CHAPTER 117 Diverticular Disease of the Colon
The earliest description of the pathology of diverticulosis traditionally has been attributed to Cruveilhier in 1849, although an earlier description by Sir Erasmus Wilson was noted in an editorial comment in Lancet in 1840.1 Occasional reports of the condition appear in the literature of the 19th century. The role of surgery in the treatment of acute diverticulitis was discussed by Mayo and associates in 1907. The presence of uncomplicated pseudodiverticula, herniations of the mucosa and submucosa through the muscularis of the colon, was defined as diverticulosis on radiologic studies by Case in 1914. The role of diet in the pathogenesis of diverticulosis was advanced in the landmark paper by Painter and Burkitt in 1971.2 Our knowledge of the epidemiology and clinical behavior of diverticular disease has grown rapidly over the past few decades, in large measure because of advances in imaging technology.
The incidence of this disorder is increasing in the Western world.3–5 In 1998, diverticular disease resulted in total medical costs of $2.499 billion (direct costs: $2.358 billion; indirect costs: $141 million) and accounted for 230,058 hospital stays (with diverticular disease as the primary diagnosis only), 147,785 outpatient hospital visits, 165,343 emergency department visits, and 2,216,519 physician office visits.6
EPIDEMIOLOGY
Because most patients are asymptomatic, the true incidence and prevalence of diverticulosis are difficult to ascertain. Autopsy series can underestimate prevalence if small diverticula are missed or not commented upon by the pathologist, whereas series using barium enema for diagnosis can overestimate the condition and lead to selection bias because the study usually is done to investigate symptoms.7 Recent studies report overall prevalence rates for diverticulosis of 12% to 49%.8 The prevalence of diverticular disease clearly increases with age, ranging from less than 10% in those younger than 40 years of age to an estimated 50% to 66% of patients 80 years of age and older.9 Diverticulosis appears to be just as common in men and women,10 although men may have a higher incidence of diverticular bleeding and women may have more episodes of obstruction or stricture.11
Diverticulosis, with its striking geographic variation, has been termed a disease of Western civilization. The disorder is extraordinarily rare in rural Africa and Asia; conversely, the highest prevalence rates are seen in the United States, Europe, and Australia.2 Within a given country, the prevalence of colonic diverticula also can vary among ethnic groups. In Singapore, the annual incidence of diverticulitis in Chinese inhabitants was 0.14 cases per million, whereas in European inhabitants, the rate was 5.41 per million.12 Japanese-born persons who migrated to and lived in Hawaii had diverticulosis at autopsy in 52% of cases—much more frequently than those remaining in Japan.13 A registry of residents of Sweden revealed that immigrants to that country from low-prevalence regions, including Asia, Africa, and the Middle East, had hospitalization rates for diverticular disease that were 30% to 50% lower than those of Swedish natives and immigrants from Western countries.14 The magnitude of this gap narrowed as time from immigration to Sweden increased, however, and the gap nearly disappeared after 10 or more years in the country.
As an individual country becomes more urbanized, an increase in diverticulosis seems to follow over time, as has been shown in Singapore and Israel.15,16 This observation may be attributable in part to a Westernization of diet with an increase in meat ingestion and a diminution of fiber intake as a country becomes more industrialized.10 Aside from age, geography, and ethnicity, other inherited and acquired risk factors have been associated with the presence of diverticulosis (Table 117-1). The role of dietary fiber is detailed later in this chapter. There is no conclusive evidence that diverticular disease is associated with colorectal cancer.
Table 117-1 Factors That Influence the Risk for Diverticulosis
| Increased Risk |
Much of the sentinel data on the natural history of diverticulosis was reported by Parks in Belfast in the 1960s and 1970s,9,17 although these data suffer from the selection bias of studying only symptomatic patients. Parks observed that patients with many diverticula were, on average, older than those with few diverticula, suggesting that the number of diverticula in a patient increases with age. In contrast, patients with total colonic involvement were, on average, younger than those with segmental disease, suggesting that the pattern of colon involvement may be determined early on and remain more or less fixed. A study of barium enemas performed an average of 4.4 years apart in patients with diverticulosis demonstrated no apparent progression of disease in most patients.18
PATHOLOGIC ANATOMY
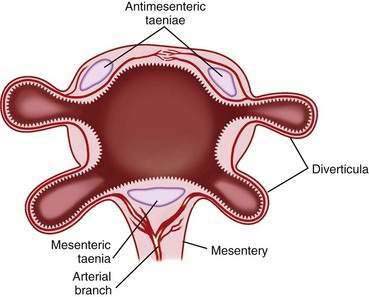
Diverticula do not arise randomly around the circumference of the colon. Rather, they originate in four distinct rows that correspond to the four sites of penetration of the bowel wall by the major branches of the vasa recta: on either side of the taenia mesocolica and on the mesenteric side of the taenia omentalis and taenia libera (Fig. 117-1). The diverticula point to the mesenteric border, and no bona fide diverticula arise from the antimesenteric intertaenial area. Diverticula maintain this fixed anatomic relationship to the taenia and are conspicuously absent from the portion of colon between the two antimesenteric taenia. Because diverticula do not involve all the layers of the colon wall, but rather are herniations of the mucosa and submucosa through a defect in the muscularis, colonic diverticula are, strictly speaking, pseudodiverticula. In this chapter, the incorrect, but traditional, terms diverticulum (singular) and diverticula (plural) are used rather than pseudodiverticulum and pseudodiverticula.
Diverticula can vary in number from one to literally hundreds. The typical size of a diverticulum is 3 to 10 mm in diameter, but they can exceed 2 cm. Giant colonic diverticula have been described, with sizes up to 25 cm. Most giant diverticula are discovered incidentally, are single, are located in the sigmoid colon, and are asymptomatic. Rarely, giant sigmoid diverticula exhibit a valve-like phenomenon by which gas can enter but not leave; they get progressively larger over a relatively short period of time, can obstruct or perforate, and thereby require surgery.19
Diverticula can occur anywhere in the colon, and the segment typically involved depends highly on geography. In Western countries, diverticula occur mainly in the left colon, with up to 90% of patients having involvement of the sigmoid; only 15% have right-sided with or without left-sided involvement.1,9,20 In contrast, right-sided involvement is predominant in Asian countries. The ascending colon is involved in about 75% of patients in Singapore15,21 and only 25% of patients have sigmoid disease. In Japan, the prevalence of right-sided diverticulosis found on barium enema doubled (from 10% to 20%) from 1982-1997, a period over which left colon involvement remained the same (∼4%).22 The structural morphology of the diverticula found on either side of the colon appears to be identical worldwide. Although the precise factors causing the segmental predominance of left colon and right colon involvement in the West and East, respectively, are not known, environmental (e.g., dietary) and genetic factors are believed to play roles. Additionally, a blending of genetic and cultural lineages could explain why many people in the United States develop pancolonic diverticulosis.
ETIOLOGY AND PATHOGENESIS
COLONIC WALL STRUCTURE
Electron microscopic studies confirm that the colonic walls in patients with diverticulosis have structurally normal muscle cells but, compared with controls, contain a greater than 200% increase in elastin deposition between the muscle cells in the taenia.23 The elastin is laid down in a contracted form, resulting in shortening of the taenia and bunching of the circular muscle. An increase in type III collagen synthesis in patients with diverticulosis also has been described, raising the possibility that age-related changes in collagen composition play an etiologic role.24
In addition to an overall increase in the collagen content, an overexpression of a tissue inhibitor of metalloproteinases has been identified in colons with diverticula.25,26 Because matrix metalloproteinases are believed to regulate deposition of extracellular matrix proteins, an increase in their regulatory molecule, tissue inhibitor, might explain the increase in elastin and collagen deposition found in diverticular colons. The importance of intestinal wall connective tissue also is underscored by the higher rate of diverticulosis reported in patients with connective tissue disorders, such as Ehlers-Danlos syndrome, Marfan’s syndrome, and scleroderma.5
MOTILITY
Early investigations using colonic manometry demonstrated higher resting, postprandial, and neostigmine-stimulated luminal pressures in patients with diverticulosis compared with controls.27,28 Based on simultaneous manometry and cineradiography, Painter proposed a theory of segmentation, postulating that contraction of the colon at haustral folds caused the colon to act not as a continuous tube but as a series of discrete “little bladders,” which led to excessively high pressures within each segment.28,29 He further suggested that the Western diet might alter colonic motility to augment hypersegmentation, thereby increasing the tendency to form diverticula.
More recently, using flexible endoscopy to accurately place manometric catheters within the sigmoid colon, the motility abnormalities previously described have been confirmed.30 Further, patients with symptomatic diverticular disease have been reported to have higher motility indices than either asymptomatic patients or persons without diverticula.31 In addition to increased contraction amplitude, another study found retropropagation of contractile waves in diverticular segments of colon, indicating that motility in these patients may be abnormal in magnitude and direction.32 An Asian study has confirmed elevated resting and stimulated luminal pressures in the presence of proximal colon diverticulosis.33
Although the demonstration of abnormal motility and elevated intraluminal pressures in the diverticular colon has been consistent, the physiologic basis for these abnormalities is less clear. Ion transport across the epithelial membrane of diverticular colons is the same as in controls.34 The number of myenteric and submucosal plexus neurons in diverticular colons are normal; however, the number of interstitial cells of Cajal (enteric pacemaker cells) is reduced.35 Increased activity of excitatory cholinergic nerves and a decreased activity of nonadrenergic, noncholinergic inhibitory nerves, the latter in part mediated by nitric oxide, have been demonstrated in diverticular colons compared with control colons.36 The magnitude of electrically stimulated contraction in diverticulosis-affected sigmoid colon is markedly reduced by antagonists of cholinergic and tachykinin neurotransmitters.37 In contrast, a study of the tachykinin neurotransmitter system showed a decreased contractility of circular muscle induced by substance P in diverticular colons compared with normal ones.38
DIETARY FIBER
The wide geographic variation of diverticular disease, with higher prevalence in countries with a Westernized diet, has long suggested a dietary factor in its pathogenesis. Low intake of dietary fiber has been strongly suspected to be the main dietary factor behind these geographic differences. Burkitt and Painter were early proponents of this theory, labeling diverticulosis a “deficiency disease” that, like scurvy, should be avoidable with dietary changes.2 In one important study, they demonstrated that persons in the United Kingdom who consumed a refined, low-fiber Western diet had stool transit times more than twice as slow and stool weights significantly less than those of rural Ugandans eating a diet very high in fiber.39 The long intestinal transit time and smaller-volume stools were believed to allow an increase in intraluminal pressure, thus predisposing to diverticular herniation, whereas bulkier stools were associated with less colonic contraction and lower wall pressures.
Although more recent studies in Western cohorts have failed to confirm this finding in humans consistently, corroborative animal data do exist. Wistar rats fed low-fiber diets developed diverticula in 45% of instances, compared with only 9% of those on high-fiber diets.40 In humans, other observational evidence exists with respect to the etiologic role of fiber in diverticulosis. In the United States, fiber intake decreased by 28% from 1909 to 1975,5 a period of dramatic increase in the prevalence of diverticular disease. In a British study, a group of vegetarians on a high-fiber diet had a lower prevalence of diverticulosis than did nonvegetarians (12% vs. 33%).41
Dietary influences for diverticulosis may have different effects on the right and left sides of the colon. Right-sided diverticular disease was shown in an Asian study to have no association with intake of fruits and vegetables or supplemental fiber, but it was strongly associated with meat consumption.42 Whether these associations apply to Westerners with right-sided diverticulosis is not known.
UNCOMPLICATED DIVERTICULOSIS
ASYMPTOMATIC DIVERTICULOSIS
A possible prophylactic benefit of a high-fiber diet has been suggested in two publications of 47,888 male health professionals who were followed over four years and in whom 385 (0.75%) new cases of symptomatic diverticular disease were identified.43,44 A dietary review found an inverse association between dietary fiber intake and the risk of subsequently developing clinically evident diverticular disease. The study also noted that fruit and vegetable fiber, or insoluble fiber, had a greater protective effect than did cereal fiber. Conversely, diets high in fat and red meat were associated with an increased risk of diverticular disease. Although prospective randomized trials are lacking, these findings suggest that patients with asymptomatic diverticulosis and SUDD (see later) might benefit from increasing their fruit and vegetable fiber intake while decreasing their fat and red meat consumption, a sensible lifestyle change that likely also provides other salutary health benefits.
For decades, it has been a widely held but weakly supported belief that patients with diverticulosis should avoid consumption of seeds, nuts, and popcorn to avoid possible plugging of diverticula and precipitating an episode of diverticulitis; this theory also was investigated in the aforementioned cohort of male health professionals.45 Not only was no increase in risk of diverticular complications found as a result of consuming nuts, corn, popcorn, or seeded fruit (strawberries or blueberries), but consumption of nuts and popcorn were inversely associated with the risk of developing diverticulitis. Although there probably is not enough evidence to warrant actively encouraging patients with diverticulosis to eat large quantities of nuts, popcorn, or seeds, neither should these foods be categorically avoided.
SYMPTOMATIC UNCOMPLICATED DIVERTICULAR DISEASE
Clinical Features
Some patients come to clinical attention because of nonspecific abdominal complaints and are found to have diverticulosis coli. A causal relationship between the diverticulosis and the abdominal symptoms often is difficult to establish. If there are features that are consistent with a diverticular source, however, and there is no evidence of a serious inflammatory condition, the disease may be defined as SUDD. Most patients with SUDD present with left lower quadrant pain; the British refer to this condition as painful diverticular disease. The pain often is exacerbated by eating and diminished by defecation or the passage of flatus. Patients also may report other symptoms of colonic dysfunction, including bloating, constipation, diarrhea, or the passage of mucus per rectum. Physical examination may be normal or may reveal fullness or mild tenderness in the left lower quadrant, but frank rebound or guarding is absent. Because rates of occult bleeding in diverticulosis are similar to those in healthy controls, a positive fecal occult blood test never should be attributed to diverticulosis.46
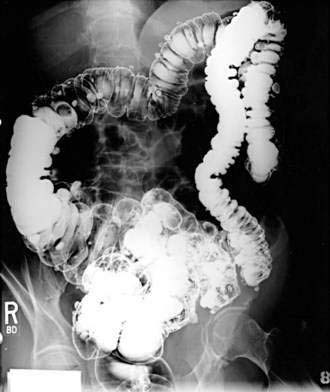
Because a barium enema can characterize the number, size, and location of diverticula, it previously had been a commonly used initial study in such patients. Barium enema, however, may be insufficient to rule out competing or associated diagnoses such as malignancy in patients with diverticulosis. In symptomatic patients in whom a double-contrast barium enema showed sigmoid diverticulosis, subsequent colonoscopy confirmed only 55% of neoplastic lesions that were suspected on the barium enema, whereas eight polyps and three malignancies were identified on colonoscopy that were missed on the barium enema (24% false-negative rate for barium enema).47
Although the barium enema may remain useful in certain cases, particularly if a colonoscopy cannot be performed safely or completely, endoscopic evaluation (Fig. 117-2) has assumed a primary role in the evaluation of most patients, particularly to exclude neoplasia. It once was believed to be unsafe to perform colonoscopy in patients with diverticulosis because of an increased risk of perforation; however, it subsequently was demonstrated that manometrically measured burst pressures for diverticula far exceed the usual pressures encountered during routine sigmoidoscopy or colonoscopy, even with the endoscope pressing against the wall or with heavy air insufflation.48 These data and many years of clinical experience have demonstrated the relative safety of using endoscopy to evaluate patients with abdominal symptoms. Caution should be exercised in patients with suspected or proved diverticulitis, however, because of a theoretical risk of perforating the wall of a diverticulum that has lost its integrity due to inflammation. In all patients, air insufflation should be minimized and excessive force in advancing the endoscope should be avoided.
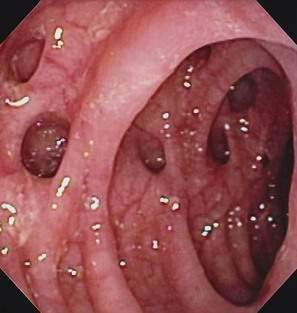
Figure 117-2. Colonoscopic view of the sigmoid colon in a patient with symptomatic uncomplicated diverticular disease.
The diverticula-laden colon can be challenging for the endoscopist to navigate because of spasm, luminal narrowing, fixation from prior inflammation and fibrosis, or confusion between luminal and diverticular openings. A number of solutions have been proposed to alleviate this problem. The use of a smaller-diameter pediatric colonoscope can be useful for difficult colons. One group has reported a success rate of more than 90% with a pediatric colonoscope in cases in which an adult colonoscope could not be passed through the sigmoid; 44% of these patients had diverticulosis.49 When colonoscopy with standard and pediatric colonoscopes were compared, the reason for failure to complete the examination was thought to be stenosing diverticular disease in 12 of 14 patients with the standard colonoscope, compared with two of eight with the pediatric colonoscope.50 A technique involving distention of the lumen with 100 to 300 mL of water, called the sigmoid floatation maneuver, was said to have facilitated colonoscopy in six technically difficult cases of severe diverticular disease.51
Occasionally, an endoscopist encounters an inverted diverticulum, where a diverticular dome protrudes into the lumen instead of out from it. These inversions often resemble polyps endoscopically, although they may be distinguished by their normal overlying mucosa, broad base, and location within a bed or row of diverticula. They are soft-appearing when manipulated with the endoscope tip or a biopsy forceps (pillow sign) and may be reducible. On barium enema, inverted diverticula appear as broad-based sessile polyps with a characteristic central umbilication,52 although it is not always possible to distinguish such a diverticulum from a polyp. When inverted diverticula are encountered, their removal should be avoided, although inadvertent colonoscopic diverticulectomy has been reported,53 and these patients had uneventful recoveries with conservative therapy.
The presenting symptoms of SUDD overlap considerably with those of irritable bowel syndrome (IBS). Some authorities have postulated that diverticula are, in fact, a late consequence of IBS. In a Danish cohort of IBS patients, one third of whom had diverticula, no difference in symptoms or prognosis was detected between those with diverticula and those without diverticula over more than five years of follow-up.54 This finding led the investigators to conclude that there is no basis to consider SUDD as a separate entity from IBS. Ritchie reported that there was a similarity of pain sensation from rectal balloon distention in patients with IBS and those with diverticulosis.55 Whether these two disorders are distinct entities is unknown and probably not clinically important, because both are treated in a similar nonspecific fashion with equally good prognoses.
Treatment
Fiber
Aside from the reported preventive effect of dietary fiber described earlier, fiber also is a mainstay of treatment for SUDD. Many uncontrolled trials of fiber in SUDD have been reported, all limited by a high placebo response rate. A randomized, double-blind trial from Oxford University showed a statistically significant decrease in bowel symptoms relative to controls in patients with SUDD who were placed on a high-fiber diet56; the separation between treatment and control groups, however, was not noted until the three-month follow-up evaluation. It is important to instruct patients to start fiber supplementation at a low dose and slowly increase the dose, because patients initially can worsen from diarrhea, gas, or bloating if the fiber dose is started too high or quickly. Because it often can take months to improve, as demonstrated in the Oxford study, the initial adverse symptoms can discourage patients from adhering to the fiber supplements if they are not counseled properly. In contrast, another study of fiber supplementation in patients with diverticular disease showed no significant improvement in overall symptoms, although decreased transit time and increased stool frequency were documented.57 Despite these conflicting data and the certainty that diverticula do not regress with an increased fiber intake, some amelioration of symptoms in patients with SUDD often can be seen with a high-fiber diet.
5-Aminosalicylic Acid
Additional medical therapies for SUDD have been studied since 2000, drawing on approaches that are effective in other colonic diseases such as inflammatory bowel disease (IBD) and IBS. 5-Aminosalicylate (5-ASA) compounds, a well established therapy for ulcerative colitis and Crohn’s disease, have been evaluated as a potential treatment for SUDD. Although patients with SUDD by definition lack severe or overt inflammation, in some patients subtle inflammation is suspected even without gross signs of diverticulitis; it is these patients that the anti-inflammatory properties of 5-ASA might benefit. Published studies that have examined the role of 5-ASA were randomized to either daily or cycled (e.g., 10 days per month) 5-ASA but were not placebo controlled.58–60 Results of each study show a significantly reduced symptom score relative to pretreatment scores, but the lack of a control arm and the known high placebo-response rates in functional bowel syndromes make the results difficult to interpret. The doses of 5-ASAs used in these studies were lower than generally are used for IBD. These results, combined with the relative safety of this medication class, make 5-ASAs a promising therapy for SUDD, although placebo-controlled trials supporting its efficacy will be needed before widespread use can be advised.
Antibiotics and Probiotics
The role of pathogenic and nonpathogenic bacteria in intestinal disease is being increasingly scrutinized. Some have postulated that a disturbance in the local microflora in and around diverticula61 might predispose to diverticulitis. If this were true, medications that alter this flora might help treat or prevent attacks of diverticulitis.
In contrast, rifaximin, a nonabsorbable antibiotic with broad-spectrum activity, mitigates some of these concerns by its solely luminal activity and by its use as an emerging potential therapy for C. difficile infection. Rifaximin has been studied in patients with SUDD and has shown promise in reducing frequency and severity of symptoms.62,63 Rifaximin has also been shown to be effective for IBS,64 possibly by eradicating concomitant small bowel bacterial overgrowth (SIBO). Whether inadvertently treating SIBO is the actual reason for the effectiveness of rifaximin in SUDD is not known.
Based on the same theory that altered local microflora is present in these patients, probiotics also have been studied in SUDD.59,65 Although some benefit has been shown, such trials are small and lack a placebo group. Although higher-quality evidence needs to be produced to support this approach, the microflora may become an important target for therapy in SUDD in the coming years; a number of trials are under way evaluating anti-inflammatory agents or probiotics.
Role of Surgery
Surgical intervention generally is not considered for patients with truly uncomplicated diverticulosis, because the risks of surgery outweigh the benefits in most cases. Some patients with subclinical or smoldering diverticulitis present with pain characteristic of diverticulitis but show no signs of systemic inflammation, such as fever or leukocytosis. In a cohort of such patients from the Mayo Clinic who underwent sigmoid resection with primary anastomosis for their symptoms without signs or laboratory markers of systemic inflammation, 76% of the resected specimens had evidence of acute or chronic diverticular inflammation.66 Sigmoid colectomy in these patients resulted in resolution of pain in 88% and complete resolution of symptoms in 76% after one year or more of follow-up. This finding underscores the importance of clinical follow-up and an open mind regarding patients with apparently uncomplicated disease whose symptoms do not improve with conservative treatment.
COMPLICATED DIVERTICULOSIS
Diverticulitis, defined as inflammation, infection, or both, associated with diverticula, is probably the most common clinical manifestation of this disorder, affecting an estimated 10% to 25% of patients with diverticula.9 It generally is believed to be the result of perforation of a single diverticulum.67 When this results in a localized phlegmon, the term uncomplicated diverticulitis is used. Complicated diverticulitis refers to cases associated with abscess, free perforation with peritonitis, fistula, or obstruction.68 Besides diverticulitis, the other major form of complicated diverticular disease is bleeding, which is discussed later in this chapter.
UNCOMPLICATED DIVERTICULITIS
Pathophysiology
The process by which a diverticulum becomes inflamed has been likened to that causing appendicitis, in which the diverticular sac becomes obstructed by inspissated stool in its neck; the fecalith abrades the mucosa of the sac, causing low-grade inflammation and further blocking drainage. Histologically, one of the earliest signs of inflammation is hyperplasia of the mucosal lymphoid tissue, with lymphoid tissue aggregation at the apex of the involved sac.69 The obstructed diverticulum predisposes to expansion of the normal bacterial flora, diminished venous outflow with localized ischemia, and altered mucosal defense mechanisms. One such alteration is a defective CD2 pathway-induced apoptosis, which has been found in lamina propria lymphocytes in patients with diverticulitis, possibly leading to an up-regulation of the local immune response in these patients similar to that seen in patients with IBD.70 Evidence also suggests that cytomegalovirus (CMV) reactivation might contribute to local inflammatory activity, because active CMV replication was found in tissue from the affected bowel segments of more than two thirds of patients with diverticulitis.71
The cascade of events initiated by fecalith obstruction, and possibly enhanced by underlying innate or acquired abnormalities, allows bacteria to breach the mucosa and extend the process transmurally, ultimately leading to perforation.72 The extent and localization of the perforation determine its clinical behavior. Microperforations can remain very well localized, contained by the pericolic fat and mesentery, and cause small pericolic abscesses. A larger perforation can allow a more extensive abscess to form, which can track longitudinally around the bowel wall. This process can lead to a large inflammatory mass, fibrosis, extension to other organs, or fistula formation. Free perforation into the peritoneum causing frank bacterial or fecal peritonitis can be life threatening, but fortunately it is uncommon, with a population incidence of 4 cases per 100,000 population per year.3,73 Hinchey and associates have described a staged grading system reflecting the severity of perforation (Table 117-2).74
Table 117-2 Hinchey Classification of Colonic Diverticular Perforation
| STAGE | DEFINITION |
|---|---|
| I | Confined pericolic abscess |
| II | Distant abscess (retroperitoneal or pelvic) |
| III | Generalized peritonitis caused by rupture of a pericolic or pelvic abscess (not communicating with the colonic lumen because of obliteration of the diverticular neck by inflammation) |
| IV | Fecal peritonitis caused by free perforation of a diverticulum (communicating with the colonic lumen) |
Clinical Features
Patients with acute diverticulitis typically present with left lower quadrant abdominal pain, reflecting the propensity for this disorder to occur in the sigmoid colon in Western countries; a redundant sigmoid colon, however, can manifest with suprapubic or right-sided pain. In contrast, Asian patients with diverticulitis have predominantly right-sided symptoms, corresponding to the location of their diverticula.75 The pain may be intermittent or constant and frequently is associated with a change in bowel habits, either diarrhea or constipation.76 Anorexia, nausea, and vomiting also can occur. Dysuria and urinary frequency can result from bladder irritation caused by the adjacent inflamed sigmoid colon.
Physical examination usually discloses localized tenderness, generally in the left lower quadrant; however, as noted, right-sided signs do not preclude the possibility of diverticulitis. Guarding and rebound tenderness may be present, as may a tender, cylindrical, palpable mass. Bowel sounds typically are depressed but may be normal in mild cases or increased in the presence of obstruction. Rectal examination can disclose tenderness or a mass, particularly with a low-lying pelvic abscess. Fever is present in most patients, whereas hypotension and shock are unusual. The white blood cell (WBC) count commonly is elevated, although one study reported a normal WBC count with no left shift in 46% of patients.76 No other laboratory abnormalities are routinely helpful, although they can help to rule out other diagnostic possibilities in select patients.
The differential diagnosis for diverticulitis is extensive. Acute appendicitis is the misdiagnosis most often made in patients with diverticulitis, particularly with right-sided disease. In Hong Kong, where awareness of the predominance of right-sided diverticulosis presumably is high, 34 of 35 patients with right-sided diverticulitis initially were believed to have acute appendicitis.75 Although appendicitis is, on average, a disease of younger patients than is acute diverticulitis, there is a wide range of ages for both. Clinical suspicion for one must remain high when diagnosing the other on clinical grounds. Other common diagnoses that need to be considered include IBD; other forms of colitis (infectious or ischemic); colorectal cancer; and gynecologic conditions such as pelvic inflammatory disease, ovarian cyst rupture, and ovarian torsion. Occasionally, diverticulitis can occur concomitantly with other diseases; in one study of patients admitted to the hospital with diverticulitis, 7% were found later also to have a colon malignancy.77
Diagnosis
Most patients with acute diverticulitis present with signs and symptoms sufficient to justify the clinical diagnosis and institution of empiric therapy. Clinical diagnosis can, however, occasionally be inaccurate, and emergency surgery for presumed diverticulitis, without the benefit of radiologic confirmation, carries a misdiagnosis rate as high as 34% to 67%.78 Therefore, radiologic studies to confirm the diagnosis of diverticulitis should be employed, particularly if invasive intervention may be required.
Plain Films
An erect chest film, together with erect and supine abdominal films, should be performed on patients with significant abdominal pain. The erect chest film has the dual purpose of detecting pneumoperitoneum, which has been reported to be present in up to 11% of patients with acute diverticulitis,79 and of assessing cardiopulmonary status in a generally elderly population with common comorbid illness. Plain abdominal films are abnormal in 30% to 50% of patients with acute diverticulitis,79,80 with findings that include bowel dilatation from obstruction or ileus, or a soft tissue density suggesting an abscess.
Contrast Enema Examinations
Contrast barium enema (Fig. 117-3) had been the diagnostic standard for diverticulitis and its complications for many years. Because the use of barium in the setting of an intestinal perforation carries a risk of barium peritonitis, only water-soluble contrast enemas, such as Gastrografin, should be used in the setting of suspected diverticulitis. A gentle, single-contrast study should be performed and terminated once findings of diverticulitis are discovered, with visualization of the entire colon deferred to a later date; air (double)-contrast studies are not indicated. Findings considered diagnostic of diverticulitis include demonstration of extravasated contrast material with or without the outlining of an abscess cavity, an intramural sinus tract, or a fistula.1,81 Extensive diverticulosis, spasm, mucosal thickening or spiking, or deformed sacs, although suggestive of diverticulitis, are not conclusive. An extraluminal mass compressing the colon is said to be the most common finding in severe diverticulitis,82 although this finding is not specific for this diagnosis. Obviously, in the absence of diverticula or associated findings, the diagnosis must be reconsidered. Contrast enema has been shown to have a sensitivity of 62% to 94% for detecting acute diverticulitis, with false-negative results in 2% to 15%.68,83
Computed Tomography
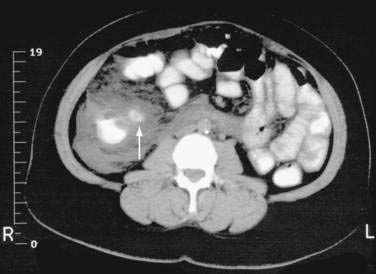
Because diverticulitis is mainly an extraluminal disease, luminal contrast studies may be inaccurate. Computed tomography (CT) (Fig. 117-4) has now replaced contrast enema as the diagnostic procedure of choice for acute diverticulitis and has the ability to image mural and extraluminal disease and to enable therapy with percutaneous drainage of abscesses. Abdominal and pelvic scanning ideally is performed with water-soluble contrast, given both orally and rectally, and with intravenous contrast when it is not contraindicated. CT criteria for diverticulitis include the presence of diverticula with pericolic infiltration of fatty tissue (often appearing as fat stranding), thickening of the colon wall, and formation of abscesses.
The earliest large series of CT findings in diverticulitis reported the finding of pericolic fat inflammation in 98%, diverticula in 84%, a colonic wall thickness greater than 4 mm in 70%, and an abscess in 35%.84 Contrast enemas in the same patients underestimated the extent of disease in 15 (41%) of 37 cases. Numerous subsequent trials comparing these two modalities in patients with suspected diverticulitis consistently have reported CT sensitivities of 93% to 98% and specificities of 75% to 100%, significantly more accurate than contrast enemas.81,85,86 CT also has been found to be highly sensitive and specific for right-sided diverticulitis and in helping to differentiate diverticulitis from colorectal cancer of the ascending colon and cecum.87,88
Although some reports show a lower sensitivity of CT for diverticulitis,89,90 it is increasingly becoming clear that, when diagnosis is in doubt or clinical deterioration occurs, CT is the best primary radiologic diagnostic modality. Conversely, in patients with mild disease and in whom the diagnosis is straightforward, CT scanning might not be necessary.
Ultrasonography
Based on its relatively low cost, convenience, and noninvasive nature, ultrasonography (US) has been advocated as a potentially useful diagnostic modality in diverticulitis. Characteristic findings implying active inflammation include bowel wall thickening, presence of diverticula or abscesses, and hyperechogenicity of the bowel wall. US has a reported sensitivity of 84% to 98% and specificity of 80% to 93%.91,92 One study of 71 patients with suspected diverticulitis who underwent US reported a negative predictive value of 100%.93 A trial comparing US with CT revealed equally good accuracy.94 US also is useful in female patients to exclude gynecologic pathology.
Treatment
When cultured, most diverticular abscesses grow mixed aerobic and anaerobic organisms, the most common single organisms being Escherichia coli, Streptococcus species, and Bacteroides fragilis.95 Therefore, oral antibiotics with broad-spectrum coverage (see later) are recommended.96
Patients with uncomplicated diverticulitis who are elderly or immunosuppressed, have severe comorbidities, or demonstrate high fever or significant leukocytosis should be hospitalized. Although data imply that early consultation with a gastroenterology subspecialist improves quality of care for the inpatient management of diverticulitis,97 this is probably not necessary for many straightforward cases. Bowel rest with either clear liquids or nothing by mouth should be instituted. Intravenous fluid therapy to restore intravascular volume, balance electrolytes, and ensure adequate urinary output should be initiated. Broad-spectrum intravenous antibiotics should be started. Recommended combination regimens include anaerobic coverage with metronidazole or clindamycin and Gram-negative coverage with an aminoglycoside, monobactam, or a third-generation cephalosporin.96 Single-agent coverage with intravenous second-generation cephalosporins or beta-lactamase inhibitor combinations, such as ampicillin/sulbactam or ticarcillin/clavulanate, are reasonable alternatives.
Symptomatic improvement with decreasing fever and leukocytosis should be observed within two to four days, at which point diet may be advanced. If improvement continues, patients may be discharged, but they should complete a seven- to 10-day course of oral antibiotics. Failure to improve with conservative medical therapy warrants a diligent search for complications, consideration of alternative diagnoses, and surgical consultation. Most patients hospitalized with acute diverticulitis respond to conservative medical therapy, but it has been estimated that 15% to 30% require surgery during the hospital admission.1,5,17,68 Surgery may be necessary when pain, fever, and leukocytosis do not respond to antibiotics and supportive care.
If surgery is planned and complicated disease, such as abscess, is ruled out, resection of the diseased segment of bowel with primary anastomosis is the most commonly performed operation, usually in a single-stage procedure. The main requirements in performing a single-stage procedure are the ability to perform bowel preparation and technical feasibility, usually determined by the extent of extramural inflammation. The entire sigmoid colon should be removed (in left-sided disease) because this is the most common location for initial disease and recurrence. The distal resection margin should be the proximal rectum, and the anastomosis should be free of tension to decrease risk of anastomotic leak.98 For uncomplicated diverticulitis, laparoscopic sigmoid colectomy has gained increasing enthusiasm99 and has significant advantages over open techniques with respect to length of stay and postoperative in-hospital morbidity.100 Discussion of the medical and surgical management of complications such as perforation, abscess, fistula, or obstruction follows.
COMPLICATED DIVERTICULITIS
Abscess
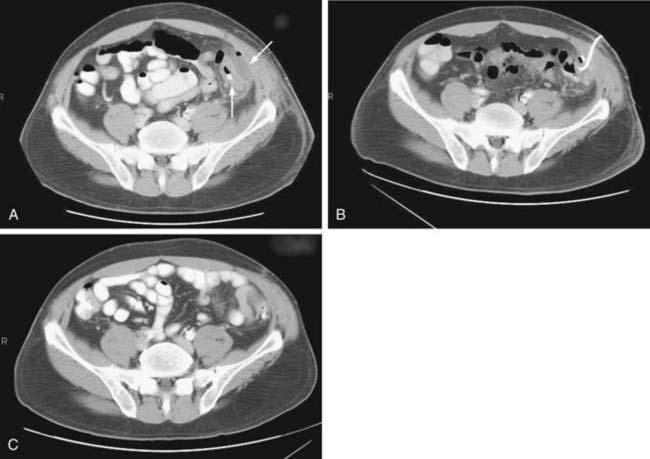
Small pericolic abscesses (Hinchey stage I) often can be treated conservatively with broad-spectrum antibiotics and bowel rest.98 In one series of patients with diverticulitis, seven of 10 patients with pericolic abscesses responded successfully to conservative treatment.101 This favorable prognosis can result from maintenance of a fistula between the abscess and the colon lumen, thus permitting spontaneous internal drainage. Continued noninterventional management of abscesses should be considered only in stable patients who demonstrate unequivocal improvements in pain, fever, tenderness, and leukocytosis over the first few days of therapy.
CT-guided percutaneous drainage of abdominal abscesses has assumed a prominent complementary role to surgery (Fig. 117-5). The immediate advantage of percutaneous catheter drainage is rapid control of sepsis and patient stabilization without the need for general anesthesia. It often eliminates the need for a multiple-stage surgical procedure with colostomy,102 instead allowing temporary palliative drainage and subsequent single-stage resection in three to four weeks. Success rates of CT-guided drainage for stabilizing patients and safely allowing subsequent single-stage procedures range from 74% to 80%.103,104 An urgent surgical procedure is required in the 20% to 25% of patients in whom the abscess is multiloculated, anatomically inaccessible, or not resolving with percutaneous drainage.
The approach to surgical intervention has evolved over the last half century. Historically, three-stage management was preferred for complicated diverticulitis of any severity: stage I involved a proximal colostomy and drainage of abscesses, stage II was for resecting the diseased bowel (colostomy maintained to protect anastomosis), and stage III was to restore bowel continuity. Although the multiple-stage surgical approach to diverticular disease was effective for managing abscesses, generally it is desirable to limit the number of laparotomies to minimize morbidity and duration of hospitalization. Additionally, only about half of patients undergoing proximal-end colostomy have the colostomy reversed because of the technical difficulties and the risks of anastomotic leakage and mortality.105
In the last few decades, two-stage management (e.g., primary resection of diseased bowel with proximal end colostomy and oversewing of the distal stump, also referred to as the Hartmann procedure, followed by reanastomosis) and, increasingly, management with a single operation (resection with primary anastomosis) have become the preferred surgical approaches. The switch to fewer operations has been made without a discernable compromise in overall outcomes relative to three-stage approaches.106 Single-stage management, which is becoming the standard for elective management of uncomplicated diverticulitis, also is being used increasingly in appropriate patients with complicated diverticulitis. To do so, bowel preparation is believed to be necessary.
When free perforation is ruled out, bowel preparation is performed before the operation with either a one-day polyethylene glycol (PEG)-electrolyte lavage or a traditional two- or three-day mechanical preparation; both types of preparation have been shown to have similar outcomes in a randomized trial.107 In the setting of urgent or emergent surgery when free perforation is a concern, bowel lavage may be performed “on table,” thereby allowing a primary anastomosis to be performed in a single stage.108,109
Regardless of approach, surgery for complicated diverticulitis has evolved into a relatively safe procedure with one of the highest success rates of any of the common gastrointestinal surgical procedures.110
Free Perforation
Peritonitis (Hinchey stages III or IV) is a surgical emergency and requires urgent operative intervention. Although uncommon in the antibiotic era, mortality from generalized peritonitis associated with diverticulitis has been reported in the 12% to 26% range.111 Early identification of free perforation is critical. CT scan can confirm the diagnosis in ambiguous cases, but an abdominal plain-film series showing free intraperitoneal air plus high clinical suspicion is sufficient to justify surgical exploration. Broad-spectrum intravenous antibiotics, such as a second- or third-generation cephalosporin and metronidazole, should be instituted immediately.
There is no clear consensus whether primary or secondary resection is more beneficial for peritonitis from diverticulitis. Retrospective studies suggest an advantage for primary over secondary resection with respect to morbidity and mortality.111,112 Two randomized trials comparing primary and secondary resection yielded conflicting results, neither appearing to be clearly superior.113,114 One decision analysis suggested that primary anastomosis with a proximal defunctioning stoma, rather than primary resection and anastomosis or the Hartmann procedure, resulted in the highest quality of life overall.115 The primary anastomosis with a proximal defunctioning stoma group had higher stoma-reversal rates than the rate of reanastomosis in Hartmann procedure patients and a lower complication rate than the primary resection and anastomosis patients.
Practically, the decision of whether to perform a primary or secondary resection is made intraoperatively based on the extent of disease, the difficulty of bowel mobilization, the degree of peritoneal contamination, and the surgeon’s expertise. In most cases of free perforation, at least two separate operations are necessary regardless of whether primary or secondary resection is performed. As noted previously, in many cases, restoration of the anastomosis is not possible and the colostomy is left indefinitely. Cases of peritonitis also require pelvic drainage, clearance of the rectum of fecal material when possible, and mobilization of the splenic flexure to perform a tension-free anastomosis. Placement of ureteral stents prophylactically can help prevent accidental ureteral injury during the operation.110 With the increasing use of the laparoscopic approach to uncomplicated diverticular disease, some centers have extended this approach to perforated disease with promising results.116,117
Fistula
When a diverticular phlegmon or abscess extends or ruptures into an adjacent organ, a fistula results. Fistulas are believed to develop in fewer than 5% of patients with diverticulitis but are present in about 20% of those who require surgery for diverticulitis.118
In a Cleveland Clinic review of 84 patients seen over 26 years with internal fistulas caused by diverticular disease, 65% were colovesical.119 There was a 2 : 1 male predominance, attributed to the protection of the bladder in women by the uterus. In one series, pneumaturia was present in 57% and fecaluria in 42%120; the latter is pathognomonic for colovesical fistula. Cystoscopy, cystography, and barium enema can be useful, although demonstration of the actual fistula often is difficult. In both of these series, single-stage operative resection with fistula closure and primary anastomosis could be performed in 75% of patients. Presumably, single-stage management is possible so often in the presence of fistulas to extracolonic organs because the fistula has effectively decompressed the inflammatory process.
Colovaginal fistulas are the next most common internal fistula after colovesical fistula, representing approximately 25% of all cases.119 The passage of stool or flatus per the vagina is pathognomonic. Frequent vaginal infections or copious or feculent vaginal discharge should prompt consideration of this complication. Many patients with colovaginal fistula have undergone a previous hysterectomy. Treatment is surgical resection of the diseased segment of colon with repair of the contiguous organ, which generally can be performed as a single-stage procedure.98
Obstruction
Obstruction can accompany diverticular disease either acutely or chronically. During an attack of acute diverticulitis, partial colonic obstruction can occur because of luminal narrowing from the pericolic inflammation, compression from abscess formation, or both. Obstruction can be confirmed with a gentle water-soluble contrast enema in a patient not suspected to have free perforation,118 while simultaneously excluding an obstructing sigmoid neoplasm. A CT scan with oral and rectal contrast also can give useful information about diverticular obstruction while also assessing for extraluminal disease. Complete obstruction is unusual. Colonic ileus or pseudo-obstruction also can occur, as can small bowel obstruction if a loop of small intestine becomes incorporated into the inflammatory mass. These conditions usually improve with effective medical therapy including antibiotics, bowel rest, and nasogastric suction.
Surgical intervention may be required for persistent obstruction from acute diverticulitis not responding to medical therapy. Ideally, a modified bowel preparation with gentle irrigation enemas or low-dose oral laxatives given over a period of a few days can be performed preoperatively,118 thereby allowing the possibility of primary anastomosis in some cases. In cases when bowel preparation is not possible, a Hartmann procedure usually is performed.
Recurrent attacks of diverticulitis, which may be subclinical, can initiate chronic stricturing of the colonic wall without ongoing inflammation. In such cases, high-grade or complete obstruction can occur. A contrast enema can help distinguish benign stricture from neoplasm. Colonoscopy also plays an important diagnostic role, and one group investigating strictures with colonoscopy was able to distinguish a benign from malignant etiology in 67% of patients.121 Strictures in which malignancy cannot be excluded despite colonoscopic and radiologic examinations should be treated by surgical resection.
A trial of endoscopic dilation therapy can reasonably be attempted in patients in whom neoplasm is believed to be sufficiently excluded and in whom acute diverticulitis is not a concern. Success rates for balloon dilation of benign colonic strictures have been reported in the 67% to 79% range.122,123 Colonic metal stents may have a role in treating obstruction complicating diverticular disease, particularly in providing temporary decompression to allow bowel preparation and subsequent single-stage resection without diversion.124
SPECIAL TOPICS RELATED TO DIVERTICULITIS
RECURRENT DIVERTICULITIS
For patients who respond well to conservative therapy, the issues of likelihood of recurrence and elective prophylactic surgical resection arise. The risk of recurrent symptoms following an attack of acute diverticulitis has been reported to range from 7% to 45%,1,5,17,68 with half of second attacks occurring within one year. Historical evidence had suggested that recurrent attacks were less likely to respond to medical therapy and had a higher mortality rate.17,68 Predicated on the notion that diverticulitis was a progressive disease, elective resection had been traditionally recommended after two attacks of diverticulitis.98,99,125 This approach is now being challenged.
Studies such as the 13-year experience of the Mayo Clinic have suggested that the risk of poor outcomes is not higher with recurrent diverticulitis (more than two attacks) than it is with the first one or two attacks.126 In fact, the mortality rate from diverticulitis in this cohort was higher in those with no prior history of diverticulitis (10%) than in those who had had prior attacks (2.5%). This difference may be due to the higher rate of free perforation in initial attacks relative to subsequent attacks. Thus, multiple recurrences do not appear to predict less-favorable outcomes. Additionally, there are some emerging medical therapies for those with diverticulitis to attempt to reduce likelihood of future attacks. As noted in the discussion of treatment for SUDD, studies have begun to show a decrease in recurrence rates of diverticulitis and symptomatic diverticular disease in patients treated with mesalamine, rifaximin, probiotics, and combinations of these agents.58–63,65,127,128 Finally, a recent decision analysis predicted that performing colectomy after the fourth attack of diverticulitis rather than after the second attack would result in fewer deaths and colostomies while having a superior cost-effectiveness.129 Recognizing this trend, published guidelines on the decision to perform colectomy after an attack of diverticulitis are now advocating evaluation on a case-by-case basis rather than empirically performing elective surgery after the second attack.130
If the choice to operate is made, most patients report having a good functional outcome and low rates of recurrent disease after elective resection for recurrent diverticulitis.131 In considering an elective sigmoid resection, relevant variables include the severity and responsiveness of the attack(s), the general health of the patient, the risk to the patient of a subsequent attack, and the risk of the resection itself. The latter factor may be lessened by the increasing use of the laparoscopic approach in many patients. Additionally, up to 10% of patients have symptomatic recurrent diverticulitis after surgical resection, and reoperation may be required in 2% to 3%.68,98,131,132 Patients undergoing resection for diverticulitis have higher recurrence rates when the sigmoid colon is used for the distal resection margin, rather than the rectum133; it is recommended to resect the entire distal colon whenever possible, forming the distal anastomosis with the proximal rectum and the proximal anastomosis with a noninflamed portion of colon.68,98
THE YOUNG PATIENT
Diverticulitis is relatively uncommon in patients younger than 40 years (2% to 5% of all patients with diverticulitis1,17) but the incidence in this age group may be rising.134 Nevertheless, because diverticulitis is uncommon in younger patients, it is often missed or mistaken for other diagnoses, such as appendicitis or IBD. Like diverticulitis in older patients, the disease is mainly sigmoid in location, although one report has described a right-sided predominance in young Israelis.135 There seems to be a significant male predominance in young patients.1,136 Attacks often are more severe, and 40% to 88% of younger patients require urgent surgery during their initial attack; recurrence and complication rates are also higher than in older patients.1,136,137
When patients with acute diverticulitis are managed nonoperatively, youth is an independent risk factor for poor outcome in subsequent course,138 possibly due to delay in diagnosis. For these reasons, some authors have advocated elective segmental colectomy in a healthy young person after one well-documented episode of diverticulitis68,125,138; others have questioned this approach.98,135,139 In the largest series to date of young patients with diverticulitis, the authors suggested that the higher incidence of surgical management in these patients relative to their older counterparts was not due to worse outcomes but rather to a higher rate of elective procedures done to prevent the expected poor outcomes.134 Thus, the latest surgical guidelines are advocating more of a case-by-case approach to elective resections for all patients with diverticulitis.130
THE ELDERLY PATIENT
Because diverticular disease is more prevalent in older populations, diverticulitis in the elderly warrants special mention. Diverticulitis can manifest with more subtle symptoms and signs in the elderly, making the diagnosis more challenging. Distinguishing diverticulitis from colorectal cancer becomes a much more important issue in older patients, because the incidence of colorectal cancer also increases with age. Though the risk of a severe initial attack of diverticulitis may be lower in older patients than in younger ones, the risk of death when diverticulitis results in perforation is more than three times higher in the elderly.140 However, it appears that the number of comorbidities is a stronger predictor of mortality from perforated diverticulitis than age.141 Because subsequent complicated disease is uncommon if the initial attack was mild,138 and because older patients are less likely than younger patients to have recurrent disease after nonoperative management,142 surgery is often delayed or set aside in older patients. Not only is surgery more risky in the elderly, but colostomy reversal is also less often performed143 and, when attempted, tends to have a higher rate of morbidity and mortality than in younger patients.144
THE IMMUNOCOMPROMISED PATIENT
Diverticulitis can manifest more subtly in immunocompromised patients and represents a more difficult diagnostic challenge than in those with a normal immune system. Although diverticulitis in such patients does not appear to be more common, it appears to have graver consequences. One study reported that 24% of nonimmunosuppressed patients needed surgery for diverticulitis, whereas 100% of immunosuppressed ones required surgery.145 Immunocompromised patients have a higher rate of free perforation (43% vs. 14%), need for surgery (58% vs. 33%), and postoperative mortality (39% vs. 2%) than do noncompromised patients.146 In solid organ (e.g., heart, lung, kidney) transplant populations, mortality from diverticulitis has been found to be extremely high, ranging from 25% to 100%.147–149 Because of this high risk, many authorities advocate elective resection after an initial episode of diverticulitis in an immunosuppressed patient.7,125
RIGHT-SIDED DIVERTICULITIS
In Asia, right-sided diverticulitis is the predominant form of diverticulitis. Especially in younger patients, the diagnosis of right-sided diverticulitis is more difficult to make than is left-sided disease and is virtually indistinguishable clinically from acute appendicitis. Clinical factors that might be helpful to distinguish diverticulitis from appendicitis in a person of Asian ethnicity who might therefore have right-sided diverticulitis are that patients with diverticulitis tend to be older and have a lower frequency of nausea and vomiting than patients with appendicitis; the characteristic progression of symptoms seen with appendicitis is absent150 (see Chapter 116).
Radiologically, right-sided diverticulitis and appendicitis also are easily confused, especially when a local abscess is present (Fig. 117-6). There is an estimated preoperative misdiagnosis rate in right-sided colon inflammatory conditions of 40% to 92%.150,151 Even with excellent imaging, the diagnosis of right-sided diverticulitis often is made at laparotomy.
When the proper diagnosis is made preoperatively, treatment of right-sided disease is the same as for left-sided diverticulitis. One study has suggested better overall responsiveness of right-sided diverticulitis to medical therapy alone, even after multiple attacks.152 The much more common complication associated with right colonic diverticula is hemorrhage, discussed later in this chapter.
SEGMENTAL COLITIS ASSOCIATED WITH DIVERTICULOSIS
A subset of patients with diverticulosis is found to have mucosal inflammation within the segment of colon containing the diverticula. Segmental colitis associated with diverticulosis (SCAD) initially was thought to be a rare form of Crohn’s colitis, but it is now increasingly recognized as a distinct, but poorly understood, manifestation of diverticular disease.153–155 SCAD primarily affects the sigmoid colon of certain patients with diverticulosis and mimics IBD in its clinical presentation. In limited study, there does not appear to be a higher risk of diverticulitis or colon cancer in these patients.155
Presenting symptoms generally are left lower quadrant cramping pain, diarrhea, and rectal bleeding. Endoscopically, in addition to diverticula, the sigmoid colonic mucosa shows erythema, friability of varying degrees, and mucosal erosions. The remaining segments of colon, including the rectum, are not visibly or histologically involved. Biopsy specimens can show chronic lymphocytic infiltration, cryptitis, crypt abscesses, and even granulomas; many cases are histologically indistinguishable from IBD.156 In fact, a subset of patients when followed endoscopically through time appear to evolve into a picture similar to ulcerative proctosigmoiditis or Crohn’s colitis. This observation should prompt a low threshold for endoscopic re-evaluation if a patient with presumed SCAD develops persistent or progressive symptoms.
Patients with SCAD generally are responsive to 5-aminosalicylic acid (5-ASA) compounds, with one series reporting over 80% of patients achieving clinical remission on 5-ASA.155 In most cases, the clinical course tends to be benign and self-limited,157 although there are reports of cases requiring sigmoid colectomy for bleeding or stricture complications.158
DIVERTICULAR HEMORRHAGE
Diverticulosis, angioectasias, colitis, neoplasms, and hemorrhoids are responsible for most cases of lower gastrointestinal bleeding in adults (see Chapter 19).20,159–162 Diverticular hemorrhage is the most common identifiable cause of significant lower gastrointestinal bleeding, accounting for 30% to 40% of cases with confirmed sources.163,164 It often is difficult, however, to make a precise determination of the source of bleeding, and conclusive evidence of the cause of bleeding is available only in a minority of cases,159,160 either by seeing an actively bleeding lesion endoscopically or by identifying extravasation from a specific site angiographically. More often, circumstantial evidence is used to suggest diverticulosis as the source of hemorrhage. Clinical features suggesting diverticular hemorrhage include copious bright red or maroon blood per rectum, the presence of diverticulosis on colonoscopy or radiologic studies, exclusion of an upper gastrointestinal source, and exclusion of alternative colonic sources. These clinical criteria, suggested by Quinn in 1960, however, are nonspecific and are met by various lesions other than diverticula, most notably angioectasias.
EPIDEMIOLOGY
Severe hemorrhage has been reported to occur in 3% to 5% of patients with diverticulosis.20,165,166 Although most diverticula are in the left colon in Western patients, the site of bleeding diverticula has been believed to be in the proximal colon in more than one half of patients.167–169 A large series of 180 Asian patients with diverticular hemorrhage reported a higher bleeding rate and greater need for surgery with right-sided compared with left-sided disease.169 Patients with pancolonic diverticulosis appear to have a higher hemorrhage rate than those with diverticula on only one side of the colon.170
PATHOPHYSIOLOGY
To study the pathogenesis of diverticular bleeding, Meyers and colleagues used sophisticated microangiographic techniques on resected colon specimens from patients with arteriographically documented bleeding diverticula.171 Three-dimensional histologic reconstructions demonstrated consistent findings of intimal thickening and medial thinning of the vasa recta as it coursed over the dome of the diverticulum. They proposed that these changes led to a segmental weakening of the artery, thus predisposing to its rupture. This arterial lesion was absent in diverticula that did not bleed. What predisposes to this arterial change and what precipitates its rupture are unknown. Inflammation does not appear to be a contributing factor, because it is not found histologically in bleeding diverticula that were resected. This absence might explain why bleeding rarely complicates diverticulitis.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been implicated in lower intestinal, and specifically diverticular, bleeding. A large prospective series of patients with lower intestinal bleeding reported a risk of bleeding with NSAIDs equal to that of duodenal ulcer.172 An overall increased risk of diverticular bleeding also was found at the four-year follow-up of a large study of health professionals who had been free of diverticulosis at baseline.173 Both aspirin and nonaspirin NSAIDs increase bleeding risk, although the magnitude of this risk appears to be higher with nonaspirin NSAIDs than with aspirin.170 In addition to having a higher rate of complications from diverticulosis, including bleeding and perforation, patients taking NSAIDs also appear to have more severe complications.174–176 Hence, the necessity for NSAIDs in patients who have an episode of diverticular bleeding should be re-evaluated.
Whether patients with diverticulosis without a prior bleeding episode should be counseled as well to avoid NSAIDs is less clear. In a multicenter trial of patients randomized to naproxen (conventional NSAID) or rofecoxib (cyclooxygenase [COX]-2 selective agent), the relative risk of lower intestinal bleeding from all causes was 0.46 in patients taking rofecoxib relative to the naproxen group.177 Whether hemorrhage specifically from diverticula is reduced by replacing NSAIDs with COX-2 selective agents is unknown, and the cost-effectiveness of this approach has not been established.
CLINICAL FEATURES
Diverticular hemorrhage typically manifests as abrupt, painless hematochezia. Because the bleeding is arterial, the volume of blood usually is moderate or large, an observation that can help distinguish diverticular hemorrhage from other common causes of rectal bleeding. Patients often pass red or maroon clots; melena is unusual. Because the bleeding is overt, neither a positive fecal occult blood test nor iron-deficiency anemia should be attributed to diverticular hemorrhage. Natural history studies report that bleeding ceases spontaneously in 70% to 80% of patients, and rebleeding rates range from 22% to 38%.165,166,169 The chance of a third bleeding episode can be as high as 50%,165 leading some to recommend surgical resection after a second bleeding episode.101
DIAGNOSIS AND TREATMENT
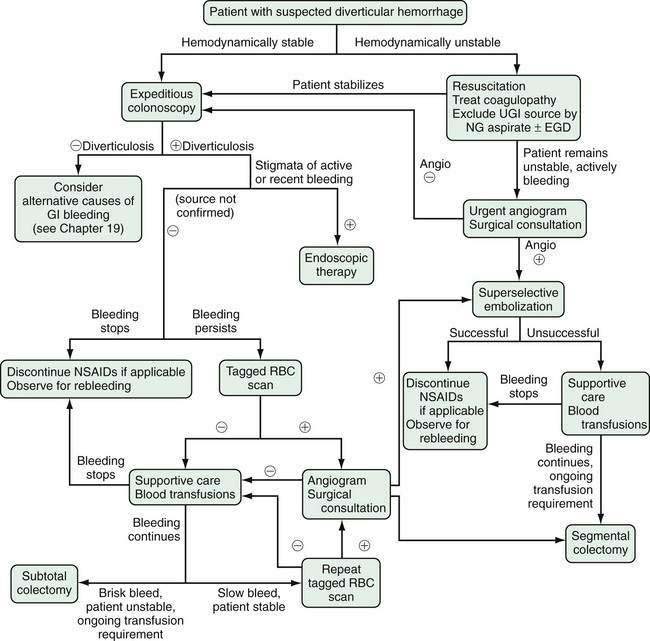
The diagnosis and treatment of patients with lower intestinal bleeding in general have been reviewed comprehensively elsewhere (see Chapter 19).130,178 The algorithm in Figure 117-7 summarizes the management of patients with bleeding diverticula. In unstable patients, volume and blood product resuscitation require immediate attention. Excluding an upper gastrointestinal source by nasogastric lavage or upper endoscopy is warranted because 10% to 15% of patients with hematochezia have an upper tract cause. If bleeding is massive or if the patient remains unstable after attempted resuscitation, early angiography to attempt bleeding localization and surgical consultation should be obtained.
A stable patient with suspected active or recent diverticular bleeding should undergo bowel preparation for a colonoscopy. The ability to identify a diverticular source, to exclude alternative diagnoses, and to provide therapy of actively bleeding lesions support colonoscopy as a primary investigation in this setting. Rapid (over three to four hours) oral or nasogastric purge with a balanced electrolyte solution provides a safe and effective bowel preparation; a dose of metoclopramide before initiation can improve tolerance to the lavage.179 If diverticulosis is not found on colonoscopy, alternative diagnoses should be entertained. If diverticula are found but bleeding has stopped and no other colonic causes are found, a presumptive diagnosis of diverticular hemorrhage is made and the patient should be instructed to avoid NSAIDs and anticoagulants, if possible. As noted, most patients with diverticular hemorrhage do not rebleed.
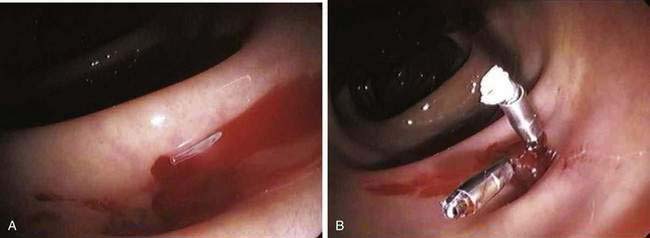
The endoscopic identification of active bleeding or stigmata of recent hemorrhage from a specific site (Fig. 117-8) is evident in about 10% to 20% of colonoscopic examinations for diverticular bleeding. The appearance of nonbleeding stigmata—visible vessel or adherent clot within a diverticulum—can permit one to estimate the future risk of hemorrhage from that site,180 although this concept has not been validated as well in the colon as it has for peptic ulcer bleeding. Identification of a bleeding site allows endoscopic therapy to be applied. The use of epinephrine injection alone181 or in combination with other therapies such as heater probe coagulation,182 bipolar coagulation,183–185 endoclips,186–188 fibrin sealant,189 and band ligation190 all have been shown in small case series to achieve hemostasis safely in patients with diverticular bleeding.177 If endoscopic therapy is not effective or durable, localizing the site facilitates directed therapy with angiography or segmental surgical resection.
With a paucity of high-quality evidence, the true effectiveness of endoscopic treatment for diverticular hemorrhage is not known. In a retrospective study, Jensen and colleagues were able to identify and treat (with epinephrine and bipolar cautery) definite bleeding sources in 10 of 48 patients with suspected diverticular bleeding.185 None of the endoscopically treated patients had recurrent bleeding or required surgery. These results were compared with 17 historical controls who had bleeding stigmata but no endoscopic therapy, nine of whom rebled and six of whom required surgery, lending indirect support to the use of endoscopic therapy for diverticular hemorrhage. Stigmata of active or recent hemorrhage are not common findings at endoscopy, however, and, therefore, endoscopic therapy only is relevant to a minority of patients.
Another group at the Mayo Clinic with an approach to lower intestinal bleeding similar to Jensen’s group reported that urgent colonoscopy (performed less than 12 hours after admission) was no more effective at identifying bleeding stigmata than colonoscopy performed later in the hospitalization.191 Another cohort of 100 patients with acute lower intestinal bleeding was randomized to urgent colonoscopy (within eight hours of hospitalization) or standard care (radiologic bleeding studies for rapid bleeds, elective colonoscopy for slow or inactive bleeds). A definite bleeding source was found in more cases in the urgent colonoscopy group than in those who received standard care, although important outcomes such as mortality, hospital stay, rebleeding, and need for surgery were the same between groups.192 Despite having little firm evidence that expedited colonoscopy improves outcomes, it still seems reasonable to perform colonoscopy within the first 12 to 48 hours of admission in most cases.
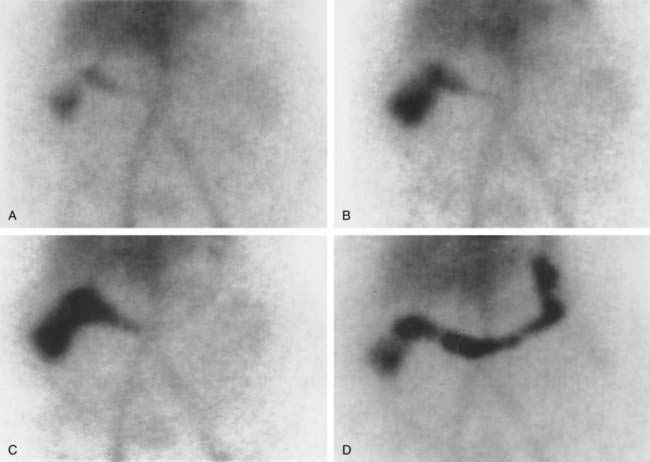
When active bleeding is present but colonoscopy fails to allow localization or treatment of a bleeding source, further evaluation with nuclear scintigraphy (tagged red blood cell scan) or angiography can be undertaken, taking into account local availability and expertise. A third radiographic option, enhanced CT, also has shown promise for identifying active lower intestinal bleeding sources, although its role has yet to be defined.193 Nuclear scintigraphy (Fig. 117-9) has many theoretical advantages in the evaluation of lower intestinal bleeding194: It is noninvasive, technically simple, relatively inexpensive, and sensitive to bleeding rates as low as 0.1 mL/min. Scintigraphy, however, can identify only the site, not the etiology of the bleeding, and it has no therapeutic potential. Furthermore, some studies have questioned its accuracy195 and have underscored a lack of proven impact on mortality, transfusion requirement, and eventual need for surgery.196 Given its sensitivity and relative simplicity, however, many centers use scintigraphy before angiography to minimize the chance of a negative angiogram and to help select a specific artery for injection of contrast.197
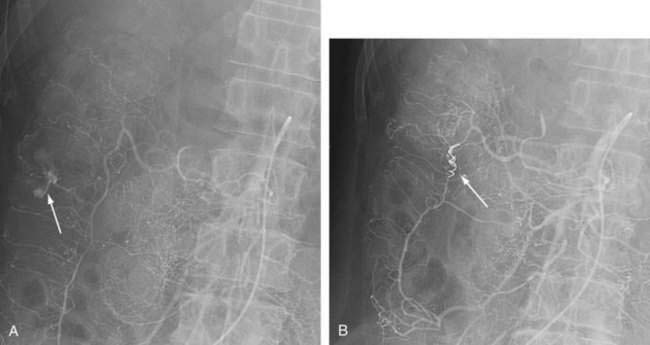
Angiography has a sensitivity for lower intestinal bleeding rates of 0.5 mL/min. Advantages of angiography are identification of the site of bleeding accurately enough to direct segmental surgical resection and therapeutic capability. Although angiographic embolization had previously been felt to carry a risk of bowel infarction, superselective embolization (Fig. 117-10), or embolization of distal arterial branches, has been demonstrated to be effective (67% to 100% with lasting hemostasis) and relatively safe (ischemia rates less than 20%).198–201 Where available, arterial embolization increasingly is becoming the nonsurgical therapy of choice when endoscopic methods are not possible.
The rebleeding rate was 6% in seven series of patients who underwent segmental resections for angiographically documented bleeding sites.202 As noted, however, it is often difficult to identify an extravasation site angiographically. One series found that angiography resulted in a successful directed resection in only 12% of patients.203 In patients with persistent, life-threatening bleeding and no identification of a likely bleeding site, a subtotal or blind colectomy may be required as a last resort. These patients have had an extremely high morbidity and mortality,204 possibly because of the multiple invasive tests leading to that end and the resultant delay in definitive management. Additionally, a blind colectomy runs the risk of not resecting the bleeding lesion when the source is more proximal and has a high risk of anastomotic failure.205 More recent literature, however, has shown morbidity and mortality rates of a subtotal colectomy not to differ from those of a blind hemicolectomy, when the site of the bleeding is not identified.205,206 Clearly, a close collaborative relationship between the gastroenterologist and the surgeon is paramount in managing such patients.
Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128:714-19. (Ref 44.)
Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2:1408-12. (Ref 39.)
Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 2003;18(Suppl 3):71-4. (Ref 8.)
Hjern F, Johansson C, Mellgren A, et al. Diverticular disease and migration—the influence of acculturation to a Western lifestyle on diverticular disease. Aliment Pharmacol Ther. 2006;23:797-805. (Ref 14.)
Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78-82. (Ref 185.)
Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288-92. (Ref 177.)
Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419-24. (Ref 163.)
Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450-4. (Ref 2.)
Painter NS, Truelove SC, Ardran GM, Tuckey M. Segmentation and the localization of intraluminal pressures in the human colon, with special reference to the pathogenesis of colonic diverticula. Gastroenterology. 1965;49:169-77. (Ref 29.)
Parks TG. Natural history of diverticular disease of the colon: a review of 521 cases. BMJ. 1969;4:639-42. (Ref 17.)
Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:53-69. (Ref 9.)
Rafferty J, Shellito P, Hyman NH, et al. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939-44. (Ref 130.)
Salem L, Veenstra DL, Sullivan SD, et al. The timing of elective colectomy in diverticulitis: a decision analysis. J Am Coll Surg. 2004;199:904-12. (Ref 129.)
Stemmermann GN, Yatani R. Diverticulosis and polyps of the large intestine: a necropsy study of Hawaii Japanese. Cancer. 1973;31:1260-70. (Ref 13.)
Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907-14. (Ref 45.)
1. Nathan BN. Who first described colonic diverticula? Can J Surg. 1991;34:203.
2. Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450.
3. Kang JY, Hoare J, Tinto A, et al. Diverticular disease of the colon—on the rise: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther. 2003;17:1189.
4. Makela J, Kiviniemi H, Laitinen S. Prevalence of perforated sigmoid diverticulitis is increasing. Dis Colon Rectum. 2002;45:955.
5. Schwesinger WH, Page CP, Gaskill HVIII, et al. Operative management of diverticular emergencies: strategies and outcomes. Arch Surg. 2000;135:558.
6. Sandler RS, Everhart JE, Donowits M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500.
7. Schoetz DJJr. Uncomplicated diverticulitis: indications for surgery and surgical management. Surg Clin North Am. 1993;73:965.
8. Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 2003;18(Suppl 3):71.
9. Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:53.
10. Jun S, Stollman N. Epidemiology of diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:529.
11. McConnell EJ, Tessier DJ, Wolff BG. Population-based incidence of complicated diverticular disease of the sigmoid colon based on gender and age. Dis Colon Rectum. 2003;46:1110.
12. Kyle J, Adesola AD, Tinckler LF, deBeaux J. Incidence of diverticulitis. Scand J Gastroenterol. 1967;2:77.
13. Stemmermann GN, Yatani R. Diverticulosis and polyps of the large intestine: a necropsy study of Hawaii Japanese. Cancer. 1973;31:1260.
14. Hjern F, Johansson C, Mellgren A, et al. Diverticular disease and migration—the influence of acculturation to a Western lifestyle on diverticular disease. Aliment Pharmacol Ther. 2006;23:797.
15. Lee YS. Diverticular disease of the large bowel in Singapore: an autopsy survey. Dis Colon Rectum. 1986;29:330.
16. Levy N, Stermer E, Simon J. The changing epidemiology of diverticular disease in Israel. Dis Colon Rectum. 1985;28:416-18.
17. Parks TG. Natural history of diverticular disease of the colon: a review of 521 cases. Br Med J. 1969;4:639.
18. Horner JL. Natural history of diverticulosis of the colon. Am J Dig Dis. 1958;3:343.
19. Levi DM, Levi JU, Rogers AI, et al. Giant colonic diverticulum: an unusual manifestation of a common disease. Am J Gastroenterol. 1993;88:139.
20. Reinus JF, Brandt LJ. Vascular ectasias and diverticulosis: common causes of lower intestinal bleeding. Gastroenterol Clin North Am. 1994;23:1.
21. Chia JG, Wilde CC, Ngoi SS, et al. Trends of diverticular disease of the large bowel in a newly developed country. Dis Colon Rectum. 1991;34:498.
22. Miura S, Kodaira S, Shatari T, et al. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum. 2000;43:1383.
23. Whiteway J, Morson BC. Elastosis in diverticular disease of the sigmoid colon. Gut. 1985;26:258.
24. Bode MK, Karttunen TJ, Makela J, et al. Type I and III collagens in human colon cancer and diverticulosis. Scand J Gastroenterol. 2000;35:747.
25. Mimura T, Bateman AC, Lee RL, et al. Up-regulation of collagen and tissue inhibitors of matrix metalloproteinase in colonic diverticular disease. Dis Colon Rectum. 2004;47:371.
26. Rosemar A, Ivarsson ML, Borjesson L, et al. Increased concentration of tissue-degrading matrix metalloproteinases and their inhibitor in complicated diverticular disease. Scand J Gastroenterol. 2007;42:215.
27. Arfwidsson S, Knock NG, Lehmann L, Winberg T. Pathogenesis of multiple diverticula of the sigmoid colon in diverticular disease. Acta Chir Scand. 1964;63:1.
28. Painter NS. The aetiology of diverticulosis of the colon with special reference to the action of certain drugs on the behaviour of the colon. Ann R Coll Surg Engl. 1964;34:98.
29. Painter NS, Truelove SC, Ardran GM, Tuckey M. Segmentation and the localization of intraluminal pressures in the human colon, with special reference to the pathogenesis of colonic diverticula. Gastroenterology. 1965;49:169.
30. Trotman IF, Misiewicz JJ. Sigmoid motility in diverticular disease and the irritable bowel syndrome. Gut. 1988;29:218.
31. Cortesini C, Pantalone D. Usefulness of colonic motility study in identifying patients at risk for complicated diverticular disease. Dis Colon Rectum. 1991;34:339.
32. Bassotti G, Battaglia E, Spinozzi F, et al. Twenty-four hour recordings of colonic motility in patients with diverticular disease: evidence for abnormal motility and propulsive activity. Dis Colon Rectum. 2001;44:1814.
33. Sugihara K, Muto T, Morioka Y. Motility study in right-sided diverticular disease of the colon. Gut. 1983;24:1130.
34. Osbak PS, Bindslev N, Poulsen SS, et al. Colonic epithelial ion transport is not affected in patients with diverticulosis. BMC Gastroenterol. 2007;7:37.
35. Bassotti G, Battaglia E, Bellone G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol. 2005;58:973.
36. Tomita R, Fujisaki S, Tanjoh K, Fukuzawa M. Role of nitric oxide in the left-sided colon of patients with diverticular disease. Hepatogastroenterology. 2000;47:692.
37. Maselli MA, Piepoli AL, Guerra V, et al. Colonic smooth muscle responses in patients with diverticular disease of the colon: effect of the NK2 receptor antagonist SR48968. Dig Liver Dis. 2004;36:348.
38. Liu L, Shang F, Markus I, Burcher E. Roles of substance P receptors in human colon circular muscle: alterations in diverticular disease. J Pharmacol Exp Ther. 2002;302:627.
39. Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972;2:1408.
40. Fisher N, Berry CS, Fearn T, et al. Cereal dietary fiber consumption and diverticular disease: a lifespan study in rats. Am J Clin Nutr. 1985;42:788.
41. Gear JS, Ware A, Fursdon P, et al. Symptomless diverticular disease and intake of dietary fibre. Lancet. 1979;1:511.
42. Lin OS, Soon MS, Wu SS, et al. Dietary habits and right-sided colonic diverticulosis. Dis Colon Rectum. 2000;43:1412.
43. Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr. 1994;60:757.
44. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128:714.
45. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300:907.
46. Nakama H, Fattah AS, Zhang B, et al. Association of diverticulosis coli and vascular ectasias and the results of fecal occult blood test. Hepatogastroenterology. 2000;47:1277.
47. Boulos PB, Karamanolis DG, Salmon PR, Clark CG. Is colonoscopy necessary in diverticular disease? Lancet. 1984;1:95.
48. Brayko CM, Kozarek RA, Sanowski RA, Howells T. Diverticular rupture during colonoscopy: fact or fancy? Dig Dis Sci. 1984;29:427.
49. Bat L, Williams CB. Usefulness of pediatric colonoscopes in adult colonoscopy. Gastrointest Endosc. 1989;35:329.
50. Kaffes AJ, Mishra A, Ding SL, et al. A prospective trial of variable stiffness pediatric vs. standard instrument colonoscopy. Gastrointest Endosc. 2003;58:685.
51. Falchuk ZM, Griffin PH. A technique to facilitate colonoscopy in areas of severe diverticular disease. N Engl J Med. 1984;310:598.
52. Glick SN. Inverted colonic diverticulum: air contrast barium enema findings in six cases. AJR Am J Roentgenol. 1991;156:961.
53. Ladas SD, Prigouris SP, Pantelidaki C, Raptis A. Endoscopic removal of inverted sigmoid diverticulum: is it a dangerous procedure? Endoscopy. 1989;21:243.
54. Otte JJ, Larsen L, Andersen JR. Irritable bowel syndrome and symptomatic diverticular disease: different diseases? Am J Gastroenterol. 1986;81:529.
55. Ritchie J. Similarity of bowel distension characteristics in the irritable colon syndrome and diverticulosis. Gut. 1977;18:A990.
56. Brodribb AJ. Treatment of symptomatic diverticular disease with a high-fibre diet. Lancet. 1977;1:664.
57. Ornstein MH, Littlewood ER, Baird IM, et al. Are fibre supplements really necessary in diverticular disease of the colon? A controlled clinical trial. BMJ. 1981;282:1353.
58. Tursi A, Brandimarte G, Daffina R. Long-term treatment with mesalamine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of colon. Dig Liver Dis. 2002;34:510.
59. Tursi A, Brandimarte G, Giorgetti GM, et al. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol. 2006;40:312.
60. Comparato G, Fanigliulo L, Cavallaro LG, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12-month follow-up. Dig Dis Sci. 2007;52:2934.
61. White J. Probiotics and their use in diverticulitis. J Clin Gastroenterol. 2006;40:S160.
62. Papi C, Ciaco A, Koch M, et al. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther. 1995;9:33.
63. Colecchia A, Vestito A, Pasqui F, et al. Efficacy of long term cyclic administration of the poorly absorbed antibiotic rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol. 2007;13:264.
64. Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557.
65. Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol. 2003;15:313.
66. Horgan AF, McConnell EJ, Wolff BG, et al. Atypical diverticular disease: surgical results. Dis Colon Rectum. 2001;44:1315.
67. Berman LG, Burdick D, Heitzman ER, Prior JT. A critical reappraisal of sigmoid peridiverticulitis. Surg Gynecol Obstet. 1968;127:481.
68. Roberts P, Abel M, Rosen L, et al. Practice parameters for sigmoid diverticulitis: the Standards Task Force American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 1995;38:126.
69. Morson BC. Pathology of diverticular disease of the colon. Clin Gastroenterol. 1975;4:37.
70. Boirivant M, Marini M, Di Felice G, et al. Lamina propria T cells in Crohn’s disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology. 1999;116:557.
71. Hollink N, Dzabic M, Wolmer N, et al. High prevalence of an active human cytomegalovirus infection in patients with colonic diverticulitis. J Clin Virol. 2007;40:116.
72. Williams RA, Davis IP. Diverticular disease of the colon. In: Haubrick WS, Schaffner F, editors. Bockus gastroenterology. Philadelphia: WB Saunders; 1995:1637.
73. Hart AR, Kennedy HJ, Stebbings WS, Day NE. How frequently do large bowel diverticula perforate? An incidence and cross-sectional study. Eur J Gastroenterol Hepatol. 2000;12:661.
74. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85.
75. Markham NI, Li AK. Diverticulitis of the right colon—experience from Hong Kong. Gut. 1992;33:547.
76. Ambrosetti P, Robert JH, Witzig JA, et al. Acute left colonic diverticulitis: a prospective analysis of 226 consecutive cases. Surgery. 1994;115:546.
77. Bahadursingh AM, Virgo KS, Kaminski DL, Longo WE. Spectrum of disease and outcome of complicated diverticular disease. Am J Surg. 2003;186:696.
78. Wexner SD, Dailey TH. The initial management of left lower quadrant peritonitis. Dis Colon Rectum. 1986;29:635.
79. Kourtesis GJ, Williams RA, Wilson SE. Surgical options in acute diverticulitis: value of sigmoid resection in dealing with the septic focus. Aust N Z J Surg. 1988;58:955.
80. Morris J, Stellato TA, Lieberman J, Haaga JR. The utility of computed tomography in colonic diverticulitis. Ann Surg. 1986;204:128.
81. Doringer E. Computerized tomography of colonic diverticulitis. Crit Rev Diagn Imaging. 1992;33:421.
82. McKee RF, Deignan RW, Krukowski ZH. Radiological investigation in acute diverticulitis. Br J Surg. 1993;80:560.
83. Shrier D, Skucas J, Weiss S. Diverticulitis: an evaluation by computed tomography and contrast enema. Am J Gastroenterol. 1991;86:1466.
84. Hulnick DH, Megibow AJ, Balthazar EJ, et al. Computed tomography in the evaluation of diverticulitis. Radiology. 1984;152:491.
85. Ambrosetti P, Jenny A, Becker C, et al. Acute left colonic diverticulitis—compared performance of computed tomography and water-soluble contrast enema: prospective evaluation of 420 patients. Dis Colon Rectum. 2000;43:1363.
86. Werner A, Diehl SJ, Farag-Soliman M, Duber C. Multi-slice spiral CT in routine diagnosis of suspected acute left-sided colonic diverticulitis: a prospective study of 120 patients. Eur Radiol. 2003;13:2596.
87. Jang HJ, Lim HK, Lee SJ, et al. Acute diverticulitis of the cecum and ascending colon: the value of thin-section helical CT findings in excluding colonic carcinoma. AJR Am J Roentgenol. 2000;174:1397.
88. Jang HJ, Lim HK, Lee SJ, et al. Acute diverticulitis of the cecum and ascending colon: thin-section helical CT findings. AJR Am J Roentgenol. 1999;172:601.
89. Johnson CD, Baker ME, Rice RP, et al. Diagnosis of acute colonic diverticulitis: comparison of barium enema and CT. AJR Am J Roentgenol. 1987;148:541.
90. Stefansson T, Nyman R, Nilsson S, et al. Diverticulitis of the sigmoid colon: A comparison of CT, colonic enema, and laparoscopy. Acta Radiol. 1997;38:313.
91. Verbanck J, Lambrecht S, Rutgeerts L, et al. Can sonography diagnose acute colonic diverticulitis in patients with acute intestinal inflammation? A prospective study. J Clin Ultrasound. 1989;17:66.
92. Zielke A, Hasse C, Nies C, et al. Prospective evaluation of ultrasonography in acute colonic diverticulitis. Br J Surg. 1997;84:385.
93. Wilson SR, Toi A. The value of sonography in the diagnosis of acute diverticulitis of the colon. AJR Am J Roentgenol. 1990;154:1199.
94. Pradel JA, Adell JF, Taourel P, et al. Acute colonic diverticulitis: prospective comparative evaluation with US and CT. Radiology. 1997;205:503.
95. Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol. 2000;49:827.
96. Chow AW. Appendicitis and diverticulitis. In: Hoeprich PD, Jordan MC, Ronald AR, editors. Infectious diseases: a treatise of infectious processes. Philadelphia: JB Lippincott; 1994:878.
97. Zarling EJ, Piontek F, Klemka-Walden L, Inczauskis D. The effect of gastroenterology training on the efficiency and cost of care provided to patients with diverticulitis. Gastroenterology. 1997;112:1859.
98. Wong WD, Wexner SD, Lowry A, et al. Practice parameters for the treatment of sigmoid diverticulitis: the Standards Task Force, American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 2000;43:290.
99. Kohler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13:430.
100. Guller U, Jain N, Hervey S, et al. Laparoscopic vs open colectomy: outcomes comparison based on large nationwide databases. Arch Surg. 2003;138:1179.
101. Ambrosetti P, Robert J, Witzig JA, et al. Incidence, outcome, and proposed management of isolated abscesses complicating acute left-sided colonic diverticulitis: a prospective study of 140 patients. Dis Colon Rectum. 1992;35:1072.
102. Saini S, Mueller PR, Wittenberg J, et al. Percutaneous drainage of diverticular abscess: an adjunct to surgical therapy. Arch Surg. 1986;121:475.
103. Stabile BE, Puccio E, van Sonnenberg E, Neff CC. Preoperative percutaneous drainage of diverticular abscesses. Am J Surg. 1990;159:99.
104. Schechter S, Eisenstat TE, Oliver GC, et al. Computerized tomographic scan-guided drainage of intra-abdominal abscesses: preoperative and postoperative modalities in colon and rectal surgery. Dis Colon Rectum. 1994;37:984.
105. Desai DC, Brennan EJJr, Reilly JF, Smink RDJr. The utility of the Hartmann procedure. Am J Surg. 1998;175:152.
106. Hackford AW, Schoetz DJJr, Coller JA, Veidenheimer MC. Surgical management of complicated diverticulitis: the Lahey Clinic experience, 1967 to 1982. Dis Colon Rectum. 1985;28:317.
107. Fleites RA, Marshall JB, Eckhauser ML, et al. The efficacy of polyethylene glycol–electrolyte lavage solution versus traditional mechanical bowel preparation for elective colonic surgery: A randomized, prospective, blinded clinical trial. Surgery. 1985;98:708.
108. Lee EC, Murray JJ, Coller JA, et al. Intraoperative colonic lavage in nonelective surgery for diverticular disease. Dis Colon Rectum. 1997;40:669.
109. Wedell J, Banzhaf G, Chaoui R, et al. Surgical management of complicated colonic diverticulitis. Br J Surg. 1997;84:380.
110. Wolff BG, Devine RM. Surgical management of diverticulitis. Am Surg. 2000;66:153.
111. Krukowski ZH, Matheson NA. Emergency surgery for diverticular disease complicated by generalized and faecal peritonitis: a review. Br J Surg. 1984;71:921.
112. Nagorney DM, Adson MA, Pemberton JH. Sigmoid diverticulitis with perforation and generalized peritonitis. Dis Colon Rectum. 1985;28:71.
113. Kronborg O. Treatment of perforated sigmoid diverticulitis: a prospective randomized trial. Br J Surg. 1993;80:505.
114. Zeitoun G, Laurent A, Rouffet F, et al. Multicentre, randomized clinical trial of primary versus secondary sigmoid resection in generalized peritonitis complicating sigmoid diverticulitis. Br J Surg. 2000;87:1366.
115. Constantinides VA, Heriot A, Remzi F, et al. Operative strategies for diverticular peritonitis: a decision analysis between primary resection ad anastomosis versus Hartmann’s procedures. Ann Surg. 2007;245:94.
116. Bretagnol F, Pautrat K, Mor C, et al. Emergency laparoscopic management of perforated sigmoid diverticulitis: a promising alternative to more radical procedures. J Am Coll Surg. 2008;206:654.
117. Franklin MEJr, Portillo G, Trevino JM, et al. Long-term experience with the laparoscopic approach to perforated diverticulitis plus generalized peritonitis. World J Surg. 2008;32:1507.
118. Rothenberger DA, Wiltz O. Surgery for complicated diverticulitis. Surg Clin North Am. 1993;73:975.
119. Woods RJ, Lavery IC, Fazio VW, et al. Internal fistulas in diverticular disease. Dis Colon Rectum. 1988;31:591.
120. McBeath RB, Schiff MJr, Allen V, et al. A 12-year experience with enterovesical fistulas. Urology. 1994;44:661.
121. Forde KA, Treat MR. Colonoscopy in the evaluation of strictures. Dis Colon Rectum. 1985;28:699.
122. Kozarek RA. Hydrostatic balloon dilation of gastrointestinal stenoses: a national survey. Gastrointest Endosc. 1986;32:15.
123. Blomberg B, Rolny P, Jarnerot G. Endoscopic treatment of anastomotic strictures in Crohn’s disease. Endoscopy. 1991;23:195.
124. Tamim WZ, Ghellai A, Counihan TC, et al. Experience with endoluminal colonic wall stents for the management of large bowel obstruction for benign and malignant disease. Arch Surg. 2000;135:434.
125. Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110.
126. Chapman JR, Dozois EJ, Wolff BG, et al. Diverticulitis: A progressive disease? Do multiple recurrences predict less favorable outcomes? Ann Surg. 2006;243:876.
127. DiMario F, Aragona G, Leandro G, et al. Efficacy of mesalamine in the treatment of symptomatic diverticular disease. Dig Dis Sci. 2005;50:581.
128. Tursi A. Acute diverticulitis of the colon—current medical therapeutic management. Expert Opin Pharmacother. 2004;5:55.
129. Salem L, Veenstra DL, Sullivan SD, et al. The timing of elective colectomy in diverticulitis: a decision analysis. J Am Coll Surg. 2004;199:904.
130. Rafferty J, Shellito P, Hyman NH, et al. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939.
131. Thorn M, Graf W, Stefansson T, Pahlman L. Clinical and functional results after elective colonic resection in 75 consecutive patients with diverticular disease. Am J Surg. 2002;183:7.
132. Frizelle FA, Dominguez JM, Santoro GA. Management of post-operative recurrent diverticulitis: a review of the literature. J R Coll Surg Edinb. 1997;42:186.
133. Thaler K, Dinnewitzer A, Mascha E, et al. Long-term outcome and health-related quality of life after laparoscopic and open colectomy for benign disease: determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Surg Endosc. 2003;17:1404.
134. Guzzo J, Hyman N. Diverticulitis in young patients: is resection after a single attack always warranted? Dis Colon Rectum. 2004;47:1187.
135. Reisman Y, Ziv Y, Kravrovitc D, et al. Diverticulitis: the effect of age and location on the course of disease. Int J Colorectal Dis. 1999;14:250.
136. Konvolinka CW. Acute diverticulitis under age forty. Am J Surg. 1994;167:562.
137. Pautrat K, Bretagnol F, Huten N, et al. Acute diverticulitis in very young patients: a frequent surgical management. Dis Colon Rectum. 2007;50:472.
138. Chautems RC, Ambrosetti P, Ludwig A, et al. Long-term follow-up after first acute episode of sigmoid diverticulitis: is surgery mandatory? A prospective study of 118 patients. Dis Colon Rectum. 2002;45:962.
139. Spivak H, Weinrauch S, Harvey JC, et al. Acute colonic diverticulitis in the young. Dis Colon Rectum. 1997;40:570.
140. Morris CR, Harvey IM, Stebbings WS, et al. Incidence of perforated diverticulitis and risk factors for death in a UK population. Br J Surg. 2008;95:876.
141. Makela JT, Kiviniemi H, Laitinen S. Prognostic factors of perforated sigmoid diverticulitis in the elderly. Dig Surg. 2005;22:100.
142. Anaya DA, Flum DR. Risk of emergency colectomy and colostomy in patients with diverticular disease. Arch Surg. 2005;140:681.
143. Maggard MA, Zingmond D, O’Connell JB, et al. What proportion of patients with an ostomy (for diverticulitis) get reversed? Am Surg. 2004;70:928.
144. Salem L, Anaya DA, Roberts KE, et al. Hartmann’s colectomy and reversal in clinical diverticulitis: a population-level assessment. Dis Colon Rectum. 2005;48:988.
145. Perkins JD, Shield CFIII, Chang FC, Farha GJ. Acute diverticulitis: comparison of treatment in immunocompromised and nonimmunocompromised patients. Am J Surg. 1984;148:745.
146. Tyau ES, Prystowsky JB, Joehl RJ, Nahrwold DL. Acute diverticulitis: a complicated problem in the immunocompromised patient. Arch Surg. 1991;126:855.
147. Lederman ED, Conti DJ, Lempert N, et al. Complicated diverticulitis following renal transplantation. Dis Colon Rectum. 1998;41:613.
148. Maurer JR. The spectrum of colonic complications in a lung transplant population. Ann Transplant. 2000;5:54.
149. Khan S, Eppstein AC, Anderson GK, et al. Acute diverticulitis in heart and lung transplant patients. Transpl Int. 2001;14:12.
150. Nirula R, Greaney G. Right-sided diverticulitis: A difficult diagnosis. Am Surg. 1997;63:871.
151. Violi V, Roncoroni L, Boselli AS, et al. Diverticulitis of the caecum and ascending colon: an unavoidable diagnostic pitfall? Int Surg. 2000;85:39.
152. Komuta K, Yamanaka S, Okada K, et al. Toward therapeutic guidelines for patients with acute right colonic diverticulitis. Am J Surg. 2004;187:233.
153. Peppercorn MA. Drug-responsive chronic segmental colitis associated with diverticula: a clinical syndrome in the elderly. Am J Gastroenterol. 1992;87:609.
154. Goldstein NS, Leon-Armin C, Mani A. Crohn’s colitis-like changes in sigmoid diverticulitis specimens is usually an idiosyncratic inflammatory response to the diverticulosis rather than Crohn’s colitis. Am J Surg Pathol. 2000;24:668.
155. Freeman HJ. Natural history and long-term clinical behavior of segmental colitis associated with diverticulosis (SCAD syndrome). Dig Dis Sci. 2008;53:2452.
156. Lamps LW, Knapple WL. Diverticular disease-associated segmental colitis. Clin Gastroenterol Hepatol. 2007;5:27.
157. Imperiali G, Terpin MM, Meucci G, et al. Segmental colitis associated with diverticula: a 7-year follow-up study. Endoscopy. 2006;38:610.
158. Makapugay LM, Dean PJ. Diverticular disease-associated chronic colitis. Am J Surg Pathol. 1996;20:94.
159. Boley SJ, DiBiase A, Brandt LJ, Sammartano RJ. Lower intestinal bleeding in the elderly. Am J Surg. 1979;137:57.
160. Potter GD, Sellin JH. Lower gastrointestinal bleeding. Gastroenterol Clin North Am. 1988;17:341.
161. Gostout CJ, Wang KK, Ahlquist DA, et al. Acute gastrointestinal bleeding: experience of a specialized management team. J Clin Gastroenterol. 1992;14:260.
162. Elta GH. Urgent colonoscopy for acute lower GI bleeding. Gastrointest Endosc. 2004;59:402.
163. Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997;92:419.
164. Peura DA, Lanza FL, Gostout CJ, Foutch PG. The American College of Gastroenterology Bleeding Registry: preliminary findings. Am J Gastroenterol. 1997;92:924.
165. McGuire HHJr, Haynes BWJr. Massive hemorrhage for diverticulosis of the colon: guidelines for therapy based on bleeding patterns observed in fifty cases. Ann Surg. 1972;175:847.
166. Zuckerman GR, Prakash C. Acute lower intestinal bleeding: II. Etiology, therapy, and outcomes. Gastrointest Endosc. 1999;49:228.
167. Casarella WJ, Kanter IE, Seaman WB. Right-sided colonic diverticula as a cause of acute rectal hemorrhage. N Engl J Med. 1972;286:450.
168. McGuire HHJr. Bleeding colonic diverticula: a reappraisal of natural history and management. Ann Surg. 1994;220:653.
169. Wong SK, Ho YH, Leong AP, Seow-Choen F. Clinical behavior of complicated right-sided and left-sided diverticulosis. Dis Colon Rectum. 1997;40:344.
170. Yamada A, Sugimoto T, Kondo S, et al. Assessment of the risk factors for colonic diverticular hemorrhage. Dis Colon Rectum. 2008;51:116.
171. Meyers MA, Alonso DR, Gray GF, Baer JW. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976;71:577.
172. Wilcox CM, Alexander LN, Cotsonis GA, Clark WS. Non-steroidal anti-inflammatory drugs are associated with both upper and lower gastrointestinal bleeding. Dig Dis Sci. 1997;42:990.
173. Aldoori WH, Giovannucci EL, Rimm EB, et al. Use of acetaminophen and nonsteroidal anti-inflammatory drugs: a prospective study and the risk of symptomatic diverticular disease in men. Arch Fam Med. 1998;7:255.
174. Goh H, Bourne R. Non-steroidal anti-inflammatory drugs and perforated diverticular disease: A case-control study. Ann R Coll Surg Engl. 2002;84:93.
175. Wilson RG, Smith AN, Macintyre IM. Complications of diverticular disease and non-steroidal anti-inflammatory drugs: a prospective study. Br J Surg. 1990;77:1103.
176. Campbell K, Steele RJ. Non-steroidal anti-inflammatory drugs and complicated diverticular disease: a case-control study. Br J Surg. 1991;78:190.
177. Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288.
178. Zuccaro GJr. Management of the adult patient with acute lower gastrointestinal bleeding: American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1998;93:1202.
179. Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia: the role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569.
180. Foutch PG, Rex DK, Lieberman DA. Diverticular bleeding: are nonsteroidal anti-inflammatory drugs risk factors for hemorrhage and can colonoscopy predict outcome for patients? Am J Gastroenterol. 1995;90:1779.
181. Bertoni G, Conigliaro R, Ricci E, et al. Endoscopic injection hemostasis of colonic diverticular bleeding: a case report. Endoscopy. 1990;22:154.
182. Johnston J, Sones J. Endoscopic heater probe coagulation of the bleeding colonic diverticulum. Gastrointest Endosc. 1986;32:160.
183. Savides TJ, Jensen DM. Colonoscopic hemostasis for recurrent diverticular hemorrhage associated with a visible vessel: a report of three cases. Gastrointest Endosc. 1994;40:70.
184. Foutch PG, Zimmerman K. Diverticular bleeding and the pigmented protuberance (sentinel clot): clinical implications, histopathological correlation, and results of endoscopic intervention. Am J Gastroenterol. 1996;91:2589.
185. Jensen DM, Machicado GA, Jutabha R, Kovacs TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342:78.
186. Hokama A, Uehara T, Nakayoshi T, et al. Utility of endoscopic hemoclipping for colonic diverticular bleeding. Am J Gastroenterol. 1997;92:543.
187. Simpson PW, Nguyen MH, Lim JK, et al. Use of endoclips in the treatment of massive colonic diverticular bleeding. Gastrointest Endosc. 2004;59:433.
188. Yen EF, Ladabaum U, Muthusamy VR, et al. Colonoscopic treatment of acute diverticular hemorrhage using endoclips. Dig Dis Sci. 2008;53:2480.
189. Andress HJ, Mewes A, Lange V. Endoscopic hemostasis of a bleeding diverticulum of the sigma with fibrin sealant. Endoscopy. 1993;25:193.
190. Farrell JJ, Graeme-Cook F, Kelsey PB. Treatment of bleeding colonic diverticula by endoscopic band ligation: An in-vivo and ex-vivo pilot study. Endoscopy. 2003;35:823.
191. Smoot RL, Gostout CJ, Rajan E, et al. Is early colonoscopy after admission for acute diverticular bleeding needed? Am J Gastroenterol. 2003;98:1996.
192. Green BT, Rockey DC, Portwood G, et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: A randomized controlled trial. Am J Gastroenterol. 2005;100:2395.
193. Yamaguchi T, Yoshikawa K. Enhanced CT for initial localization of active lower gastrointestinal bleeding. Abdom Imaging. 2003;28:634.
194. O’Neill BB, Gosnell JE, Lull RJ, et al. Cinematic nuclear scintigraphy reliably directs surgical intervention for patients with gastrointestinal bleeding. Arch Surg. 2000;135:1076.
195. Hunter JM, Pezim ME. Limited value of technetium 99m–labeled red cell scintigraphy in localization of lower gastrointestinal bleeding. Am J Surg. 1990;159:504.
196. Kan JH, Funaki B, O’Rourke BD, et al. Delayed 99mTc-labeled erythrocyte scintigraphy in patients with lower gastrointestinal tract hemorrhage: effect of positive findings on clinical management. Acad Radiol. 2003;10:497.
197. Alavi A, Ring EJ. Localization of gastrointestinal bleeding: superiority of 99mTc sulfur colloid compared with angiography. AJR Am J Roentgenol. 1981;137:741.
198. DeBarros J, Rosas L, Cohen J, et al. The changing paradigm for the treatment of colonic hemorrhage: Superselective angiographic embolization. Dis Colon Rectum. 2002;45:802.
199. Gordon RL, Ahl KL, Kerlan RK, et al. Selective arterial embolization for the control of lower gastrointestinal bleeding. Am J Surg. 1997;174:24.
200. Gady JS, Reynolds H, Blum A. Selective arterial embolization for control of lower gastrointestinal bleeding: recommendations for a clinical management pathway. Curr Surg. 2003;60:344.
201. Khanna A, Ognibene SJ, Koniaris LG. Embolization as first-line therapy for diverticulosis-related massive lower gastrointestinal bleeding: evidence from a meta-analysis. J Gastrointest Surg. 2005;9:343.
202. Browder W, Cerise EJ, Litwin MS. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204:530.
203. Cohn SM, Moller BA, Zieg PM, et al. Angiography for preoperative evaluation in patients with lower gastrointestinal bleeding: are the benefits worth the risks? Arch Surg. 1998;133:50.
204. Setya V, Singer JA, Minken SL. Subtotal colectomy as a last resort for unrelenting, unlocalized, lower gastrointestinal hemorrhage: Experience with 12 cases. Am Surg. 1992;58:295.
205. Farner R, Lichliter W, Kuhn J, et al. Total colectomy versus limited colonic resection for acute lower intestinal bleeding. Am J Surg. 1999;178:587.
206. Renzulli P, Maurer CA, Netzer P, et al. Subtotal colectomy with primary ileorectoscopy is effective for unlocalized, diverticular hemorrhage. Lagenbeck’s Arch Surg. 2002;387:67.