Chapter 17 Does Early Treatment Influence the Long-Term Outcome of Epilepsy?
Seizure Recurrence following a First Seizure
Studies assessing the prognosis for patients presenting with their first seizure or seizures have tended to focus on seizure recurrence rates. For patients presenting with a single seizure, many observational studies, both prospective and retrospective, have assessed seizure recurrence rates, many of which have been summarized in a systematic review and meta-analysis reported by Berg and Shinnar.1 Thirteen first seizures studies were included in this systematic review, and the overall seizure recurrence risk was 46% (44 to 49) and for the subset of 5 prospective studies was 40% (37 to 43). The 2-year recurrence risk was 42% (39 to 44) across all studies and 36% (32 to 39) for the subset of prospective studies. There was a significantly higher recurrence risk for patients with partial onset seizures, an abnormal EEG, and neurological abnormality (learning difficulty, neurological signs, etc.). Thus, for the majority of patients presenting with a single seizure, the risk of a recurrence is low, and given the potential side effects of antiepileptic drugs, antiepileptic drug treatment has not been routinely recommended.
The most reliable method to assess the influence of antiepileptic drug treatment on recurrence rates and other outcomes is the randomized controlled trial. Such a study would randomly allocate patients to treatment or no treatment (or immediate or deferred treatment) and ideally should be large enough to allow subgroup analyses, most commonly in regression analyses, allowing the identification of patient characteristics that influence seizure recurrence risks and the magnitude of any treatment effect. To date, five published randomized controlled trials have examined the impact of antiepileptic drug treatment following a first seizure,2–6 and the key characteristics of these trials are summarized in Table 17-1.
TABLE 17–1 Randomized Controlled Trials Comparing Antiepileptic Drug Treatment Versus No Treatment Following a First Seizure
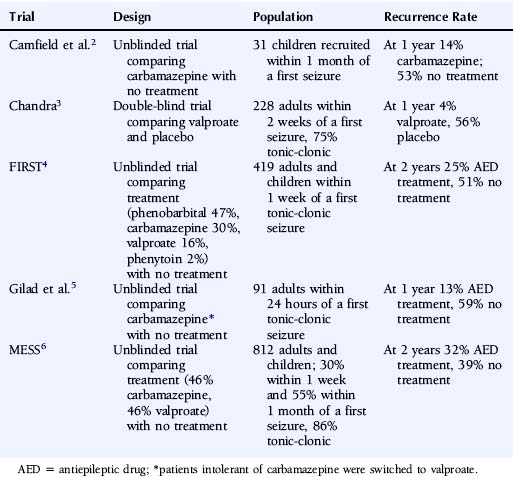
These trials consistently show a lower seizure recurrence rate with antiepileptic drug treatment compared to no treatment or placebo. There are, however, substantial differences among these trials in the estimated recurrence risks, with the 1- to 2-year recurrence risk ranging from 4 to 32% for AED-treated patients and 39 to 59% for untreated patients. These differences are most likely explained by differences in study design and patient populations in terms of factors such as time between seizure and randomization, seizure type, and age. Only two trials recruited sufficient patients to examine patient predictors of outcome. In the FIRST trial4,7 age <16 years, secondarily generalized seizures, remote symptomatic seizures, and epileptiform changes on the EEG were associated with a higher risk of recurrence, whereas acute treatment with benzodiazepines was associated with a lower risk of recurrence.
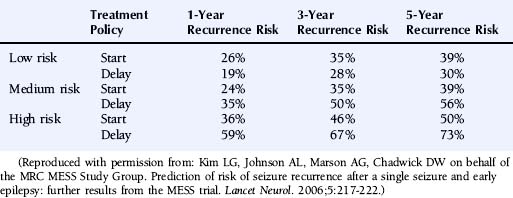
The multicenter study of early epilepsy and single seizures (MESS trial)6 recruited 812 patients following a single seizure and 631 following two or more seizures. Regression modeling showed that the number of seizures before randomization, an abnormal EEG, and the presence of a neurological abnormality (learning disability or neurological signs) increased the risk of a seizure recurrence.8 This allowed the creation of a prognosis index to stratify patients in the groups with a low, medium, or high risk of seizure recurrence as summarized in Table 17-2. The recurrence risks estimated for these groups are given in Table 17-3.
| Prognostic Index | |
|---|---|
| Starting Value | |
| Single seizure | 0 |
| Two or three seizures | 1 |
| Four or more seizures | 2 |
| Add if Present | |
| Neurological disorder of deficit | 1 |
| Abnormal EEG | 1 |
| Risk Classification Group | Final Score |
| Low risk | 0 |
| Medium risk | 1 |
| High risk | 2–4 |
Reproduced with permission from: Kim LG, Johnson AL, Marson AG, Chadwick DW on behalf of the MRC MESS Study Group. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurol. 2006;5:217-222.
Seizure Recurrence following a Second Seizure
The risk of unprovoked seizures following a second seizure has not been examined in a prospective population-based study of patients untreated with antiepileptic drugs. Antiepileptic drugs became accepted practice for patients with epilepsy before robust epidemiological methods had been applied to examine the natural history of untreated epilepsy. It would now, of course, be considered unethical to deny a population of patients a treatment that is believed to be effective in order to examine this. The risk of seizure recurrence following two unprovoked seizures has been examined by Hauser et al.,9 who prospectively followed up 204 patients, 87% of whom were on antiepileptic drug treatment following their second seizure. The risk of a third seizure following a second was 32% (21 to 43) at 3 months, 41% (29 to 53) at 6 months, 57% (45 to 70) at 12 months, and 73% (59 to 87) at 4 years. The risk of a third seizure was higher in patients with remote symptomatic seizures than those with idiopathic or cryptogenic seizures, hazard ratio 1.9 (1.0 to 3.4). Other factors such as seizure type, EEG abnormality, or neurological abnormality were not significantly associated with a third seizure. The risk of a fourth seizure following a third was 31% (16 to 46) at 3 months, 48% (32 to 64) at 6 months, 61% (44 to 77) at 1 year and 76% (60 to 90) at 3 years. Shinnar et al. found similar results in a cohort of 407 children prospectively followed after their first seizure.10
Thus, in contrast to patients with a first seizure, the risk of an additional seizure following a second or third unprovoked seizure is high, even with the use of antiepileptic drugs. Given this finding in their paper, Hauser and colleagues recommend that patients with two or more unprovoked seizures should start antiepileptic drug treatment, although this study, by design, is unable to define the magnitude of any effect that antiepileptic drug treatment might have on seizure recurrence rates. To address this question, we need data from randomized controlled trials comparing treatment with no treatment for a cohort of patients with newly diagnosed epilepsy. No such trial has ever been undertaken and would now be considered unethical and would certainly contravene the Declaration of Helsinki.11 At present, therefore, for patients with newly diagnosed epilepsy, we have no robust data that defines the magnitude of any treatment effect of antiepileptic drugs, both for the short-term outcomes (seizure recurrence) discussed earlier, as well as for the longer-term outcomes that are discussed later in this chapter. Despite this, consensus seems to hold that the majority of patients should be offered treatment following two or more seizures, particularly if they have occurred over a relatively short period of time (6 to 12 months). There has continued to be uncertainty regarding the appropriate recommendation for patients with seizures of minor symptomatology (e.g., simple partial seizures), and patients with long periods of time between seizures. Such patients (631) were entered into the MESS study6 when both clinician and patient were uncertain about the need for antiepileptic drug treatment.
Longer-Term Outcomes
Given that up to 30% of patients with epilepsy are drug refractory12 and experience continued seizures and a diminished quality of life, any intervention proven to improve the natural history, by reducing the severity of epilepsy and improving the probability of a long-term remission from seizures, could have important consequences for a large number of patients.13,14 Thus it is important to understand whether the early use of antiepileptic drugs might influence the natural history of epilepsy.
Some have suggested that epilepsy might be a self-facilitating process in which seizures beget seizures,15,16 whereby each seizure causes further neuronal damage and reorganization that increases the probability of the next seizure. This idea was suggested by Gower in 1881, when he suggested that “the tendency of the disease is toward self perpetuation; each attack facilitating the occurrence of the next by increasing the instability of nerve elements.”17
Elwes et al.18 recruited a cohort of 183 patients presenting with a new diagnosis of epilepsy and retrospectively assessed the time interval between successive tonic-clonic seizures prior to their clinic visit. They observed that the time interval between successive seizures became progressively shorter, and the authors suggest that this finding supports early epilepsy being an accelerating process. However, this study may be confounded by its retrospective design, selection bias, seizure type, and perhaps seizure clustering and does not provide evidence of seizures begetting seizures.
Other studies have showed that patients with more seizures or a higher seizure frequency prior to starting antiepileptic drug treatment had a lower rate of remission from seizures.19,20 This finding was again interpreted as indicating that seizures predispose to further seizures and used as an argument for early treatment. However, the number of seizures prior to starting treatment is likely to be correlated with seizure type, with patients with complex partial seizures experiencing a greater number of seizures before starting treatment and having a poorer prognosis, as was the case in these studies. Thus although seizure type and the number of seizures prior to starting treatment may be important prognostic factors, these studies do not necessarily provide evidence of seizures begetting seizures.
Two studies have been undertaken in developing countries, one from Kenya21 and one from Ecuador,22 in which people with untreated epilepsy were ascertained by field workers going door to door. These patients were then offered entry into a randomized controlled trial comparing carbamazepine and phenobarbital; 192 entered the trial in Ecuador, and 302 entered the trial in Kenya. Despite having untreated epilepsy, sometimes for many years, in both studies 53% of patients were seizure free from the 6th to the 12th month of follow-up, which is comparable to outcomes in randomized controlled trials in newly diagnosed populations.23,24 This finding would suggest that the failure to treat epilepsy early does not worsen the prognosis of epilepsy once treatment is started.
There is some animal data that suggests that some antiepileptic drugs may exhibit antiepileptogenic properties. Studies in kindled rats suggest that valproate, phenobarbital, diazepam, vigabatrin, topiramate, levetiraceta, and lamotrigine might have antiepileptogenic properties,25 although the appropriateness of the kindling model for assessing antiepileptogenic properties has been criticized.26 Post-status epilepticus models suggest that topiramate and valproate may have antiepileptogenic properties, although results have been conflicting. Although these models may be appropriate to examine potential antiepileptogenic effects and might equate to patients presenting with prolonged status epilepticus, they do not readily equate to the patient presenting with a single seizure or early epilepsy.
Assessing the Impact of Antiepileptic Drug Treatment on Longer-Term Outcomes
The longer-term seizure outcome most commonly examined is seizure remission rates, which is one of the primary outcomes recommended by the International League Against Epilepsy for trials of antiepileptic drug monotherapy.27 One approach is to use survival methods to assess the time taken from randomization to achieve a specified period of remission (e.g., 1 or 2 years). This approach allows an assessment as to which treatment policy is associated with achieving this outcome soonest, most commonly expressed as a hazard ratio or risk ratio. In addition, this approach allows an estimate of the proportion of patients that have achieved a remission at specific points in time.
Three reports assessing longer-term outcomes in randomized controlled trials have been published.28–31 Camfield et al.2 reported a trial that recruited 31 children within 1 month of a first seizure who were allocated to immediate treatment with carbamazepine or deferred treatment. In 2001 they attempted to collect 15-year follow-up data28 and were able to trace 26 of the original 31 participants, 16 of the 17 control group patients, and 10 of the 14 patients allocated carbamazepine. Four patients allocated carbamazepine, and five patients in the control group had remained free of seizures since randomization. Of the 10 patients originally allocated carbamazepine, eight had a 2-year terminal remission at 15-year follow-up, compared to 14 of the 16 allocated to the control group, RR 0.73 (0.24 to 2.2). Thus the number of patients with either a recurrence or in a 2-year terminal remission at 15-year follow-up was similar. The authors concluded that the long-term clinical course of epilepsy is not improved by treatment following a first unprovoked seizure. However, with 31 patients, this study did not have the power to exclude the possibility of an important treatment effect, as supported by the confidence intervals around the RR for a 2-year terminal remission at 15-year follow-up, 0.73 (0.24 to 2.22), which indicates that these results could be in keeping with doubling this terminal remission rate with early antiepileptic drug treatment.
For the FIRST trial, two reports give longer-term follow-up data, the first of which provides results for the first 7 years of follow-up. Eighty-seven percent of patients allocated immediate treatment and 83% allocated deferred treatment achieved a 1-year remission from seizures during follow-up. The estimated hazard ratio for time to achieve a 1-year remission was 1.2 (0.95 to 1.37), indicating that patients allocated immediate treatment may be 20% more likely to enter a 1-year remission, although this result was not statistically significant as the confidence interval crosses one.
The latest report from the FIRST trial provides data following 10 to 13 years of follow-up and provides results for 2-year remission rates.30 For the whole cohort, 87% of those allocated immediate treatment and 78% of those allocated deferred treatment had achieved a 2-year remission. When the analysis was restricted to patients with 9 or more years follow-up data available, 92% of patients allocated immediate treatment and 93% allocated deferred treatment achieved a 2-year remission. The report estimates that during the first 3 months of follow-up 57% allocated deferred treatment and 72% allocated immediate treatment entered a 2-year remission. At 5 years follow-up, the report estimated that 79% allocated deferred treatment and 84% allocated immediate treatment had achieved a 2-year remission, whereas at 12 years of follow-up, 86% allocated deferred treatment and 85% allocated immediate treatment achieved a 2-year remission. Again, these results indicate that patients allocated immediate treatment might enter a 2-year remission sooner, but by 12 years of follow-up, the estimates are similar for both groups. Estimates for the differences between groups at these time points with 95% confidence intervals are not provided; hence, from the published data, we cannot be certain whether the possibility of important differences existing has been excluded.
The largest trial to examine the influence of early antiepileptic drug treatment on longer-term outcomes following a single seizure or early epilepsy is the MESS trial.6 This trial recruited 1443 patients, 812 following a single seizure and 631 following two or more seizures, the latter representing a group with infrequent seizures or seizures with minor symptomatology for whom there was uncertainty about the need for antiepileptic drug treatment.
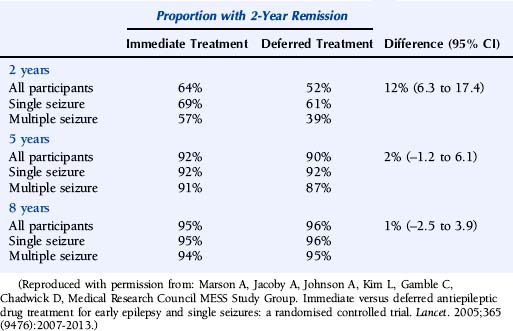
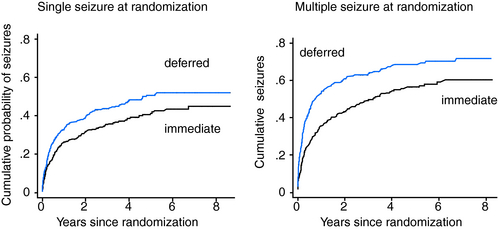
The results for time to a 2-year remission are summarized in Table 17-4 and Figure 17-1. Results for the whole cohort recruited to the MESS trial show that at 2 years, 64% of the immediate-treatment group and 52% of the deferred-treatment group achieved an immediate 2-year remission, whereas at 5 years 92% of patients allocated immediate treatment had achieved a 2-year remission compared to 90% in the deferred-treatment group. The difference between the two groups at 5 years was 2% (–1.2 to 6.1). The confidence intervals around this estimate are narrow and exclude the possibility of an important effect of treatment. At 8 years, 95% allocated immediate treatment had achieved a 2-year remission compared to 96% in the deferred-treatment group. The difference between the two groups was 1% (–2.5 to 3.9), which again excludes the possibility of an important treatment effect. Thus, in keeping with results from the FIRST trial group, MESS confirms that patients starting treatment following a single or few seizures will enter a 2-year remission sooner than those allocated deferred treatment, but treatment has no influence on the proportion of patients entering a 2-year period of remission after 5 years or longer of follow-up.
Adverse Effects and Quality-of-Life Consequences of Immediate versus Deferred Treatment
The MESS study reported data for adverse effects and for quality-of-life outcomes. Patients in the immediate-treatment group were more likely to report at least on adverse effect—39% versus 31%, difference 8% (4 to 14). The adverse events were largely those potentially associated with antiepileptic drug treatment. There were 54 deaths, 31 in the immediate-treatment group and 23 in the deferred-treatment group, 6 of which were classified as sudden unexpected death in epilepsy, four in the immediate-and two in the deferred-treatment group. Of the 527 eligible adults recruited into MESS in the U.K. 441 provided quality-of-life data at baseline and at 2 years.32 Overall there was no quality-of-life advantage for either treatment policy, with results suggesting a trade-off between the adverse consequences of more seizure recurrences in the deferred-treatment group and a higher rate of antiepileptic drug adverse events in the immediate-treatment group.
1. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41(7):965-972.
2. Camfield P, Camfield C, Dooley J, Smith E, Garner B. A randomized study of carbamazepine versus no medication after a first unprovoked seizure in childhood. Neurology. 1989;39(6):851-852.
3. Chandra B. First seizure in adults: to treat or not to treat. Clin Neurol Neurosurg. 1992;94(Suppl.):S61-S63.
4. First Seizure Trial Group (FIR.S.T. Group). Randomized clinical trial on the efficacy of antiepileptic drugs in reducing the risk of relapse after a first unprovoked tonic clonic seizure. Neurology. 1993;43(3 Pt 1):478-483.
5. Gilad R, Lampl Y, Gabbay U, Eshel Y, Sarova-Pinhas I. Early treatment of a single generalized tonic-clonic seizure to prevent recurrence. Arch Neurol. 1996;53(11):1149-1152.
6. Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet. 2005;365(9476):2007-2013.
7. Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. Neurology. 1997;49(4):991-998.
8. Kim LG, Johnson TL, Marson AG, Chadwick DW, MRC MESS Study group. Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS Trial. Lancet Neurol. 2006;5(4):317-322.
9. Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338(7):429-434.
10. Shinnar S, Berg AT, O’Dell C, Newstein D, Moshe SL, Hauser WA. Predictors of multiple seizures in a cohort of children prospectively followed from the time of their first unprovoked seizure. Ann Neurol. 2000;48(2):140-147.
11. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Edinburgh, Scotland: World Medical Association, 2000.
12. Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346(8968):140-144.
13. Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38(3):353-362.
14. Jacoby A, Baker GA, Steen N, Potts P, Chadwick DW. The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. community study. Epilepsia. 1996;37(2):148-161.
15. Reynolds EH. Do anticonvulsants alter the natural course of epilepsy? Treatment should be started as early as possible. BMJ. 1995;310(6973):176-177.
16. Reynolds EH. Early treatment and prognosis of epilepsy. Epilepsia. 1987;28(2):97-106.
17. Gower WR. Epilepsy and Other Chronic Convulsive Disorders. London: Churchill, 1881.
18. Elwes RD, Johnson AL, Shorvon SD, Reynolds EH. The prognosis for seizure control in newly diagnosed epilepsy. N Engl J Med. 1984;311(15):944-947.
19. Elwes RD, Johnson AL, Shorvon SD, Reynolds EH. The prognosis for seizure control in newly diagnosed epilepsy. N Engl J Med. 1984;311(15):944-947.
20. Shorvon SD, Reynolds EH. Early prognosis of epilepsy. BMJ. 1982;285(6356):1699-1701.
21. Feksi AT, Kaamugisha J, Sander JW, Gatiti S, Shorvon SD. Comprehensive primary health care antiepileptic drug treatment programme in rural and semi-urban Kenya. ICBERG (International Community-based Epilepsy Research Group). Lancet. 1991;337(16):406-409. (8738)
22. Placencia M, Sander JW, Shorvon SD, et al. Antiepileptic drug treatment in a community health care setting in northern Ecuador: a prospective 12-month assessment. Epilepsy Res. 1993;14(3):237-244.
23. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1000-1015.
24. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1016-1026.
25. Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002;50(1-2):105-123.
26. Pitkanen A, Halonen T. Prevention of epilepsy. Trends Pharmacol Sci. 1998;19(7):253-255.
27. Commission on antiepileptic drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia. 1998;39(7):799-803.
28. Camfield P, Camfield C, Smith S, Dooley J, Smith E. Long-term outcome is unchanged by antiepileptic drug treatment after a first seizure: a 15-year follow-up from a randomized trial in childhood. Epilepsia. 2002;43(6):662-663.
29. Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. Neurology. 1997;49(4):991-998.
30. Leone MA, Solari A, Beghi E, FIRST Group. Treatment of the first tonic-clonic seizure does not affect long-term remission of epilepsy. Neurology. 2006;67(12):2227-2229.
31. Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet. 2005;365(9476):2007-2013.
32. Jacoby A, Gamble C, Doughty J, Marson A, Chadwick D, on behalf of the Medical Research Council MESS Study Group. Quality of life outcomes of immediate or delayed treatment of early epilepsy and single seizures. Neurology. 2007;68(15):1188-1196.