Chapter 56 Disseminated Intravascular Coagulation
2 Why is DIC important?
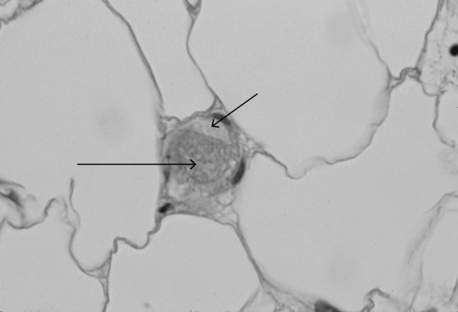
DIC is a common cause of concurrent thrombocytopenia and prolonged clotting times (activated partial thromboplastin time [aPTT] and prothrombin time [PT]) in hospitalized patients. It can also be an independent predictor of mortality. DIC leads to fibrin and platelet deposition in small vessels, which can cause tissue ischemia and result in organ dysfunction (Figure 56-1). The consumptive coagulopathy can also lead to clinically significant bleeding.
4 In critical care patients, what conditions are associated with DIC?
 Malignancy: adenocarcinoma, acute promyelocytic leukemia (incidence approaches 100%)
Malignancy: adenocarcinoma, acute promyelocytic leukemia (incidence approaches 100%)
 Obstetric: hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; retained uterine placental or fetal tissue; placental abruption or previa; amniotic fluid embolus
Obstetric: hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; retained uterine placental or fetal tissue; placental abruption or previa; amniotic fluid embolus
 Vascular: vasculitis, abdominal aortic aneurysm, cavernous hemangiomas
Vascular: vasculitis, abdominal aortic aneurysm, cavernous hemangiomas
 Miscellaneous: burns, anaphylaxis, transfusion reaction, snake bite, acute pancreatitis, transplant rejection, intravenous anti-D immunoglobulin
Miscellaneous: burns, anaphylaxis, transfusion reaction, snake bite, acute pancreatitis, transplant rejection, intravenous anti-D immunoglobulin
5 How does DIC present clinically?
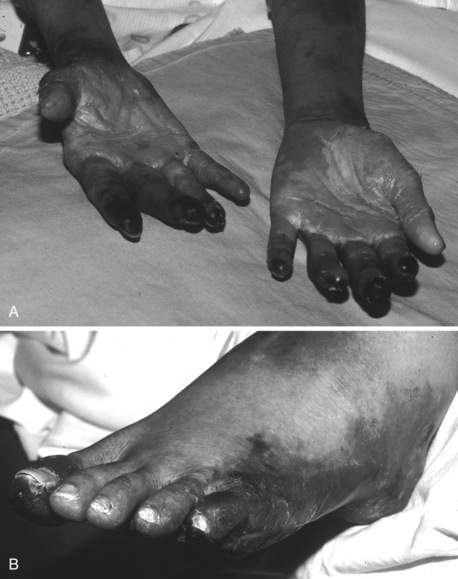
In acutely ill hospitalized patients, DIC usually presents with prolongation of the PT and aPTT along with decreased fibrinogen and platelets; systemic bleeding may or may not be present. Typically, bleeding manifests as ecchymoses, purpura, petechiae; it also occurs at surgical incisions or insertion sites of vascular access catheters. Mucosal and urinary bleeding are common, whereas pulmonary, gastrointestinal, and central nervous system bleeding occur less frequently. Because of widespread intravascular coagulation, tissue ischemia can occur, resulting in cyanosis, delirium, oliguria, hypoxia, and frank tissue necrosis (Figure 56-2).
7 Is the peripheral blood smear useful in the diagnosis of DIC?
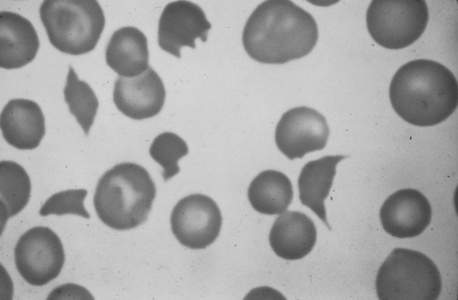
The peripheral blood smear from a patient typically shows mild to moderate thrombocytopenia. The finding of schistocytes (red blood cell fragments created by intravascular hemolysis) (Figure 56-3) is neither sensitive nor specific, and schistocytes are present in only 10% to 50% of cases of acute DIC. If present in DIC, there are usually only one to four per high-power field, in contrast to thrombotic thrombocytopenic purpura where they are much more abundant.
11 How should blood products be administered in cases of acute DIC?
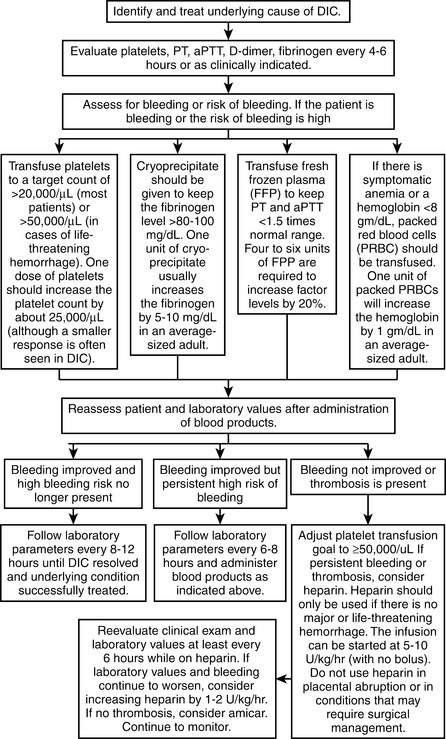
Platelet transfusions should be reserved for patients with signs of clinical bleeding; a platelet goal of >30,000/mcL with minor bleeding or >50,000/mcL with major bleeding is reasonable in DIC, except in patients with recent major neurosurgical procedures where platelet counts of >100,000/mcL may be required. Cryoprecipitate may be administered to keep the fibrinogen level >80 to 100 mg/dL. Fresh frozen plasma should be given only if clinically significant bleeding is present or if a significant risk for bleeding exists (e.g., for invasive procedures); it should not be used solely to correct a prolonged PT or aPTT. It should also be noted that international normalized ratio values of up to 1.7 are not associated with an increased risk for bleeding. Paradoxically, blood products are not associated with a worsening of DIC. See Figure 56-4 for additional details.
18 What new treatments may be available in the future for DIC?
Key Points Disseminated intravascular coagulation
1. Definition: DIC is a syndrome involving the activation of both coagulation and fibrinolysis, resulting in the intravascular deposition of fibrin and the consumption of coagulation proteins and platelets, which commonly leads to bleeding.
2. Importance: DIC can lead to organ dysfunction and is associated with high mortality.
3. Diagnosis: No single laboratory test can be used to diagnose DIC. Instead, a combination of a prolonged aPTT and PT, decreased fibrinogen, decreased platelets, increased D-dimer or FDPs, and schistocytes on a blood smear in an appropriate clinical context may suggest the diagnosis.
4. Treatment: Management of DIC should focus primarily on treatment of the underlying disorder.
1 Abraham E., Reinhart K., Opal S., et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247.
2 Abraham E., Reinhart K., Svoboda P., et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit Care Med. 2001;29:2081–2089.
3 Asakura H., Ontachi Y., Mizutani T., et al. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29:1164–1168.
4 Bick R.L. Disseminated intravascular coagulation: objective clinical and laboratory diagnosis, treatment, and assessment of therapeutic response. Semin Thromb Hemost. 1996;22:69–88.
5 Hermida J., Montes R., Paramo J.A., et al. Endotoxin-induced disseminated intravascular coagulation in rabbits: effect of recombinant hirudin on hemostatic parameters, fibrin deposits, and mortality. J Lab Clin Med. 1998;131:77–83.
6 Kitchens C.S. Thrombocytopenia and thrombosis in disseminated intravascular coagulation (DIC). Hematology Am Soc Hematol Educ Program. 2009;2009:240–246.
7 Levi M., Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:586–592.
8 Manios S.G., Kanakoudi F., Maniati E. Fulminant meningococcemia. Heparin therapy and survival rate. Scand J Infect Disease. 1971;3:127–133.
9 Schmaier A.H. Disseminated intravascular coagulation. N Engl J Med. 1999;341:1937–1938.
10 Segal J., Dzik W. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413.
11 Stouthard J.M., Levi M., Hack C.E., et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742.
12 Toussaint S., Gerlach H. Activated protein C for sepsis. N Engl J Med. 2009;361:2646–2652.
13 Yamakawa K., Fujimi S., Mohri T., et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care. 2011;15:R123.