexistent, medical treatment for a prosthetic hip infection was apparently successful; however, follow-up was limited.
Regardless of the therapeutic regimen chosen, an effort to ensure compliance and close follow-up for potential relapse (or perhaps reinfection), which can occur many years after an apparent cure, will maximize the chances for a good outcome.
201 |
Infections Due to Mixed Anaerobic Organisms |
Anaerobes comprise the predominant class of bacteria of the normal human microbiota (formerly termed “the normal human flora”) that reside on mucous membranes and predominate in many infectious processes, particularly those arising from mucosal surfaces. These organisms generally cause disease subsequent to the breakdown of mucosal barriers and the leakage of the microbiota into normally sterile sites. Infections resulting from contamination by the microbiota are usually polymicrobial and involve both aerobic and anaerobic bacteria. However, the difficulties encountered in handling specimens in which anaerobes may be important and the technical challenges entailed in cultivating and identifying these organisms in clinical microbiology laboratories continue to leave the anaerobic etiology of an infectious process unproven in many cases. Therefore, an understanding of the types of infections in which anaerobes can play a role is crucial in selecting appropriate microbiologic tools to identify the organisms in clinical specimens and in choosing the most appropriate treatment, including antibiotics and surgical drainage or debridement of the infected site.
This chapter focuses on infections caused by nonsporulating anaerobic bacteria. It does not address clostridial infections and syndromes, which are covered elsewhere (Chaps. 161 and 179).
DEFINITIONS
Anaerobic bacteria are organisms that require reduced oxygen tension for growth, failing to grow on the surface of solid media in 10% CO2 in air. (In contrast, microaerophilic bacteria can grow in an atmosphere of 10% CO2 in air or under anaerobic or aerobic conditions, although they grow best in the presence of only a small amount of atmospheric oxygen, and facultative bacteria can grow in the presence or absence of air). Most clinically relevant anaerobes, such as Bacteroides fragilis, Prevotella melaninogenica, and Fusobacterium nucleatum, are relatively aerotolerant. Although they can survive for sustained periods in the presence of up to 2–8% oxygen, they generally do not multiply in this environment. A smaller number of pathogenic anaerobic bacteria (which are also part of the microbiota) die after brief contact with oxygen, even in low concentrations.
ANAEROBES OF THE HUMAN MICROBIOTA
Most human mucocutaneous surfaces harbor a rich indigenous normal microbiota composed of aerobic and anaerobic bacteria. These surfaces are dominated by anaerobic bacteria, which often account for 99.0–99.9% of the culturable microbiota and range in concentration from 109/mL in saliva to 1012/mL in gingival scrapings and the colon. It is interesting that anaerobes inhabit many areas of the body that are exposed to air: skin, nose, mouth, and throat. Anaerobes are thought to reside in the portions of these sites that are relatively well protected from oxygen, such as gingival crevices. New technologies based on analyses of microbial DNA have expanded our knowledge of these bacterial populations. For example, in an analysis of 13,555 prokaryotic ribosomal RNA gene sequences from the colon, most bacteria identified were considered uncultivated and novel microorganisms. Two immense projects based on these new technologies, the Human Microbiome Project funded by the U.S. National Institutes of Health and MetaHIT financed by the European Commission, aim to characterize the normal microbiota of healthy individuals.
The major reservoirs of anaerobic bacteria are the mouth, lower gastrointestinal tract, skin, and female genital tract (Table 201-1). In the oral cavity, the ratio of anaerobic to aerobic bacteria ranges from 1:1 on the surface of a tooth to 1000:1 in the gingival crevices. Prevotella and Porphyromonas species comprise much of the indigenous oral anaerobic microbiota. Fusobacterium and Bacteroides (non–B. fragilis group) are present in lower numbers. Anaerobic bacteria are not found in appreciable numbers in the normal stomach and upper small intestine. In the distal ileum, the microbiota begins to resemble that of the colon. In the colon, the ratio of anaerobes to facultative species is high; for example, there are 1011–1012 organisms/g of stool, and >99% of these organisms are anaerobic, with an anaerobe-to-aerobe ratio of ~1000:1. The predominant anaerobes in the human intestine belong to the phyla Bacteroidetes and Firmicutes and include a number of Bacteroides species (e.g., members of the B. fragilis group, such as B. fragilis, B. thetaiotaomicron, B. ovatus, B. vulgatus, B. uniformis, and Parabacteroides distasonis) as well as various clostridial, peptostreptococcal, and fusobacterial species. In the female genital tract, there are ~109 organisms/mL of secretions, with an anaerobe-to-aerobe ratio of 1:1 to 10:1. The predominant anaerobes in the female genital tract are Prevotella, Bacteroides, Fusobacterium, Clostridium, and the anaerobic Lactobacillus species. the skin microbiota contains anaerobes as well, the predominant species being Propionibacterium acnes and, in lower numbers, other species of propionibacteria and peptostreptococci.
|
ANAEROBIC HUMAN MICROBIOTA: AN OVERVIEW |
Commensal bacteria in general and commensal anaerobes in particular have been implicated as crucial mediators of physiologic, metabolic, and immunologic functions in the mammalian host. One of the most important roles that anaerobes serve as components of the normal colonic microbiota is the promotion of resistance to colonization; the presence of anaerobic bacteria effectively interferes with colonization by potentially pathogenic bacterial species through the depletion of oxygen and nutrients, the production of enzymes and toxic end products, and the modulation of the host’s intestinal innate immune response. For example, B. thetaiotaomicron stimulates Paneth cells to produce RegIIIγ, a bactericidal lectin that can result in killing of gram-positive bacteria. The normal colonic microbiota plays an important role in protection against Clostridium difficile–associated diarrhea or colitis—a toxin-mediated, potentially life-threatening disease that results when C. difficile spores in the colon transform into toxin-producing vegetative forms after antibiotic elimination of critical components of the competing colonic microbiota.
Bacteroides and other intestinal bacteria ferment carbohydrates and produce volatile fatty acids that are reabsorbed and used by the host as an energy source. The anaerobic intestinal microbiota is also responsible for the production of secreted products that promote human health (e.g., vitamin K and bile acids useful in fat absorption and cholesterol regulation).
Moreover, the anaerobic intestinal microbiota influences the development of an intact mucosa and of mucosa-associated lymphoid tissue. Colonization of germ-free mice with a single species, B. thetaiotaomicron, affects the expression of various host genes and corrects deficiencies of nutrient uptake, metabolism, angiogenesis, mucosal barrier function, and enteric nervous system development. The symbiosis factor polysaccharide A (PSA) of B. fragilis influences the normal development and function of the mammalian immune system and protects mice against colitis in a model of inflammatory bowel disease. It has also been shown that PSA can confer protection both prophylactically and therapeutically, restraining inflammatory processes at an extraintestinal site (the central nervous system [CNS]) and ameliorating disease in a mouse model of multiple sclerosis. Anaerobes can stimulate specific lymphocyte populations of the small and large intestine and can influence immunologic balance (including TH1/TH2 balance) as well as the number of TH17 and regulatory T cells in gut tissues.
Clearly, the gut microbiota confers many benefits, and its dysregulation may play a role in the pathogenesis of diseases characterized by inflammation and aberrant immune responses, such as inflammatory bowel disease, rheumatoid arthritis, multiple sclerosis, asthma, and type 1 diabetes. Furthermore, the gut microbiota has been associated with obesity and metabolic syndrome. An interesting association between certain microbes found in the microbiota and testosterone production has been suggested as well.
ETIOLOGY
Thousands of species of anaerobic bacteria have been identified as components of the complete human microbiota, with each individual colonized by hundreds of these species. Despite the complex array of bacteria in the normal microbiota, relatively few species are isolated commonly from human infections. Anaerobic infections occur when the harmonious relationship between the host and the host’s microbiota is disrupted. Any site in the body is susceptible to infection with these indigenous organisms when a mucosal barrier or the skin is compromised by surgery, trauma, tumor, ischemia, or necrosis, all of which can reduce local tissue redox potentials. Because the sites that are colonized by anaerobes contain many species of bacteria, disruption of anatomic barriers allows contamination of normally sterile sites by many organisms, resulting in mixed infections involving multiple species of anaerobes in combination with synergistically acting facultative or microaerophilic organisms.
Severe mixed infections of the head and neck may arise from an abscessed tooth infected with commensal microbiota of the mouth. Examples of infections arising from an oral source are chronic sinusitis, chronic otitis media, Ludwig’s angina, and periodontal abscesses. Brain abscesses and subdural empyema are also commonly associated with the oral microbiota. Oral anaerobes are usually responsible for pleuropulmonary diseases such as aspiration pneumonia, necrotizing pneumonia, lung abscess, and empyema.
Anaerobes from the intestine play an important role in various intraabdominal infections, such as peritonitis and intraabdominal abscesses (Chap. 159). Colonic contents are the source of microorganisms in the case of these infections, which usually follow disruption of intestinal continuity and contamination of the peritoneal cavity. Anaerobic bacteria are isolated frequently in female genital tract infections, such as salpingitis, pelvic peritonitis, tuboovarian abscess, vulvovaginal abscess, septic abortion, and endometritis (Chap. 163). In addition, these bacteria are often found in bacteremia and in infections of the skin, soft tissues, and bones.
Predominant among the anaerobic gram-positive cocci that produce disease are the peptostreptococci; the species of this genus that are most commonly involved in infections are P. micros, P. magnus, P. asaccharolyticus, P. anaerobius, and P. prevotii. Clostridia (Chap. 179) are anaerobic spore-forming gram-positive rods that are isolated from wounds, abscesses, sites of abdominal infection, and blood. Gram-positive anaerobic non-spore-forming bacilli are uncommon as etiologic agents of human infection. P. acnes, a component of the skin microbiota and a rare cause of foreign-body infections, is one of the few nonclostridial gram-positive rods associated with infections. The principal anaerobic gram-negative bacilli found in human infections belong to the B. fragilis group and to Fusobacterium, Prevotella, and Porphyromonas species.
The most important potential anaerobic pathogens found in the upper airways and isolated from clinical specimens of oral and pleuropulmonary infections are the Fusobacterium species F. necrophorum, F. nucleatum, and F. varium; P. melaninogenica; the Prevotella oralis group; Porphyromonas gingivalis; Porphyromonas asaccharolytica; Peptostreptococcus species; and the Bacteroides ureolyticus group.
The B. fragilis group contains the anaerobic pathogens most frequently isolated from clinical infections. Members of this group are part of the normal bowel microbiota; they include several distinct species, such as B. fragilis, B. thetaiotaomicron, B. vulgatus, B. uniformis, B. ovatus, and P. distasonis. B. fragilis is the most important clinical isolate, although it is isolated in lower numbers than some other Bacteroides species from cultures of the commensal fecal microbiota.
In female genital tract infections, organisms normally colonizing the vagina (e.g., Prevotella bivia and Prevotella disiens) are the most common isolates. However, B. fragilis is not uncommon.
PATHOGENESIS
Anaerobic bacterial infections usually occur when an anatomic barrier is disrupted and constituents of the local microbiota enter a site that was previously sterile. Because of the specific growth requirements of anaerobic organisms and their presence as commensals on mucosal surfaces, conditions must arise that allow these organisms to penetrate mucosal barriers and enter tissue with a lowered oxidation-reduction potential. Therefore, tissue ischemia, trauma, surgery, perforated viscus, shock, and aspiration provide environments conducive to the proliferation of anaerobes. The introduction of many bacterial species into otherwise sterile sites leads to a polymicrobial infection in which certain organisms predominate. Three major factors are involved in the pathogenesis of anaerobic infections: bacterial synergy, bacterial virulence factors, and mechanisms of abscess formation. The ability of different anaerobic bacteria to act synergistically during polymicrobial infection contributes to the pathogenesis of anaerobic infections. It has been postulated that facultative organisms function in part to lower the oxidation-reduction potential in the microenvironment, allowing the propagation of obligate anaerobes. Anaerobes can produce compounds such as succinic acid and short-chain fatty acids that inhibit the ability of phagocytes to clear facultative organisms. In experimental models, facultative and obligate anaerobes synergistically potentiate abscess formation.
Virulence factors associated with anaerobes typically confer the ability to evade host defenses, adhere to cell surfaces, produce toxins and/or enzymes, or display surface structures such as capsular polysaccharides and lipopolysaccharide (LPS) that contribute to pathogenic potential. The ability of an organism to adhere to host tissues is important to the establishment of infection. Some oral species adhere to the epithelium in the oral cavity. P. melaninogenica actually attaches to other microorganisms. P. gingivalis, a common isolate in periodontal disease, has fimbriae that facilitate attachment. Some Bacteroides strains appear to be piliated, a characteristic that may account for their ability to adhere.
The most extensively studied virulence factor of the nonsporulating anaerobes is the capsular polysaccharide complex of B. fragilis. This organism is unique among anaerobes in its potential for virulence during growth at normally sterile sites. Although it constitutes only 0.5–1% of the normal colonic microbiota, B. fragilis is the anaerobe most commonly isolated from intraabdominal infections and bacteremia. In an animal model of intraabdominal sepsis, the capsular polysaccharide was identified as the major virulence factor of B. fragilis; this polymer plays a specific, central role in the induction of abscesses. A series of detailed biologic and molecular studies of this virulence factor showed that B. fragilis produces at least eight distinct capsular polysaccharides, far more than the number reported for any other encapsulated bacterium. B. fragilis can exhibit distinct surface polysaccharides either alone or in combination by regulating the expression of these different capsules in an on–off manner through a reversible inversion of DNA segments within the promoters for operons containing the genes required for polysaccharide synthesis. Structural analysis of two of these polysaccharides, PSA and PSB, revealed that each polymer consists of repeating units with positively charged free amino groups and negatively charged groups. This structural feature is rare among bacterial polysaccharides, and the ability of PSA—and, to a lesser extent, PSB—to induce abscesses in animals depends on this zwitterionic charge motif. Intraabdominal abscess induction is related to the capacity of this polysaccharide to stimulate macrophages to release cytokines and chemokines—in particular, interleukin (IL) 8, IL-17, and tumor necrosis factor α (TNF-α)—from resident peritoneal cells through a Toll-like receptor 2–dependent mechanism. The release of cytokines and chemokines results in the chemotaxis of polymorphonuclear neutrophils (PMNs) into the peritoneum, where they adhere to mesothelial cells induced by TNF-α to upregulate their expression of intercellular adhesion molecule 1 (ICAM-1). PMNs adherent to ICAM-1-expressing cells probably represent the nidus for an abscess. PSA also activates T cells to produce certain cytokines, including IL-17 and interferon γ, that are necessary for abscess formation.
B. fragilis produces other virulence factors that allow it to predominate in disease. This organism synthesizes pili, fimbriae, and hemagglutinins that aid in attachment to host cell surfaces. In addition, Bacteroides species produce many enzymes and toxins that contribute to pathogenicity. Enzymes such as neuraminidase, protease, glycoside hydrolases, and superoxide dismutases are all produced by B. fragilis. Anaerobic bacteria produce a number of exoproteins that can enhance the organisms’ virulence. The collagenase produced by P. gingivalis may enhance tissue destruction. An association of B. fragilis strains positive for the enterotoxin BFT with clinical episodes of diarrhea in children and adults has been suggested. BFT is a metalloprotease that is cytopathic for intestinal epithelial cells and induces fluid secretion and tissue damage in ligated intestinal loops of experimental animals. Recent evidence from mouse models indicates that enterotoxin-producing strains of B. fragilis may play a role in colon carcinoma.
Exotoxins produced by clostridial species, including botulinum toxins, tetanus toxin, C. difficile toxins A and B, and five toxins produced by Clostridium perfringens, are among the most virulent bacterial toxins in mouse lethality assays. Anaerobic gram-negative bacteria such as B. fragilis possess LPSs (endotoxins) that are 100–1000 times less biologically potent than endotoxins associated with aerobic gram-negative bacteria. This relative biologic inactivity may account for the lower frequency of disseminated intravascular coagulation and purpura in Bacteroides bacteremia than in facultative and aerobic gram-negative bacillary bacteremia. An exception is the LPS from Fusobacterium, which may account for the severity of Lemierre’s syndrome (see “Complications of Anaerobic Head and Neck Infections,” below).
EPIDEMIOLOGY
Difficulties in the performance of appropriate cultures, contamination of cultures by components of the normal microbiota, and the lack of readily available, reliable culture techniques have made it impossible to obtain accurate data on incidence or prevalence. However, anaerobic infections are encountered frequently in hospitals with active surgical, trauma, and obstetric and gynecologic services. Depending on the institution, anaerobic bacteria account for 0.5–12% of all cases of bacteremia.
CLINICAL MANIFESTATIONS
Anaerobic Infections of the Mouth, Head, and Neck Anaerobic bacteria are commonly involved in infections of the mouth, head, and neck (Chap. 44). The predominant isolates are components of the normal microbiota of the upper airways—mainly the Bacteroides oralis group, pigmented Prevotella species, P. asaccharolytica, Fusobacterium species, peptostreptococci, and microaerophilic streptococci.
Soft tissue infections of the oral-facial area may or may not be odontogenic. Odontogenic infections—primarily dental caries and periodontal disease (gingivitis and periodontitis)—are common and have both local consequences (especially tooth loss) and the potential for life-threatening spread to the deep fascial spaces of the head and neck. Infections of the mouth can arise from either supragingival or subgingival dental plaque composed of bacteria colonizing the tooth surface. Supragingival plaque formation begins with the adherence of gram-positive bacteria to the tooth surface. This form of plaque is influenced by salivary and dietary components, oral hygiene, and local host factors. Supragingival plaque can lead to dental caries and, with further invasion, to pulpitis (endodontic infection) that can further perforate the alveolar bone, causing periapical abscess. Subgingival plaque is associated with periodontal infections (e.g., gingivitis, periodontitis, and periodontal abscess) that can further disseminate to adjacent structures such as the mandible, causing osteomyelitis of the maxillary sinuses. Periodontitis may also result in spreading infection that can involve adjacent bone or soft tissues. In the healthy periodontium, the sparse microbiota consists mainly of gram-positive organisms such as Streptococcus sanguinis and Actinomyces species. In the presence of gingivitis, there is a shift to a greater proportion of anaerobic gram-negative bacilli in the subgingival microbiota, with predominance of Prevotella intermedia. In well-established periodontitis, the complexity of the microbiota increases further. The predominant isolates are P. gingivalis, P. intermedia, Aggregatibacter actinomycetemcomitans, Treponema denticola, and Tannerella forsythensis.
Necrotizing Ulcerative Gingivitis Gingivitis may become a necrotizing infection (trench mouth, Vincent’s stomatitis) (Chap. 44). The onset of disease is usually sudden and is associated with tender bleeding gums, foul breath, and a bad taste. The gingival mucosa, especially the papillae between the teeth, becomes ulcerated and may be covered by a gray exudate, which is removable with gentle pressure. Patients may become systemically ill, developing fever, cervical lymphadenopathy, and leukocytosis.
![]() Noma (cancrum oris) is a necrotizing infection of the oral mucous membranes. It is characterized by destruction of soft tissue and bone and evolves rapidly from gingival inflammation to orofacial gangrene. Noma occurs most frequently in young children (1–4 years of age) with malnutrition or systemic disease. This infection occurs worldwide but is most common in sub-Saharan Africa.
Noma (cancrum oris) is a necrotizing infection of the oral mucous membranes. It is characterized by destruction of soft tissue and bone and evolves rapidly from gingival inflammation to orofacial gangrene. Noma occurs most frequently in young children (1–4 years of age) with malnutrition or systemic disease. This infection occurs worldwide but is most common in sub-Saharan Africa.
Acute Necrotizing Infections of the Pharynx These infections usually occur in association with ulcerative gingivitis. Symptoms include an extremely sore throat, foul breath, and a bad taste accompanied by fever and a sensation of choking. Examination of the pharynx demonstrates that the tonsillar pillars are swollen, red, ulcerated, and covered with a grayish membrane that peels easily. Lymphadenopathy and leukocytosis are common. The disease may last for only a few days or, if not treated, may persist for weeks. Lesions begin unilaterally but may spread to the other side of the pharynx or the larynx. Aspiration of the infected material by the patient can result in lung abscesses.
Peripharyngeal Space Infections These infections arise from the spread of organisms from the upper airways to potential spaces formed by the fascial planes of the head and neck. The etiology is typically polymicrobial and represents the normal microbiota of the mucosa of the originating site.
Peritonsillar abscess (quinsy) is a complication of acute tonsillitis caused mainly by a mixed flora containing anaerobes (e.g., F. necro-phorum and Peptostreptococcus species) and the facultative anaerobe group A Streptococcus (Chap. 44). Of cases of submandibular space infection (Ludwig’s angina), 80% are caused by infection of the tissues surrounding the second and third molar teeth. This infection results in marked local swelling of tissues, with pain, trismus, and superior and posterior displacement of the tongue. Submandibular swelling of the neck can impair swallowing and cause respiratory obstruction. In some cases, tracheotomy is life-saving. Cervicofacial actinomycosis (Chap. 200) is caused by a branching, gram-positive, non-spore-forming, strict/facultative anaerobe that is a part of the normal oral microbiota. This chronic disease is characterized by abscesses, draining sinus tracts, fistula, bone destruction, and fibrosis. It can easily be mistaken for malignancy or granulomatous disease. Actinomycosis less frequently involves the thorax, abdomen, pelvis, and CNS.
Sinusitis and Otitis Anaerobic bacteria have been implicated in chronic sinusitis but play little role in acute sinusitis. In several studies on chronic sinusitis, anaerobic bacteria were found in 0–52% of cases, depending on the method used to collect specimens. Polymicrobial infection is common, and the predominant anaerobic isolates are pigmented Prevotella, Fusobacterium, Peptostreptococcus, and P. acnes. Aerobic gram-negative bacilli and S. aureus have also been implicated in chronic sinusitis. Anaerobic bacteria have been isolated in a large percentage of cases of chronic suppurative otitis media in children. The role of anaerobes in acute otitis media is less clear.
Complications of Anaerobic Head and Neck Infections Contiguous cranial spread of these infections can result in osteomyelitis of the skull or mandible or in intracranial infections such as brain abscess and subdural empyema. Caudal spread can produce mediastinitis or pleuropulmonary infection. Hematogenous complications can also result from anaerobic infections of the head and neck. Bacteremia, which occasionally is polymicrobial, can lead to endocarditis or other distant infections. Lemierre’s syndrome (Chap. 44), which has been uncommon in the antimicrobial era, is an acute oropharyngeal infection with secondary septic thrombophlebitis of the internal jugular vein and frequent metastasis, most commonly to the lung. F. necrophorum is the usual cause. This infection typically begins with pharyngitis, which is followed by local invasion in the lateral pharyngeal space, with resultant internal jugular vein thrombophlebitis. A typical clinical triad includes pharyngitis, a tender/swollen neck, and noncavitating pulmonary infiltrates.
CNS Infections CNS infections associated with anaerobic bacteria are brain abscess, epidural abscess, and subdural empyema. Anaerobic meningitis is rare and is usually related to parameningeal collection or shunt infection. If optimal bacteriologic techniques are used, as many as 85% of brain abscesses yield anaerobic bacteria. Most anaerobic brain abscesses arise by direct extension from a site of otorhinolaryngeal infection such as otitis, sinusitis, or tooth infection. Hematogenous dissemination from a distant infected site, usually intraabdominal or pelvic, can occur. Common isolates are Peptostreptococcus, Fusobacterium, Bacteroides, Prevotella, Propionibacterium, Eubacterium, Veillonella, and Actinomyces species. Facultative or microaerophilic streptococci and coliforms are often part of a mixed infecting flora in brain abscesses.
Pleuropulmonary Infections Anaerobic pleuropulmonary infections result from the aspiration of oropharyngeal contents by patients with predisposing conditions such as dysphagia due to neurologic or esophageal disorders or transiently impaired consciousness due to conditions such as alcohol or drug abuse, seizures, head trauma, and cerebrovascular accidents. Clinical syndromes that are associated with anaerobic pleuropulmonary infection produced by aspiration include aspiration pneumonitis, which can be complicated by necrotizing pneumonia, lung abscess, and empyema. Many of these infections have an indolent course that may serve as a clinical clue differentiating them, for example, from pneumococcal pneumonia, which often presents with abrupt onset, shaking chills, and rapid progression.
The anaerobes most common in pleuropulmonary infections are indigenous to the oral cavity, especially the gingival crevice, and include pigmented and nonpigmented Prevotella, Peptostreptococcus, and Bacteroides species and F. nucleatum. Many of these infections are of mixed aerobic-anaerobic etiology, and the predominant aerobes isolated from community-acquired aspiration pneumonias are microaerophilic streptococci such as Streptococcus milleri. Studies using in-depth culture techniques in patients with community-acquired lung abscess showed aerobic and microaerophilic streptococci to be the most common pathogens (60% of patients) and anaerobes to be the second most common (26%). In a study on aspiration pneumonia from a long-term care facility, the most common isolates were gram-negative bacilli (49%), anaerobes (16%), and S. aureus (12%). Nosocomial aspiration pneumonia commonly involves a mixture of anaerobes and gram-negative bacilli or S. aureus.
ASPIRATION PNEUMONITIS Bacterial aspiration pneumonitis must be distinguished from two other clinical syndromes associated with aspiration that are not of bacterial etiology. One syndrome results from aspiration of solids, usually food. Obstruction of major airways typically results in atelectasis and moderate nonspecific inflammation. Therapy consists of removal of the foreign body. The second aspiration syndrome is more easily confused with bacterial aspiration. Mendelson’s syndrome, a chemical pneumonitis, results from regurgitation of stomach contents and aspiration of chemical material, usually acidic gastric juices. Pulmonary inflammation—including the destruction of the alveolar lining, with transudation of fluid into the alveolar space—occurs with remarkable rapidity. Typically this syndrome develops within hours, often following anesthesia when the gag reflex is depressed. The patient becomes tachypneic, hypoxic, and febrile. The leukocyte count may rise, and the chest x-ray may evolve from normal to a complete bilateral “whiteout” within 8–24 h. Sputum production is minimal. The pulmonary signs and symptoms can resolve quickly with symptom-based therapy or can culminate in respiratory failure, with the subsequent development of bacterial superinfection over a period of days. Antibiotic therapy is not indicated unless bacterial infection supervenes.
In contrast to these syndromes, bacterial aspiration pneumonitis develops over a period of several days or weeks rather than hours. Patients who enter the hospital with this syndrome typically have been ill for several days and generally report low-grade fever, malaise, and sputum production. In some patients, weight loss and anemia reflect a more chronic process. Usually the history reveals factors predisposing to aspiration, such as alcohol overdose or residence in a nursing home. Examination sometimes yields evidence of periodontal disease. Sputum characteristically is not malodorous unless the process has been under way for at least a week. A mixed bacterial flora with many PMNs is evident on Gram’s staining of sputum. Expectorated sputum is unreliable for anaerobic cultures because of inevitable contamination by the normal oral microbiota. Reliable specimens for culture can be obtained by transtracheal or transthoracic aspiration—techniques that are rarely used at present. Culture of protected-brush specimens or bronchoalveolar lavage fluid obtained by bronchoscopy is controversial.
Chest x-rays show consolidation in dependent pulmonary segments: in the basilar segments of the lower lobes if the patient has aspirated while upright and in either the posterior segment of the upper lobe (usually on the right side) or the superior segment of the lower lobe if the patient has aspirated while supine.
NECROTIZING PNEUMONITIS This form of anaerobic pneumonitis is characterized by numerous small abscesses that spread to involve several pulmonary segments. The process can be indolent or fulminating. This syndrome is less common than either aspiration pneumonitis or lung abscess and includes features of both types of infection.
ANAEROBIC LUNG ABSCESSES (See also Chap. 154) These abscesses result from subacute anaerobic pulmonary infection. The clinical syndrome typically involves a history of constitutional signs and symptoms (including malaise, weight loss, fever, night sweats, and foul-smelling sputum), perhaps over a period of weeks (Chap. 153). Patients who develop lung abscesses characteristically have dental infection and periodontitis, but lung abscesses in edentulous patients have been reported. Abscess cavities may be single or multiple and generally occur in dependent pulmonary segments (Fig. 201-1). Anaerobic abscesses must be distinguished from lesions associated with tuberculosis, neoplasia, and other conditions.
FIGURE 201-1 Chest radiograph of right-lower-lobe lung abscess in a 60-year-old alcoholic patient. (From GL Mandell [ed]: Atlas of Infectious Diseases, Vol VI. Philadelphia, Current Medicine Inc, Churchill Livingstone, 1996; with permission.)
Septic pulmonary emboli may originate from intraabdominal or female genital tract infections and can produce anaerobic pneumonia and abscess.
EMPYEMA Empyema is a manifestation of long-standing anaerobic pulmonary infection complicated by bronchopleural fistula. The clinical presentation, which includes foul-smelling sputum, resembles that of other anaerobic pulmonary infections. Patients may report pleuritic chest pain and marked chest-wall tenderness.
Empyema may be masked by overlying pneumonitis and should be considered especially in cases of persistent fever despite antibiotic therapy. Diligent physical examination and the use of ultrasound to localize a loculated empyema are important diagnostic tools. The collection of a foul-smelling exudate by thoracentesis is typical. Cultures of infected pleural fluid yield an average of 3.5 anaerobic and 0.6 facultative or aerobic bacterial species. Drainage is required. Defervescence, a return to a feeling of well-being, and resolution of the process may require several months.
Extension from a subdiaphragmatic infection may also result in anaerobic empyema.
Intraabdominal Infections Intraabdominal infections—mainly peritonitis and abscesses—are usually polymicrobial and represent the normal intestinal (especially colonic) microbiota. These infections most often follow a breach in the mucosal barrier resulting from appendicitis, diverticulitis, neoplasm, inflammatory bowel disease, surgery, or trauma. On average, four to six bacterial species are isolated per specimen submitted to the microbiology laboratory, with a predominance of enteric aerobic/facultative gram-negative bacilli, anaerobes, and streptococci/enterococci. The most common isolates are Escherichia coli (found in ≥50% of patients) and B. fragilis (30–50%). Other anaerobes commonly isolated from this type of infection include Peptostreptococcus, Prevotella, and Fusobacterium species. The involvement of clostridia can lead to severe infections. The dominance of four to six bacterial species out of the more than 500 colonic mucosal species is related both to the virulence factors of these species and to the inability of clinical laboratories to culture many other species residing in the colonic mucosa.
Disease originating from proximal-bowel perforation reflects the microbiota of this site, with a predominance of aerobic and anaerobic gram-positive bacteria and Candida.
Neutropenic enterocolitis (typhlitis) has been associated with anaerobic infection of the cecum but—in the setting of neutropenia (Chap. 104)—may involve the entire bowel. Patients usually present with fever; abdominal pain, tenderness, and distention; and watery diarrhea. The bowel wall is edematous with hemorrhage and necrosis. The primary pathogen is thought by some authorities to be Clostridium septicum, but other clostridia and mixed anaerobes have also been implicated. More than 50% of patients developing early clinical signs can benefit from antibiotic therapy and bowel rest. Surgery is sometimes required to remove gangrenous bowel. See Chap. 159 for a complete discussion of intraabdominal infections.
Enterotoxigenic B. fragilis has been associated with watery diarrhea in a few young children and adults. In case–control studies of children with undiagnosed diarrheal disease, enterotoxigenic B. fragilis was isolated from significantly more children with diarrhea than children in the control group.
Pelvic Infections The vagina of a healthy woman is a major reservoir of anaerobic and aerobic bacteria. In the normal microbiota of the female genital tract, anaerobes outnumber aerobes by a ratio of ~10:1 and include anaerobic gram-positive cocci and Bacteroides species (Table 201-1). Anaerobes are isolated from most women with genital tract infections that are not caused by a sexually transmitted pathogen. The major anaerobic pathogens are B. fragilis, P. bivia, P. disiens, P. melaninogenica, anaerobic cocci, and Clostridium species. Anaerobes are frequently encountered in pelvic inflammatory disease, pelvic abscess, endometritis, tubo-ovarian abscess, septic abortion, and postoperative or postpartum infections. These infections are often of mixed etiology, involving both anaerobes and coliforms; pure anaerobic infections without coliform or other facultative bacterial species occur more often in pelvic than in intraabdominal sites. Septic pelvic thrombophlebitis may complicate the infections and lead to repeated episodes of septic pulmonary emboli. See Chap. 163 for a complete discussion of pelvic inflammatory disease.
Anaerobic bacteria have been thought to be contributing factors in the etiology of bacterial vaginosis. This syndrome of unknown etiology is characterized by a profuse malodorous discharge and a change in the bacterial ecology that results in replacement of the Lactobacillus-dominated normal microbiota with an overgrowth of bacterial species including Gardnerella vaginalis, Prevotella species, Mobiluncus species, peptostreptococci, and genital mycoplasmas. A study based on 16S rRNA identification found other anaerobes that were predominant in cases but not in controls: Atopobium, Leptotrichia, Megasphaera, and Eggerthella. Pelvic infections due to Actinomyces species have been associated with the use of intrauterine devices (Chap. 200).
Skin and Soft Tissue Infections Injury to skin, bone, or soft tissue by trauma, ischemia, or surgery creates a suitable environment for anaerobic infections. These infections are most frequently found in sites prone to contamination with feces or with upper airway secretions—e.g., wounds associated with intestinal surgery, decubitus ulcers, or human bites. Moreover, anaerobes have been isolated from cutaneous abscesses, rectal abscesses, and axillary sweat gland infections (hidradenitis suppurativa). Anaerobes also are often cultured from foot ulcers of diabetic patients. The deep soft-tissue infections associated with anaerobic bacteria are crepitant cellulitis, synergistic cellulitis, gangrene, and necrotizing fasciitis (Chaps. 156 and 179).
These soft tissue or skin infections are usually polymicrobial. A mean of 4.8 bacterial species are isolated, with an anaerobe-to-aerobe ratio of ~3:2. The most frequently isolated organisms include Bacteroides, Peptostreptococcus, Clostridium, Enterococcus, and Proteus species. The involvement of anaerobes in these types of infections is associated with a higher frequency of fever, foul-smelling lesions, gas in the tissues, and visible foot ulcer.
Anaerobic bacterial synergistic gangrene (Meleney’s gangrene), a rare infection of the superficial fascia, is characterized by exquisite pain, redness, and swelling followed by induration. Erythema surrounds a central zone of necrosis. A granulating ulcer forms at the original center as necrosis and erythema extend outward. Symptoms are limited to pain; fever is not typical. These infections usually involve a combination of Peptostreptococcus species and S. aureus; the usual site of infection is an abdominal surgical wound or the area surrounding an ulcer on an extremity. Treatment includes surgical removal of necrotic tissue and antimicrobial administration.
Necrotizing fasciitis, a rapidly spreading destructive disease of the fascia, is usually attributed to group A streptococci (Chap. 173) but can also be a mixed infection involving anaerobes and aerobes, usually occurring after surgeries and in patients with diabetes or peripheral vascular disease. The most frequently isolated anaerobes in these infections are Peptostreptococcus and Bacteroides species. Gas may be found in the tissues. Similarly, myonecrosis can be associated with mixed anaerobic infection. Fournier’s gangrene consists of cellulitis involving the scrotum, perineum, and anterior abdominal wall, with mixed anaerobic organisms spreading along deep external fascial planes and causing extensive loss of skin.
Bone and Joint Infections Although actinomycosis (Chap. 200) accounts on a worldwide basis for most anaerobic infections in bone, organisms including peptostreptococci or microaerophilic cocci, Bacteroides species, Fusobacterium species, and Clostridium species can also be involved in osteomyelitis (Chap. 158). These infections frequently arise adjacent to soft tissue infections. Many patients with osteomyelitis due to anaerobic bacteria have evidence of an anaerobic infection elsewhere in the body; most commonly, infected adjacent soft-tissue sites are the source of the organisms involved. Examples are diabetic foot ulcers and decubitus ulcers that may be complicated by mixed aerobic-anaerobic osteomyelitis. Hematogenous seeding of bone is uncommon. Prevotella and Porphyromonas species are detected in infections involving the maxilla and mandible, whereas Clostridium species have been reported as anaerobic pathogens in cases of osteomyelitis of the long bones following fracture or trauma. Fusobacteria have been isolated in pure culture from sites of osteomyelitis adjacent to the perinasal sinuses. Peptostreptococci and microaerophilic cocci have been reported as significant pathogens in infections involving the skull, mastoid, and prosthetic implants placed in bone. In patients with osteomyelitis, the most reliable culture specimen is a bone biopsy sample free of normal uninfected skin and subcutaneous tissue.
In contrast to anaerobic osteomyelitis, most cases of anaerobic arthritis (Chap. 157) involve a single isolate, and most cases are secondary to hematogenous spread. The most common isolates are Fusobacterium species. Most of the patients involved have uncontrolled peritonsillar infections progressing to septic cervical venous thrombophlebitis (Lemierre’s syndrome) and resulting in hematogenous dissemination with a predilection for the joints. Unlike anaerobic osteomyelitis, anaerobic pyoarthritis in most cases is not polymicrobial and may be acquired hematogenously. Anaerobes are important pathogens in infections involving prosthetic joints; in these infections, the causative organisms (such as Peptostreptococcus species and P. acnes) are part of the normal skin microbiota.
Bacteremia Transient bacteremia is a well-known event in healthy individuals whose anatomic mucosal barriers have been injured (e.g., during dental extractions or dental scaling). These bacteremic episodes, which are often due to anaerobes, have no pathologic consequences. However, anaerobic bacteria are found in cultures of blood from clinically ill patients when proper culture techniques are used. Anaerobes have accounted for 5% (range at various institutions, 0.5–12%) of cases of clinically significant bacteremia. The incidence of anaerobic bacteremia decreased from the 1970s through the early 1990s. This change may have been related to the administration of antibiotic prophylaxis before intestinal surgery, the earlier recognition of localized infections, and the empirical use of broad-spectrum antibiotics for presumed infection. Recent reports present conflicting data regarding rates of anaerobic bacteremia. A study from the Mayo Clinic compared three periods (1993–1996, 1997–2000, and 2001–2004) and found a 74% increase in the mean incidence of anaerobic bacteremia; this finding contrasts with a 45% decrease in incidence from 1977 to 1988 at the same institution. In contrast, a report from Switzerland compared two periods (1997–2001 and 2002–2006) and found decreases in both the number of anaerobe-positive blood cultures and the proportion of all blood culture isolates that were anaerobes.
The majority of anaerobic bacteremias are due to gram-negative bacilli—mainly the B. fragilis group, with B. fragilis most commonly isolated (60–80% of cases). Other organisms causing bacteremia include Clostridium species (10%), Peptostreptococcus species (10%), and Fusobacterium species (5%).
Once the organism in the blood has been identified, both the portal of bloodstream entry and the underlying problem that probably led to seeding of the bloodstream can often be deduced from an understanding of the organism’s normal site of residence. For example, mixed anaerobic bacteremia including B. fragilis usually implies a colonic pathology with mucosal disruption from neoplasia, diverticulitis, or some other inflammatory lesion. Debilitating diseases such as malignancies, diabetes, organ transplantation, and abdominal and pelvic surgeries are among the predisposing factors for anaerobic bacteremia. In a retrospective nested case–control study, diabetes was identified as a risk factor for anaerobic bacteremia when the source of bacteremia was unknown. The initial manifestations are determined by the portal of entry and reflect the localized condition. When bloodstream invasion occurs, patients can become extremely ill, with rigors and hectic fevers. The clinical picture may be quite similar to that seen in sepsis involving aerobic gram-negative bacilli. Although complications of anaerobic bacteremia (e.g., septic thrombophlebitis and septic shock) have been reported, their incidence in association with anaerobic bacteremia is low. Anaerobic bacteremia is potentially fatal and requires rapid diagnosis and appropriate therapy. Reported case–fatality rates are high, ranging from 25% to 44%, and appear to increase with the age of the patient (with reported rates of >66% among patients >60 years old), with the isolation of multiple species from the bloodstream, and with the failure to surgically remove a focus of infection. The attributable mortality rate for bacteremia associated with the B. fragilis group was examined in a matched case–control study. Patients with B. fragilis–group bacteremia had a significantly higher mortality rate (28% vs 8%), with an attributable mortality rate of 19.3% and a mortality risk ratio of 3.2.
Endocarditis and Pericarditis (See also Chap. 155) Endocarditis due to anaerobes is uncommon. However, anaerobic streptococci, which are often classified incorrectly, are responsible for this disease more frequently than is generally appreciated. Gram-negative anaerobes are unusual causes of endocarditis. Signs and symptoms of anaerobic endocarditis are similar to those of endocarditis due to facultative organisms. Mortality rates of 21–43% have been reported for anaerobic endocarditis.
Anaerobes, particularly B. fragilis and Peptostreptococcus species, are uncommonly found in infected pericardial fluids. Anaerobic pericarditis is associated with a mortality rate of >50%. Anaerobes can reach the pericardial space by hematogenous spread, by spread from a contiguous site of infection (e.g., heart or esophagus), or by direct inoculation arising from trauma or surgery.
DIAGNOSIS
There are three critical steps in the diagnosis of anaerobic infection: (1) proper collection of specimens; (2) rapid transport of the specimens to the microbiology laboratory, preferably in anaerobic transport media; and (3) proper handling of the specimens by the laboratory. Specimens must be collected by meticulous sampling of infected sites, with avoidance of contamination by the normal microbiota. When such contamination is likely, the specimen is unacceptable. Examples of specimens unacceptable for anaerobic culture include sputum collected by expectoration or nasal tracheal suction, bronchoscopy specimens, samples collected directly through the vaginal vault, urine collected by voiding, and feces. Specimens appropriate for anaerobic culture include sterile body fluids such as blood, pleural fluid, peritoneal fluid, cerebrospinal fluid, and aspirates or biopsy samples from normally sterile sites. As a general rule, liquid or tissue specimens are preferred; swab specimens should be avoided.
Because even brief exposure to oxygen may kill some anaerobic organisms and result in failure to isolate them in the laboratory, air must be expelled from the syringe used to aspirate the abscess cavity, and the needle must be capped with a sterile rubber stopper. It is also important to remember that prior antibiotic therapy reduces the cultivability of these bacteria. Specimens can be injected into transport bottles containing a reduced medium or taken immediately in syringes to the laboratory for direct culture on anaerobic media. Delays in transport may lead to a failure to isolate anaerobes due to exposure to oxygen or overgrowth of facultative organisms, which may eliminate or obscure any anaerobes that are present. All clinical specimens from suspected anaerobic infections should be subjected to Gram’s staining and examined for organisms with characteristic morphology. It is not unusual for organisms to be observed on Gram’s staining but not isolated in culture.
Because of the time and difficulty involved in the isolation of anaerobic bacteria, diagnosis of anaerobic infections must frequently be based on presumptive evidence. There are few clinical clues to the probable presence of anaerobic bacteria at infected sites. The involvement of certain sites with lowered oxidation-reduction potential (e.g., avascular necrotic tissues) and the presence of an abscess favor the diagnosis of an anaerobic infection. When infections occur in proximity to mucosal surfaces normally harboring an anaerobic microbiota, such as the gastrointestinal tract, female genital tract, or oropharynx, anaerobes should be considered as potential etiologic agents. A foul odor is often indicative of anaerobes, which produce certain organic acids as they proliferate in necrotic tissue. Although these odors are nearly pathognomonic for anaerobic infection, the absence of odor does not exclude an anaerobic etiology. The presence of gas in tissues is highly suggestive, but not diagnostic, of anaerobic infection. Because anaerobes often coexist with other bacteria and cause mixed or synergistic infection, Gram’s staining of exudate frequently reveals multiple morphotypes suggestive of anaerobes. Sometimes these organisms have morphologic characteristics associated with specific species.
When cultures of obviously infected sites or purulent material yield no growth, streptococci only, or a single aerobic species (such as E. coli) and Gram’s staining reveals a mixed flora, the involvement of anaerobes should be suspected; the implication is that the anaerobic microorganisms have failed to grow because of inadequate transport and/or culture techniques. Failure of an infection to respond to antibiotics that are not active against anaerobes (e.g., aminoglycosides and—in some circumstances—penicillin, cephalosporins, or tetracyclines) suggests an anaerobic etiology.
|
TREATMENT |
ANAEROBIC INFECTIONS |
Successful therapy for anaerobic infections requires the administration of a combination of appropriate antibiotics, surgical resection, debridement of devitalized tissues, and drainage either surgically or percutaneously (guided by an imaging technique such as CT, MRI, or ultrasound). Any anatomic breach must be closed promptly, closed spaces drained, tissue compartments decompressed, and an adequate blood supply established. Abscess cavities should be drained as soon as fluctuation or localization occurs.
ANTIBIOTIC THERAPY AND RESISTANCE
The antibiotics used to treat anaerobic infections should be active against both aerobic and anaerobic organisms because many of these infections are of mixed etiology. Antibiotic regimens can usually be selected empirically on the basis of the type of infection, the species of the organisms usually present in such cases, the results of Gram’s staining, and a knowledge of antimicrobial resistance patterns (Chap. 170 and Table 201-2). Other factors influencing the selection of antibiotics include need for bactericidal activity and for penetration into certain organs (such as the brain), toxicity, and impact on the normal microbiota. Antibiotics active against clinically relevant anaerobes can be grouped into four categories based on their predicted activity (Table 201-2). Nearly all the drugs listed have toxic side effects, which are described in detail in Chap. 170.
|
ANTIMICROBIAL THERAPY FOR INFECTIONS INVOLVING COMMONLY ENCOUNTERED ANAEROBIC GRAM-NEGATIVE RODS |
Antibiotic susceptibility testing of anaerobic bacteria has been difficult and controversial. Because of the slow growth rate of many anaerobes, the lack of standardized testing methods and of clinically relevant standards for resistance, and the generally good results obtained with empirical therapy, there has been limited interest in testing these organisms for antibiotic susceptibility. However, one study of antibiotic-treated patients with Bacteroides isolates from blood found mortality rates of 45% among those whose isolates were deemed resistant to the agent used and 16% among those whose isolates were deemed sensitive. It is accepted that testing is important for patients with serious or prolonged infections or in cases in which antibiotics have not had an impact. Testing is also helpful in monitoring the activity of new drugs and recording current resistance patterns among anaerobic pathogens. The antibiotics with the greatest activity against nearly all anaerobic bacteria include carbapenems, β-lactam/β-lactamase inhibitor combinations, metronidazole, and chloramphenicol.
![]() Antibiotic resistance in anaerobic bacteria is an increasing problem. Resistance rates vary with the institution and the geographic region. In recent years, the activity of clindamycin, cefoxitin, cefotetan, and moxifloxacin has decreased against B. fragilis and related strains (B. distasonis, B. ovatus, B. the taiotaomicron, B. uniformis, B. vulgatus). Multidrug-resistant B. fragilis has recently been reported. Nearly all organisms in the B. fragilis group (>97%) are resistant to penicillin G. The cephamycins cefoxitin and cefotetan display greater activity against this group, but rates of resistance have increased, with current figures at ~10% in the United States and higher in Argentina (28%) and Europe (17%). Rates of resistance to β-lactam agents among anaerobes other than Bacteroides are lower but are highly variable. β-Lactam/β-lactamase inhibitor combinations such as ampicillin/sulbactam, ticarcillin/clavulanic acid, and piperacillin/tazobactam are usually good therapeutic options against β-lactamase-producing anaerobes, including the B. fragilis group. Although resistance rates reported from most countries are still low, several studies have documented nonsusceptibility to ampicillin/sulbactam in 0.5–3% of isolates in the United States, 3–10% in Europe, and 1–8% in Argentina. Recently, up to 48% of B. fragilis isolates in Taiwan were found to be nonsusceptible to ampicillin/sulbactam, and a significant increase in resistance to this combination was also identified among other Bacteroides, Prevotella, and Fusobacterium species.
Antibiotic resistance in anaerobic bacteria is an increasing problem. Resistance rates vary with the institution and the geographic region. In recent years, the activity of clindamycin, cefoxitin, cefotetan, and moxifloxacin has decreased against B. fragilis and related strains (B. distasonis, B. ovatus, B. the taiotaomicron, B. uniformis, B. vulgatus). Multidrug-resistant B. fragilis has recently been reported. Nearly all organisms in the B. fragilis group (>97%) are resistant to penicillin G. The cephamycins cefoxitin and cefotetan display greater activity against this group, but rates of resistance have increased, with current figures at ~10% in the United States and higher in Argentina (28%) and Europe (17%). Rates of resistance to β-lactam agents among anaerobes other than Bacteroides are lower but are highly variable. β-Lactam/β-lactamase inhibitor combinations such as ampicillin/sulbactam, ticarcillin/clavulanic acid, and piperacillin/tazobactam are usually good therapeutic options against β-lactamase-producing anaerobes, including the B. fragilis group. Although resistance rates reported from most countries are still low, several studies have documented nonsusceptibility to ampicillin/sulbactam in 0.5–3% of isolates in the United States, 3–10% in Europe, and 1–8% in Argentina. Recently, up to 48% of B. fragilis isolates in Taiwan were found to be nonsusceptible to ampicillin/sulbactam, and a significant increase in resistance to this combination was also identified among other Bacteroides, Prevotella, and Fusobacterium species.
Carbapenems (ertapenem, doripenem, meropenem, and imipenem) are equally active against anaerobes, with <1% of B. fragilis strains showing resistance in the United States and Europe. Higher rates of carbapenem nonsusceptibility are being reported from some countries (5% in Germany, 8% [to doripenem] in Canada, and 7–12% in Taiwan).
Metronidazole is active against gram-negative anaerobes, including the B. fragilis group; resistance, although rare (<1%), has been reported in both Europe and the United States. Resistance to metronidazole is more common among gram-positive anaerobes, including P. acnes, Actinomyces species, lactobacilli, and anaerobic streptococci. Clindamycin is active against many anaerobes. However, rates of resistance to clindamycin among the B. fragilis group have increased in the United States from 3% in 1982 to 16% in 1996 and 26% in 2000, with rates as high as 40–50% in some series. Resistance to clindamycin among non-Bacteroides anaerobes is much less common (<10%).
Tigecycline is active against some anaerobic bacteria, including Peptostreptococcus, Propionibacterium, Prevotella, Fusobacterium, and most Bacteroides species. Its efficacy for treatment of intraabdominal infections was comparable to that of imipenem in two phase 3 double-blind clinical trials. This drug is therefore recommended as single-agent treatment for complicated intraabdominal infections, but resistance (~6%) among Bacteroides and non-Bacteroides species has been reported.
Fluoroquinolones such as moxifloxacin have shown potential in the treatment of mixed aerobic-anaerobic infections. A survey in the United States found a 38% rate of resistance to moxifloxacin among the B. fragilis group; in Europe 14–30% of isolates were nonsusceptible to this drug, as were 7–25% of anaerobes isolated from blood cultures in Taiwan. Despite excellent in vitro activity against all clinically important anaerobes, chloramphenicol is less desirable than other active drugs for the treatment of anaerobic infection because of documented clinical failures.
If a patient fails to respond to one of the category 1 or category 2 drugs (Table 201-2), consideration should be given to alternative therapy and to determination of the resistance patterns among Bacteroides isolates.
INFECTIONS AT SPECIFIC SITES
In clinical situations, specific regimens must be tailored to the initial site of infection. The duration of therapy also depends on the infection site; the reader is referred to specific chapters on sites of infection for recommendations.
Infections above the diaphragm usually reflect the orodental microbiota, which does not include the B. fragilis group. β-Lactamase production has been reported in anaerobic strains that are usually isolated from infections originating above the diaphragm. Up to 60% of clinical isolates classified as Prevotella or Porphyromonas species, non–B. fragilis species of Bacteroides, or Fusobacterium species reportedly produce β-lactamase; thus all β-lactam drugs (penicillins and cephalosporins) are poor options. Because most of these infections have a mixed etiology that includes microaerophilic and aerobic streptococci, antibiotics that cover both aerobic and anaerobic bacteria are recommended. The recommended regimens include clindamycin, a β-lactam/β-lactamase inhibitor combination, or metronidazole in combination with a drug active against microaerophilic and aerobic streptococci.
Bronchoscopy in lung abscess is indicated only to rule out airway obstruction and does not enhance drainage; in any event, it should be delayed until the antimicrobial regimen has begun to affect the disease process so that the procedure does not spread the infection. Surgery is almost never indicated because of the danger of spilling the abscess contents into the lungs.
Chloramphenicol has been used successfully against anaerobic CNS infections at doses of 30–60 mg/kg per day, with the exact dose depending on the severity of illness. However, penicillin G and metronidazole also cross the blood–brain barrier and are bactericidal for many anaerobic organisms.
Anaerobic infections arising below the diaphragm (e.g., colonic and intraabdominal infections) must be treated specifically with agents active against Bacteroides species (Table 201-2). In intraabdominal sepsis (Chap. 159), the use of antibiotics effective against penicillin-resistant anaerobes has clearly reduced the incidence of postoperative infections and serious infectious complications. Specifically, a drug from category 1 (Table 201-2) must be included for broad-spectrum coverage. Single agents suitable for this purpose include the carbapenems, cefoxitin, and β-lactam/β-lactamase inhibitor combinations. A two-drug regimen is an alternative, with one drug active against coliforms and the other against anaerobes (e.g., a third-generation cephalosporin or a quinolone with metronidazole). In addition, if the clinician suspects that gram-positive facultative organisms such as enterococci are involved, therapeutic regimens should include ampicillin or vancomycin. Although clindamycin and cefotetan were previously considered acceptable options for intraabdominal infections involving anaerobes, these drugs are no longer recommended because of escalating rates of resistance in the B. fragilis group. Ampicillin/sulbactam is not recommended because of high rates of resistance among community-acquired strains of E. coli rather than because of resistance in anaerobic bacteria.
A meta-analysis of 40 randomized or quasi-randomized controlled trials of 16 antibiotic regimens for secondary peritonitis showed equivalent clinical success for all regimens.
Cases of anaerobic osteomyelitis in which a mixed flora is isolated from a bone biopsy specimen should be treated with a regimen that covers all isolates. When an anaerobic organism is recognized as a major or sole pathogen infecting a joint, the duration of treatment should be similar to that used for arthritis caused by aerobic bacteria (Chap. 157). Therapy includes the management of underlying disease states, the administration of appropriate antimicrobial agents, temporary joint immobilization, percutaneous drainage of effusions, and (usually) the removal of infected prostheses or internal fixation devices. Surgical drainage and debridement procedures such as sequestrectomy are essential for the removal of necrotic tissue that can sustain anaerobic infections.
The outcome of anaerobic bacteremia is significantly better in patients either initially given or switched to appropriate therapy on the basis of known antibiotic susceptibilities.
FAILURE OF THERAPY
Anaerobic infections that fail to respond to treatment or that relapse should be reassessed. Consideration should be given to additional surgical drainage or debridement. Superinfections with resistant gram-negative facultative or aerobic bacteria should be ruled out. The possibility of drug resistance must be entertained; if resistance is involved, repeat cultures may yield the pathogen.
SUPPORTIVE MEASURES
Other supportive measures in the management of anaerobic infections include careful attention to fluid and electrolyte balance (since extensive local edema may lead to hypoalbuminemia), hemodynamic support for septic shock, immobilization of infected extremities, maintenance of adequate nutrition during chronic infections by parenteral hyperalimentation, relief of pain, and anticoagulation for thrombophlebitis. For patients with severe anaerobic infections of soft tissues, hyperbaric oxygen therapy is advocated by some experts, but its value has not been proven in controlled trials.
SECTION 8 |
MYCOBACTERIAL DISEASES |
202 |
Tuberculosis |
Tuberculosis (TB), which is caused by bacteria of the Mycobacterium tuberculosis complex, is one of the oldest diseases known to affect humans and a major cause of death worldwide. Recent population genomic studies suggest that M. tuberculosis may have emerged ~70,000 years ago in Africa and subsequently disseminated along with anatomically modern humans, expanding globally during the Neolithic Age as human density started to increase. Progenitors of M. tuberculosis are likely to have affected prehominids. This disease most often affects the lungs, although other organs are involved in up to one-third of cases. If properly treated, TB caused by drug-susceptible strains is curable in the vast majority of cases. If untreated, the disease may be fatal within 5 years in 50–65% of cases. Transmission usually takes place through the airborne spread of droplet nuclei produced by patients with infectious pulmonary TB.
ETIOLOGIC AGENT
Mycobacteria belong to the family Mycobacteriaceae and the order Actinomycetales. Of the pathogenic species belonging to the M. tuberculosis complex, which comprises eight distinct subgroups, the most common and important agent of human disease is M. tuberculosis. The complex includes M. bovis (the bovine tubercle bacillus—characteristically resistant to pyrazinamide, once an important cause of TB transmitted by unpasteurized milk, and currently the cause of a small percentage of human cases worldwide), M. caprae (related to M. bovis), M. africanum (isolated from cases in West, Central, and East Africa), M. microti (the “vole” bacillus, a less virulent and rarely encountered organism), M. pinnipedii (a bacillus infecting seals and sea lions in the Southern Hemisphere and recently isolated from humans), M. mungi (isolated from banded mongooses in southern Africa), M. orygis (described recently in oryxes and other Bovidae in Africa and Asia and a potential cause of infection in humans), and M. canetti (a rare isolate from East African cases that produces unusual smooth colonies on solid media and is considered closely related to a supposed progenitor type).