Non-mechanical disorders
Pathology
Osseous disorders
Fractures and luxations
A considerable variety of fractures and dislocations follow trauma between the occiput and the first thoracic vertebra. Most are caused by car accidents, with falls and sports accidents next in frequency.1–3 Half of all patients present with neurological problems.4
Early diagnosis is important. The cervical fracture is often combined with another spinal fracture and therefore the entire vertebral column must be X-rayed.5 Plain radiographs are the first way in which diagnosis is established. Multidirectional CT is particularly advantageous in patients with facet injuries. CT seems to add most additional information in laminar or posterior column injuries, fractures of the vertebral body or in atlantoaxial subluxations.6
Fractures and dislocations of the atlantoaxial complex
Fractures of the axis are common. In 14–17.5% of all fractures of the cervical spinal column the lesion lies at the axis.7 Most frequent are odontoid fractures. They are classified as type I – avulsion of the tip of the odontoid process, type II – fracture through the base and type III – fracture through the vertebral body.8,9 These are followed in frequency by those of the vertebral body, the pedicle or the lateral mass. Less common is hangman’s fracture – a bilateral fracture through the pars interarticularis of the axis. Neurological damage is not frequent in odontoid and hangman’s fractures but quite common in the other miscellaneous fractures of the axis.10
Dislocations of the atlas are not uncommon and may lead to serious neurological damage.11 Fractures of the atlas are rare and seldom cause neurological problems.
In order of frequency the following fractures are found: bilateral fracture of the posterior arch, comminuted fracture of the ring of the atlas – traumatic spondylolisthesis or Jefferson’s fracture, and unilateral fracture of the lateral mass.12
Fractures of the lower cervical spine
Most fractures in the cervical spine occur below C2. They range from fractures of the articular process to fractures of the vertebral body, lamina, spinous process and pedicle. Fractures are commonly classified in six groups depending (in order of frequency) on the forces that have acted on the cervical spine: compressive flexion, distractive flexion, compressive extension, vertical compression, distractive extension and lateral flexion.13 Neurological problems most often occur when the fracture is combined with luxation.14,15 Pathological fractures following a minor injury or a sudden effort give rise to the same clinical picture but the history is much less indicative and may even be misleading. If neurological injury is present, the diagnosis of a probable fracture or dislocation is made more simple.
Clay-shoveller’s fracture
This is a fracture of one or more spinous processes in the lower cervical or upper thoracic spine (mostly C7, sometimes C6 or T1).16
A traction fracture may occur as the result of strong muscular force from the trapezius transmitted to the spinous processes through the musculature. It happens suddenly. A crack is felt followed by severe pain at the base of the neck and between the shoulders. The same event may occur in motor vehicle accidents where a strong flexion force is applied to the neck, and thus also in whiplash injuries.17,18
Bony tumours
Primary bone tumours are uncommon. They represent only 0.4% of all tumours and cervical localization accounts for only 4.2% of the primary bone tumours of the spine. This is very much less than in the thoracic or lumbar spine.19
The symptoms may vary and include local heat, tenderness, neuralgic pain, root palsy, torticollis-like limitation of neck movements20 and myelopathy, although early in development the symptoms may mimic ordinary soft tissue lesions. Severe pain at night is often a hallmark of neoplasm.21
The presence of one or more inconsistencies during history and/or functional examination is a warning sign and puts the examiner on guard (see Box 1). It is again mainly the clinical approach that suggests a serious disorder. Radiography – usually the first additional examination – is not always helpful, as it appears that more than 30% of the cancellous bone of the vertebral body must be destroyed before a plain X-ray becomes positive.22–24 More refined imaging such as CT scan, technetium scan, angiography and MRI confirms the diagnosis. A radiograph and CT scan of the chest or abdomen may be necessary in patients with unknown primary sites.
Benign tumours
The most common benign neoplasms affecting the cervical spine are, in order of frequency: osteoid osteoma, osteoblastoma, haemangioma, aneurysmal bone cyst, eosinophilic granuloma, giant cell tumour and osteochondroma. They can be found at any level, except C1, and are most common at C2, C4 and C7 levels.25–28
Osteoid osteoma
This is the most frequent benign tumour in the cervical spine20 and appears in young adults, mostly males.29,30 It affects the cervical spine less frequently than the lumbar spine and is located in the pedicles and vertebral arches.31
Osteoblastoma
This tumour affects the cervical spine as frequently as it does the thoracic spine. It is more common in the lumbar spine.25 Young adults – males more frequently – are affected and the posterior elements of the vertebra are involved, which may lead to radiculopathy and myelopathy.
Haemangioma
This occurs most often in women in their fourth decade.33 It is common and usually has an asymptomatic development – one-quarter of all cases are in the cervical spine.
Aneurysmal bone cyst
This is seen most frequently in children and young adults, mostly females (under 30 years old).35 Twenty-five per cent of spinal aneurysmal bone cysts are located in the cervical spine.28 It is a destructive tumour and is mostly localized in the neural arch but may also invade the vertebral body. As it expands, it may lead to root pain and even to compression of the spinal cord.
Excision and/or curettage and stabilization with bone grafts are indicated.36
Eosinophilic granuloma
The fracture may heal spontaneously. However, when the flattening is significant, neurological symptoms may follow; these are reversible when treatment is started without delay. Open biopsy, followed by immobilization and irradiation may be necessary.37,38
Giant cell tumour
This is more frequent in the sacrum and the lumbar spine but may also affect the cervical spine. It seems to occur in younger patients (between 20 and 40 years old), especially women.39 It leads to destruction of the vertebral body and can later involve the posterior part of the vertebra.
It may cause pain in the neck but can also give rise to radicular symptoms.
Malignant tumours
The primary malignant tumours represent 6.3% of all primary bone tumours of the spine and occur mainly from middle age on, much more frequently in men than in women. They are found at all levels, except C1.26
Multiple myeloma
The treatment of choice is radiation and/or systemic chemotherapy. The outlook is poor.
Chordoma
This is an uncommon, locally invasive, slow-growing malignant neoplasm that arises from the vertebral or suboccipital remnants of the embryonic notochord. In 33–38% it occurs in the upper cervical vertebrae, especially C2, and is found most often in men aged between 50 and 70 years.41,42
The tumour often extends anteriorly into the soft tissues and may then result in dysphagia, upper respiratory obstruction and Horner’s syndrome.43 Posterior extension may be accompanied by neurological complications, such as epidural spinal cord compression or cervical radiculopathy.44,45
Solitary plasmacytoma
This is a myeloma (plasma cell neoplasm) in a single vertebral body. The patient is over 60 years and complains of slowly progressing neck pain with muscle spasm. The prognosis is much more favourable than in patients with multiple myeloma.46 Collapse of the vertebral body and cord compression may result.
Metastases
Secondary deposits are the most common malignant tumours of the cervical spine, although this part of the vertebral column is the least affected, occurring in 8–20% of patients with known metastatic disease.47 Breast, lung, prostate, colon, kidney and thyroid are the most frequent sites of primaries.48 Metastases in the spine may pass unnoticed for a considerable time and are sometimes discovered during routine radiography.
In symptomatic cases, pain is the earliest and most prominent feature in 90%.49 Localized pain that starts spontaneously and becomes gradually worse, especially at night, is the most common picture of spinal metastases. It is axiomatic that a cancer patient who develops neck pain harbours a spinal metastasis until proven otherwise. If a patient presents with neck pain but has a history of a primary tumour, for example breast cancer, even a long time ago, metastases must be taken into consideration. Tumour-related pain is predominantly nocturnal or early morning pain and generally improves with activity during the day. This pain may be caused by inflammatory mediators or tumour stretching the periost of the vertebral body.50
The clinical features differ depending on whether the lesion is localized at the upper cervical spine (C1-C3), the lower cervical spine (C4-C7) or the upper thoracic spine (T1-T3).51
Upper thoracic metastases
If vertebral metastases are suspected, further investigations are arranged.
Plain radiographs are often ordered as the first test to evaluate a patient with cancer who has neck pain, but are relatively poor screening tests for metastases. Visualization of a radiolucent defect on plain radiographs requires a 30% destruction of the vertebral body. Additionally, metastatic tumour often infiltrates the bone marrow of the vertebral body without destroying the cortical bone. Bone scan (99mTc-MDP) is more sensitive than plain radiographs for detecting spinal metastases. The advantage of bone scan is the ability to screen the entire skeleton with a single image. However, the sensitivity is not 100%: patients with rapidly progressive, destructive tumours may not be detected and bone scan is relatively insensitive for multiple myeloma and tumours confined to the bone marrow.52 It also has a low specificity for tumour: fractures, degenerative disease, and benign disorders of the spine (Schmorl’s nodes, haemangioma) all may be positive.53 Since MRI is widely available, it has become the most sensitive and specific modality for imaging spinal metastases. Sagittal screening images of the entire spine reveal bone, epidural and paraspinal tumour. The extent and degree of spinal cord compression can be readily appreciated.54
Rheumatoid arthritis and ankylosing spondylitis
Rheumatoid arthritis
Rheumatoid arthritis is as frequent in the cervical spine as it is uncommon in other parts of the spine and the atlantoaxial complex especially becomes affected.55 The frequency of involvement of the cervical spine is between 25% and 80%56 and the longer a patient has rheumatoid arthritis the more chance it has to reach the cervical level.57 As in other joints the bony, cartilaginous and ligamentous structures are destroyed and laxity and deformation result.
Anterior atlantoaxial subluxation is the most frequent complication – it occurs in 49% of patients58 and represents 65% of all cervical subluxations59 – and results from laxity of the transverse ligament of the atlas and/or the ligaments of the dens (alar ligaments/apical ligament).60 The subluxation is mostly anterior but may be lateral and occasionally posterior. When, on a flexion/extension radiograph, the distance between the anterior arch of the atlas and the odontoid process of the axis exceeds 3 mm, laxity is probable. More than 10 mm indicates gross instability61 and requires surgical stabilization.62 The spinal cord is seriously threatened at this stage, especially during flexion movements. This may in the end lead to spasticity, hyperreflexia (deep tendon reflexes), weakness, sensory loss and urinary problems. More recent reports show a tendency to rely more on the posterior atlantodental interval, the distance between the posterior arch of the atlas and the dens, which should be a minimum 14 mm.63
If the destructive process reaches both the occipitoatlantal and atlantoaxial joint complexes, the odontoid process of the axis may protrude through the foramen magnum – cranial settling or vertical odontoid subluxation64,65 which happens in 38% of rheumatoid patients.66 Later in the development, the atlantoaxial instability tends to decrease again,67,68 leaving the patient with only odontoid subluxation. The lower cranial nerves, the cardiorespiratory centre and pyramidal tracts may all become compressed. Mikulowski et al report that 10% of rheumatoid arthritis patients may die of compression of the brainstem which is unrecognized before death.69
It is therefore important to do a radiographic evaluation of the cervical spine in all patients with rheumatoid arthritis, even when they are asymptomatic. Collins et al found that only 50% of patients with abnormalities on their radiographs had symptoms of their cervical disease.70
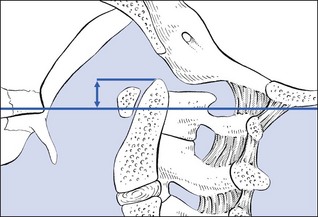
The degree of odontoid protrusion can be measured on a lateral radiograph. A line is drawn between the posterior edge of the hard palate and the inferior border of the occiput (the McGregor line). If the tip of the odontoid process lies more than 4.5 mm above this line, cranial settling is present (Fig. 1). If these landmarks are not clear, the Fischgold and Metzger measurement can be used: on an anteroposterior open-mouth tomogram the digastric line is drawn (between the points where the mastoid processes join the base of the skull), and the tip of the dens should lie 1 cm or more below this, to be classed as normal.71
Subaxial subluxation is less frequent (10–20% of patients) but may arise at several levels,72,73 especially C2–C3 and C3–C4. The laxity then occurs at the zygoapophyseal and uncovertebral joints74 and may result in compression of nerve root and/or vertebral artery, the latter leading to vertebrobasilar insufficiency and its consequent clinical features.
Many patients are asymptomatic. There may be a discrepancy between the degree of destruction or instability and the symptoms. Patients may be seen with slight instability and neurological problems only, whereas others may have significant laxity without neurological symptoms.75,76 Neurological dysfunction occurs in 7–34% of patients and may include brainstem, spinal cord and nerve roots.77
The disorder may cause moderate pain in the neck. Involvement of the upper cervical joints gives rise to pain often felt in the upper neck, radiating bilaterally to the occipital, temporal, auricular and retro-orbital regions. Occasionally the patient may complain of a clunking sensation with flexion movements (Sharp’s and Purser’s sign).78
On clinical examination a full articular pattern is found with a soggy or empty end-feel. This characteristic sensation immediately draws attention to the disorder. A clunking sensation on neck flexion indicates excessive movement between C1 and C2, as happens in atlantoaxial subluxation. The Sharp–Purser test may help to diagnose the condition; it appears to have a sensitivity of 69% and a specificity of 96% compared with radiological evidence.79 The patient sits on a chair. The examiner stands to one side and places the index finger on the spinous process of C2. The palm of the other hand lies on the patient’s forehead. An attempt is made to slide the head posteriorly and, if a slide between the spinous processes of C1 and C2 is felt, the test is positive.
Ankylosing spondylitis
When, late in its development, the disease reaches the cervical spine, this leads to increasing stiffness and limitation in all directions and, in the end, possibly to ankylosis in flexion. This happens in 75% of patients with a history of more than 16 years.80 The end-feel is hard and may ultimately become bone-to-bone. As long as this bone-hard end-feel is not present the pain can be controlled with slow gradual stretching manūuvres at regular intervals. Once this bony end-feel has occurred, any forcing of the joints is futile.
Complications may occur, such as atlantoaxial instability81 and fractures.82
Infections
Infectious disease affecting the cervical spine is very uncommon compared to the thoracic and lumbar spines. Tuberculosis in the cervical spine occurs in less than 5% of spinal involvement and infection by pyogenic organisms (i.e. discitis, osteomyelitis) is extremely rare but may lead to severe neurological problems.86,87 Infections of the spinal canal present as epidural, subdural or intramedullary abscesses. Parapharyngeal infections may lead to upper cervical ligamentous laxity.88
Intraspinal tumours
Extradural tumours
These constitute almost one-quarter of all primary spinal tumours. The majority are metastatic89,90 (see Fig. 2) and invade the vertebral bodies or arches: carcinoma of the bronchus is the most common primary but other sources are the breast, prostate, gastrointestinal tract, thyroid and kidney.91
These tumours must be differentiated from benign and malignant bony tumours of the spine (see pp. e14–e16), although any intradural neoplasm may also perforate the dura mater to become extradural. Pyogenic and tuberculous abscesses in the epidural space give rise to similar symptoms and signs.
These space-occupying lesions in the spinal canal very commonly lead to radicular symptoms and signs: root pain quickly follows the original local neck pain and motor and sensory deficit supervenes. Very soon in their development, the spinal cord becomes compressed and transverse myelopathy occurs with bladder dysfunction and further progressive sensory and motor deficit (see pp. 165–168).
Intradural extramedullary tumours
These form almost two-thirds of all intradural neoplasms,92 of which meningeomas and neuromas are the most common. Neuromas (schwannomas and neurofibromas) are benign, slowly growing peripheral nerve-sheath tumours originating from Schwann cells. Schwannomas are composed entirely of Schwann cells, whilst neurofibromas contain Schwann cells, fibroblasts, perineurial cells, mast cells and axons in an extracellular matrix. Neuromas in the cervical spine are not as frequent as they are in the thoracic spine but are more frequent than in the lumbar spine.93 Sometimes the growing tumour develops a large extradural component that expands towards the outside of the spinal canal. These so-called dumbbell neuromas present a special entity and account for 15% of all cervical neuromas.94
These tumours show a tendency to present with radicular symptoms very much resembling those caused by a disc protrusion. The root pain is quite constant, slightly influenced by activity and worse at night.95 Of all patients with symptoms consistent with disc herniation, 1% have an intraspinal tumour.96
The ease of differential diagnosis from a cervical disc lesion may vary. In some it is quite easy, while in others it may be extremely difficult. Any of the following features should arouse suspicion (Table 1).
Table 1
| Discoradicular interactions | Neurofibroma/schwannoma | |
| Symptoms | No root pain under 35 years | At any age |
| Root pain stabilizes after 2 months | Root pain continues to worsen after 6 months | |
| Secondary posterolateral evolution | Primary posterolateral onset | |
| Coughing may hurt in the scapular area | Coughing always hurts down the arm | |
| Unilateral root pain | Unilateral root pain may become bilateral | |
| Paraesthesia felt distally | Paraesthesia is felt all over the body | |
| Signs | Articular signs | No articular signs |
| Slight segmental weakness | Unusual weakness | |
| Symmetrical cord signs | Asymmetrical cord signs |
• Absence of articular signs: because the lesion is extra-articular, neck movements are negative. It should be noted, however, that this may also happen later in the evolution of a disc protrusion compressing the nerve root.
• Unusual weakness is strongly suspicious: this means weakness out of proportion to other symptoms and signs, or affecting muscles that are not usually involved or in muscles derived from more than one segment.
• Spinal cord signs may occur (spasticity, incoordination, incontinence, sensory loss) but are rarely symmetrical.
Differential diagnosis should be made with intramedullary and extradural tumours, and non-neoplastic conditions such as syrinx, spondylotic myeloradiculopathy, multiple sclerosis or spinal arteriovenous malformation.97
Radiographs may remain normal for a very long time. CT, myelography and particularly MRI are very useful diagnostic procedures.98
The treatment of choice is surgery and the prognosis is excellent.99,100
Intramedullary tumours
Intramedullary spinal cord tumours are rare lesions, most frequently seen in children and young adults.101 The majority are ependyomas and astrocytomas. Most are of low histological grade and follow indolent clinical courses, their presentation pattern depending upon the tumour’s anatomical location, which poses diagnostic challenges. Most lesions occur in the cervicothoracic cord.102 The commonest symptom is pain which may be diffuse in nature. Anterior horn cell and corticospinal tract involvement lead to upper limb weakness with small hand muscle wasting and upper motor neurone signs in the legs.103
The traditional treatment of intramedullary tumours is biopsy and dural decompression, followed by radiotherapy. In recent times, modern neurosurgical techniques including ultrasonic aspiration, laser, evoked potential monitoring and intraoperative ultrasonography have become available facilitating safer attempts at radical excision.104 Aggressive tumour resection reduces chances of local tumour recurrence but carries risks of major postoperative neurological impairment.105
Neurological conditions with positive signs on neck examination
• Mononeuritis of the long thoracic nerve: active elevation of the arm is grossly limited
• Mononeuritis of the spinal accessory nerve: active elevation of the arm is slightly limited
• Mononeuritis of the suprascapular nerve: resisted abduction and external rotation of the shoulder are weak
These conditions are discussed extensively in the online chapter Nerve lesions and entrapment neuropathies of the upper limb.
Shoulder girdle disorders causing cervicoscapular pain
In both cases the complete shoulder girdle examination must be done and is diagnostic. These conditions are discussed in detail in Chapter 23.
References
1. Hadley, MN, Browner, C, Sonntag, VKH, Axis fractures: a comprehensive review of management and treatment in 107 cases. Neurosurgery 1985; 17:281. ![]()
2. Weiss, MH, Mid- and lower cervical spine injuries. Neurosurgery. Wilkins, RH, Rengachary, SS, eds. Neurosurgery; vol 2. McGraw-Hill, New York, 1985:1708.
3. Reiss, SJ, Raque, GH, Shields, CB, et al, Cervical spine fractures with major associated trauma. Neurosurgery 1986; 18:327. ![]()
4. Graham, B, Van Peteghem, K, Fractures of the spine in ankylosing spondylitis. Diagnosis, treatment and complications. Spine 1989; 14:803. ![]()
5. Ducker, TB. Comments. Neurosurgery. 1986; 18:330.
6. Clark, CR, Igram, CM, El-Khoury, GY, Ehara, S, Radiographic evaluation of cervical spine injuries. Spine. 1988;13(7):742. ![]()
7. Clark, CR, White, AAIII, Cooper, P. Fractures of the dens: a multicenter study. Honolulu, Hawaii: Presented at the 35th Annual Meeting of Congress of Neurological Surgeons; 1985.
8. An, H, Cervical spine trauma. Spine. 1998;23(24):2713–2729. ![]()
9. Anderson, LD, D’Alonzo, RT, Fractures of the odontoid process of the axis. J Bone Joint Surg 1974; 56A:1663–1674. ![]()
10. Hadley, MN, Browner, C, Sonntag, VKH, Miscellaneous fractures of the second cervical vertebra. Barrow Neurol Inst Q 1985; 1:34. ![]()
11. Bohlman, HH, Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg 1979; 61B:1119. ![]()
12. Garfin, SR, Rothman, RH. Traumatic spondylolisthesis of the axis (hangman’s fracture). In: Sherk HH, Dunn EJ, Eismont FJ, et al, eds. The Cervical Spine. 2nd ed. Philadelphia: Lippincott; 1989:223.
13. Allen, BL, Ferguson, RI, Lehman, TR, O’Brien, RP, A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine 1982; 7:1–27. ![]()
14. Sonntag, VKH, Management of bilateral locked facets of the cervical spine. Neurosurgery 1981; 150:150. ![]()
15. Sonntag, VKH, The early management of cervical spine injuries. Arizona Med 1982; 10:644. ![]()
16. White, AA, III., Panjabi, MM. Clinical Biomechanics of the Spine. Philadelphia: Lippincott; 1978.
17. Wong, WB, Panjabi, MM, White, AAIII. Mechanisms of injury in the cervical spine. Basic concepts, biomechanical modeling, experimental evidence, and clinical applications. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:95.
18. Meyer, PR, Heim, S. Surgical stabilization of the cervical spine. In: Meyer PR, ed. Surgery of Spine Trauma. New York: Churchill Livingstone; 1989:414.
19. Weinstein, JN, Surgical approach to spine tumours. Orthopedics 1989; 12:897–905. ![]()
20. Levine, AM, Boriani, S, Donati, D, Campanacci, M, Benign tumours of the cervical spine. Spine. 1992;17(10):399–406. ![]()
21. Abdu, WA, Provencher, M, Primary bone and metastatic tumours of the cervical spine. Spine. 1998;23(24):2767–2777. ![]()
22. Edelstyn, GA, Gillespie, PG, Grebbel, FS, The radiologica demonstration of skeletal metastases: experimental observations. Clin Radiol 1967; 18:158. ![]()
23. Vanzanten, TEG, Tevle, GJJ, Golding, RP, Heidendal, GAK, CT and nuclear medicine imaging in vertebral metastases. Clin Nucl Med 1985; 14:334. ![]()
24. Raskas, DS, Graziano, GP, Herzenberg, JE, Heidelberger, KP, Hensinger, RN, Osteoid osteoma of the spine. J Spinal Disord 1992; 5:204–211. ![]()
25. Bohlman, HH, Sachs, BL, Carter, JR, Riley, L, Robinson, RA, Primary neoplasms of the cervical spine. Diagnosis and treatment of twenty-three patients. J Bone Joint Surg 1986; 68A:483–493. ![]()
26. Di Lorenzo, N, Delfini, R, Ciappetta, P, Cantore, G, Fortuna, A, Primary tumors of the cervical spine: surgical experience with 38 cases. Surg Neurol 1992; 38:12–18. ![]()
27. Liu, H, Liu, Z, Surgical treatment of cervical spine tumors. Chin Med J 1992; 105:564–566. ![]()
28. Levine, AM, Boriani, S, Donati, D, Campanacci, M, Benign tumors of the cervical spine. Spine 1992; 17:399–406. ![]()
29. Pettine, KA, Klassen, RA, Osteoid osteoma and osteoblastoma of the spine. J Bone Joint Surg 1986; 68A:354–361. ![]()
30. Kan, P, Schmidt, MH. Osteoid osteoma and osteoblastoma of the spine. Neurosurg Clin N Am. 2008; 19(1):65–70.
31. Maiuri, F, Signorelli, C, Lavano, A, et al, Osteoid osteomas of the spine. Surg Neurol 1986; 25:375. ![]()
32. Marsh, BW, Bonfiglio, M, Brady, LP, Enneking, WF, Benign osteoblastoma: range of manifestations. J Bone Joint Surg. 1975;57A(1):1. ![]()
33. Healey, M, Herz, DA, Pearl, L, Spinal hemangiomas. Neurosurgery 1983; 13:689. ![]()
34. Graham, JJ, Yang, WC, Vertebral hemangioma with compression fracture and paraparesis treated with preoperative embolization and vertebral resection. Spine 1984; 9:97. ![]()
35. Stillwell, WT, Fielding, JW, Aneurysmal bone cyst of the cervicodorsal spine. Clin Orthop Rel Res 1984; 187:144. ![]()
36. Hay, MC, Paterson, D, Taylor, TK, Aneurysmal bone cysts of the spine. J Bone Joint Surg. 1978;60A(3):406. ![]()
37. Fowles, JV, Bobechko, WP, Solitary eosinophilic granuloma in bone. J Bone Joint Surg. 1970;52(2):238. ![]()
38. Makely, JT, Carter, JR, Eosinophilic granuloma of bone. Clin Orthop Rel Res 1986; 204:37. ![]()
39. Dahlin, DC, Giant-cell tumor of vertebrae above the sacrum. Cancer 1977; 39:1350. ![]()
40. Malat, J, Virapongse, C, Levine, A, Solitary osteochondroma of the spine. Spine. 1986;11(6):625. ![]()
41. Huvos, AG. Bone Tumors: Diagnosis, Treatment and Prognosis. Philadelphia: Saunders; 1979.
42. O’Neill, P, Bell, BA, Miller, JD, Jacobson, I, Guthrie, W, Fifty years of experience with chordomas in southeast Scotland. Neurosurgery 1985; 16:166. ![]()
43. Boriani, S, Sundaresan, N, Weinstein, JN. Primary malignant tumors of the cervical spine. In: Clark CR, Ducker TB, Dvorak J, et al, eds. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:643–657.
44. Rich, TA, Schiller, A, Suit, HD, Mank, HJ, Clinical and pathologic review of 48 cases of chordoma. Cancer 1985; 56:182. ![]()
45. Boriani, S, Chevalley, E, Weinstein, JN, et al, Chordoma of the spine above sacrum – treatment and outcome in 21 cases. Spine 1996; 21:1569–1571. ![]()
46. McLain, RF, Weinstein, JN, Solitary plasmocytomas of the spine: a review of 84 cases. J Spinal Disord. 1989;2(2):69. ![]()
47. Riley, LH, 3rd., Frassica, DA, Kostuik, JP, Frassica, FJ, Metastatic disease to the spine: diagnosis and treatment. Instr Course Lect 2000; 49:471–477. ![]()
48. Sciubba, DM, Gokaslan, ZL, Diagnosis and management of metastatic spine disease. Surg Oncol 2006; 15:141–151. ![]()
49. White, AP, Kwon, BK, Lindskog, DM, Friedlaender, GE, Grauer, JN, Metastatic disease of the spine. J Am Acad Orthop Surg 2006; 14:587–598. ![]()
50. Gokaslan, ZL, York, JE, Walsh, GL, et al, Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg 1998; 89:599–609. ![]()
51. Atanasin, JP, Badatcheff, F, Pidhorz, L, Metastatic lesions of the cervical spine, a retrospective analysis of 20 cases. Spine. 1993;18(10):1279–1284. ![]()
52. Gosfield, E, Alavi, A, Kneeland, B, Comparison of radionuclide bone scans and magnetic resonance imaging in detecting spinal metastases. J Nucl Med 1993; 34:2191–2198. ![]()
53. Taoka, T, Mayr, NA, Lee, HJ, et al, Factors influencing visualization of vertebral metastases on MR imaging versus bone scintigraphy. AJR Am J Roentgenol 2001; 176:1525–1530. ![]()
54. Schiff, D, O’Neill, BP, Wang, CH, et al, Neuroimaging and treatment implications of patients with multiple epidural spinal metastases. Cancer 1998; 83:1593–1601. ![]()
55. Grantham, SA, Lipson, SJ. Rheumatoid arthritis and other non-infectious inflammatory diseases. In: Sherk HH, ed. The Cervical Spine. 2nd ed. Philadelphia: Lippincott; 1989:564.
56. Rajangam, K, Thomas, IM, Frequency of cervical spine involvement in rheumatoid arthritis. J Indian Med Assoc 1995; 93:138–139. ![]()
57. Oda, T, Fujiwara, K, Yonenobu, K, Azuma, B, Ochi, T, Natural course of cervical spine lesions in rheumatoid arthritis. Spine 1995; 20:1128–1135. ![]()
58. Morizono, Y, Sakou, T, Kawaida, H, Upper cervical involvement in rheumatoid arthritis. Spine 1987; 12:721–725. ![]()
59. Boden, SD, Clark, CR. Rheumatoid arthritis of the cervical spine. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:693–703.
60. Stevens, JC, Cartlidge, NE, Saunders, M, et al, Atlanto-axial subluxation and cervical myelopathy in rheumatoid arthritis. Q J Med 1971; 40:391. ![]()
61. Fielding, JW, Cochran van, GB, Lawsing, JF, 3rd., Holh, M, Tears of the transverse ligament of the atlas, a clinical and biomechanical study. J Bone Joint Surg 1974; 56A:1683–1691. ![]()
62. Clark, CR, Goetz, DD, Menezes, AH, Arthrodesis of the cervical spine in rheumatoid arthritis. J Bone Joint Surg 1989; 71A:381–392. ![]()
63. Boden, SD, Dodge, LD, Bohlman, HH, Rechtine, GR, Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg 1993; 75A:1282–1297. ![]()
64. Dirheimer, Y. The Craniovertebral Region in Chronic Inflammatory Rheumatic Diseases. Berlin: Springer; 1977.
65. Rasker, JJ, Cosh, JA, Radiological study of cervical spine and hand in patients with rheumatoid arthritis of 15 years’ duration; an assessment of the effects of corticosteroid treatment. Ann Rheumatol Dis 1978; 37:529. ![]()
66. Reiter, MF, Boden, SD, Inflammatory disorders of the cervical spine. Spine. 1998;23(24):2755–2766. ![]()
67. Casey, A, Crockard, H, Geddes, J, Stevens, J, Vertical translocation: The enigma of the disappearing atlantodens interval in patients with myelopathy and rheumatoid arthritis. Part I: Clinical, radiological and neuropathological features. J Neurosurg 1997; 87:856–862. ![]()
68. Casey, A, Crockard, H, Stevens, J, Vertical translocation. Part II: Outcomes after surgical treatment of rheumatoid cervical myelopathy. J Neurosurg 1997; 87:863–869. ![]()
69. Mikulowski, P, Wollheim, FA, Rotmil, P, Olsen, I, Sudden death in rheumatoid arthritis with atlanto-axial dislocation. Acta Med Scand 1975; 198:445–451. ![]()
70. Collins, DN, Barnes, CL, Fitz, RL, Cervical spine instability in rheumatoid patients having total hip or knee arthroplasty. Clin Orthop Rel Res 1991; 272:127–135. ![]()
71. Grantham, SA, Lipson, SJ. Rheumatoid arthritis in the cervical spine. In: Sherk HH, ed. The Cervical Spine. 2nd ed. Philadelphia: Lippincott; 1989:567.
72. Paimela, L, Laasonen, L, Kankaanpaa, E, Leirisalo-Repo, M, Progression of cervical spine changes in patients with early rheumatoid arthritis. J Rheumatol 1997; 24:1280–1284. ![]()
73. Yonezawa, T, Tsuji, H, Matsui, H, Hirano, N, Subaxial lesions in rheumatoid arthritis. Radiographic factors suggestive of lower cervical myelopathy. Spine 1995; 20:208–215. ![]()
74. Ball, J, Sharp, J, Rheumatoid arthritis of the cervical spine. Modern Trends in Rheumatology. Hill, AGS, eds. Modern Trends in Rheumatology; vol 2. Butterworth, London, 1971:117.
75. Meijers, KAE, van Beusekam, GT, Luyendijk, W, Duijfjes, F, Dislocation of the cervical spine with cord compression in rheumatoid arthritis. J Bone Joint Surg 1974; 56B:668. ![]()
76. Shaw, DA, Cartlidge, NE, Cervical myelopathy in rheumatoid arthritis. Acta Neurol Belg 1976; 76:279. ![]()
77. Clark, CR, Degenerative conditions of the spine: differential diagnosis and non-surgical treatment. The Adult Spine, Principles and Practice. Frymoyer, JW, eds. The Adult Spine, Principles and Practice; vol 2. Raven Press, New York, 1991:1154.
78. Sharp, J, Purser, DW, Spontaneous atlanto-axial dislocation in ankylosing spondylitis and rheumatoid arthritis. Ann Rheumatol Dis 1961; 20:47. ![]()
79. Uitvlugt, G, Indenbaum, S, Clinical assessment of atlantoaxial instability using the Sharp–Purser test. Arthritis Rheum 1988; 31:918. ![]()
80. Wade, W, Saltzstein, R, Mainman, D, Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil 1989; 70:39. ![]()
81. Ramos, C, Gomez, A, Guzman, JL, et al, Frequency of atlantoaxial subluxation and neurologic involvement in patients with ankylosing spondylitis. J Rheumatol 1995; 22:2120–2125. ![]()
82. Olerud, D, Frost, A, Bring, J, Spinal fractures in patients with ankylosing spondylitis. Eur Spine J 1996; 5:51–55. ![]()
83. Montgomery, WW, Peroue, PM, Schall, LA, Arthritis of cricoarytenoid joint. Ann Otol Rhinol Laryngol 1955; 64:1025. ![]()
84. Wojtulewski, JA, Sturrock, RD, Branfoot, AC, Hart, FD, Crycoarytenoid arthritis in ankylosing spondylitis. BMJ 1973; ii:145. ![]()
85. Cyriax, JH. Textbook of Orthopaedic Medicine. vol I, Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
86. Forsythe, M, Rothman, RH, New concepts in the diagnosis and treatment of infections of the cervical spine. Orthop Clin North Am 1978; 9:1039. ![]()
87. Hsu, LCS, Yau, ACM. Tuberculosis. In: Sherk HH, ed. The Cervical Spine. 2nd ed. Philadelphia: Lippincott; 1989:544.
88. Currier, BL, Heller, JG, Eismont, FJ. Cervical spinal infections. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:659–690.
89. Simeone, F. Intraspinal neoplasms. In: Rothman R, Simeone F, eds. The Spine. Philadelphia: Saunders; 1992:1515–1528.
90. Sundaresan, N, Sachdev, VP, Holland, JF, et al, Surgical treatment of spinal cord compression from epidural metastasis. J Clin Oncol 1995; 13:2330–2335. ![]()
91. Fornasier, VL, Horne, JG, Metastases to the vertebral column. Cancer 1975; 36:490. ![]()
92. Nittner, K, Spinal meningiomas, neurinomas and neurofibromas – hourglass tumors. Handbook of Clinical Neurology. Vinken, PJ, Bruyn, GW, eds. Handbook of Clinical Neurology; vol 20. Elsevier, New York, 1976:177.
93. Russell, DS, Rubinstein, U. Pathology of Tumours of the Nervous System. London: EA Pall; 1977.
94. Lot, G, George, B, Cervical neuromas with extradural components: surgical management in a series of 57 patients. Neurosurgery 1997; 41:813–820. ![]()
95. Austin, GM, The significance and nature of pain in tumors of the spinal cord. Surg Forum 1959; 10:782. ![]()
96. Epstein, JA, Common errors in the diagnosis of herniation of the intervertebral disk. Indust Med 1970; 39:488. ![]()
97. Zeidman, SM, Ellenbogen, RG, Ducker, TB. Intradural tumors. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:587–601.
98. Fine, MJ, Kricheff, II, Freed, D, Epstein, FJ, Spinal cord ependymomas: MR imaging features. Radiology 1995; 197:655–658. ![]()
99. Schultheiss, R, Gullotta, G, Resection of relevant nerve roots in surgery of spinal neurinomas without persisting neurological deficit. Acta Neurochir (Wien) 1993; 122:91–96. ![]()
100. Lot, G, George, B, Cervical neuromas with extradural components: surgical management in a series of 57 patients. Neurosurgery 1997; 41:813–820. ![]()
101. Nadkarni, TD, Rekate, HL, Pediatric intramedullary spinal cord tumors. Critical review of the literature. Child Nerv Syst 1999; 15:17–28. ![]()
102. Epstein, FJ, Farmer, J-P, Freed, D, Adult intramedullary astrocytomas of the spinal cord. J Neurosurg 1992; 77:355–359. ![]()
103. Mihehan, KJ, Shaw, EG, Scheithauer, BW, Davis, DL, Onofrio, BM, Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg 1995; 83:590–595. ![]()
104. Ahmed, TS, Oliver, M, Blackburn, N, Insidious onset neck pain – a symptom not to be dismissed. Ann R Coll Surg Engl 2007; 89:W6–W8. ![]()
105. Hausmann, ON, Kirsch, EC, Tolnay, M, Gratzl, O, Intramedullary spinal cord tumours: a clinical outcome and radiological follow-up study. Swiss Med Wkly 2001; 131:582–587. ![]()