Diuretic agents
After reading this chapter, the reader will be able to:
1. Define terms pertaining to diuretic agents
2. Describe renal function, filtration, reabsorption, and acid-base balance
3. List and describe the various groups of diuretics
4. List some indications for diuretic therapy
5. List the most common adverse effects associated with the use of diuretics
Congestive heart failure (CHF)
Failure of the heart to pump the blood adequately, resulting in lung congestion and tissular edema.
Drug that increases urine output.
Swelling resulting from abnormal accumulation of fluid in intercellular spaces of the body.
Mechanism by which hydrostatic pressure forces fluid out of the glomerular capillaries and into the renal ducts.
Abnormally decreased volume of blood circulating in the body.
Renal lithiasis in which calcium deposits form in the renal parenchyma, resulting in reduced kidney function and the presence of blood in the urine.
Microscopic functional unit of the kidney, responsible for filtering and maintaining fluid balance; each kidney has approximately 2 million nephrons.
Damage to the ear, specifically the cochlea or auditory nerve and sometimes the vestibulum, by a toxin.
Return to the blood of most of the water, sodium, amino acids, and sugar that were removed during filtration; occurs mainly in the proximal tubule of the nephron.
Effect of two chemicals on an organism is greater than effect of either chemical individually.
Amount of urine produced in 24 hours; normal urine output averages 30 to 60 mL/hr.
The main purpose of diuretics, or agents that increase urine output, is to eliminate excess fluid from the body. Introduced into medicine in 1958, diuretics are drugs that increase the excretion of solutes and water by directly increasing urine output. Generally, the primary goal of diuretic therapy is to reduce extracellular fluid volume to decrease blood pressure or to rid the body of excess interstitial fluid. Chapter 19 summarizes the essentials of the clinical pharmacology of diuretics, briefly reviewing renal function with an emphasis on acid-base balance. The major groups of diuretics, their modes of action, and common interactions and side effects are summarized. These groups include osmotic diuretics, carbonic anhydrase inhibitors, thiazides, loop diuretics, and potassium-sparing agents.
Renal structure and function
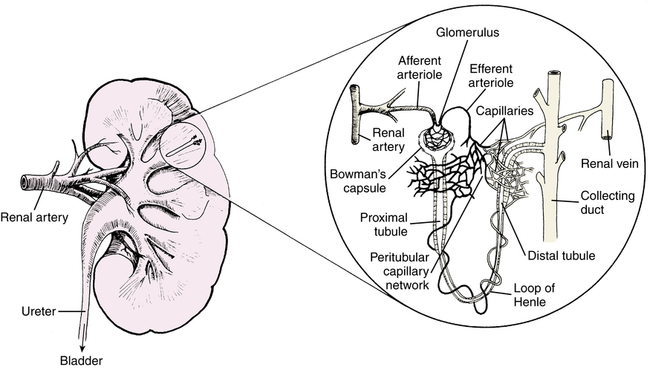
Figure 19-1 illustrates the kidney and a nephron, which is the functional unit of the kidney, similar to the alveolus in the lung. The nephron is composed of the glomerulus, proximal tubule, loop of Henle, distal tubule, and collecting duct. Almost 75% of the almost 1 million nephrons may need to be compromised before renal disease is apparent. The renal artery branches into the afferent arteriole, which enters and forms the capillary tuft of the glomerulus. This blood flow leaves in the efferent arteriole, which forms the capillary network around the tubules and loop of Henle. This capillary network rejoins to form the renal vein.
Electrolyte filtration and reabsorption
The ions listed in Box 19-1 are filtered and exchanged in the tubules.
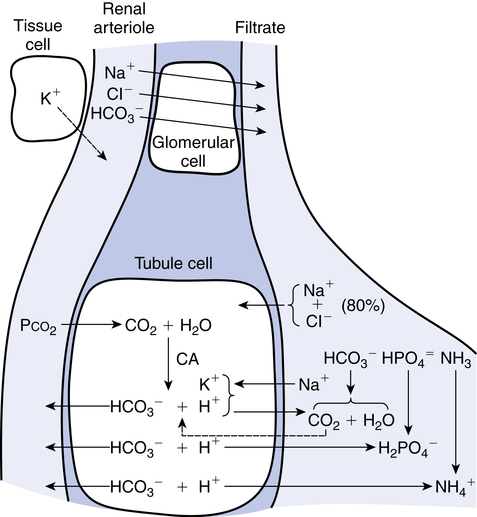
• Sodium: About 70% of Na+ in the filtrate is reabsorbed in the proximal tubules; 20%, in the loops of Henle; and about 10%, in the distal tubules. There is an exchange of Na+ for H+ or K+ in the distal tubules.
• Potassium: Most filtered K+ is reabsorbed in the proximal tubules. K+ found in the urine is that secreted by the distal tubule.
• Chloride and bicarbonate: Cl− and HCO3− are passively reabsorbed in the proximal and distal tubules.
Acid-base balance
Because a fundamental function of the kidney is the control of buffering substances, especially HCO3−, diuretics may cause acid-base imbalances to occur as they increase water loss. Figure 19-2 illustrates the hydrogen and bicarbonate pathways that regulate pH. The filtration and reabsorption of Na+, Cl−, and HCO3−, described previously, can be seen in Figure 19-2.
< ?xml:namespace prefix = "mml" />
Diuretic groups
The primary therapeutic goal of diuretic use is to reduce the ECFV. NaCl output must exceed NaCl intake. Diuretics primarily prevent Na+ entry into the tubule cell. Diuretics need to access the tubule fluid to exert their action. Once in the tubule fluid, the nephron site at which the diuretic acts determines its effect. The site of action also determines which electrolytes, other than Na+, are affected. All diuretics except spironolactone exert their effects from the luminal side of the nephron.1
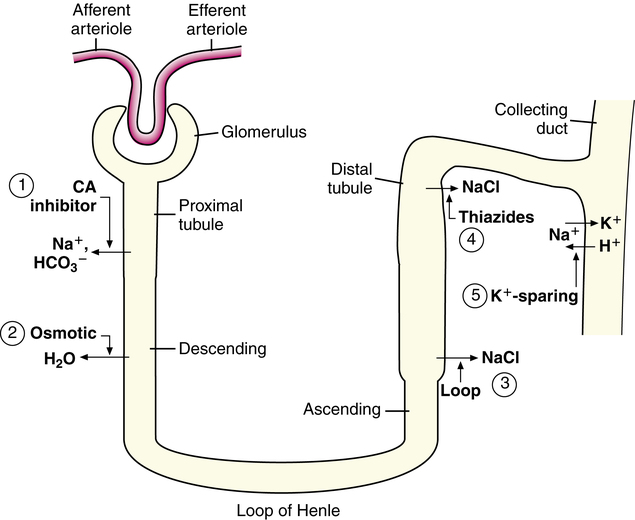
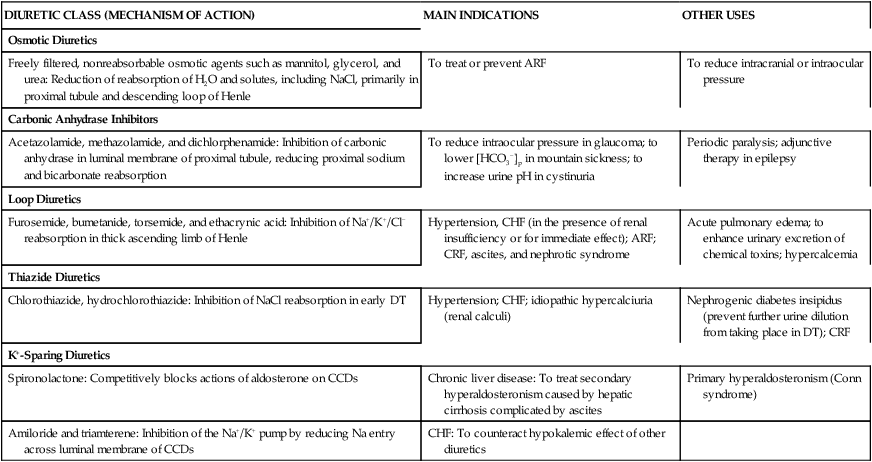
Five major groups of diuretics are described in this chapter. Figure 19-3 illustrates the site of action, and Table 19-1 summarizes the mechanism of action and the indications for use of each of the five major groups of diuretics.2–5
TABLE 19-1
Site and Mechanism of Action, Main Indications, and Other Uses of Diuretics
| DIURETIC CLASS (MECHANISM OF ACTION) | MAIN INDICATIONS | OTHER USES |
| Osmotic Diuretics | ||
| Freely filtered, nonreabsorbable osmotic agents such as mannitol, glycerol, and urea: Reduction of reabsorption of H2O and solutes, including NaCl, primarily in proximal tubule and descending loop of Henle | To treat or prevent ARF | To reduce intracranial or intraocular pressure |
| Carbonic Anhydrase Inhibitors | ||
| Acetazolamide, methazolamide, and dichlorphenamide: Inhibition of carbonic anhydrase in luminal membrane of proximal tubule, reducing proximal sodium and bicarbonate reabsorption | To reduce intraocular pressure in glaucoma; to lower [HCO3−]p in mountain sickness; to increase urine pH in cystinuria | Periodic paralysis; adjunctive therapy in epilepsy |
| Loop Diuretics | ||
| Furosemide, bumetanide, torsemide, and ethacrynic acid: Inhibition of Na+/K+/Cl− reabsorption in thick ascending limb of Henle | Hypertension, CHF (in the presence of renal insufficiency or for immediate effect); ARF; CRF, ascites, and nephrotic syndrome | Acute pulmonary edema; to enhance urinary excretion of chemical toxins; hypercalcemia |
| Thiazide Diuretics | ||
| Chlorothiazide, hydrochlorothiazide: Inhibition of NaCl reabsorption in early DT | Hypertension; CHF; idiopathic hypercalciuria (renal calculi) | Nephrogenic diabetes insipidus (prevent further urine dilution from taking place in DT); CRF |
| K+-Sparing Diuretics | ||
| Spironolactone: Competitively blocks actions of aldosterone on CCDs | Chronic liver disease: To treat secondary hyperaldosteronism caused by hepatic cirrhosis complicated by ascites | Primary hyperaldosteronism (Conn syndrome) |
| Amiloride and triamterene: Inhibition of the Na+/K+ pump by reducing Na entry across luminal membrane of CCDs | CHF: To counteract hypokalemic effect of other diuretics |
Because hypertension affects one-third of adults in the United States,6 the diuretics of most immediate relevance to respiratory and critical care clinicians are those used to treat hypertension and congestive heart failure (CHF). There is evidence that diuretic-based therapy is effective in reducing morbidity and mortality among elderly hypertensive patients.7–10 Diuretics are also used to aid in the treatment of other conditions associated with fluid retention, such as corticosteroid therapy and certain renal and liver diseases.
Osmotic diuretics
Osmotic diuretics (Table 19-2) are freely filtered at the glomerulus but are not reabsorbed. These agents remain in the tubule lumen and impair the ability of the proximal tubule and thick ascending limb of Henle to reabsorb NaCl. The net result is that osmotic substances are potent diuretics that lead to increased excretion of water and NaCl. The resultant increased delivery of sodium and chloride to the distal tubule results in increased exchange of Na+ for K+, producing a net potassium loss in urine.
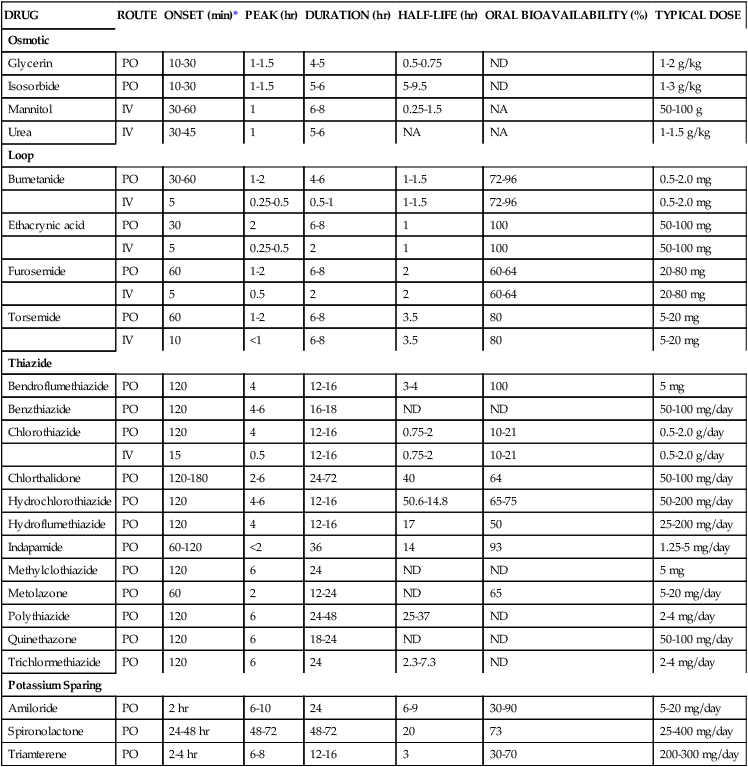
TABLE 19-2
| DRUG | ROUTE | ONSET (min)* | PEAK (hr) | DURATION (hr) | HALF-LIFE (hr) | ORAL BIOAVAILABILITY (%) | TYPICAL DOSE |
| Osmotic | |||||||
| Glycerin | PO | 10-30 | 1-1.5 | 4-5 | 0.5-0.75 | ND | 1-2 g/kg |
| Isosorbide | PO | 10-30 | 1-1.5 | 5-6 | 5-9.5 | ND | 1-3 g/kg |
| Mannitol | IV | 30-60 | 1 | 6-8 | 0.25-1.5 | NA | 50-100 g |
| Urea | IV | 30-45 | 1 | 5-6 | NA | NA | 1-1.5 g/kg |
| Loop | |||||||
| Bumetanide | PO | 30-60 | 1-2 | 4-6 | 1-1.5 | 72-96 | 0.5-2.0 mg |
| IV | 5 | 0.25-0.5 | 0.5-1 | 1-1.5 | 72-96 | 0.5-2.0 mg | |
| Ethacrynic acid | PO | 30 | 2 | 6-8 | 1 | 100 | 50-100 mg |
| IV | 5 | 0.25-0.5 | 2 | 1 | 100 | 50-100 mg | |
| Furosemide | PO | 60 | 1-2 | 6-8 | 2 | 60-64 | 20-80 mg |
| IV | 5 | 0.5 | 2 | 2 | 60-64 | 20-80 mg | |
| Torsemide | PO | 60 | 1-2 | 6-8 | 3.5 | 80 | 5-20 mg |
| IV | 10 | <1 | 6-8 | 3.5 | 80 | 5-20 mg | |
| Thiazide | |||||||
| Bendroflumethiazide | PO | 120 | 4 | 12-16 | 3-4 | 100 | 5 mg |
| Benzthiazide | PO | 120 | 4-6 | 16-18 | ND | ND | 50-100 mg/day |
| Chlorothiazide | PO | 120 | 4 | 12-16 | 0.75-2 | 10-21 | 0.5-2.0 g/day |
| IV | 15 | 0.5 | 12-16 | 0.75-2 | 10-21 | 0.5-2.0 g/day | |
| Chlorthalidone | PO | 120-180 | 2-6 | 24-72 | 40 | 64 | 50-100 mg/day |
| Hydrochlorothiazide | PO | 120 | 4-6 | 12-16 | 50.6-14.8 | 65-75 | 50-200 mg/day |
| Hydroflumethiazide | PO | 120 | 4 | 12-16 | 17 | 50 | 25-200 mg/day |
| Indapamide | PO | 60-120 | <2 | 36 | 14 | 93 | 1.25-5 mg/day |
| Methylclothiazide | PO | 120 | 6 | 24 | ND | ND | 5 mg |
| Metolazone | PO | 60 | 2 | 12-24 | ND | 65 | 5-20 mg/day |
| Polythiazide | PO | 120 | 6 | 24-48 | 25-37 | ND | 2-4 mg/day |
| Quinethazone | PO | 120 | 6 | 18-24 | ND | ND | 50-100 mg/day |
| Trichlormethiazide | PO | 120 | 6 | 24 | 2.3-7.3 | ND | 2-4 mg/day |
| Potassium Sparing | |||||||
| Amiloride | PO | 2 hr | 6-10 | 24 | 6-9 | 30-90 | 5-20 mg/day |
| Spironolactone | PO | 24-48 hr | 48-72 | 48-72 | 20 | 73 | 25-400 mg/day |
| Triamterene | PO | 2-4 hr | 6-8 | 12-16 | 3 | 30-70 | 200-300 mg/day |
Carbonic anhydrase inhibitors
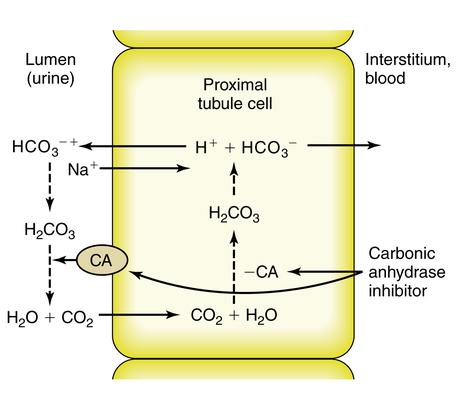
CAIs also inhibit NaCl reabsorption at the proximal tubule. The decreased osmotic gradient for water reabsorption results in increased delivery of NaHCO3, NaCl, and water from the proximal tubule (Figure 19-4). Much of the NaCl is reabsorbed in the thick ascending loop of Henle. The net result is a moderate increase in sodium and bicarbonate in the urine along with increased water excretion. The potential for metabolic acidosis coupled with their weak diuretic properties limit the use of CAIs as the first-line treatment for patients who require more aggressive management of their hypervolemic status.
Loop diuretics
Loop diuretics (see Table 19-2) are often called “high ceiling” diuretics because they can cause up to 20% of the filtered load of NaCl and water to be excreted in the urine. They inhibit the reabsorption of NaCl at the thick ascending limb of Henle, where about 20% of filtered NaCl is usually reabsorbed.11 Use of loop diuretics leads to increased Na+, K+, Cl−, and water excretion.
When administered intravenously, loop diuretics produce an acute hemodynamic effect independent of their diuretic properties.12 Within 5 minutes of the administration of intravenous loop diuretics to cardiac patients, an acute vasodilatory effect is observed. This effect is manifested by a decrease in pulmonary capillary wedge pressure, blood pressure, and systemic vascular resistance. The effect seems to be derived from the renal release of vasodilating prostaglandins.13,14
The acute hemodynamic effect has also been reported to activate the sympathetic nervous system, resulting in an adverse hemodynamic profile characterized by increased afterload and diminished cardiac function before the onset of diuresis.15 This effect is also short-lived and dissipates with the onset of diuresis. Because the diuretic effect may last several hours, several doses per day may be required to maintain a net diuretic effect for 24 hours. Patients requiring frequent bolus doses may benefit from continuous infusion.
Administration of loop diuretics to patients with renal dysfunction results in less total drug reaching the site of action within the nephron, and the administration of larger doses is required to achieve a therapeutic effect.16–18 In these patients, differences exist among the effects of furosemide, bumetanide, and torsemide. Furosemide may have a more prolonged effect in patients with renal dysfunction. However, patients may be resistant to furosemide compared with bumetanide. Because loop diuretics are the most potent diuretics, they are effective at very low creatinine clearance levels (a low creatinine clearance level indicates kidney disease). Loop diuretics as single agents should be considered as first-line therapy in patients with creatinine clearance values less than 40 mL/min. If this dose is inadequate to produce diuresis within 20 minutes, the dose can be doubled every 20 minutes until a response occurs or until a maximum dose is reached. Various studies have reported a ceiling effect to furosemide of approximately 250 mg.19,20 Increasing the dose above this ceiling dose may not produce an increased response.
Although patients with renal dysfunction require larger doses to deliver diuretics into the urine, the remaining nephrons in these patients continue to function normally. Overall, sodium excretion may be limited as a result of diminished sodium filtration. To overcome this relative resistance, an effective response may occur by administering a large enough effective dose several times a day. Certain disease states result in a diminished response that does not improve by administering larger doses. Although the mechanism for this effect is unknown, it has been reported in patients with congestive heart failure, cirrhosis, or nephrotic syndrome.21 In these patients, multiple doses should be given rather than larger single doses. This finding implies a modest ceiling dose of loop diuretics in patients with CHF and cirrhosis.
Thiazide diuretics
Thiazide diuretics (see Table 19-2) block NaCl reabsorption at the distal tubule.21,22 Thiazide diuretics are of moderate potency because only about 5% to 10% of filtered NaCl is reabsorbed in the distal tubule. However, they are considered the first line of therapy for mild hypertension. Thiazide diuretics are effective to a creatinine clearance of approximately 30 mL/min. Thiazide diuretics have a limited dose-response curve compared with loop diuretics. This limited dose-response curve results in a narrow difference between maximal and minimal effective doses. Doses greater than 50 mg may not produce greater diuresis, but they may predispose the patient to increased toxicity.
Doses greater than 50 mg may, however, be useful in the treatment of hypertension. The use of thiazide diuretics in the treatment of hypertension produces an effect initially as a result of diuresis-induced decreases in blood volume.2,21
Long-term benefits of thiazide diuretics in hypertension are most likely not due to a diuretic response. One proposed mechanism is decreased peripheral vascular resistance.23 Although the exact mechanism of action is unknown, as mentioned, hypertensive patients may respond to thiazide diuretic doses greater than 50 mg/day.
Potassium-sparing diuretics
Potassium-sparing diuretics include spironolactone, amiloride, and triamterene (see Table 19-2). These agents are weak diuretics that block sodium reabsorption by slightly different mechanisms of action. Amiloride and triamterene block the Na+ channels in the luminal membrane of the principal cells of the cortical collecting ducts, whereas spironolactone is a competitive aldosterone antagonist at the cytosolic receptor level. On the basis of its mechanism of action, spironolactone is specifically used for conditions known to have elevated aldosterone concentrations, such as hyperaldosteronism (primary and secondary), cirrhosis and ascites, adrenal hyperplasia, and renal artery stenosis. The most common use is in patients with cirrhosis and ascites. Because the duration of effect of spironolactone is 1 or more days, the dose should be increased every 3 or 4 days until the desired level of diuresis is attained.24
In the distal tubule, sodium is typically exchanged for potassium and hydrogen. Blocking this exchange is what makes these agents potassium-sparing diuretics. Although frequently used in combination with thiazide diuretics to produce better diuresis and to diminish potassium loss, the rationale for this is controversial. Only about 5% of patients receiving thiazide diuretics become potassium depleted.21 In addition, potassium-sparing agents may produce hyperkalemia, which is a more life-threatening situation than potassium depletion.
Triamterene is a short-acting agent requiring multiple doses per day. Triamterene must be converted to an active metabolite by the liver, and this agent may be a poor choice in patients with liver dysfunction.25
Diuretic combinations
Various diuretic combinations may be used in an attempt to obtain additive or synergistic effects in patients who respond poorly to one agent. By using agents with different sites of action within the nephron, the diuretic response may be enhanced. The most common combination is of a loop diuretic and a thiazide.26 Although not consistently effective, combinations occasionally may result in pronounced diuresis.27
Drug interactions
Because diuretics are commonly prescribed in combination with other medications, knowledge of drug interaction plays an important role in the selection of the diuretic agent. Clinicians who prescribe diuretics need to be informed of associated comorbidities, such as diabetes, renal disease, hepatic disease, or gout. Table 19-3 summarizes some of the most common drug interaction side effects associated with diuretic agents.
TABLE 19-3
Drug Interactions and Their Potential Side Effects Associated with Use of Diuretics
| INTERACTING DRUG | POTENTIAL SIDE EFFECT |
| Angiotensin-converting enzyme inhibitors AND K+-sparing diuretics | Hyperkalemia and cardiac irritability |
| Aminoglycosides AND loop diuretics | Ototoxicity and nephrotoxicity |
| Digoxin AND thiazide and loop diuretics | Hypokalemia |
| β blockers AND thiazide diuretics | Hyperglycemia, hyperlipidemia, hyperuricemia |
| Steroids AND thiazide and loop diuretics | Increased risk of hypokalemia |
| Carbamazepine or chlorpropamide AND thiazide diuretics | Increased risk of hyponatremia |
Adverse effects
Although diuretics have been used successfully for more than 40 years, they have the potential to cause adverse effects (Table 19-4). Most complications associated with diuretic use can be anticipated as an extension of their pharmacologic activity, with hypovolemia and electrolyte and acid-base abnormalities being the most common. Rare side effects that need immediate medical attention include the following:
TABLE 19-4
Common Side Effects of Diuretic Therapy
| DRUG | EFFECT |
| Osmotic diuretics | Acute expansion of ECFV and increased risk of pulmonary edema |
| Acute hyperkalemia | |
| Nausea and vomiting; headache | |
| Loop diuretics | Depletions: Hypokalemia; hypomagnesemia; hyponatremia; hypovolemia |
| Retention: Hyperuricemia | |
| Metabolic: Hyperglycemia (insulin resistance) | |
| Metabolic alkalosis (partly secondary to ECFV reduction) | |
| Ototoxicity and diarrhea (mainly with ethacrynic acid) | |
| Thiazide diuretics | Depletions: Hypokalemia, hyponatremia, hypovolemia |
| Retentions: Hyperuricemia secondary to enhanced urate reabsorption; hypercalcemia secondary to enhanced Ca2+ reabsorption | |
| Metabolic alkalosis (hypochloremia) | |
| Metabolic: Hyperglycemia (insulin resistance), hyperlipidemia | |
| Hypersensitivity (fever, rash, purpura, anaphylaxis) | |
| Interstitial nephritis | |
| K+-sparing diuretics | Spironolactone: Hyperkalemia, gynecomastia, hirsutism, menstrual irregularities, testicular atrophy (with prolonged use) |
| Amiloride: Hyperkalemia, glucose intolerance in diabetic patients | |
| Triamterene: Hyperkalemia; megaloblastic anemia in patients with liver cirrhosis | |
| Carbonic anhydrase inhibitors | Metabolic acidosis (secondary to HCO3− depletion) |
| Drowsiness, fatigue, CNS depression, paresthesia |
CNS, Central nervous system; ECFV, extracellular fluid volume.
• Blood in the urine or stools
• Painful or difficult urination
• Pinpoint red spots on the skin
• Ringing or buzzing in the ears
• Severe stomach pain with nausea and vomiting
Other adverse effects are even rarer or idiosyncratic and cannot be anticipated or prevented. There is a particular concern with the suggested association between long-term diuretic therapy and the risk of developing renal cell carcinoma.28
Hypovolemia
Because diuretics promote sodium and fluid excretion, elimination may exceed intake, resulting in hypovolemia. Hypovolemia should be suspected if dizziness, extreme thirst, excessive dryness of the mouth, decreased urine output, dark-colored urine, or constipation is observed. Certain situations may predispose a patient to hypovolemia (Box 19-2). Diuretic-induced hypovolemia should be treated by discontinuation of the diuretic. Mild cases of hypovolemia may respond to liberalization of sodium intake, whereas more severe cases require intravenous volume replacement.
Hypokalemia
Preserving potassium balance has emerged as one of the most important factors in the management of hypertension.29 Potassium is exchanged for sodium in the distal convoluted tubule and collecting duct. Any diuretic that increases sodium delivery to these regions may potentially induce hypokalemia. In addition to a direct potassium loss, diuretic-induced volume depletion produces reabsorption of sodium via release of aldosterone in the distal tubule in an effort to bolster intravascular volume. This additional sodium reabsorption also contributes to potassium excretion. Dietary sodium intake and chloride depletion may also influence potassium excretion.
Diuretic-induced hypokalemia apparently is dose-related, with loop diuretics having a lower incidence than thiazide diuretics.30 Although studies have tried to identify the incidence of diuretic-induced hypokalemia, it is impossible to predict whether a particular patient will develop hypokalemia.31–33 The issue of potassium supplementation is also controversial. Who to treat, when to treat, and how to treat hypokalemia all are unresolved questions. At the center of this unresolved issue is whether hypokalemia poses a risk for arrhythmias or sudden cardiac death. Supplemental potassium should be considered in patients with a history of cardiac disease, patients with symptoms indicating hypokalemia, patients with a serum potassium level less than 3.0 mEq/L, and patients receiving digitalis therapy.34 Potassium-sparing diuretics may induce a hyperkalemic state in 8.6% of patients receiving spironolactone and in 23% of patients receiving a potassium-sparing diuretic and potassium supplementation.33,35
Glucose changes
Loop and thiazide diuretics have been associated with hyperglycemia. The average increase in serum glucose is 6.5 to 9.6 mg/dL, although cases of diabetic ketoacidosis have also been reported.36,37 The severity of glucose elevation in these reports was related to the dose of diuretic used and to the decrease in potassium levels. Although the cause of hyperglycemia is not completely understood, several possible etiologies have been postulated, including decreased pancreatic insulin release and insulin resistance with impaired uptake of glucose in response to insulin.
Ototoxicity
Loop diuretics may cause a dose-related ototoxicity consisting of tinnitus and clinical or subclinical hearing loss. Ototoxicity results from anatomic and chemical abnormalities produced within the inner ear.34 Ototoxicity is related to the blood level of these agents. Rapid infusion and drug accumulation with large parenteral doses in renal failure both predispose patients to ototoxicity. Reducing the infusion rate or administering the drug orally may alleviate the hearing loss.38,39 Most ototoxicity is reversible; however, cases of irreversible hearing loss have occurred. Ethacrynic acid has a higher likelihood of causing irreversible hearing loss.39,40 Limited data on bumetanide indicate that it may have a lower incidence of ototoxicity than furosemide and ethacrynic acid.
Special situations
Pregnancy, lactation, and children
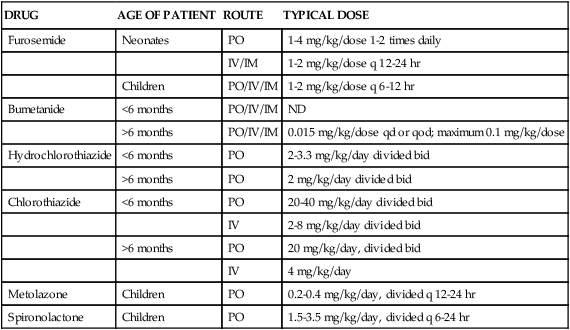
Children can safely take diuretics because the side effects are similar to the side effects in adults. However, they may require smaller doses of the drug (Table 19-5). Furosemide is one of the most effective and least toxic diuretics used in pediatric practice. However, long-term use of loop diuretics in children should be carefully evaluated because of the risk of nephrocalcinosis and potential decrease in bone mass density.41,42
TABLE 19-5
Pediatric Dosages of Commonly Prescribed Diuretics
| DRUG | AGE OF PATIENT | ROUTE | TYPICAL DOSE |
| Furosemide | Neonates | PO | 1-4 mg/kg/dose 1-2 times daily |
| IV/IM | 1-2 mg/kg/dose q 12-24 hr | ||
| Children | PO/IV/IM | 1-2 mg/kg/dose q 6-12 hr | |
| Bumetanide | <6 months | PO/IV/IM | ND |
| >6 months | PO/IV/IM | 0.015 mg/kg/dose qd or qod; maximum 0.1 mg/kg/dose | |
| Hydrochlorothiazide | <6 months | PO | 2-3.3 mg/kg/day divided bid |
| >6 months | PO | 2 mg/kg/day divided bid | |
| Chlorothiazide | <6 months | PO | 20-40 mg/kg/day divided bid |
| IV | 2-8 mg/kg/day divided bid | ||
| >6 months | PO | 20 mg/kg/day, divided bid | |
| IV | 4 mg/kg/day | ||
| Metolazone | Children | PO | 0.2-0.4 mg/kg/day, divided q 12-24 hr |
| Spironolactone | Children | PO | 1.5-3.5 mg/kg/day, divided q 6-24 hr |
IM, Intramuscular; IV, intravenous; N/D, no data; PO, oral.
Modified from Bestic M, Reed M: Pharmacology review: common diuretics used in the preterm and term infant, Neoreviews 6:392, 2005.
Acute respiratory distress syndrome
A pathophysiologic landmark of acute respiratory distress syndrome (ARDS) is the presence of noncardiogenic pulmonary edema. The inflammatory process associated with ARDS explains the increase of the endothelial permeability that causes intravascular water leakage into the interstitial and alveolar spaces. Although balancing the risks of increased pulmonary edema versus the risks of decreased vital organ perfusion has proven to be a difficult task for clinicians, a reduction in pulmonary capillary wedge pressure (PCWP) has been associated with increased survival in ARDS patients.43 The ARDS Network published the results of the Fluid And Catheter Treatment Trial (FACTT),44 in which patients who were not in shock and who were managed with a protocolized fluid management plus furosemide (conservative fluid management arm) had significantly more ventilator-free days, more ICU-free days, and lower mortality than those in the liberal fluid management arm.
Chronic lung disease in preterm infants
Lung disease in premature infants is complicated by the presence of pulmonary edema. A more recent meta-analysis concluded that in preterm infants older than 3 weeks diagnosed with chronic lung disease (CLD), administration of a single dose of aerosolized furosemide may transiently improve pulmonary mechanics. However, routine or sustained use of aerosolized loop diuretics in infants with (or developing) CLD cannot be recommended based on existing evidence.45