CHAPTER 236 Distal Entrapment Syndromes
Carpal Tunnel, Cubital Tunnel, Peroneal, and Tarsal Tunnel
An entrapment neuropathy is defined as a pressure or pressure-induced injury to a segment of a peripheral nerve secondary to anatomic or pathologic structures. It may be due to a whole host of causes. Some patients have a predilection for entrapment neuropathies related to congenital narrowing of the nerve’s osseous tunnel or thickening of an overlying retinaculum. Inflammation or edema of adjacent structures, such as tendons, may reduce the size of the passageway for the nerve, and mechanical forces on the nerve can result in nerve compression. The effect of nerve compression is mediated by ischemia and edema; compression of the nerve results in disruption of the blood-nerve barrier and dysfunction of the intraneural circulation. In the early stages of compression, morphometric changes may not be seen, but as the ischemia persists, segmental demyelination occurs. At this point the injury is often reversible with treatment. Prolonged compression results in edema, which may result in epineurial fibrosis and further thickening of the nerve. Damage to the myelin sheath and axonal disruption are end stages of chronic compression that result in irreversible nerve damage.1–3 Not all nerve fibers are equally susceptible to pressure; larger fibers are more susceptible than small fibers, and fascicular location within the nerve may also affect vulnerability, depending on the force vectors applied.2,4,5
Median Nerve
Carpal tunnel decompression is the most common operation for peripheral nerves, with more than 366,000 procedures performed in 1996 alone.6 The prevalence of electrophysiologically confirmed, symptomatic carpal tunnel syndrome (CTS) is approximately 3% in women and 2% in men, with a peak prevalence in women older than 55 years.7 Studies have shown prevalence estimates as high as 16% in the general population.8
The earliest description of CTS was by Sir James Paget in 1854, and in 1880, Putnam published a series of 30 patients with pain and paresthesias in the median nerve distribution in the hand, as well as nocturnal numbness and pain that could be relieved with vigorous shaking of the affected hand.9,10 It was not until 1933 that the first report was published on the surgical treatment of CTS.9,11 Since then, much progress has been made in the diagnosis and treatment of this disorder.
The underlying pathologic process of CTS is thought to be increased pressure within the carpal tunnel. Any condition that results in decreased space in the carpal tunnel or increased volume of structures contained within it may result in CTS. When the pressure within the tunnel exceeds perfusion pressure into the nerve, adequate circulation and nutrition to the nerve fibers are compromised.12 At 30 mm Hg, axonal transport is impaired, and between 30 and 40 mm Hg, paresthesias and neurophysiologic changes are seen.13 Axonal block can be seen at 50 mm Hg, and at 60 mm Hg, complete intraneural ischemia occurs, with resultant sensory and motor block.14 The median nerve is thought to respond to these effects over time with endoneurial edema, demyelination, distal axonal degeneration, and fibrosis, with intervening periods of regrowth of axons and remyelination.
Anatomy
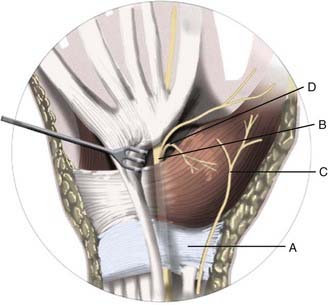
The carpal tunnel is a fibro-osseous passageway in the anterior aspect of the wrist that is formed by the carpal bones and flexor retinaculum, also known as the transverse carpal ligament (TCL) (Fig. 236-1). The floor of the carpal tunnel is composed of the volar radiocarpal ligament and other bridging ligaments interconnecting the pisiform and hook of the hamate medially and the tubercles of the scaphoid and trapezium laterally. The TCL forms the roof by attaching medially to the pisiform and hook of the hamate and laterally to the scaphoid tuberosity and crest of the trapezium.15,16 The carpal tunnel is approximately 4 to 6 cm in length and contains the median nerve and its vascular bundle, four tendons each of the flexor digitorum superficialis (FDS) and profundus (FDP) muscles, and the tendon of the flexor pollicis longus muscle. A persistent median artery or anomalous muscles and tendons may also run in the carpal tunnel.17 The TCL is approximately 3 to 4 cm in width and 2.5 to 3.5 mm in thickness. The ulnar nerve (UN) and ulnar artery run superficially on the ulnar side of the TCL, and the tendon of the flexor carpi radialis is enveloped by two layers of the TCL on the radial side of the wrist.18
The median nerve arises from contributions of the lateral and medial cords and travels with the brachial artery and vein in the medial part of the arm. The median nerve courses through the cubital fossa and passes between the two heads of the pronator teres to enter the forearm. On a linear path through the forearm, the nerve travels on the deep surface of the FDS. The median palmar cutaneous nerve arises from the radial side of the median nerve approximately 5 cm proximal to the TCL and travels superficial to the carpal tunnel to provide sensory innervation to the thenar eminence. Immediately proximal to the carpal tunnel, the median nerve runs superficial to the FDS and deep to the palmaris longus tendon, and within the carpal tunnel, the median nerve runs parallel to the second and third FDS tendons.18,19 Most often, the recurrent motor branch arises from the median nerve distal to the TCL, but anatomic variations do exist. Lanz classified these variations into three main groups: extraligamentous (50%), subligamentous (30%), and transligamentous (20%).20 The recurrent motor branch provides innervation to the opponens pollicis, abductor pollicis brevis, and flexor pollicis brevis muscles. An important anatomic variant is the Riche-Cannieu anastomosis, in which innervation of thenar muscles is provided by the UN.21
The median nerve then divides into multiple palmar digital branches that provide motor innervation to the lateral two lumbricals and sensory innervation to the palmar surfaces and the distal dorsal portions of the thumb, index finger, middle finger, and lateral half of the ring finger.15,16 The median nerve is vulnerable to compression at two particular sites in the carpal tunnel. The first site is the proximal edge of the TCL and the second is adjacent to the hook of the hamate.22
Clinical Findings
Patients with CTS most often complain of pain and paresthesias in the median nerve distribution in the hand, particularly after strenuous wrist movements or at nighttime. The pain is often described as burning and may radiate proximally. Nocturnal occurrence of pain has been postulated by Sunderland to occur because of venous stasis within the upper extremity related to hypotonia during sleep.12 Frequently, the patient will describe improvement of symptoms by shaking the affected hand, which may support the venous stasis theory.
Important questions for the patient include the duration, quality, and severity of symptoms, as well as aggravating/relieving activities. Certain conditions should be considered during the patient interview (Table 236-1): pregnancy (particularly the third trimester), renal failure/dialysis, rheumatoid arthritis, hypothyroidism, acromegaly, and amyloidosis. A thorough past medical and surgical history must be taken, including medical comorbid conditions and a history of trauma or fractures of the upper extremity. The social history should include questions about repetitive wrist motion with work or hobbies, but no conclusive evidence correlates repetitive stress with the development of CTS. Certain sports are associated with the development of CTS. Wheelchair athletes, archers, bicyclers, bodybuilders, football players, golfers, and wrestlers are prone to CTS.23 Screening questions for alcoholism should also be asked. The patient’s family history should be obtained as well. Familial CTS often occurs through an autosomal dominant inheritance pattern, and other conditions associated with CTS include mucopolysaccharidoses, mucolipidoses, and hereditary susceptibility to pressure palsy.
TABLE 236-1 Common Conditions Associated with Carpal Tunnel Syndrome
| Metabolic/endocrine | Diabetes mellitus |
| Pregnancy | |
| Hypothyroidism | |
| Acromegaly | |
| Renal failure | |
| Pyridoxine (vitamin B6) deficiency | |
| Autoimmune/inflammatory | Rheumatoid arthritis |
| Amyloidosis | |
| Sarcoidosis | |
| Tenosynovitis | |
| Anatomic | Persistent median artery |
| Anomalous tendons or muscles | |
| Congenital stenosis of the carpal tunnel | |
| Fracture and/or dislocation at the wrist | |
| Infectious | Septic arthritis |
| Lyme disease | |
| Tuberculosis | |
| Histoplasmosis | |
| Neoplasm | Nerve sheath tumor |
| Ganglion cyst |
On examination, the entire shoulder and limb should be inspected for atrophy or asymmetry. Thenar atrophy is important to identify because it is a clue to the severity of CTS, but it may be absent in severe cases in patients with a Riche-Cannieu anastomosis.21 Coexistent hypothenar or first dorsal interosseous muscle atrophy may signify compression at the thoracic outlet or an ulnar entrapment neuropathy. Palpation is performed over the entire length of the median nerve from the brachial plexus to the hand to check for masses, points of tenderness, and adjacent bony abnormalities (Table 236-2). Motor examination should include all median-innervated muscles. In CTS, motor strength should be normal in the pronator teres, flexor carpi radialis, the entire FDS, FDP going to the index and middle fingers, and the flexor pollicis longus. In the hand, the lumbrical muscles of the index and middle fingers should be tested by checking extension at the proximal interphalangeal joint, and abduction of the thumb with palpation of the abductor pollicis brevis muscle must be performed. A full sensory examination of the median nerve also needs to be performed. In particular, the presence of sensory abnormalities in the distribution of the palmar cutaneous branch of the median nerve implies that the median nerve lesion is above the wrist (see Table 236-2 for potential proximal entrapment sites). Semmes-Weinstein monofilament and vibratory testing has been reported in the literature to be more sensitive and specific than two-point discrimination.24–26
TABLE 236-2 Possible Entrapment Sites of the Median Nerve
| Arm | Struthers’ ligament/supracondylar process of the humerus |
| Lacertus fibrosus (bicipital aponeurosis) | |
| Forearm | Pronator teres (between the two heads) |
| Hand | Flexor digitorum superficialis (sublimis bridge) |
| Carpal tunnel |
Certain provocative maneuvers can be used when examining a patient with suspected CTS. A positive finding occurs when the maneuver/position elicits symptoms in the distribution of the median nerve in the hand. The Tinel sign is performed by tapping over the carpal tunnel; sensitivity for the Tinel sign in patients with CTS ranges from 45% to 75%, and its specificity ranges from 40% to 67%.25–31 The Phalen test is performed by having the patient flex the wrist as far as possible and holding that position for 60 seconds, and the sensitivity and specificity of this test range from 4% to 86% and 48% to 54%, respectively.24,25,28,29,32 The pressure provocation test (Durkan compression test) is performed by the examiner placing a thumb over the carpal tunnel and exerting downward pressure for 30 seconds. This test has a significantly better sensitivity and specificity of 82% to 89% and 90% to 99%, respectively.25,33–35 Other provocative maneuvers reported in the literature are the reverse Phalen test, the Gilliat (tourniquet) test, and the ultrasonic stimulation test.
Diagnostic Evaluation
Electrophysiologic testing can provide important objective information to support the diagnosis of CTS. Palmar sensory latency, measured by stimulating sensory fibers in the palm and recording over the wrist, is the most sensitive test for CTS.36 Distal motor latency is usually prolonged but may be normal in 25% of patients with other signs and symptoms of CTS. Sensory nerve action potentials (SNAPs) are either unrecordable or of low amplitude at the wrist. Electromyographic recording of the abductor pollicis brevis or opponens pollicis may reveal spontaneous fibrillation potentials and positive sharp waves, as well as an increased incidence of long-duration, polyphasic motor unit potentials. These electrophysiologic data are compared with recordings from the ipsilateral ulnar and radial nerves because the contralateral median nerve may be affected by subclinical CTS. Electrodiagnostic studies are also helpful in grading the severity of CTS. In mild CTS, the SNAP or mixed nerve action potential (NAP) is often prolonged, and SNAP amplitude may be below the lower limit of normal. In moderate CTS, there are findings of mild CTS plus prolongation of median motor distal latency. In severe CTS, median motor and sensory distal latencies are prolonged, with absent SNAPs or mixed NAPs or absent or reduced thenar compound motor action potentials, or both. Fibrillations, reduced recruitment, and changes in motor unit potential are often seen in severe cases.37
Imaging can be a useful adjunct in the diagnosis of CTS. Plain films of the wrist can identify bony abnormalities such as fractures. Refinement of ultrasound techniques has allowed direct visualization of neural structures and associated sites of constriction, compression, or both. An entrapped peripheral nerve may appear hypoechoic, swollen, or flattened or exhibit any combination of these features.38,39 Ultrasonography has been shown to be highly sensitive and specific in patients with clinical and electrophysiologic signs of CTS,40 as well as in patients with clinical signs of CTS but negative electrodiagnostic studies.41
Improvements in magnetic resonance imaging (MRI) have resulted in greater sensitivity in the detection of peripheral nerve inflammation. Increased signal intensity within inflamed peripheral nerves may be seen on short tau inversion recovery (STIR) images or fat-suppressed T2-weighted spin echo images. Nerve thickening or nerve enlargement on MRI can also signify inflammation.42,43 Magnetic resonance neurography, a technique based on enhancing signal differences between nerves and surrounding tissues, has increasingly been used for the diagnosis of entrapment neuropathies.44 This technique is useful in demonstrating nerve position in relation to an adjacent joint placed in varying degrees of flexion. These images may suggest adhesion of nerve to surrounding tissue.45 These MRI techniques may be useful in the diagnosis of CTS in patients with normal electrophysiologic studies or in those with an underlying systemic neuropathy altering the electrophysiologic results.46,47 MRI evidence of nerve enlargement can be demonstrated at the level of the pisiform bone, where its diameter is 1.6 to 3.5 times that at the level of the distal radioulnar joint.48–50 MRI is also useful in the detection of mass lesions. It is unlikely to be cost-effective in the diagnosis of routine CTS, but it may be useful in patients with complicated manifestations.
Conservative Treatment
CTS is usually a progressive condition, but a course of conservative therapy should be completed before surgical intervention. Wrist splints, physical therapy, lifestyle modification, ultrasound therapy, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroids (either oral or direct carpal tunnel injection) are several conservative options available, but efficacy has yet to be proved for many of these approaches. Splinting of the affected wrist is the most commonly used nonoperative treatment and is supported by both anatomic and clinical studies. Anatomically, a wrist splint places the wrist in the neutral position, which has been shown to create the least amount of pressure or friction, or both, within the carpal tunnel.51–54 Splinting has also been shown to temporarily improve functional deficits and the severity of symptoms.51,53,55,56 Walker and colleagues compared nighttime with round-the-clock use of wrist splints for CTS and found that although full-time splinting provided better relief of symptoms and neurological improvement, splints limited the patient’s ability to perform normal daily activities.53 Piazzini and associates recently performed an extensive review of the literature regarding the efficacy of conservative treatment of CTS. Their findings were the following: (1) locally injected steroids produce a significant, but temporary improvement; (2) wrist splints are effective, particularly if used full-time; (3) steroids are more effective than NSAIDs or diuretics; (4) ultrasound therapy may be effective, and laser therapy shows variable results; and (5) exercise therapy and pyridoxine (vitamin B6) are both ineffective.57 Randomized controlled studies have also shown that surgical decompression of the carpal tunnel is superior to both wrist splinting and steroids in terms of relief of symptoms and neurophysiologic outcome.58,59 In regard to CTS in the setting of pregnancy, symptoms often resolve on delivery.60,61
Surgical Techniques
Open Technique
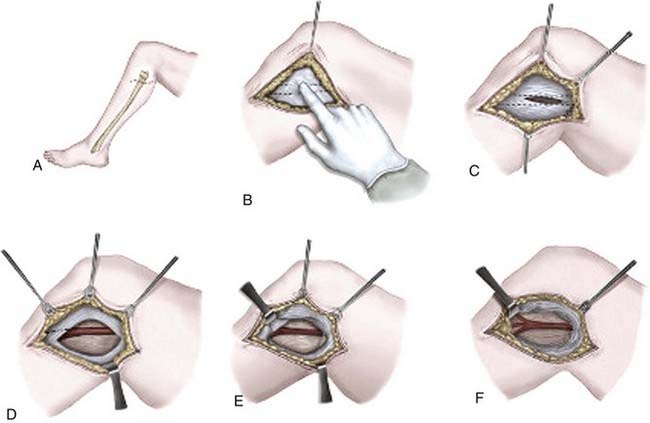
![]() The goal of the open approach is complete division of the TCL and decompression of the median nerve, with preservation of the palmar cutaneous and recurrent motor branches of the median nerve. This procedure is typically performed on an outpatient basis with local anesthesia and, in some cases, mild sedation administered by an anesthesiologist. Other anesthetic options are general anesthesia, regional block, or a Bier block.62 The patient is positioned supine with the arm abducted and forearm supinated on a hand table or arm board. Use of a tourniquet is optional. After careful skin preparation and draping, the wrist is often placed on a roll to provide wrist extension. Loupe magnification and headlights are useful adjuncts. A 3- to 4-cm (1.5 to 3 cm in a “mini-open” approach—see Fig. 236-2 and Video 236-1) straight or slightly curvilinear incision is marked starting at the distal wrist crease and ending at a point intercepting an imaginary line (Kaplan’s) drawn from the distal border of the extended thumb to the pisiform prominence, in line with the long axis of the radial side of the ring finger. The incision is placed ulnar to or in line with the tendon of the palmaris longus and the major thenar skin crease. The incision may have to be extended distally for better exposure in large hands. After infiltration of the proposed incision with local anesthetic, an incision is made with a No. 15 scalpel. Deep to the skin, subcutaneous fat and the palmar fascia are encountered, with care taken to protect the palmar cutaneous branch of the median nerve, which is not consistently visualized. A small self-retaining retractor such as an Alm or a small bur-hole retractor is placed, and meticulous hemostasis is maintained with bipolar electrocautery. The TCL is encountered deep to the palmar fascia; occasionally, the thenar and hypothenar muscles may obscure the ligament. The TCL is divided at its midpoint in layers with either a No. 15 scalpel or small scissors, and a fine instrument such as a Jacobson, McCabe, or a flat dissector is used to elevate each layer. The recurrent motor branch of the median nerve may be transligamentous or subligamentous. Once the median nerve is visualized, the TCL is incised both proximally and distally. Proximally, the skin is elevated to permit visualization 2 to 3 cm into the forearm. The proximal TCL and a distal portion of the antebrachial fascia are incised. The distal TCL is incised until the deep palmar fat pad is visualized. The proximal and distal portions of the incisions are probed with either the surgeon’s fifth finger or a Penfield No. 4 dissector.36 At this point the carpal tunnel may be explored for tumor, ganglion cysts, muscle anomalies, or any other structural abnormality. Neurolysis of the median nerve is not generally recommended. Before closure, the wound is inspected for hemostasis and any bleeding points are coagulated with bipolar electrocautery; if used, the tourniquet should be released at this point. The wound is irrigated and then reapproximated with several absorbable subcutaneous sutures. The skin is closed with either absorbable or nonabsorbable monofilament in either a running or mattress configuration. A bulky hand dressing is then applied, and the patient is encouraged to perform gentle range-of-movement exercises as soon as possible.63 Postoperative splinting is not usually recommended because splinting has not been shown to improve wound healing, reduce postoperative pain, or diminish scar tenderness.64
The goal of the open approach is complete division of the TCL and decompression of the median nerve, with preservation of the palmar cutaneous and recurrent motor branches of the median nerve. This procedure is typically performed on an outpatient basis with local anesthesia and, in some cases, mild sedation administered by an anesthesiologist. Other anesthetic options are general anesthesia, regional block, or a Bier block.62 The patient is positioned supine with the arm abducted and forearm supinated on a hand table or arm board. Use of a tourniquet is optional. After careful skin preparation and draping, the wrist is often placed on a roll to provide wrist extension. Loupe magnification and headlights are useful adjuncts. A 3- to 4-cm (1.5 to 3 cm in a “mini-open” approach—see Fig. 236-2 and Video 236-1) straight or slightly curvilinear incision is marked starting at the distal wrist crease and ending at a point intercepting an imaginary line (Kaplan’s) drawn from the distal border of the extended thumb to the pisiform prominence, in line with the long axis of the radial side of the ring finger. The incision is placed ulnar to or in line with the tendon of the palmaris longus and the major thenar skin crease. The incision may have to be extended distally for better exposure in large hands. After infiltration of the proposed incision with local anesthetic, an incision is made with a No. 15 scalpel. Deep to the skin, subcutaneous fat and the palmar fascia are encountered, with care taken to protect the palmar cutaneous branch of the median nerve, which is not consistently visualized. A small self-retaining retractor such as an Alm or a small bur-hole retractor is placed, and meticulous hemostasis is maintained with bipolar electrocautery. The TCL is encountered deep to the palmar fascia; occasionally, the thenar and hypothenar muscles may obscure the ligament. The TCL is divided at its midpoint in layers with either a No. 15 scalpel or small scissors, and a fine instrument such as a Jacobson, McCabe, or a flat dissector is used to elevate each layer. The recurrent motor branch of the median nerve may be transligamentous or subligamentous. Once the median nerve is visualized, the TCL is incised both proximally and distally. Proximally, the skin is elevated to permit visualization 2 to 3 cm into the forearm. The proximal TCL and a distal portion of the antebrachial fascia are incised. The distal TCL is incised until the deep palmar fat pad is visualized. The proximal and distal portions of the incisions are probed with either the surgeon’s fifth finger or a Penfield No. 4 dissector.36 At this point the carpal tunnel may be explored for tumor, ganglion cysts, muscle anomalies, or any other structural abnormality. Neurolysis of the median nerve is not generally recommended. Before closure, the wound is inspected for hemostasis and any bleeding points are coagulated with bipolar electrocautery; if used, the tourniquet should be released at this point. The wound is irrigated and then reapproximated with several absorbable subcutaneous sutures. The skin is closed with either absorbable or nonabsorbable monofilament in either a running or mattress configuration. A bulky hand dressing is then applied, and the patient is encouraged to perform gentle range-of-movement exercises as soon as possible.63 Postoperative splinting is not usually recommended because splinting has not been shown to improve wound healing, reduce postoperative pain, or diminish scar tenderness.64
Results from the open carpal tunnel release are generally excellent. From the Louisiana State University Health Sciences Center (LSUHSC) series of 376 carpal tunnel releases, 89% of the patients were satisfied with their results. Improvement in pain was seen in 87% of patients, improvement in paresthesias in 92%, improvement in numbness in 56%, and improvement in weakness in 42% of patients. Major symptoms persisted in 6% of patients, and complications included wound infections, reflex sympathetic dystrophy, and hematoma.65
Endoscopic Techniques
Endoscopic carpal tunnel release (ECTR) has been performed since the late 1980s, and several different endoscopic techniques involving either a uniportal or biportal approach have been developed since. The Agee66 and Okutsu67,68 methods use the uniportal approach, whereas the Chow69 and Brown70 techniques use the biportal method. For both types of approaches, a tourniquet and either local anesthesia or a Bier block are used. A small incision is made at or just proximal to the distal wrist crease on the ulnar side of the palmaris longus tendon. The antebrachial fascia is exposed and divided bluntly. An elevator is placed deep to the antebrachial fascia and superficial to the flexor tendons. An obturator and slotted cannula are then inserted into the carpal tunnel while staying superficial to the median nerve and flexor tendons. In the two-portal technique, the obturator and cannula are brought through the skin approximately 4 cm distal to the distal wrist crease, the obturator is removed, and an endoscope is placed through the distal opening. The cannula is slotted to allow passage of a blade. The TCL is divided in either a proximal-to-distal or a distal-to-proximal manner. In the uniportal technique, the endoscope camera follows the blade. With these endoscopic techniques no attempt is usually made to visualize the median nerve. Once the TCL has been completely divided, the cannula is removed, the tourniquet is deflated, and after hemostasis is obtained, the skin incision or incisions are closed with simple skin stitches.71,72 Potential advantages include a shorter recovery time, less postoperative pain, and reduced wound complications. Drawbacks include a steep learning curve; less visibility, which may result in incomplete sectioning of the TCL and increased neurovascular injury; and increased cost associated with endoscopic instruments.
Hankins and coauthors reported a large case series of patients who underwent ECTR with the Brown biportal technique. Of the 14,722 patients included in this series, 82.6% had complete resolution of symptoms, 14.7% had some resolution, and 2.6% had no improvement and required open revision.73
Although ECTR has a growing number of proponents, open carpal tunnel release (OCTR) is still the approach used by most peripheral nerve surgeons.9
During the past 2 decades there has been quite a bit of controversy over the question whether OCTR or ECTR is superior. The Cochrane Collaboration recently published a systematic review of carpal tunnel surgery in which they looked at 33 studies involving CTS. Fourteen studies reported results pertaining to return to work or normal daily activity and found a mean difference of 0 to 25 days in favor of the endoscopic approach. In terms of reported complications, ETCR was associated with more transient nerve dysfunction such as neurapraxia, numbness, and paresthesias, whereas OCTR was found to have more wound complications. From 6 published studies that included revision rates, the relative risk of needing revision surgery was determined to be higher in the endoscopic group. Revision surgery was performed in 12 of 513 ECTR procedures versus 5 of 370 OCTR procedures (relative risk, 1.2; confidence interval, 0.5 to 3.1). The authors concluded that there is no strong evidence supporting replacement of the standard OCTR and that application of ECTR should be guided by patient and surgeon preference. Four of the studies included compared ECTR and OCTR with a modified incision.74–77 Pain score, symptom severity, and functional status initially favored ECTR but equilibrated by 8 to 12 weeks postoperatively.76,77 Use of a modified incision appeared to increase the need for revision surgery in comparison to ECTR.74 This review also found no evidence supporting internal neurolysis, epineurotomy, tenosynovectomy, or flexor retinaculum lengthening.78
Another important issue in the surgical treatment of CTS is simultaneous versus staged treatment of bilateral CTS. The potential advantage of simultaneous carpal tunnel release is a reduction in total disability time and reduced surgical cost. However, the major disadvantage of simultaneous procedures is the compromised ability of the patient to perform self-care. Studies have compared these two approaches and found no significant difference in total disability time and return to work; however, simultaneous procedures cost approximately 60% of staged procedures and potentially require fewer follow-up visits.79,80 Our practice is to stage carpal tunnel releases and treat the more affected hand first, followed 2 to 6 weeks later by treatment of the other hand.
Ulnar Nerve
The second most common entrapment neuropathy is UN entrapment at the elbow. Like CTS, UN entrapment neuropathy may be debilitating and demoralizing because of pain and impaired hand function. The first report of surgical treatment of UN compression at the elbow is attributed to Henry Earle in 1816, who sectioned the UN proximal to the elbow to treat severe pain in the UN distribution. In the 19th and early 20th century, several reports were published describing UN palsy after elbow trauma. In 1898, Curtis reported the first anterior subcutaneous UN transposition in a patient who experienced ulnar neuritis after a bilateral condylar fracture. In 1922, Buzzard described chronic neuritis at the elbow and attributed it to excessive use of the arm and hand in a flexed position.81 Another important advance in UN surgery occurred in 1942 with the description of anterior submuscular UN transposition by Learmonth. In 1956, Feindel and Stratford proposed the designation “cubital tunnel” to describe the site of UN compression at the elbow and compared it with median nerve compression at the wrist.82,83
Anatomy
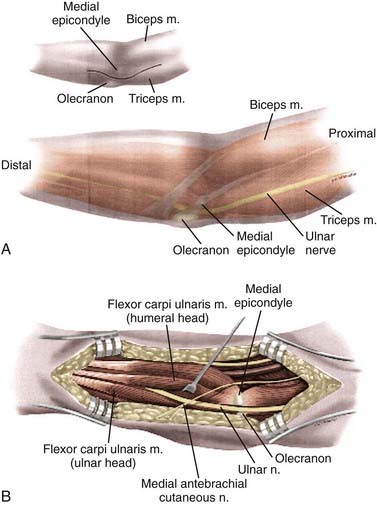
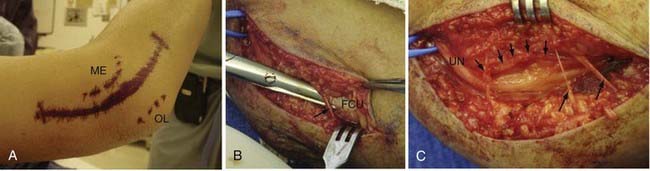
The UN is composed of contributions from C7, C8, and T1 and is the terminal continuation of the medial cord of the brachial plexus (Fig. 236-3). The UN enters the medial part of the arm by traveling in a groove between the coracobrachialis and triceps. The nerve initially travels into the arm with the axillary artery but diverges posteriorly and medially from the brachial artery. The UN pierces the medial intermuscular septum near the midpoint of the arm and then travels along the anterior aspect of the medial head of the triceps. In some cases, the UN will run within the triceps muscle. As the UN approaches the postcondylar groove, it may traverse a thickened fascial structure known as the arcade of Struthers approximately 8 cm proximal to the elbow. The nerve enters the postcondylar groove posterior to the medial epicondyle and then gives off articular branches to the elbow. The UN courses between the medial epicondyle and olecranon within the cubital tunnel, the most common site of entrapment.84 The roof of the cubital tunnel is formed by the cubital tunnel retinaculum or arcuate ligament of Osborne, which extends from the tip of the olecranon to the medial epicondyle. The fibers of the retinaculum are oriented in transverse fashion and become taut with elbow flexion. The floor is formed by the capsule of the elbow joint and the medial collateral ligament; the walls are formed by the medial epicondyle and olecranon. The distal extent of the retinaculum fuses with the common aponeurosis of the flexor carpi ulnaris (FCU) muscle, also referred to as Osborne’s fascia, another common site of entrapment.83,85,86 The UN enters the forearm between the ulnar and humeral heads of the FCU and provides muscular branches for innervation of this muscle. Approximately 3 cm distal to the cubital tunnel, the UN pierces the flexor pronator aponeurosis, another potential site of entrapment.86,87 The UN continues down the forearm and gives off muscular branches to the ulnar half of the FDP muscle. The palmar cutaneous branch of the UN originates approximately 16 cm proximal to the ulnar styloid and provides sensation to the distal ulnar aspect of the forearm.88 In the distal part of the forearm, the dorsal cutaneous branch of the UN passes medial to the FCU and enters the dorsal ulnar portion of the hand. The UN enters the hand through Guyon’s canal, which is a fibro-osseous tunnel between the pisiform and hook of the hamate. The floor of this canal is the pisohamate ligament, and the roof is the superficial volar carpal ligament. Guyon’s canal is another potential site of entrapment (Table 236-3), and within the canal the UN divides into a superficial and a deep branch. The superficial branch of the UN innervates the palmaris brevis muscle and continues to the fourth and fifth fingers as a sensory nerve. The deep or motor branch of the UN innervates the hypothenar muscles, the interosseous muscles, and the third and fourth lumbricals and ends by supplying the adductor pollicis and medial head of the flexor pollicis brevis.89
| Arm | Struthers’ arcade/medial intermuscular septum |
| Forearm | Postcondylar groove |
| Flexor carpi ulnaris/Osborne’s fascia (band) | |
| Hand | Guyon’s canal |
Clinical Findings
Patients with UN entrapment at the elbow often complain of numbness, tingling, and pain in the fourth and fifth fingers, as well as elbow pain and hand weakness. Motor weakness may precede sensory disturbances because of the predominance of motor fibers within the UN. Loss of hand dexterity, a feeling of hand clumsiness, and frequent dropping of objects are other common symptoms. Patients with certain jobs such as carpentry, painting, and music are typically more susceptible to the development of UN symptoms because of prolonged elbow flexion.90 An increased incidence of cubital tunnel syndrome is also seen in patients engaging in baseball, cycling, weightlifting, karate, cross-country skiing, and wrestling.23
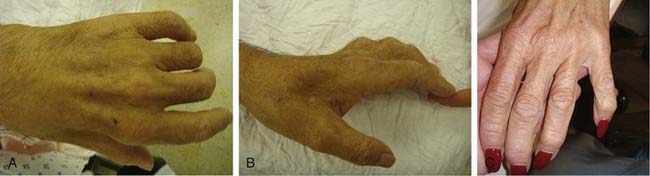
On examination, sensation is diminished in the ulnar distribution of the hand, particularly the palmar and dorsal surfaces of the fifth finger. Sensory testing of the dorsal medial portion of the hand is important; preserved sensation in this area with sensory deficits in the ulnar distribution of the fingers may suggest entrapment at Guyon’s canal (the dorsal cutaneous branch distribution is spared). Frequently, intrinsic hand weakness is present. The lumbrical muscle to the fifth finger and the abductor digiti minimi muscle are the earliest affected. Weakness of the FCU and FDP occur late in the disease. Muscle atrophy can occur quickly in UN entrapment syndromes; wasting of the first dorsal interosseous and hypothenar muscles is easily identifiable (Fig. 236-4). The metacarpal bones may be prominent because of interosseous atrophy. In advanced cases, the fourth and fifth fingers will appear clawed as a result of weakness of the lumbricals to those fingers. The fifth finger may be abducted away from the other fingers at rest, a finding known as the Wartenberg sign; patients with this sign often complain of catching the fifth finger when placing the affected hand in a pocket. This occurs when the third volar interosseous muscle is weak and allows the extensor digiti minimi to abduct the fifth finger during extension.91 Palpation of the elbow may reveal tenderness over the UN. A positive Tinel sign over the elbow will cause paresthesias in the fifth finger most often, and the overall sensitivity of this test in patients with cubital tunnel syndrome is around 70%. A more sensitive provocative test is the pressure-flexion test, in which the elbow is flexed and pressure applied over the cubital tunnel for 30 seconds, with paresthesias being produced in the distribution of the UN. The sensitivity of the pressure-flexion test has been reported to be 91%.92 The elbow should also be flexed and extended while feeling over the UN for subluxation.93 A thorough examination for thoracic outlet syndrome and C8 radiculopathy must be performed because these conditions may mimic ulnar entrapment neuropathy (Table 236-4). Amyotrophic lateral sclerosis (ALS) usually involves bilateral weakness and atrophy of the hand intrinsic muscles (with preserved sensation) but may be manifested initially by unilateral involvement. A bulbar examination should be performed to look for tongue fasciculations, although absence of such findings does not exclude the spinal form of ALS.93,94
TABLE 236-4 Differential Diagnosis of Cubital Tunnel Syndrome
| Spinal cord | Cervical spondylotic myelopathy |
| Cervical syrinx | |
| Cervical spinal cord tumor | |
| Nerve root | Motoneuron disease |
| C8 or T1 radiculopathy | |
| Peripheral nerve | Brachial plexopathy (lower trunk or medial cord) |
| Ulnar nerve sheath tumor | |
| Ulnar nerve compression at the arcade of Struthers | |
| Ulnar nerve entrapment at Guyon’s canal | |
| Other | Peripheral neuropathy |
Diagnostic Evaluation
Electrophysiologic studies usually provide reliable information for the diagnosis and localization of ulnar entrapment neuropathy. Entrapment of the UN at the elbow results in prolonged motor and sensory latency across the elbow but normal latency in the distal part of the forearm. Motor conduction velocities of less than 50 m/sec across the elbow also suggest entrapment at the elbow. It is important to recognize, however, that significant cubital tunnel syndrome can exist in the absence of noninvasive conductive abnormalities across the elbow. “Inching” studies, especially those done operatively, may demonstrate significant abnormalities.93 Electromyography of ulnar-innervated muscles may show reduced voluntary motor units, fibrillations, increased insertional activity, and other electrophysiologic signs of denervation.95 Operative inching studies indicate that abnormal conduction almost always begins proximal to the olecranon notch and is maximal through this bony canal.
Plain radiographs of the elbow are important to search for fracture or deformity when there is a history of trauma. At the cubital tunnel, the UN appears on axial T1-weighted MRI as a round hypointense structure surrounded by fat, posterior to the medial epicondyle. In cubital tunnel syndrome, the nerve may appear to have increased signal intensity on images acquired with T2-weighted or STIR sequences. UN subluxation or dislocation can be seen on axial images acquired during elbow flexion.48,96
Ultrasonography can also be applied to the UN with some sensitivity and specificity. In one series, compression of the UN at the cubital tunnel was associated with higher nerve cross-sectional areas on ultrasound; a cutoff point of 0.10 cm2 or higher yields a sensitivity of 93% and specificity of 98%.97 This is not a universally applicable or accepted method of diagnosis because the nerve can be narrowed or scarred or have other defects and yet still be obviously involved.
Conservative Treatment
Patients with mild sensory symptoms and no evidence of motor weakness should undergo a course of conservative treatment before surgical intervention. These patients should avoid activities that exacerbate their symptoms, such as prolonged elbow flexion and pressure over the cubital tunnel. Splinting (e.g., with a neoprene sleeve), with the goal of limiting elbow flexion, can also be effective in reducing or alleviating symptoms, particularly in patients with intermittent symptoms.98,99
Operative Treatment
Several surgical options are available to treat compression of the UN at the elbow: simple decompression (with or without medial epicondylectomy), anterior subcutaneous transposition, intramuscular transposition, and submuscular transposition (Fig. 236-5).
Simple or in situ decompression of the UN, which involves unroofing of the cubital tunnel, is the easiest and most commonly used option. This procedure may be performed with the patient under local anesthesia with mild sedation, but some surgeons prefer to use regional or general anesthesia. The patient is positioned supine, and the arm is placed on a hand table. The shoulder is abducted to 90 degrees, the arm is extended, and the forearm is supinated. A small roll under the shoulder may help in maintaining this position. The entire upper extremity is prepared, and an extremity drape with a sterile stocking is used. A 6- to 8-cm curvilinear incision is planned over the course of the UN as it traverses the elbow adjacent to the medial epicondyle, with 3 to 4 cm of the incision projecting proximal and 3 to 4 cm distal to the elbow. After infiltration with local anesthetic, an incision is made with a No. 15 scalpel blade to expose the subcutaneous tissues. During subcutaneous dissection, care must be taken to preserve branches of the medial brachial and antebrachial cutaneous nerves because injury may result in neuroma formation.99 The deep fascia overlying the UN is incised with either scissors or a No. 15 scalpel blade. The UN is identified and exposed proximal to the postcondylar groove. Dissection is carried distally through the postcondylar groove, and the fibrofascial cubital retinaculum overlying the nerve is divided. As mentioned before, this is most often the site of maximal compression. The UN is followed distally by dividing Osborne’s fascia, the proximal edge of the FCU muscle, and at this point care must be taken to protect the muscle branches of the nerve. The distal skin edge can be elevated for distal visualization of the nerve. Exposure of the UN is also carried proximal to the postcondylar groove to inspect for possible compression at the arcade of Struthers and the medial intermuscular septum. The proximal skin edge should also be elevated for better visualization. The proximal and distal extent of the exposure is probed for any constrictive bands. With simple decompression, external neurolysis (i.e., circumferential dissection) of the UN is not performed. Once decompression is completed, the elbow is flexed and extended to look for nerve subluxation. If significant subluxation is present, some surgeons believe that a transposition procedure is warranted; however, we never perform a transposition at the same time because it rarely proves to be needed. The wound is irrigated and inspected for hemostasis. Hemostasis is easily achieved with bipolar coagulation. The wound is closed with interrupted absorbable subcutaneous/fascial suture, and the skin is closed with absorbable or nonabsorbable monofilament suture in either a running or mattress configuration. A soft compressive dressing is placed and the patient is given a sling to wear for comfort only. Early mobilization of the arm is encouraged.100
If either an anterior subcutaneous or submuscular transposition is planned, complete external neurolysis of the UN must be performed.93 To elevate the nerve from the postcondylar groove, articular branches and small vessels tethering the UN often need to be divided. Fortunately, the nerve tolerates such dissection in the vast majority of cases. A distal segment of the medial intermuscular septum must be excised to prevent tethering or compression of the transposed UN. In the case of anterior subcutaneous transposition, the nerve is brought anterior to the medial epicondyle, and a fascial sling is created to hold the nerve in place. Distally, a portion of the FCU should be divided to prevent kinking of the UN. In the case of submuscular transposition, the origin of the flexor-pronator mass is isolated and divided in a step-cut or Z-plasty configuration, with a proximal cuff of muscle and fascia left intact. Laterally, care must be taken to avoid the brachial artery and median nerve. The proximal attachment of the volar FCU to the ulna needs to be detached to prevent distal kinking of the UN.93 The UN is brought anteriorly and placed deep to the flexor-pronator mass. The nerve must not be kinked or compressed over its new course. The flexor-pronator mass is then reapproximated by using the step cut to provide lengthening. Reattachment is performed with multiple 0-grade sutures. Wound closure is similar to that for the simple decompression, but occasionally a wound drain may be needed. A soft compressive dressing is applied, and the arm is placed in a sling for approximately 3 weeks. Gentle range-of-movement exercises are encouraged as soon as possible.22
In situ decompression is sometimes supplemented with medial epicondylectomy. First introduced by King and Morgan in 1959,101 this technique involves performing total or subtotal medial epicondyle osteotomy, thereby allowing the UN to move anterior to the axis of rotation of the elbow. Proponents also cite the medial epicondyle as a source of UN irritation. We suspect that the effectiveness of epicondylectomy may be related to the in situ nerve decompression that is performed at the same time. Complications of this procedure can include medial elbow stiffness or instability, or both, particularly if too much of the epicondyle is resected.22,102
In 2007, Zlowodzki and coauthors103 published a meta-analysis of four randomized, controlled trials that compared in situ decompression and anterior transposition.104–107 Two of these studies used the submuscular transposition method,105,106 and two used the subcutaneous technique.104,107 A total of 261 patients with an average follow-up of 21 months were included in this study. The results of this analysis found no significant difference in clinical outcome or postoperative nerve conduction velocity between in situ decompression, subcutaneous transposition, and submuscular transposition.103
Significantly fewer complications occurred in patients who underwent in situ decompression than in those who underwent subcutaneous UN transposition. Bartels and coworkers reported a complication rate of 9% in the in situ group and 30% in the anterior subcutaneous transposition group.104 These authors also compared the costs associated with simple decompression and anterior subcutaneous transposition by using data accumulated from a randomized, controlled trial (the results of this study were included in the aforementioned meta-analysis). The anterior subcutaneous transposition procedure has a total median cost 2.5 times that of simple decompression. Costs related to sick leave had the most impact on this difference.108 Submuscular UN transposition was shown to result in longer recovery times and higher wound complication rates than in situ decompression was. Gervasio and colleagues demonstrated that patients who underwent in situ decompression returned to work, on average, 9 days earlier than those who underwent submuscular transposition (21 versus 30 days).106 In the study by Biggs and Curtis, 4 of 21 patients who underwent submuscular transposition and 2 of 23 who underwent in situ decompression experienced superficial wound infections; 3 of 21 patients who underwent submuscular transposition and 0 of 23 who underwent in situ decompression experienced deep wound infections.105 Furthermore, in situ UN decompression is routinely performed with local anesthesia, thereby avoiding potential complications associated with general anesthesia.
Proponents of submuscular transposition argue that devascularization of the UN after transposition is rarely clinically evident. Davis and Bulluss retrospectively demonstrated no deterioration in symptoms or UN function after submuscular transposition. Furthermore, 82.5% of these patients (33/40) undergoing initial surgery improved at least one grade based on the LSUMC grading system.109
Recently, the endoscope has been used to decompress the UN at the elbow. In 1999, Tsai and coauthors published a series of 85 cubital tunnel releases with endoscopic assistance. Through a 2- to 3-cm incision over the course of the UN at the elbow, the authors were able to decompress up to 10 cm proximal and 10 cm distal to the medial epicondyle. In 36 patients with abnormal two-point discrimination preoperatively, 23 (64%) showed improvement after surgery. No significant difference was found between preoperative and postoperative motor function at a mean follow-up period of 32 months. Two patients subsequently required transposition procedures for recurrent symptoms.110 Ahcan and Zorman published a series of patients who underwent endoscopic release of a 20-cm segment of UN via a 3.5-cm incision overlying the cubital tunnel, with good or excellent results achieved in 91% of patients.111
In 2006, Krishnan and coauthors published a series of 11 patients who underwent endoscopically assisted anterior transposition of the UN with a unique endoscope-retractor system. Through a 1.5- to 2-cm incision over the ulnar sulcus, the UN was freed circumferentially proximally through the medial intermuscular septum and distally through Osborne’s ligament. The nerve was transposed subcutaneously and a fascial flap was created to maintain this position. Using the Bishop scoring system, 7 patients (63.7%) had excellent results, 3 (27.3%) had good results, and 1 patient (9.1%) had fair results with a mean follow-up time of 15.5 months. There were no complications, nor did any case have to be converted to an open procedure.112
Common Peroneal Nerve
The common peroneal nerve (CPN) is the most frequently injured nerve in the lower extremity and is involved in nearly 25% of all traumatic peripheral nerve injuries.113 Because of its superficial location, the CPN is most vulnerable just distal to the knee, where it courses laterally around the fibular neck. Furthermore, the CPN has a higher fascicle number and lower connective tissue content at the fibular neck than does the CPN within the popliteal fossa, which increases the nerve’s susceptibility to stretch or compression injury.114,115
Anatomy
Division of the CPN into the superficial peroneal nerve (SPN) and deep peroneal nerve (DPN) occurs within the peroneus longus muscle. The DPN pierces the lateral intermuscular septum to enter the anterior compartment. It courses inferiorly with the tibial artery and vein along the anterior aspect of the interosseous membrane. This nerve provides innervation to the muscles of the anterior compartment, namely, the tibialis anterior, extensor digitorum longus, extensor hallucis longus, and peroneus longus. Thus, the DPN is responsible for ankle dorsiflexion, ankle eversion, and toe extension. The DPN terminates in the dorsum of the foot into a lateral branch that innervates the extensor digitorum brevis and a medial branch that provides cutaneous innervation of the first web space. The SPN descends in the lateral compartment and provides innervation to the peroneus longus and brevis muscles, as well as sensory branches providing cutaneous innervation to the distal lateral aspect of the calf. In the distal part of the calf, the SPN pierces the fascia and divides into the medial and intermediate dorsal cutaneous nerves, which provide sensory innervation to the dorsum of the foot.15,16,116,117
Clinical Findings
In absence of a specific traumatic event, the most common cause of CPN entrapment is due to habitual leg crossing. Such patients usually cross the same leg, which causes repetitive injury to the CPN at the fibular neck; in these patients a skin dimple may even develop at the site of trauma.5,118–120 Patients who lose a significant amount of weight, as in the case of cancer or an eating disorder, may also be prone to injury to the CPN at the fibular neck (slimmer’s palsy).121,122 An increased incidence is seen in certain professions that require frequent squatting (e.g., roofers, carpet layers, strawberry pickers). Women may experience this condition after prolonged squatting during childbirth,5,123 and iatrogenic injury can occur as a result of improper cushioning or positioning of the leg, particularly in the dorsal lithotomy or lateral decubitus positions.124 Any contact sport may result in trauma to the CPN at the fibular neck.23
Diagnostic Evaluation
Electrophysiologic evaluation of CPN neuropathy is important to confirm the diagnosis and exclude other conditions that mimic CPN entrapment at the knee, such as L5 radiculopathy or a more proximal lesion of the CPN (Table 236-5). SNAPs of the SPN may be reduced or absent but should be spared in the case of L5 radiculopathy. Another electrophysiologic technique is to record from the extensor digitorum brevis or tibialis anterior while stimulating the CPN above and below the fibular neck to look for focal slowing, temporal dispersion, or conduction block.5,125 Electromyography should be performed on both peroneal-innervated muscles and non–peroneal, L5-innervated muscles such as the tibialis posterior (TP) and flexor digitorum longus (FDL). The short head of the biceps femoris should also be studied because it is the only peroneal-innervated muscle proximal to the peroneal tunnel; an electrical abnormality suggests a lesion proximal to the popliteal fossa.5,125
TABLE 236-5 Differential Diagnosis of Common Peroneal Nerve Entrapment
| Nerve root | L5 radiculopathy |
| Peripheral nerve | Sciatic nerve entrapment (piriformis syndrome) |
| Sciatic nerve injury | |
| Anterior tarsal tunnel syndrome | |
| Tumor | Pelvic plexus or sciatic nerve neoplasm |
| Ganglion cyst | |
| Nerve sheath tumor | |
| Other | Anterior compartment syndrome |
| Peripheral neuropathy |
Imaging of the knee with plain films must be performed to look for fracture or deformity. MRI can depict the CPN within the peroneal tunnel, but it is most useful in demonstrating space-occupying lesions.126,127 Ultrasound is useful for detection of not only intraneural ganglia128 but also entrapment.129
Conservative Treatment
Conservative therapy is effective for most cases of CPN entrapment. Exacerbating factors such as habitual leg crossing should be strictly avoided. In patients with footdrop, treatment with an ankle-foot orthosis is important to prevent falls and ankle sprains; physical therapy exercises are essential to prevent contractures. Patients who have recently lost a great deal of weight may benefit from protective padding over the fibular neck.130 Surgical decompression is often recommended for patients who show little or no improvement after 3 to 4 months.131
Operative Treatment
Operative decompression of the CPN is relatively straightforward and well tolerated. General anesthesia is used most often (we prefer local anesthesia with mild sedation), and a tourniquet is optional. The patient is positioned laterally with the affected leg uppermost and flexed at the knee. A curvilinear incision is marked in an oblique orientation over the fibular neck above the anticipated course of the CPN (Fig. 236-6). After infiltration with local anesthetic, an incision is made with a No. 15 scalpel blade. Care is taken to preserve any significant cutaneous nerve branches, such as the lateral sural cutaneous and posterior femoral cutaneous nerves. The deep fascia is incised over the usually palpable CPN. The CPN is often surrounded by a significant amount of adipose tissue and is identified and protected. Exposure of the nerve is carried both proximally and distally. The CPN is followed around the fibular neck as it passes between the two heads of the peroneus longus. The fascia overlying the peroneus longus is divided and the nerve followed distally. A fascial band is often identified overlying the nerve in this location and must be divided. The origins of the DPN and SPN should be visualized. The proximal and distal extents of the exposure should be probed for occult sites of compression or entrapment. Intraoperative NAPs may be performed at this point, as well as an external neurolysis. In patients with severe damage to the nerve and absent NAPs, nerve grafting may be required. The wound is irrigated and hemostasis is attained with bipolar coagulation. The subcutaneous tissues are reapproximated with interrupted absorbable suture, and the skin is closed with either absorbable or nonabsorbable monofilament in a running or mattress configuration. A soft compressive dressing is placed, and early mobilization is encouraged.132
In 2004, Kim and associates published a large series of patients who underwent surgery for CPN lesions at LSUHSC.133 Over a 32-year period, 318 knee-level CPN lesions were managed surgically, 51% of which were stretch/contusion injuries, 12% were lacerations, 9% involved entrapment, 7% were due to compression, and others included iatrogenic injuries, tumors, and gunshot wounds. Neurolysis was performed in patients with recordable NAPs across the injured segment. Of the 121 patients in whom neurolysis was performed, 107 (88%) recovered useful function. In patients in whom NAPs were absent, the injured segment was removed and grafted. Functional outcomes were better in patients who required shorter grafts; 75% of patients who had grafts smaller than 6 cm achieved grade 3 or better function as compared with 38% in the 6- to 12-cm group and 16% in the 13- to 24-cm group. In the event of persistent footdrop after surgery, one may consider referral to an orthopedist for TP tendon transfer. This procedure can be highly effective for footdrop caused by CPN injury, particularly in men younger than 30 years.134
Posterior Tibial Nerve
Compression of the posterior tibial nerve as it passes through the tarsal tunnel (TT) may result in a condition known as tarsal tunnel syndrome (TTS). TTS may be caused by bony impingement, including changes related to ankle trauma, or by space-occupying lesions, such as ganglion cysts, neurilemomas and other tumors, tenosynovitis, accessory or hypertrophic muscles, or varicosities.135 Bilateral TT symptoms can occur with systemic diseases such as rheumatoid arthritis, gout, diabetes, and myxedema.2
Anatomy
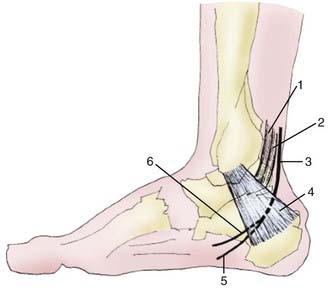
The TT is a continuation of the deep posterior compartment of the calf into the posteromedial aspect of the ankle and the medial plantar aspect of the foot (Fig. 236-7). The TT is made up of two main compartments: an upper (tibiotalar) and a lower (talocalcaneal) compartment. The floor of the upper compartment is formed by the posterior aspect of the tibia and the talus, and the roof is formed by a deep aponeurosis. The posterior tibial neurovascular bundle (including the posterior tibial nerve) runs through this space with the tendons of the TP, FDL, and flexor hallucis longus. The lower compartment of the TT contains the abductor hallucis muscle.136 The tibial nerve passes within the upper compartment of the TT posterior to the tendons of the TP and FDL and the posterior tibial artery and vein. The medial and inferior calcaneal nerves may arise proximal to, within, or distal to the TT.137
Clinical Findings
Most often, a patient with TTS will have foot pain that is neuritic and burning in character. Symptoms associated with TTS are variable and nonspecific, with patients complaining of poorly localized pain and paresthesias along the medial aspect of the heel and plantar aspect of the foot and toes (first, second, and third most commonly). Symptoms are usually worse with weight bearing and relieved with rest. Some patients will experience nocturnal exacerbations. Certain medical conditions, such as rheumatoid arthritis and diabetes, are associated with TTS. Hockey, hiking, and running are sports associated with TTS.23 The ankle and foot must be examined both by inspection and by palpation. Ankle deformity or swelling may suggest a traumatic cause. A positive Tinel sign will reproduce paresthesias in the digits distal to the point of percussion; manual pressure over the TT may also reproduce symptoms. Sensory innervation is variable on the sole of the foot, but deficits may be found in the distribution of the calcaneal, medial plantar, and lateral plantar nerves.
Diagnostic Evaluation
Electrodiagnostic tests may be useful in the diagnosis of TTS, but the correlation between preoperative electrodiagnostic testing and surgical outcome is unclear.138,139 Tibial motor nerve conduction may exhibit prolonged distal onset latency when recorded over the abductor hallucis and abductor digit minimi. Mixed nerve conduction studies of the medial and lateral plantar nerves may demonstrate prolonged peak latency or slowed velocity, and sensory nerve conduction of the two nerves may be slowed or absent across the tarsal tunnel.140
Plain radiographs of the affected ankle may be useful for identification of fracture or deformity. As with most other peripheral nerves, MRI is best for identifying space-occupying lesions, but the anatomy of the TT can also be characterized. The anatomy of the TT is generally well demonstrated by T1- and T2-weighted MRI of the hindfoot and midfoot, particularly axial and sagittal images.2,136 Ultrasound also can demonstrate TT anatomy, as well as space-occupying lesions.136,141,142
Conservative Treatment
A period of conservative therapy should be attempted before surgical intervention. Lifestyle and activity modification should be instituted, such as weight loss and avoidance of ill-fitting shoes or high heels. Some patients may benefit from a trial of immobilization, orthotics, or physical therapy. Antiepileptic, anti-inflammatory, antidepressant, and narcotic pain medications may help with chronic pain complaints. Nerve blocks or local steroid injection can be useful adjuncts as well.143
Surgical Treatment
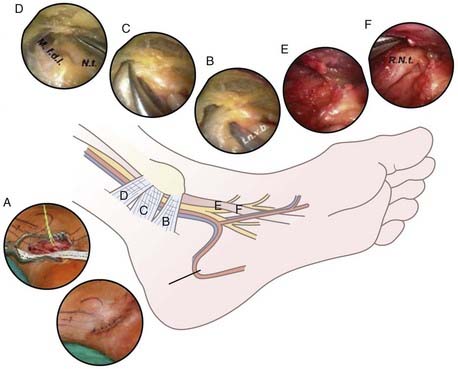
Open exploration of the TT is the preferred surgical technique, but endoscopic techniques have been developed.112,144 Success rates for surgical decompression of the TT have been reported to be between 44% and 93%, with success being defined as resolution or improvement of symptoms, no requirement for pain medications, and the ability to return to work.139,145,146 A curvilinear incision is started 4 cm proximal to the medial malleolus while staying posterior to the medial malleolus, extends distally toward the midaspect of the plantar surface of the foot, and curves anteriorly at the heel. The deep fascia over the neurovascular bundle is divided proximal to the TT, and division is continued distally as the fascia thickens to form the flexor retinaculum. The fascia covering the abductor hallucis brevis signifies the end of the TT. The medial and lateral plantar nerves are identified and followed into their two separate tunnels. Both tunnels are released by dividing the fascial origin of the abductor hallucis brevis, which forms their roof. Any calcaneal branches are identified and decompressed. The posterior tibial vessels are elevated and the tibial nerve and its branches are inspected. Complete external neurolysis is usually performed114,139 (Fig. 236-8).
Mullick and Dellon recently reported their long-term outcomes after decompression of the TT. The series included 87 procedures with a mean follow-up of 3.6 years. Significant improvement was seen in motor and sensory function. Using unspecified postoperative assessment techniques, there were 82% excellent (resolution of symptoms), 11% good (slight residual numbness and tingling, able to return to work, no pain medications), 5% fair (residual symptoms requiring pain medications, unable to return to work), and 2% poor results (no improvements).139 Revision surgery for TTS carries a less favorable outcome. Barker and coauthors reported a series of 44 patients who underwent revision by neurolysis, resection of scar neuroma, or occasional neurectomy, with a primary outcome measure of self-reported patient satisfaction. At a mean follow-up time of 2.2 years, 54% reported excellent results; 24%, good results; 13%, fair results; and 9%, poor results.147 Kim and Murovic reported a series of patients who underwent revision surgery for TTS at LSUHSC. Of the 10 patients who underwent external neurolysis of the posterior tibial nerve, only 4 showed improvement (40%); of the 5 patients who underwent internal neurolysis of the posterior tibial nerve, 2 (40%) had satisfactory results. Seven patients from the series underwent neurectomy of the posterior tibial nerve, all of whom reported improvement in pain; none of these patients experienced ulceration of the sole at a mean follow-up time of 3.2 years.114
For diabetic sensory neuropathy of the lower extremity, Dr. Dellon has advocated external neurolysis of the CPN at the knee, peroneal branches at the anterior aspect of the ankle, and the posterior tibial nerve along with calcaneal, medial, and lateral plantar branches at the TT (the Dellon triple decompression technique). A multicenter prospective study of this technique in diabetic patients reported a reduction in the prevalence of foot ulceration in 665 patients without previous ulceration from 15% to 0.6%; in 44 patients with a previous history of foot ulceration, the prevalence of ulceration was reduced from 50% to 2.2%. The authors claim that this triple decompression technique also improves sensation and reduces foot pain in diabetics with sensory neuropathy.148 This controversial approach has not yet been subjected to a prospective, randomized trial and has been stated to be of unproven value by the American Academy of Neurology.149
Double Crush Syndrome
First postulated by Upton and McComas in 1973,150 double crush syndrome is defined as coexistence of compressive lesions in series along the course of a peripheral nerve, with one lesion rendering the nerve susceptible to distal or proximal compression. Explanations for this phenomenon include impaired axonal flow, ischemia, and altered nerve elasticity, which lessen the nerve’s resiliency. Intrinsic neuropathies additionally affect the nerve’s susceptibility to injury. This syndrome can occur along any peripheral nerve, and common examples include cervical radiculopathy and CTS, thoracic outlet syndrome and CTS, and cubital tunnel syndrome and Guyon’s canal syndrome.22,151–153 This is a controversial entity whose existence and prevalence have been subject to debate.
Barker AR, Rosson GD, Dellon AL. Outcome of neurolysis for failed tarsal tunnel surgery. J Reconstr Microsurg. 2008;24:111.
Bartels RH, Termeer EH, van der Wilt GJ, et al. Simple decompression or anterior subcutaneous transposition for ulnar neuropathy at the elbow: a cost-minimization analysis—part 2. Neurosurgery. 2005;56:531.
Bartels RH, Verhagen WI, van der Wilt GJ, et al. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow: part 1. Neurosurgery. 2005;56:522.
Dellon AL. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy: update 2006. Acta Neurochir Suppl. 2007;100:149.
Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643.
Hankins CL, Brown MG, Lopez RA, et al. A 12-year experience using the Brown two-portal endoscopic procedure of transverse carpal ligament release in 14,722 patients: defining a new paradigm in the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2007;120:1911.
Huang JH, Samadani U, Zager EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150.
Huang JH, Zager EL. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397.
Humphreys DB, Novak CB, Mackinnon SE. Patient outcome after common peroneal nerve decompression. J Neurosurg. 2007;107:314.
Kim DH, Murovic JA, Tiel RL, et al. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery. 2004;54:1421.
Kim S, Choi JY, Huh YM, et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy—what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity. Eur Radiol. 2007;17:509.
Kim S, Choi JY, Huh YM, et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy—what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 1. Overview and lower extremity. Eur Radiol. 2007;17:139.
Krishnan KG, Pinzer T, Schackert G. A novel endoscopic technique in treating single nerve entrapment syndromes with special attention to ulnar nerve transposition and tarsal tunnel release: clinical application. Neurosurgery. 2006;59:ONS89.
Piazzini DB, Aprile I, Ferrara PE, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21:299.
Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. [update of Cochrane Database Syst Rev. 2004;(4):CD003905; PMID: 15495070]. Cochrane Database Syst Rev. 2007;4:CD003905.
Toth C. Peripheral nerve injuries attributable to sport and recreation. Neurol Clin. 2008;26:89.
Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5.
Zlowodzki M, Chan S, Bhandari M, et al. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591.
1 Dahlin LB. Aspects on pathophysiology of nerve entrapments and nerve compression injuries. Neurosurg Clin N Am. 1991;2:21.
2 Hochman MG, Zilberfarb JL. Nerves in a pinch: imaging of nerve compression syndromes. Radiol Clin North Am. 2004;42:221.
3 Schmidek AK, Winograd JM. Surgical management of median nerve compression at the wrist by open technique, 5th ed. Schmidek HH, Roberts D, editors. Operative Neurosurgical Techniques: Indications, Methods, and Results, Vol 2. Philadelphia: WB Saunders. 2006.
4 Brown WF, Watson BV. Quantitation of axon loss and conduction block in peroneal nerve palsies. Muscle Nerve. 1991;14:237.
5 Campbell WW. Diagnosis and management of common compression and entrapment neuropathies. Neurol Clin. 1997;15:549.
6 Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;138:1.
7 Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153.
8 Ferry S, Pritchard T, Keenan J, et al. Estimating the prevalence of delayed median nerve conduction in the general population. Br J Rheumatol. 1998;37:630.
9 Lo SL, Raskin K, Lester H, et al. Carpal tunnel syndrome: a historical perspective. Hand Clin. 2002;18:211.
10 Putnam JJ. A series of cases of parasthesias, mainly of the hand, or periodic recurrence, and possibly of vasomotor origin. Arch Med. 1880;4:147.
11 Learmonth JR. The principle of decompression in the treatment of certain diseases of the peripheral nerves. Surg Clin North Am. 1933;13:905.
12 Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976;39:615.
13 Gelberman RH, Szabo RM, Williamson RV, et al. Tissue pressure threshold for peripheral nerve viability. Clin Orthop Relat Res. 1983;178:285.
14 Lundborg G, Gelberman RH, Minteer-Convery M, et al. Median nerve compression in the carpal tunnel—functional response to experimentally induced controlled pressure. J Hand Surg Am. 1982;7:252.
15 Drake R, Vogl W, Mitchell AW. Gray’s Anatomy for Students. Philadelphia: Elsevier; 2005.
16 Gray H, Williams PL. Gray’s Anatomy, 37th ed. New York: Churchill Livingstone; 1989.
17 Rotman MB, Donovan JP. Practical anatomy of the carpal tunnel. Hand Clin. 2002;18:219.
18 Kim S, Choi JY, Huh YM, et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy—what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 2. Upper extremity. Eur Radiol. 2007;17:509.
19 Omer GEJr. Median nerve compression at the wrist. Hand Clin. 1992;8:317.
20 Lanz U. Anatomical variations of the median nerve in the carpal tunnel. J Hand Surg Am. 1977;2:44.
21 Refaeian M, King JC, Dumitru D, et al. Carpal tunnel syndrome and the Riche-Cannieu anastomosis: electrophysiologic findings. Electromyogr Clin Neurophysiol. 2001;41:377.
22 Slutsky DJ, Hentz VR. Peripheral Nerve Surgery: Practical Applications in the Upper Extremity. Philadelphia: Churchill Livingstone; 2006.
23 Toth C. Peripheral nerve injuries attributable to sport and recreation. Neurol Clin. 2008;26:89.
24 Koris M, Gelberman RH, Duncan K, et al. Carpal tunnel syndrome. Evaluation of a quantitative provocational diagnostic test. Clin Orthop Relat Res. 1990;251:157.
25 Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am. 2000;25:120.
26 Szabo RM, Gelberman RH, Dimick MP. Sensibility testing in patients with carpal tunnel syndrome. J Bone Joint Surg Am. 1984;66:60.
27 Borg K, Lindblom U. Increase of vibration threshold during wrist flexion in patients with carpal tunnel syndrome. Pain. 1986;26:211.
28 Buch-Jaeger N, Foucher G. Correlation of clinical signs with nerve conduction tests in the diagnosis of carpal tunnel syndrome. J Hand Surg Br. 1994;19:720.
29 Gunnarsson LG, Amilon A, Hellstrand P, et al. The diagnosis of carpal tunnel syndrome. Sensitivity and specificity of some clinical and electrophysiological tests. J Hand Surg Br. 1997;22:34.
30 Seror P. Tinel’s sign in the diagnosis of carpal tunnel syndrome. J Hand Surg Br. 1987;12:364.
31 Spindler HA, Dellon AL. Nerve conduction studies and sensibility testing in carpal tunnel syndrome. J Hand Surg Am. 1982;7:260.
32 Gerr F, Letz R. The sensitivity and specificity of tests for carpal tunnel syndrome vary with the comparison subjects. J Hand Surg Br. 1998;23:151.
33 Durkan JA. A new diagnostic test for carpal tunnel syndrome. J Bone Joint Surg Am. 1991;73:535.
34 Durkan JA. The carpal-compression test. An instrumented device for diagnosing carpal tunnel syndrome. Orthop Rev. 1994;23:522.
35 Tetro AM, Evanoff BA, Hollstien SB, et al. A new provocative test for carpal tunnel syndrome. Assessment of wrist flexion and nerve compression. J Bone Joint Surg Br. 1998;80:493.
36 Huang JH, Zager EL. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397.
37 Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997;20:1477.
38 Bianchi S, Montet X, Martinoli C, et al. High-resolution sonography of compressive neuropathies of the wrist. J Clin Ultrasound. 2004;32:451.
39 Martinoli C, Bianchi S, Pugliese F, et al. Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound. 2004;32:438.
40 Yesildag A, Kutluhan S, Sengul N, et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910.
41 Koyuncuoglu HR, Kutluhan S, Yesildag A, et al. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005;56:365.
42 Kijowski R, Tuite M, Sanford M. Magnetic resonance imaging of the elbow. Part II: abnormalities of the ligaments, tendons, and nerves. Skeletal Radiol. 2005;34:1.
43 Rosenberg ZS, Beltran J, Cheung YY, et al. The elbow: MR features of nerve disorders. Radiology. 1993;188:235.
44 Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet. 1993;341:659.
45 Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643.
46 Britz GW, Haynor DR, Kuntz C, et al. Carpal tunnel syndrome: correlation of magnetic resonance imaging, clinical, electrodiagnostic, and intraoperative findings. Neurosurgery. 1995;37:1097.
47 Grant GA, Britz GW, Goodkin R, et al. The utility of magnetic resonance imaging in evaluating peripheral nerve disorders. Muscle Nerve. 2002;25:314.
48 Andreisek G, Crook DW, Burg D, et al. Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features. Radiographics. 2006;26:1267.
49 Bordalo-Rodrigues M, Amin P, Rosenberg ZS. MR imaging of common entrapment neuropathies at the wrist. Magn Reson Imaging Clin N Am. 2004;12:265.
50 Horch RE, Allmann KH, Laubenberger J, et al. Median nerve compression can be detected by magnetic resonance imaging of the carpal tunnel. Neurosurgery. 1997;41:76.
51 Brininger TL, Rogers JC, Holm MB, et al. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:1429.
52 Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg Am. 1989;14:624.
53 Walker WC, Metzler M, Cifu DX, et al. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81:424.
54 Weiss ND, Gordon L, Bloom T, et al. Position of the wrist associated with the lowest carpal-tunnel pressure: implications for splint design. J Bone Joint Surg Am. 1995;77:1695.
55 Kruger VL, Kraft GH, Deitz JC, et al. Carpal tunnel syndrome: objective measures and splint use. Arch Phys Med Rehabil. 1991;72:517.
56 Manente G, Torrieri F, Di Blasio F, et al. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle Nerve. 2001;24:1020.
57 Piazzini DB, Aprile I, Ferrara PE, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21:299.
58 Gerritsen AA, de Vet HC, Scholten RJ, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA. 2002;288:1245.
59 Hui AC, Wong S, Leung CH, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology. 2005;64:2074.
60 Mondelli M, Rossi S, Monti E, et al. Long term follow-up of carpal tunnel syndrome during pregnancy: a cohort study and review of the literature. Electromyogr Clin Neurophysiol. 2007;47:259.
61 Mondelli M, Rossi S, Monti E, et al. Prospective study of positive factors for improvement of carpal tunnel syndrome in pregnant women. Muscle Nerve. 2007;36:778.
62 Gebhard RE, Al-Samsam T, Greger J, et al. Distal nerve blocks at the wrist for outpatient carpal tunnel surgery offer intraoperative cardiovascular stability and reduce discharge time. Anesth Analg. 2002;95:351.
63 Singh SK, Midha R. Carpal tunnel syndrome. In: Midha R, Zager EL, editors. Surgery of Peripheral Nerves: A Case-Based Approach. New York: Thieme, 2008.
64 Huemer GM, Koller M, Pachinger T, et al. Postoperative splinting after open carpal tunnel release does not improve functional and neurological outcome. Muscle Nerve. 2007;36:528.
65 Spinner RJ. Median nerve. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries. 2nd ed. Philadelphia: WB Saunders; 2008:139.
66 Agee JM, McCarroll HRJr, Tortosa RD, et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17:987.
67 Okutsu I, Ninomiya S, Hamanaka I, et al. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg Am. 1989;71:679.
68 Okutsu I, Ninomiya S, Takatori Y, et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5:11.
69 Chow JC. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy. 1989;5:19.
70 Brown MG, Keyser B, Rothenberg ES. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17:1009.
71 Arle JE, Zager EL. Surgical treatment of common entrapment neuropathies in the upper limbs. Muscle Nerve. 2000;23:1160.
72 Jimenez DF, Loftus T. Endoscopic carpal tunnel release. In: Midha R, Zager EL, editors. Surgery of Peripheral Nerves: A Case-Based Approach. New York: Thieme, 2008.
73 Hankins CL, Brown MG, Lopez RA, et al. A 12-year experience using the Brown two-portal endoscopic procedure of transverse carpal ligament release in 14,722 patients: defining a new paradigm in the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2007;120:1911.
74 Eichhorn J, Dietrich K. Open versus endoscopic carpal tunnel release. Results of a prospective study. Chir Praxis. 2003;61:279.
75 Mackenzie DJ, Hainer R, Wheatley MJ. Early recovery after endoscopic vs. short-incision open carpal tunnel release. Ann Plast Surg. 2000;44:601.
76 Rab M, Grunbeck M, Beck H, et al. Intra-individual comparison between open and 2-portal endoscopic release in clinically matched bilateral carpal syndrome. J Plast Reconstr Aesthet Surg. 2006;59:730.
77 Wong KC, Hung LK, Ho PC, et al. Carpal tunnel release. A prospective, randomised study of endoscopic versus limited-open methods. J Bone Joint Surg Br. 2003;85:863.
78 Scholten RJ, Mink van der Molen A, Uitdehaag BM, et al. Surgical treatment options for carpal tunnel syndrome. [update of Cochrane Database Syst Rev. 2004;(4):CD003905; PMID: 15495070]. Cochrane Database Syst Rev. 2007;4:CD003905.
79 Acharya AD, Auchincloss JM. Return to functional hand use and work following open carpal tunnel surgery. J Hand Surg Br. 2005;30:607.
80 Amadio PC. What’s new in hand surgery. J Bone Joint Surg Am. 2007;89:460.
81 Buzzard E. Some varieties of traumatic and toxic ulnar neuritis. Lancet. 1922;1:317.
82 Bartels RH. History of the surgical treatment of ulnar nerve compression at the elbow. Neurosurgery. 2001;49:391.
83 Feindel W, Stratford J. The role of the cubital tunnel in tardy ulnar palsy. Can J Surg. 1958;1:287.
84 Khoo D, Carmichael SW, Spinner RJ. Ulnar nerve anatomy and compression. Orthop Clin North Am. 1996;27:317.
85 Adelaar RS, Foster WC, McDowell C. The treatment of the cubital tunnel syndrome. J Hand Surg Am. 1984;9:90.
86 Amadio PC, Beckenbaugh RD. Entrapment of the ulnar nerve by the deep flexor-pronator aponeurosis. J Hand Surg Am. 1986;11:83.
87 Gonzalez MH, Lotfi P, Bendre A, et al. The ulnar nerve at the elbow and its local branching: an anatomic study. J Hand Surg Br. 2001;26:142.
88 McCabe SJ, Kleinert JM. The nerve of Henle. J Hand Surg Am. 1990;15:784.
89 Polatsch DB, Melone CPJr, Beldner S, et al. Ulnar nerve anatomy. Hand Clin. 2007;23:283.
90 Szabo RM, Kwak C. Natural history and conservative management of cubital tunnel syndrome. Hand Clin. 2007;23:311.
91 Freeland AE, Barrett GR, Wheeless GS. Correction of abduction. Deformity of the small finger caused by avulsion of the insertion of the third volar interosseous muscle. Am J Sports Med. 1985;13:273.
92 Novak CB, Lee GW, Mackinnon SE, et al. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19:817.
93 Kim DH. Ulnar nerve. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries. Philadelphia: WB Saunders, 2008.
94 Kuwabara S, Sonoo M, Komori T, et al. Dissociated small hand muscle atrophy in amyotrophic lateral sclerosis: frequency, extent, and specificity. Muscle Nerve. 2008;37:426.
95 Pirouzmand F, Midha R. Ulnar nerve entrapment at the elbow. In: Midha R, Zager EL, editors. Surgery of the Peripheral Nerves: A Case-Based Approach. New York: Thieme, 2008.
96 Beltran J, Rosenberg ZS. Diagnosis of compressive and entrapment neuropathies of the upper extremity: value of MR imaging. AJR Am J Roentgenol. 1994;163:525.
97 Wiesler ER, Chloros GD, Cartwright MS, et al. Ultrasound in the diagnosis of ulnar neuropathy at the cubital tunnel. J Hand Surg Am. 2006;31:1088.
98 Dellon AL, Hament W, Gittelshon A. Nonoperative management of cubital tunnel syndrome: an 8-year prospective study. Neurology. 1993;43:1673.
99 Sarris I, Gobel F, Gainer M, et al. Medial brachial and antebrachial cutaneous nerve injuries: effect on outcome in revision cubital tunnel surgery. J Reconstr Microsurg. 2002;18:665.
100 Huang JH, Samadani U, Zager EL. Ulnar nerve entrapment neuropathy at the elbow: simple decompression. Neurosurgery. 2004;55:1150.
101 King T, Morgan FP. Late results of removing the medial humeral epicondyle for traumatic ulnar neuritis. J Bone Joint Surg Br. 1959;41:51.
102 Osterman AL, Spiess AM. Medial epicondylectomy. Hand Clin. 2007;23:329.
103 Zlowodzki M, Chan S, Bhandari M, et al. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591.
104 Bartels RH, Verhagen WI, van der Wilt GJ, et al. Prospective randomized controlled study comparing simple decompression versus anterior subcutaneous transposition for idiopathic neuropathy of the ulnar nerve at the elbow: part 1. Neurosurgery. 2005;56:522.
105 Biggs M, Curtis JA. Randomized, prospective study comparing ulnar neurolysis in situ with submuscular transposition. Neurosurgery. 2006;58:296.
106 Gervasio O, Gambardella G, Zaccone C, et al. Simple decompression versus anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: a prospective randomized study. [erratum appears in Neurosurgery. 2005 Feb;56(2):409]. Neurosurgery. 2005;56;:108.
107 Nabhan A, Ahlhelm F, Kelm J, et al. Simple decompression or subcutaneous anterior transposition of the ulnar nerve for cubital tunnel syndrome. J Hand Surg Br. 2005;30:521.
108 Bartels RH, Termeer EH, van der Wilt GJ, et al. Simple decompression or anterior subcutaneous transposition for ulnar neuropathy at the elbow: a cost-minimization analysis—part 2. Neurosurgery. 2005;56:531.
109 Davis GA, Bulluss KJ. Submuscular transposition of the ulnar nerve: review of safety, efficacy and correlation with neurophysiological outcome. J Clin Neurosci. 2005;12:524.
110 Tsai TM, Chen IC, Majd ME, et al. Cubital tunnel release with endoscopic assistance: results of a new technique. J Hand Surg Am. 1999;24:21.
111 Ahcan U, Zorman P. Endoscopic decompression of the ulnar nerve at the elbow. J Hand Surg Am. 2007;32:1171.
112 Krishnan KG, Pinzer T, Schackert G. A novel endoscopic technique in treating single nerve entrapment syndromes with special attention to ulnar nerve transposition and tarsal tunnel release: clinical application. Neurosurgery. 2006;59:ONS89.
113 Noble J, Munro CA, Prasad VS, et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116.
114 Kim DH, Murovic JA. Lower extremity nerve injuries. In: Kim DH, Midha R, Murovic JA, et al, editors. Kline and Hudson’s Nerve Injuries. Philadelphia: WB Saunders, 2008.
115 Macnicol MF. The Problem Knee, 2nd ed. Boston: Butterworth-Heinemann; 1995.
116 Lollis SS, Nikas DC. Entrapment neuropathies of the lower extremities, 5th ed. Schmidek HH, Roberts D, editors. Operative Neurosurgical Techniques: Indications, Methods, and Results, Vol 2. Philadelphia: WB Saunders. 2006.
117 Ryan W, Mahony N, Delaney M, et al. Relationship of the common peroneal nerve and its branches to the head and neck of the fibula. Clin Anat. 2003;16:501.
118 Carney LR. The dimple sign in peroneal palsy. Neurology. 1967;17:922.
119 Nagler SH, Rangell L. Peroneal palsy caused by crossing the legs. JAMA. 1947;133:755.
120 Woltman H. Crossing the legs as a factor in the production of peroneal palsy. JAMA. 1929;93:670.
121 Cruz-Martinez A, Arpa J, Palau F. Peroneal neuropathy after weight loss. J Peripher Nerv Syst. 2000;5:101.
122 Sotaniemi KA. Slimmer’s paralysis—peroneal neuropathy during weight reduction. J Neurol Neurosurg Psychiatry. 1984;47:564.
123 Reif ME. Bilateral common peroneal nerve palsy secondary to prolonged squatting in natural childbirth. Birth. 1988;15:100.
124 Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5.
125 Aminoff MJ. Electromyography in Clinical Practice, 3rd ed. New York: Churchill Livingstone; 1998.
126 Iverson DJ. MRI detection of cysts of the knee causing common peroneal neuropathy. Neurology. 2005;65:1829.
127 Kim S, Choi JY, Huh YM, et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy—what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image: part 1. Overview and lower extremity. Eur Radiol. 2007;17:139.
128 Visser LH. High-resolution sonography of the common peroneal nerve: detection of intraneural ganglia. Neurology. 2006;67:1473.
129 Lo YL, Fook-Chong S, Leoh TH, et al. High-resolution ultrasound as a diagnostic adjunct in common peroneal neuropathy. Arch Neurol. 2007;64:1798.
130 Shapiro BE, Preston DC. Entrapment and compressive neuropathies. Med Clin North Am. 2003;87:663.
131 Harbaugh K, Midha R. Peroneal nerve entrapment. In: Midha R, Zager EL, editors. Surgery of the Peripheral Nerves: A Case-Based Approach. New York: Thieme, 2008.
132 Humphreys DB, Novak CB, Mackinnon SE. Patient outcome after common peroneal nerve decompression. J Neurosurg. 2007;107:314.
133 Kim DH, Murovic JA, Tiel RL, et al. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery. 2004;54:1421.
134 Yeap JS, Birch R, Singh D. Long-term results of tibialis posterior tendon transfer for drop-foot. Int Orthop. 2001;25:114.
135 Fernandez E, Pallini R, Lauretti L, et al. Neurosurgery of the peripheral nervous system: entrapment syndromes of the lower extremity. Surg Neurol. 1999;52:449.
136 Delfaut EM, Demondion X, Bieganski A, et al. Imaging of foot and ankle nerve entrapment syndromes: from well-demonstrated to unfamiliar sites. Radiographics. 2003;23:613.
137 Govsa F, Bilge O, Ozer MA. Variations in the origin of the medial and inferior calcaneal nerves. Arch Orthop Trauma Surg. 2006;126:6.
138 Gondring WH, Shields B, Wenger S. An outcomes analysis of surgical treatment of tarsal tunnel syndrome. Foot Ankle Int. 2003;24:545.
139 Mullick T, Dellon AL. Results of decompression of four medial ankle tunnels in the treatment of tarsal tunnels syndrome. J Reconstr Microsurg. 2008;24:119.
140 Patel AT, Gaines K, Malamut R, et al. Usefulness of electrodiagnostic techniques in the evaluation of suspected tarsal tunnel syndrome: an evidence-based review. Muscle Nerve. 2005;32:236.
141 Martinoli C, Bianchi S, Gandolfo N, et al. Ultrasound of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20:S199.
142 Peer S, Kovacs P, Harpf C, et al. High-resolution sonography of lower extremity peripheral nerves: anatomic correlation and spectrum of disease. J Ultrasound Med. 2002;21:315.
143 Spinner RJ, Tiel RL. Tarsal tunnel syndrome. In: Midha R, Zager EL, editors. Surgery of Peripheral Nerves: A Case-Based Approach. New York: Thieme, 2008.
144 Day FN3rd, Naples JJ. Endoscopic tarsal tunnel release: update 96. J Foot Ankle Surg. 1996;35:225.
145 Cimino WR. Tarsal tunnel syndrome: review of the literature. Foot Ankle. 1990;11:47.
146 Pfeiffer WH, Cracchiolo A3rd. Clinical results after tarsal tunnel decompression. J Bone Joint Surg Am. 1994;76:1222.
147 Barker AR, Rosson GD, Dellon AL. Outcome of neurolysis for failed tarsal tunnel surgery. J Reconstr Microsurg. 2008;24:111.
148 Dellon AL. Neurosurgical prevention of ulceration and amputation by decompression of lower extremity peripheral nerves in diabetic neuropathy: update 2006. Acta Neurochir Suppl. 2007;100:149.
149 Chaudhry V, Stevens JC, Kincaid J, et al. Practice Advisory: utility of surgical decompression for treatment of diabetic neuropathy: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2006;66:1805.
150 Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359.
151 Hurst LC, Weissberg D, Carroll RE. The relationship of the double crush to carpal tunnel syndrome (an analysis of 1,000 cases of carpal tunnel syndrome). J Hand Surg Br. 1985;10:202.
152 Osterman AL. The double crush syndrome. Orthop Clin North Am. 1988;19:147.
153 Simpson RL, Fern SA. Multiple compression neuropathies and the double-crush syndrome. Orthop Clin North Am. 1996;27:381.