Disorders of the inert structures
Capsular pattern
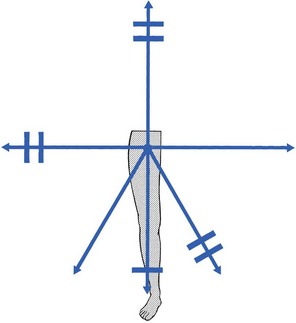
The capsular pattern at the hip joint is gross limitation of medial rotation, abduction and flexion, less limitation of extension, and little or no limitation of adduction and lateral rotation (Fig. 47.1). In advanced arthritis, abduction and internal rotation are impossible and associated with obvious limitation of flexion and extension.
A capsular pattern in the hip joint of a child or adolescent always implies a serious problem. The slightest limitation of movement should be reason enough to put the child on bed rest and start diagnostic procedures to detect the cause. Weight bearing is prohibited until the reason for the capsulitis is discovered (see online chapter Hip disorders in children).
Traumatic arthritis
This results more often from overuse of a stiffened and arthrotic joint than from a single injury. When it occurs in a child, transitory arthritis or the beginning of Perthes’ disease should always be suspected (see online chapter Hip disorders in children).
Monoarticular steroid-sensitive arthritis
Rheumatoid conditions
In rheumatoid conditions, the hip is affected only late in the evolution of the disease.
In polymyalgia rheumatica, both shoulders and hips are involved early in the course of the disease. Systemic therapy quickly relieves signs and symptoms.2
Septic arthritis
As a rule, this is caused by haematogenous dissemination, although it can also be the result of an intra-articular injection. Septic arthritis at the hip is not only a disaster for the joint but can also be life-threatening.3
Local aspiration should be carried out and systemic antibiotic therapy should be administered at once.4
Monoarticular arthritis in middle-aged people
This condition was described by Cyriax5 (his p. 386). For no special reason, a middle-aged patient experiences aching at the anterior aspect of the thigh during exertion. Clinical examination of the hip shows only a slight capsular pattern, with some limitation of internal rotation and flexion. There is pain at the end of range and the end-feel is elastic. The radiograph reveals nothing but a normal hip joint. The condition continues unchanged for months. Intra-articular injection with triamcinolone seems to be ineffective, but the lesion responds very well to stretching of the capsule, which relieves the pain quickly and permanently (see p. 633–634 for technique).
Osteoarthrosis
Aetiology
It is widely accepted that the most likely causative factor in the development of arthrosis of the hip is the incapacity of (parts of) the hip to withstand mechanical stresses. In the literature, a distinction is made between primary and secondary arthrosis.6
When the osteoarthrosis results from an undetermined abnormality of the cartilage or the subchondral bone, the condition is called idiopathic or primary. Primary osteoarthrosis is extremely rare – in more than 90% of cases, previous abnormalities in the hip joint can be demonstrated.7–9
Cartilage fibrillation
Strain may induce loss of proteoglycan molecules in the collagen network.10 This results in an increase in hydration and loss of tensile strength, and initiates the fibrillation of the cartilage.11,12
Subchondral bone
The health of articular cartilage depends largely on the mechanical qualities of its bony subchondral bed: the greater the discontinuity in elasticity between cartilage and subchondral bone, the more shear stress will occur.13 The presence of stiffened subchondral bone thus increases the likelihood of the progression of cartilage lesions.14
Capsule of the joint
Inflammation of the synovial membrane can also be responsible for the degeneration of cartilage: connective tissue activating peptides and catabolites are set free during inflammatory reactions and have a negative influence on cartilage and subchondral bone.15,16
In addition, rigidity of the capsule, so often seen in the early stages of arthrosis,17 plays a role in its progression: because of the loss of laxity, the normal gliding movement of the cartilage surfaces is altered, which imposes a change in load distribution and thus increases stresses on particular areas of the joint.
Synovial fluid
The composition of the synovial fluid may enhance the development of osteoarthrosis: a decrease in viscoelastic quality provokes more friction between the joint surface. It is well to remember that immobilization also decreases synovial fluid hyaluronan levels and may thus contribute to the progression of osteoarthrosis.18
Muscular dysfunction
Muscular dysfunction and disturbed neuromuscular balance are quite common in osteoarthrosis of the hip. They cause the joint to work under abnormal conditions and may play a role in the development or continuation of hip osteoarthrosis.19 A pattern of tightness and overactivity of the psoas, adductors, tensor fasciae latae and rectus femoris is typical in arthrosis of the hip joint, whereas the gluteals show a tendency towards weakness and inhibition.20,21
The continuous interaction between the changing structures of the joint imposes a physiological imbalance, which starts the process of degeneration. Vigorous and persistent attempts to repair the degenerative changes aggravate the already disordered joint function and set up a vicious circle. Hypervascularity, weakening of the subchondral bone, fatigue fractures, localized zones of collapse, flattening of the femoral head and formation of osteophytes then become inevitable (Fig. 47.3). This whole process can lead to rapid destruction of the joint; however, this is not always the case and spontaneous clinical and radiological improvements can occur.22
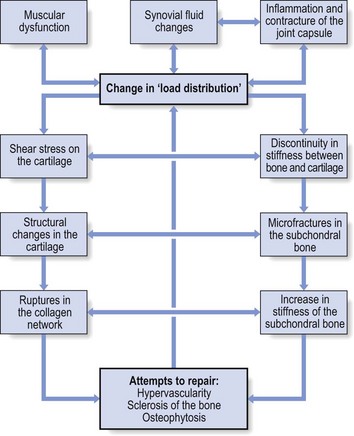
Fig 47.3 The aetiology of osteoarthrosis.
Symptoms and signs
Symptoms
In the early stage, pain is only present during and after exercise but later it becomes continuous and can even disturb sleep. It is suggested that pain at night in coxarthrosis is associated with an increase in intracapsular pressure and subsequent joint contracture.23 If the patient mentions twinges during normal walking, the disorder is considered to be complicated by impacted loose bodies (see later).
Signs
The examination often reveals a capsular pattern, with internal rotation the most limited and some limitation of flexion, extension and abduction, but this is certainly not always so. Many cases of hip osteoarthrosis present with other movement restriction patterns: for example, gross limitation of both internal and external rotation.24 As a rule, there is considerable difference between the clinical signs of an early osteoarthrosis and those found in advanced cases.
In advanced instances, gross limitation is found, with loss of all rotational movement. In extreme cases, a ‘hinge joint’ develops, allowing only flexion and extension in an oblique plane: the femur moves laterally when flexion is forced. The end-feel is hard and marked coarse crepitus can be palpated. Muscle tightness can sometimes be detected by performing muscle length test procedures such as those described by Janda and Lewit.25–27
Radiography
‘Osteoarthrosis of the hip’ must be a clinical diagnosis and it is unwise to rely entirely on the radiograph for estimation of functional incapacity and for deciding on optimal treatment. First, there is a considerable lack of correspondence between the degree of pain, the mobility of the joint and the radiograph appearances.28 Second, the patient may suffer from other lesions at or around the arthrotic hip. These lesions – loose body, psoas or gluteal bursitis (see pp. 642–647) – are not radiographically visible. If a radiological examination is performed without a full history and proper clinical examination of the hip, such conditions will be missed and the painless arthrosis will be blamed for the pain.
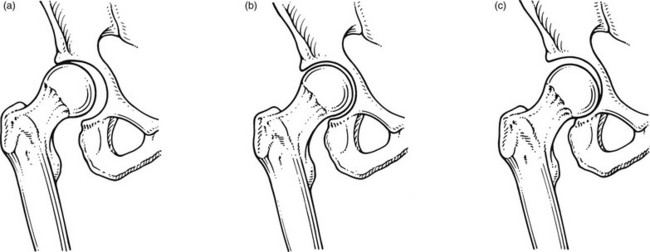
• Most osteoarthrotic hips show superolateral migration of the femoral head with localized erosion of cartilage at the lateral border of the labrum and a widening of the inferomedial part of the joint.29 Cameron and MacNab suggest that this is the form of osteoarthrosis that is primarily related to capsular restrictions and responds well to capsular stretching.30
• A medial–axial migration occurs in about 10–15% of cases. This presentation is usually associated with gross osteophytosis at the lower border of the femur and labrum.
• Another 10–15% of cases are non-migratory hip osteoarthrosis, associated with superior or concentric loss of cartilage space and concentric formation of osteophytes (Fig. 47.4).
Treatment
Early treatment of osteoarthrosis is vital. There is evidence that reduced motion of the hip (from capsular tightening and muscular imbalance) further increases the degenerative process in cartilage and subchondral bone. Several studies have demonstrated the beneficial effect of exercise on pain and disability.31 The treatment of choice is therefore early stretching of the joint (grade B mobilizations). Treatment with injections has only a limited indication. In later stages or in quickly developing osteoarthrosis, conservative treatment is useless and surgery is indicated (Box 47.1).
Capsular stretching
It is generally believed that early stretching of a tight capsule may prevent joint damage or at least slow further progression.32 Therefore, stretching is the treatment of choice in the early stage of the disease. The decision to use it depends largely on the clinical findings: early arthrosis with a slight capsular end-feel usually responds quite well to such treatment. Stretching is of no use in advanced arthrosis with gross limitation of movement, a hard end-feel and coarse crepitus because these are the clinical indications of gross cartilaginous destruction and formation of large osteophytes.
Technique: forced flexion
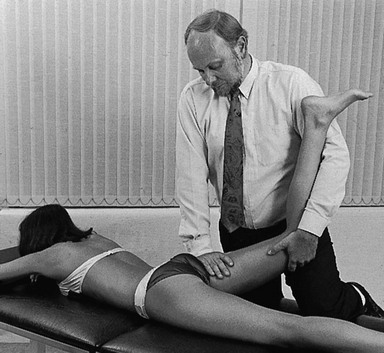
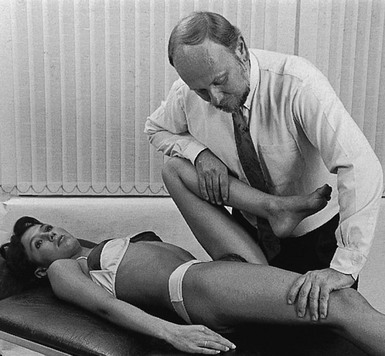
The patient adopts a half-lying position on the couch. The therapist stands on the affected side, level with the patient’s knee. The hip is flexed as far as is comfortably possible. One hand rests on the anterior aspect of the knee, while the other presses the opposite thigh down towards the couch. With steadily increasing pressure applied to the knee, the thigh is forced towards flexion (Fig. 47.5). This position is maintained for as long as the patient can bear it: e.g. 1 minute. The pressure is then slowly released to give the patient a break and the same procedure is repeated.
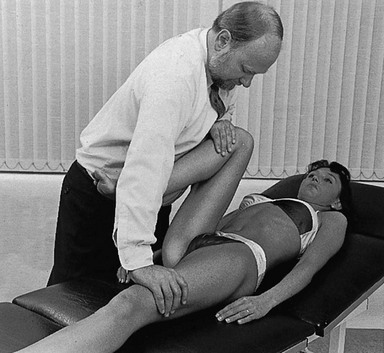
Fig 47.5 Forced flexion of the right hip.
Technique: forced extension – 1
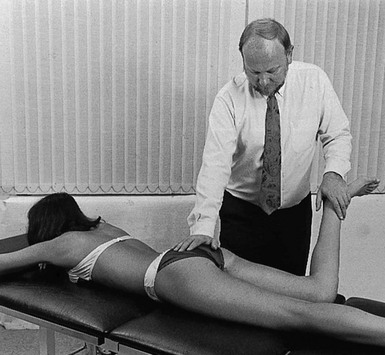
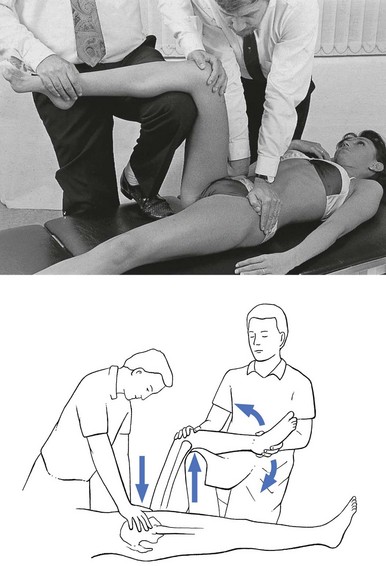
The patient lies prone on a high couch. The therapist stands level with the thighs. To prevent stress on the lumbar spine, it is vital to keep the patient’s pelvis on the couch when extension of the hip is forced. Therefore, with one hand, the therapist presses on the lower part of the buttock, which will obstruct movements at the lower back and the sacroiliac joint. With the other hand, the therapist grasps the thigh from the medial side, just above the patella. Both arms are kept rigid. Bending the body sideways, the therapist then pulls the patient’s thigh upwards, while the patient’s pelvis is pushed forcefully downwards (Fig. 47.6).
Technique: forced extension – 2
Some elderly people have difficulty lying on their stomach for any length of time and an alternative method can be used. The patient lies flat on the back, with the head supported with a cushion. The painless hip is now forced as far into flexion as possible, which tilts the pelvis and makes the other thigh lift off the couch. Sufficient extension can now be forced when sustained downward pressure is applied just above the patella (Fig. 47.7).
Technique: forced medial rotation
The patient adopts a prone-lying position on a high couch. The knee at the affected side is bent at a right angle. The therapist stands on the affected side and level with the pelvis. The ipsilateral hand is pressed on to the ilium. The other hand is placed at the medial malleolus and forces the hip into medial rotation. The pelvis rotates and moves the iliac crest backwards on the opposite side. Pressure applied on the pelvic region now forces the iliac crest downwards again. When the thigh on the affected side is held firmly in the rotated position, this downward pressure will considerably increase the outward stress on the affected hip (Fig. 47.8).
Traction
Traction (either manual or mechanical) is an alternative technique to stretch the joint capsule.33–35
Traction I
Traction is performed by leaning backwards with straight arms (Fig. 47.9). Once the therapist feels the patient relaxing the muscles, a jerk can be tried by pulling the arms towards the body. At this point, slight separation of the femoral head from the acetabulum can be felt.
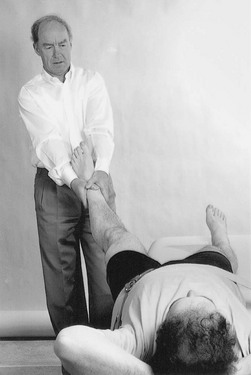
Fig 47.9 Manual traction.
Traction II
The therapist sits or stands at the level of the pelvis. The leg should be flexed to at least 90° and slightly laterally rotated. Both hands take hold of the upper part of leg (Fig. 47.10a). Traction is performed in the direction of the acetabulum: inferiorly, laterally and anteriorly.
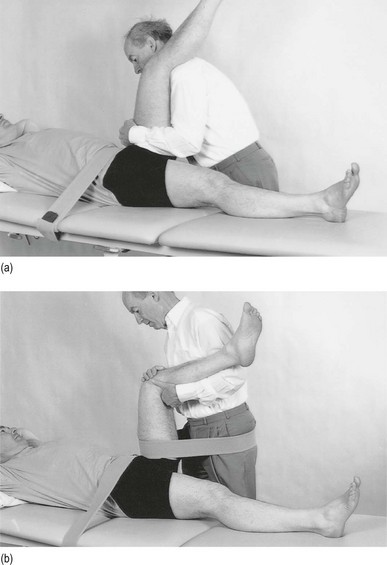
Fig 47.10 Mechanical traction.
Use of a band makes it possible to lessen the effort of the therapist a great deal. Both hands then hold the leg in position at the knee (Fig. 47.10b). Traction is achieved by leaning backwards.
Muscular re-education
In order to correct the pattern of muscular dysfunction and a disturbed neuromuscular balance, selective activation of inhibited, weak muscles and stretching of tight, shortened muscles is advocated by several authors.38–41 The second measure is of even greater importance because tight, hyperactive muscles interfere with the activation of inhibited muscles. These muscles (usually psoas, tensor fasciae latae and rectus femoris) are stretched slowly without straining the joint.
Activation of inhibited muscles is achieved by exercises with low loads to prevent overflow into other muscles. It is also advisable to perform exercises as closely as possible to their functional manner. For this purpose, closed kinetic chain exercises are advocated because the weight-bearing component effectively stimulates mechanoreceptors around the joint, so improving muscular contractions.42
Intra-articular injections
If the patient suffers from a subacute exacerbation because of traumatic arthritis superimposed on a stiffened arthrotic capsule, one intra-articular injection with 50 mg of triamcinolone will relieve the traumatic inflammation and the pain but increased mobility will not follow. To avoid arthropathy, this injection should not be repeated.43
Also, intra-articular injections with hyaluronic acid seem to be a safe and effective method of treatment for patients with advanced hip arthrosis and may lead to a significant reduction in pain scores, disability scores and analgesic use.44–46
Surgery
Total hip replacement is indicated in advanced osteoarthrosis. It is one of the most commonly performed operations in the United States, with over 280 000 procedures reported annually.47 The benefits of total hip replacement in terms of reduced pain and improved function and quality of life for patients with advanced coxarthrosis have been well documented in the literature.48 The prosthesis is made up of two parts: an acetabular component made of a metal shell with a plastic inner socket (the socket portion) that replaces the acetabulum, and a femoral component made of metal (the stem portion) that replaces the femoral head.
The life expectancy of the prosthesis is between 15 and 20 years.49 Aseptic loosening with or without osteolysis is the major problem and accounts for 71% of the revisions, but the incidence had decreased three times during the past 15 years to less than 3% at 10 years in Sweden.50 However, long-term durability of the acetabular components remains a major concern.51
Concerns regarding high rates of failure among young, active patients and a desire to preserve bone for future revision operations led to the development of hip resurfacing arthroplasty. This differs from total hip replacement in that the femoral head is resurfaced rather than resected, thereby preserving femoral bone stock, which could theoretically decrease morbidity and improve patient outcomes associated with future revision operations.52,53
Non-capsular pattern
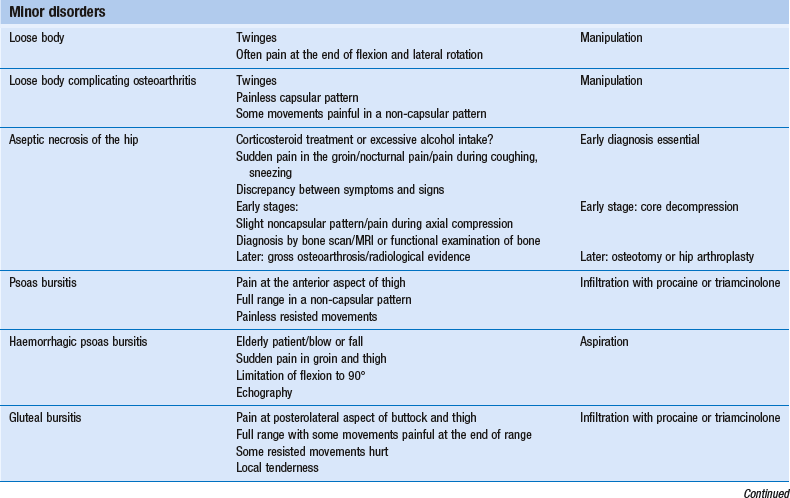
Serious lesions in the buttock are characterized by an interesting pattern of physical signs, called the ‘buttock sign’, summarized in Box 47.2. Non-capsular lesions of the hip itself comprise loose bodies, bursitis and aseptic necrosis of the femoral head.
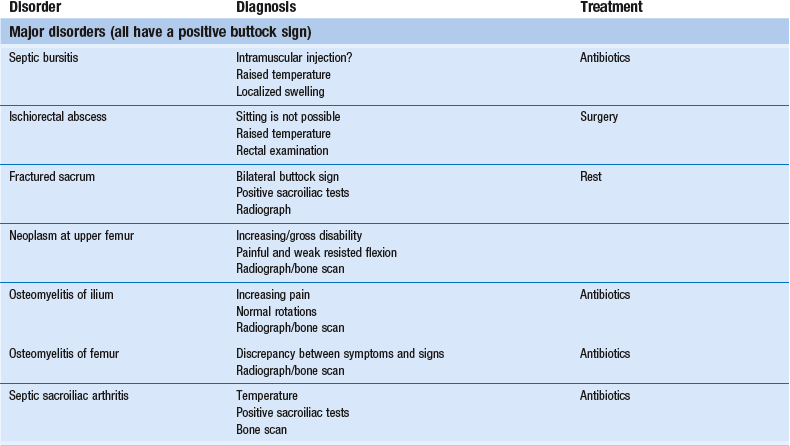
Disorders with a positive ‘buttock sign’
This clinical syndrome, described by Cyriax5 (his p. 375), always indicates a major lesion in the buttock.
The buttock sign is characterized by more limitation and/or pain on passive hip flexion with a flexed knee than with an extended knee (i.e. straight leg raising; Fig. 47.11). The other passive movements at the hip joint are limited in a non-capsular way. This strange pattern immediately draws attention to the gluteal region. If the hip joint itself were affected, straight leg raising would not be limited, except in those gross articular patterns in which flexion cannot reach 90°. If the nerve roots, the sciatic nerve or the hamstrings were affected, hip flexion with a flexed knee would not be painful or limited because it does not stretch these structures. The fact that both movements are limited and painful implicates other structures in the gluteal region.
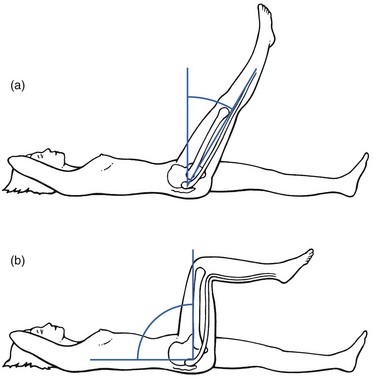
Fig 47.11 The buttock sign: passive hip flexion is more limited and/or painful than straight leg raising.
Checking for the buttock sign is very important in pain syndromes at the gluteal region. Because there will probably be nothing characteristic about the pain, only a comparison between the results of the straight leg raising test and passive hip flexion can detect serious disorders in the buttock.54–56 When this typical combination of signs emerges, a very careful examination of passive and resisted movements must follow. Passive movements disclose a non-capsular pattern, almost always with a full range of medial rotation. The end-feel of the limited movements is ‘empty’: as a consequence of the increasing pain, the examiner has to stop the movement, even though it is felt that the end of range has not been reached. Some resisted movements are painful and weak as well because they increase the tension on the affected tissues. As a rule, resisted extension and internal rotation are the most painful. Palpation may disclose a painful swelling.
Septic bursitis
Clinical examination
The patient’s gait is hobbling, as if the weight can hardly be borne on the affected leg.57 This major disability contrasts markedly with the minor degree of discomfort and is the first warning for the examiner.
Ischiorectal abscess
Occasionally, an anorectal abscess points towards the ischiorectal fossa instead of to the rectal region – an ischiorectal abscess.58
Sitting is impossible. The patient limps badly and even putting the foot to the ground causes considerable pain.59 The hip is held constantly in slight flexion but further flexion is prevented by increasing pain, as is straight leg raising, indicating the buttock sign. Apart from fever, other toxic symptoms may be present. The abscess may be felt during bidigital rectal examination with the index finger in the rectum and the thumb external.
The treatment is surgical and consists of prompt incision and adequate drainage.60
Fractured sacrum
Sacral fractures are associated with pain, swelling, ecchymosis and tenderness on palpation. In the presence of neurological symptoms, the diagnosis is usually not difficult. Neurological damage is not present, however, if the fracture line lies through the ala. Because of the position of the sacroiliac ligaments, the fracture remains stable and the diagnosis is then frequently missed.61 The patient may ascribe discomfort to local bruising and sometimes continues to be mobile.
In a spontaneous sacral insufficiency fracture in an elderly woman the diagnosis is more difficult.62,63
Because the sacrum is curved, diagnosis on a radiograph is not always easy. It is therefore advisable to obtain anteroposterior and lateral tomograms.64
If the fracture is markedly displaced or associated with neurological deficit, surgical reduction and stabilization must be performed. In uncomplicated fractures, treatment consists of aspiration of the haematoma and rest for 4–6 weeks, although bony union without deformity seems to take place whether the patient rests or not.65
Neoplasm at the upper femur
Metastases or primary tumours in the upper femur give rise to increasing pain in buttock and thigh. After a short development of the disease process, the functional disability may be so gross that it prevents the patient bearing weight on the affected side. There is also pain at rest.66
Septic sacroiliac arthritis
This disease affects young patients. Predisposing factors are immunosuppression, drug addiction and childbirth.67,68 In addition to demonstrating the positive buttock sign, the sacroiliac distraction test is extremely painful. Fever and general illness are present,69 together with local tenderness over the joint.
Radiological examination remains negative70 in the early stage but computed tomography (CT) or bone scan usually confirms the diagnosis.71
Aseptic necrosis of the hip
Aseptic necrosis (osteonecrosis, ischaemic necrosis and avascular necrosis are synonymous) of the hip is an osteoarticular disorder characterized by bone marrow ischaemia and death of trabecular bone of the femoral head. As bone repair occurs, weight-bearing bone becomes mechanically weakened and flattened and may eventually collapse. Secondarily, this leads to quickly developing osteoarthritis of the hip. In children it is called Perthes’ disease (see online chapter Hip disorders in children).
Aseptic necrosis of the hip in adults was first described in the German literature.73 Two types – post-traumatic (complicating fractures or dislocations) and idiopathic – exist. The latter has been the subject of many experimental and clinical studies over recent decades. All observations show an increasing incidence,74 although this is probably due to improved diagnostic techniques. The fact that the disease is not uncommon was shown by Streda in 1971,75 who found that 68% of patients with current osteoarthritis had pre-existent osteonecrosis.
Pathogenesis
It has been accepted for years that non-traumatic osteonecrosis of the hip results from progressive ischaemia, caused by interruption of the arterial supply of the femoral head.76 Experimental and clinical studies, however, indicate that other mechanisms can induce bone necrosis. The fact that aseptic necrosis occurs most frequently in patients treated with high-dose corticosteroids77 and in those with a history of alcohol abuse78 suggests a direct toxic effect on the osteocytes.79 This results in local inflammatory exudate and increased intraosseous bone marrow pressure. Elevation of intraosseous pressure is transmitted to small venules and capillaries within the bone, causing a decrease in blood flow to the bone. This uncompensated increase in intraosseous pressure is thought to result in irreversible circulatory disturbances and subsequent tissue damage, which further magnifies the initial result.80 The problem with osteonecrosis is that the radiological evidence only appears months after the process has started: necrosis of bone is only the irreversible end result of severe and prolonged ischaemia. Furthermore, it is not the necrosis itself but the reaction of the tissue to the ischaemia that shows up on a radiograph. For these reasons, a standard radiograph cannot assist in early diagnosis. However, diagnosis and treatment at an early stage are extremely important if gross damage to the femoral head is to be avoided.
Diagnosis
Bone scan
A bone scan usually shows increased uptake of the radionuclide from the very beginning of the disease – the sensitivity is about 70%.81 Both sides should be compared.
Magnetic resonance imaging (MRI)
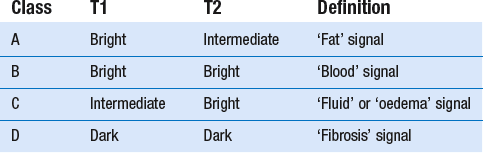
Nowadays, MRI is the most sensitive non-invasive method for the diagnosis of aseptic necrosis, which is diagnosed when a peripheral band of low signal intensity is present on all imaging sequences, typically in the superior portion of the femoral head, outlining a central area of marrow. This peripheral band is most apparent on T1-weighted sequences.82–85 Because the two hips are frequently involved, it is necessary to image both, not just the symptomatic one. The sensitivity of MRI is between 75 and 100%.
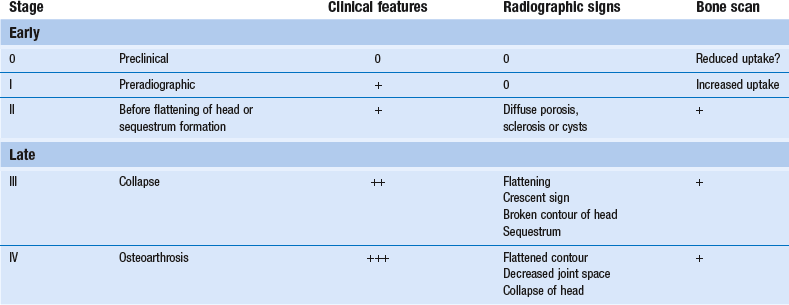
Staging
There are two staging classifications of aseptic necrosis, one based on radiographs (Table 47.1) (Ficat and Arlet86) and the other on MRI signal intensities87 (Table 47.2).
Stage I
The earliest clinical manifestations are sudden groin pain (sometimes referred to the L3 dermatome), often pain at night, and increasing pain on weight bearing.89 Coughing may hurt.
Clinical examination normally shows a full range of movement. Sometimes there is pain in a non-capsular pattern: external rotation and flexion hurt at the end of range.90 This could suggest the presence of a psoas bursa or an impacted loose body. Sometimes a forceful axial and upward blow on the heel also provokes groin pain.
Standard anteroposterior and lateral radiographs are entirely normal but a bone scan shows increased uptake of the radionuclide.91 The MRI is positive.
Stage IV
MRI staging of aseptic necrosis is based on the signal intensity of the centre of the marrow inside the dark line of necrosis.87 Radiographically occult aseptic necrosis will generally be depicted on MRI as any of classes A–C. Unlike radiographic staging, MRI classes have little predictive value regarding the prognosis for collapse of the femoral head. However, MRI size and position of the necrotic lesion are related to prognosis.
Treatment
The most important factor in effective treatment of non-traumatic osteonecrosis of the hip remains early diagnosis.92
While patients with advanced aseptic necrosis usually end up having hip arthroplasty, some of those whose lesion has been diagnosed early (at pre-collapse stage) have been managed with hip salvage surgery.93 A variety of drugs have also been used, such as lipid-lowering drugs, anticoagulants, vasodilators and bisphosphonates.94–96 Their use has been considered on the basis of specific physiological risk factors for osteonecrosis, such as lipid emboli, adipocyte hypertrophy, venous thrombosis, increased intraosseous pressure and resorption of bone.
Early decompression of bone (core decompression) is the treatment of choice in the early stages, before irreversible damage has taken place. The rationale for the use of core decompression is based on the concept that increased intramedullary pressure is involved in the pathogenesis of avascular necrosis. The aim of the proponents of core decompression is to decrease the intramedullary pressure and thus arrest or reverse the process of avascular necrosis before it is evident.97 Cortical bone grafting can be added to the core decompression in an effort to provide structural support for the subchondral bone and articular cartilage, and to prevent collapse during the repair process. A meta-analysis of 24 reports analysing 1206 hips treated by core decompression with or without cancellous bone grafting revealed an overall clinical success rate of 63.5% (range 33–95%). Less than 33% of the hips required a replacement or salvage procedure during the follow-up period.98
In stage III, an osteotomy can be performed to rotate the necrotic or collapsing segment of the hip out of the weight-bearing zone, replacing it with a segment of articular cartilage of the femoral head supported by healthy viable bone. In addition to the biomechanical effect, osteotomy may also reduce venous hypertension and decrease intramedullary pressure.99
Symptoms, signs, investigations and treatment of aseptic necrosis of the hip are summarized in Box 47.3.
Stress fracture of the femoral neck
Stress fractures of the femoral neck are not uncommon. The disorder presents in athletes,100 military recruits101,102 and the elderly103 with specific symptoms and findings. However, the diagnosis can be easily missed.
Patients present with unremitting, localized hip and groin pain without a history of significant trauma or unusual increase in daily activity. As in the initial stages of aseptic necrosis of the hip, there is a discrepancy between the obvious symptoms (localized pain and limping) and the minimal clinical signs. There is a non-capsular pattern with full and painless range of flexion and extension but considerable pain at the end of internal and external rotation of the hip. Resisted movements are painless.104 The initial radiographic features may prove negative.
An early diagnosis of spontaneous stress fracture of the femoral neck may be made when the scintigraphic examination shows increased focal radionuclide uptake.105 MRI seems to be the most accurate diagnostic tool in the early detection of a femoral neck stress fracture.106
Internal derangement of the hip
Cyriax5 suggested that a small piece of exfoliated articular cartilage, secondary to trauma or osteoarthrosis, becomes loose in the joint. When the fragment lies inside the capsular fold, level with the femoral neck, it is harmless and painless, but when it is pinched at the acetabular edge, sudden twinges result.
During arthroscopy, Dorfmann and Boyer sometimes found a small focus of synovitis on the anterior and anteroinferior aspects of the femoral neck in patients with symptoms of internal derangement. They hypothesized that this was probably due to impingement of the capsule by the psoas muscle tendon.107
Recent arthroscopic studies suggest that most internal derangement may be the result of impingement of acetabular labral tears.108 The latter are caused by repetitive contact between a widened femoral neck and the acetabular rim.109 Most tears are radial flaps located in the anterior section of the acetabulum and are more tag-like than massive.110 Anatomical studies have shown that the labrum is richly innervated with free nerve endings, capable of nociception. This may cause a direct painful response from the nipped flap.111
Symptoms
The pathognomonic sensation in internal derangement of the hip is the presence of a ‘twinge’ – a sharp, severe pain in the groin or the trochanteric area. It appears suddenly and unexpectedly during ordinary walking or descending stairs. The patient feels a pain shooting down the thigh to the knee and the leg feels as if it ‘might let the patient down’. Sometimes the pain disappears immediately or the patient has to stand on the good leg for a while.112 This painful twinge may be repeated on each step, some 100 metres later or not at all for the first few weeks. If patients have many twinges a day, they will be severely disabled, because they happen at any moment without warning and nothing can be done to prevent them.
Signs
Examination of a joint not complicated by osteoarthrosis shows a full range of movement, with some discomfort at the end of one or two movements – as a rule, external rotation and flexion. As these two movements are also painful in psoas bursitis (see later), the differential diagnosis will be supplied by the existence of twinges and the slightly different end-feel. Some authors report successful diagnosis of impingement lesions with the so-called Thomas test.113,114 This involves flexion and external rotation of the hip, then allowing the extremity to abduct. The hip is then moved into extension, internal rotation and adduction. A positive test result is indicated by a palpable or audible click and the production of pain.
Current modalities for imaging the hip joint, including arthrography and MRI, are poor at directly identifying a labral tear (sensitivity is between 13 and 24%).115 Indirect MRI findings of labral tears include a large α angle, an osseous bump formation at the femoral neck, a deep acetabulum and posteroinferior or anterosuperior cartilage lesions.116
Treatment
Technique: reduction of a loose body – 1
The patient lies supine on a low couch. An assistant presses on both anterior superior iliac spines in order to fix the pelvis firmly to the couch during the whole procedure (Fig. 47.12). To prevent local pain, a thick layer of foam may be placed between the hands and iliac crests. The pressure on the iliac spines should be directed downwards and cranially to withstand the force of the traction.
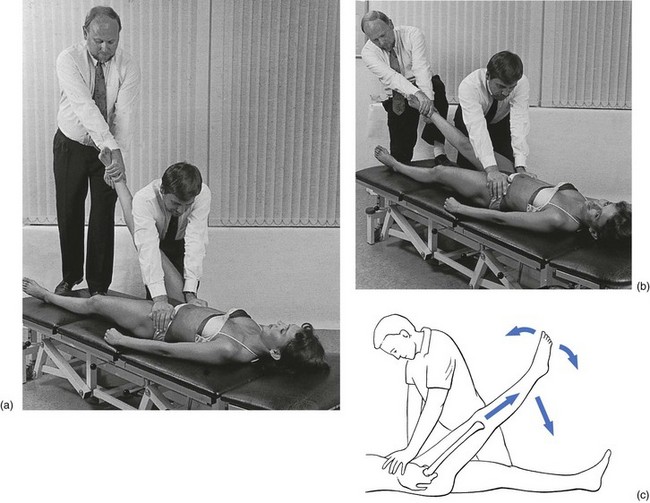
Fig 47.12 Reduction of a loose body in the hip joint – technique 1: start (a) and end (b) of the manipulation.
The contralateral hand encircles the heel. The other hand is placed at the distal side of the leg, level with the malleoli (Fig. 47.13). This position of the hands is vital in protecting the ligaments of the ankle when rotation is performed. The manipulator raises the outstretched leg to 70 or 80° and leans backwards, inducing as much traction as possible. As soon as the muscles are felt to relax, the manipulator steps gradually off the couch, meanwhile rotating the leg with a jerk to full medial rotation and back to the neutral position as the leg is extended. This manœuvre is repeated three or four times during extension.
The ipsilateral hand is used to grasp the heel. The foot is held at a right angle and the other hand encircles the medial border of the foot (Fig. 47.14). The manipulator leans backwards after having lifted the patient’s leg to about 70°. Again, when the muscles relax, a movement backwards is made and then downwards, meanwhile rotating the leg laterally. At the end of the rotational movement, a sharp jerk is added and the leg is turned back into the neutral position. This rotational movement is performed three or four times as the leg is extended.
Attention should be paid to two important technical points:
• The rotation must have the largest possible amplitude. Thus, starting at least from the neutral position, the leg has to be rotated until the end-feel is reached. It is then rotated back to the initial position, to restart the same procedure.
• Traction should not be lost during extension. Care should be taken to maintain strong traction throughout the whole manipulation until the joint is extended completely. Therefore, the manipulator keeps one foot on the couch, in order to maintain sufficient traction.
Technique: reduction of a loose body – 2
The patient adopts a supine-lying position on a low couch. As in the previous manipulation, an assistant holds the pelvis down on the couch, putting all the body weight on the anterior superior iliac spines. The manipulator places the contralateral foot on the couch, just beyond the patient’s buttock, and puts the back of the patient’s bent knee over the thigh. The contralateral hand holds the knee and the other hand grasps the ankle (Fig. 47.15).
Psoas bursitis
The psoas bursa is one of the largest bursae in the human body.117 Located between the lesser trochanter, the musculotendinous portion of the iliopsoas muscle and the anterior capsule of the hip joint, it can be up to 7 cm long and 4 cm wide.118
As the bursa is derived from the second and third lumbar segments, pain is usually felt in the groin, anterior thigh, knee and leg. It appears during walking and during specific movements: for example, crossing the legs.119 Untreated, the disorder can persist for years.
The lesion is generally overlooked and the symptoms are usually blamed on the slight arthrosis visible on radiograph rather than the (invisible) psoas bursa.120 Clinical examination, however, shows a non-capsular pattern: lateral rotation is painful and the end-feel soft, and flexion is slightly painful at the end of range. Sometimes there is also pain at the end of extension or adduction.121 An accessory test is provided by passive adduction in flexion, which is the most painful movement because it squeezes the bursa. This test also provokes considerable stretching of the sacroiliac ligaments and tissues in the buttock, so it is essential to ascertain that the reproduced pain is in the groin and is not some vague sensation of pulling in the buttock. Resisted movements are strong and painless. The bursa may be palpably enlarged, as is sometimes the case in rheumatoid conditions.119,122,123
Differential diagnosis should be made from a loose body in the hip and early aseptic necrosis. In the former the patient complains of sudden twinges and in the latter there will often be a gross discrepancy between the functional incapacity and the moderate signs. Ultrasonography is the best confirmatory diagnostic test.124
The diagnosis is also confirmed when a diagnostic infiltration with local anaesthetic is found to abolish the clinical features. This injection, perhaps repeated once or twice, is very often therapeutic too.125
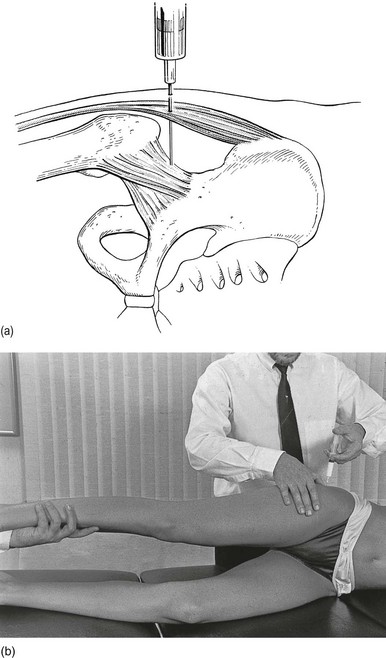
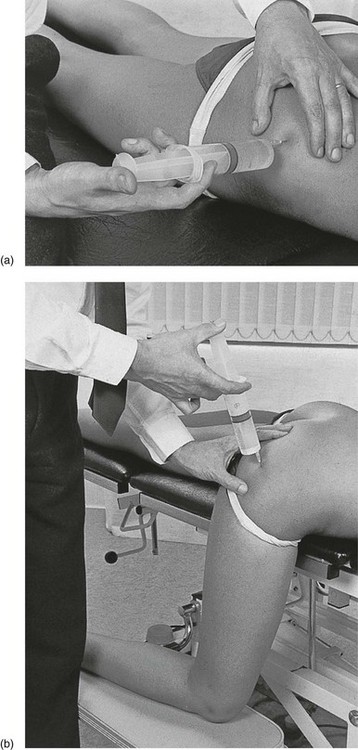
Technique: infiltration
A needle 7 cm long is fitted to a 50 mL syringe, filled with procaine 0.5%. The needle is inserted at the identified spot, and thrust in at a 45° medial and upward direction (Fig. 47.16).
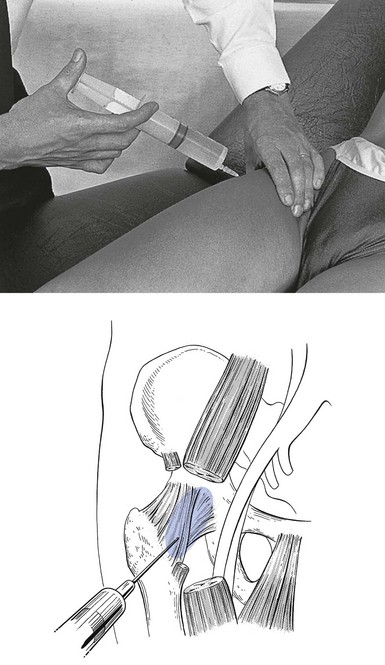
Fig 47.16 Infiltration of the psoas bursa.
Results
Diagnostic infiltration often has a lasting therapeutic result, regardless of the duration of symptoms. If necessary, the infiltration can be repeated once or twice, at weekly intervals. If no lasting relief is afforded and the injection only acts temporarily, 50 mg of triamcinolone should be injected at the same site at the next attendance.126
Haemorrhagic psoas bursitis
As in haemorrhagic bursitis at the shoulder, this condition sometimes occurs in the elderly. The patient states that the hip was hurt during a sideways slip of the leg, which was immediately followed by extreme pain at the front of the thigh and knee. Spontaneous haemorrhage complicating a pigmented villonodular synovitis of the bursa has also been reported.127
Examination shows a gross non-capsular pattern, with up to 90° limitation of flexion and gross limitation of lateral rotation and extension, but almost a full range of medial rotation.128 Resisted movements are full-range and painless. Sometimes a tense, tender swelling can be palpated in the groin. Aspiration confirms the diagnosis and is also the required treatment.
Gluteal bursitis
This syndrome, also referred to as ‘greater trochanteric pain syndrome’, is one of the most frequent causes of pseudoradicular pain in the limb.129 The condition most frequently occurs in late-middle-aged females and shows little tendency to spontaneous cure.130 Patients are usually in their forties or fifties and complain of pain at the gluteal or tronchanteric area, spreading to the outer or posterior thigh and down to the calf muscles and outer malleolus.131 Unlike the pain caused by a disc lesion, the symptoms are not related to sitting but to walking, prolonged standing or transitioning to a standing position.132 They are also exacerbated by sitting with the affected leg crossed and by climbing stairs.133 The patient will not mention the twinges that are so typical of an impacted loose body but may state that a particular movement causes a sharp pain down to the leg. Sometimes the patient has nocturnal pain when lying on the affected side. Sitting with the painful leg crossed over the other also hurts.134 Coughing is painless. The combination of long-standing leg pain, related to hip movements and without symptoms typical of disc lesions, suggests the existence of gluteal bursitis.135
There is a full range of movement but some movements hurt in a non-capsular way at the end of range. The end-feel is normal. Some resisted movements may be painful because they squeeze the tender bursa. A typical pattern is pain on passive external rotation and passive abduction and resisted external rotation or resisted abduction.136,137 Pain on resisted hip movements may be explained by compression of the bursa. However, recent histopathologic findings show that tendinopathy and bursa pathology may coexist in greater trochanteric pain syndrome.138
Most of the affected bursae are located near the greater trochanter and between the gluteus maximus and gluteus medius (Fig. 47.17).
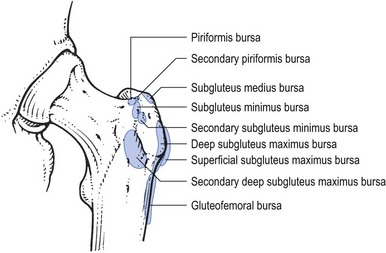
Fig 47.17 The bursae at the greater trochanter.
Localization depends on the findings on palpation, although sometimes the approximate area can be deduced from the general examination: when full passive internal rotation together with resisted external rotation causes pain, the bursa at the insertion of the piriformis is affected; pain on passive abduction and passive flexion, together with resisted abduction, indicates that the bursa between the gluteus medius and gluteus maximus is at fault.139 Caution is required in drawing conclusions from palpation. Because the gluteal region is an awkward area and the affected tissues lie deeply, tenderness can be elicited in a normal buttock if the pressure applied is considerable. It is therefore always wise to compare the unaffected side with the affected one.
During the last few decades there has been increasing use of ultrasonography and MRI for visualizing lesions of the greater trochanteric area. It should be mentioned, however, that several studies identified gluteal abnormalities in asymptomatic hips,140–142 sometimes in as many as 60%.143 It is therefore unwise to rely entirely on the result of these tests to establish the diagnosis.144
The diagnosis is settled by local anaesthesia. If, after infiltration, the movements known to hurt are tested again and are no longer painful, the right point has been found.145 This diagnostic injection often has a lasting result; therefore the patient should re-attend after a week for re-examination. If there is considerable and lasting relief, the injection is repeated two or three times at weekly intervals. If there is no therapeutic result, 50 mg of triamcinolone is injected in the same site. This injection may also have to be repeated once or twice but this time at an interval of 2 weeks.146 Sometimes an intractable case is encountered; though injections relieve the symptoms for 2 or 3 weeks, the latter subsequently recur; as operative exploration of the buttock is a very difficult procedure and rarely effects a cure, it is best to admit defeat and hope a spontaneous remission will take place.147
Technique: injection – 1
When the patient has evidence of a highly localized bursitis, the lateral approach is used (Fig. 47.18). The patient lies prone and the physician sits or stands on the affected side. A 7 cm needle is fitted to a syringe containing 50 mL of procaine 0.5%. The tender area is localized and the needle inserted horizontally until it reaches the ilium. It is then withdrawn by 1 or 2 cm and a large area is infiltrated using the classic technique, employing withdrawals and reinsertions at different angles.
Trochanteric bursitis
The trochanteric bursa (Fig. 47.19) lies at the lateral aspect of the trochanter tip, between the iliotibial tract and the bone, level with the insertion of the gluteus medius and the tensor fasciae latae. It can become inflamed after a local blow,148 although trochanteric bursitis may also result from overuse in long-distance runners.149 The incidence of trochanteric bursitis peaks between the fourth and sixth decades of life and is more common in women (male : female ratio 1 : 10).150
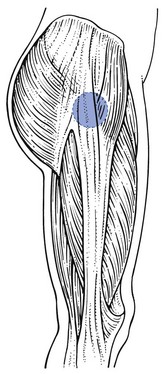
Fig 47.19 Location of the trochanteric bursa.
Clinical examination shows a non-capsular pattern: external rotation is very painful and sometimes limited, with a soft end-feel; however, when external rotation is retested with the hip and the knee extended, there is no pain at all. Passive abduction may also be painful. The resisted movements are painless, except sometimes abduction.151 Palpation reveals a tender area at the lateral aspect of the trochanter.
Treatment consists of infiltration with procaine.
Technique: infiltration
A condition similar to acute subdeltoid bursitis can occur in the trochanteric bursa. The patient complains of serious and rapidly increasing pain at the trochanteric area. Clinical examination shows a non-capsular pattern, with limited external rotation and an extremely tender trochanteric area. The radiograph often reveals calcification.152 Treatment consists of local infiltration of the whole bursal wall with triamcinolone, which, as in acute subdeltoid bursitis, affords immediate and lasting relief.
Ischial bursitis
The ischial bursa is painfully squeezed between the ischial tuberosity and the hard surface of a chair during sitting. Because the condition is not very common, it will not be the first thing to occur to the clinician in the presence of ischial pain. When a patient complains of pain in the buttock, coming on during sitting and easing as soon as he or she is standing, the first condition to come to mind is a disc lesion at a low lumbar level. However, in ischial bursitis, the patient experiences pain as soon as the buttocks touch the chair, whereas in a discodural lesion the pain gradually increases during sitting or the moment the patient stands again. If the routine lumbar, sacroiliac and hip examinations are negative, this condition should be considered.153 The only clinical finding is local tenderness at the ischial tuberosity. The diagnosis can be confirmed by introducing some local anaesthetic but the injection seldom gives lasting relief. Treatment consists of one or two infiltrations with 20 mg of triamcinolone and avoidance of further compression.
References
1. Leopold, SS, Battista, V, Oliverio, JA, Safety and efficacy of intraarticular hip injection using anatomic landmarks. Clin Orthop Relat Res 2001; 391:192–197. ![]()
2. Healey, LA. Polymyalgia rheumatica. In: McCarthy DJ, ed. Arthritis and Allied Conditions. 9th ed. Philadelphia: Lea & Febiger; 1979:681.
3. Goldenberg, DL, Cohen, AS, Acute infectious arthritis. A review of patients with non-gonococcal joint infections (with emphasis on therapy and prognosis). JAMA 1976; 60:369. ![]()
4. Goldenberg, DL, Brandt, KD, Cohen, AS, et al, Treatment of septic arthritis. Comparison of needle aspiration and surgery as initial modes of joint drainage. Arthritis Rheum 1975; 18:83. ![]()
5. Cyriax, JH, 8th ed. Textbook of Orthopaedic Medicine; vol. I. Baillière Tindall, London, 1982.
6. Murray, RO, The aetiology of primary osteoarthrosis of the hip. Br J Radiol 1965; 38:810–824. ![]()
7. Solomon, L, Patterns of osteoarthrosis of the hip. J Bone Joint Surg. 1976;58B(2):176–183. ![]()
8. Harris, WH, Etiology of osteoarthritis of the hip. Clin Orthop Rel Res 1986; 213:20–33. ![]()
9. Visuri, T, Stress osteopathy of the femoral head: 10 military recruits followed for 5–11 years. Acta Orthop Scand 1997; 68:138–141. ![]()
10. Radin, EL, Martin, RB, Burr, DB, et al. Mechanical factors influencing cartilage damage. In: Peyron JG, ed. Osteoarthrosis. Current Clinical and Fundamental Problems. Paris: CIBA-Geigy; 1986:90–99.
11. Byers, PD, Contempomi, CA, Farkas, TA, A postmortem study of the hip joint. Am Rheum Dis 1970; 29:15. ![]()
12. Meachim, G, Emery, IH, Cartilage fibrillation in shoulder and hip joints of Liverpool necropsies. J Anat 1973; 116:61. ![]()
13. Radin, EL, Rose, RM, Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop 1986; 213:34–40. ![]()
14. Mankin, HJ, Dorfman, H, Lipiello, L, Zarins, A, Biomechanical and metabolic abnormalities in articular cartilage from human osteoarthritic hips. J Bone Joint Surg 1970; 52A:424–434. ![]()
15. Castors, CW, Dorstewitz, EL, Smith, SF, Ritchie, JC, Connective tissue activation. I. The nature, specificity and distribution of connective tissue activating peptides. Arthritis Rheum 1971; 14:41–54. ![]()
16. Dingle, JT, Saklatvala, J, Hembry, R, et al, A cartilage catabolic factor from synovium. Biochem J 1979; 184:177–180. ![]()
17. Christmann, OD, Biomechanical aspects of degenerative joint disease. Clin Orthop 1969; 64:77–85. ![]()
18. Pitsillides, AA, Skerry, TM, Edwards, JC, Joint immobilization reduces synovial fluid hyaluronan concentration and is accompanied by changes in the synovial intimal cell populations. Rheumatology. 1999;38(11):1108–1112. ![]()
19. Strange, FSC. The Hip. London: Heinemann; 1965.
20. Long, W, Dorr, LD, Healy, B, et al, Functional recovery of noncemental total hip arthroplasty. Clin Orthop Rel Res 1993; 288:73–77. ![]()
21. Nakamura, T, Susuki, K, Muscular changes in osteoarthritis of the hip and knee. Nippon Seikeigeka Gukki Zasshi. 1992;66(5):467–475. ![]()
22. Nillson, BE, Danielsson, LG, Jerker Hernborg, SA, Clinical feature and natural course of coxarthrosis and gonarthrosis. Scand J Rheum Suppl. 1980;(43 suppl):13–21. ![]()
23. Robertsson, O, Wingstrand, H, Onnerfalt, R, Intracapsular pressure and pain in coxarthrosis. J Arthroplasty. 1995;10(5):632–635. ![]()
24. Bijl, D, Dekker, J, van Baar, ME, et al, Validity of Cyriax’s concept capsular pattern for the diagnosis of osteoarthritis of hip and/or knee. Scand J Rheumatol. 1998;27(5):347–351. ![]()
25. Janda, V. Muscle Function Testing. London: Butterworths; 1983.
26. Lewit, K. Manipulative Therapy in Rehabilitation of the Locomotor System, 2nd ed. Oxford: Butterworth Heinemann; 1991.
27. Vogt, L, Banzer, W, Dynamic testing of the motor stereotype in prone hip extension from the neutral position. Clin Biomech 1997; 12:122–127. ![]()
28. Bierma-Zeinstra, S, Bohnen, A, Ginai, A, et al, Validity of American College of Rheumatology criteria for diagnosing hip osteoarthrosis in primary care research. J Rheumatol. 1999;26(5):1129–1133. ![]()
29. Ledingham, J, Dawson, S, Preston, B, et al, Radiographic patterns and associations of osteoarthrosis of the hip. Ann Rheum Dis. 1992;51(10):1111–1116. ![]()
30. Cameron, H, MacNab, I, Observations on osteoarthritis of the hip joint. Clin Orthop Rel Res 1975; 108:31–40. ![]()
31. Van Baar, ME, Dekker, J, Oostendorp, RA, et al, The effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a randomized clinical trial. J Rheumatol. 1998;25(12):2432–2439. ![]()
32. Van Baar, ME, Assendelft, WJ, Dekker, J, et al, Effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review of randomised clinical trials. Arthritis Rheum. 1999;42(7):1361–1369. ![]()
33. Kaltenborn, F. Manual Mobilisation of the Extremity Joints, 4th ed. Oslo: Olaf Norlis Bokhandel; 1989.
34. Lewit, K. Manipulative Therapy in Rehabilitation of the Locomotorsystem, 2nd ed. Oxford: Butterworth Heinemann; 1991.
35. Mink, AJF, ter Veer, HJ, Vorselaars, JACTh. Extremiteiten. Funktie-onderzoek en Manuele Therapie. Houten, NL: Uitg, Bohn; Scheltema & Holkema; 1990.
36. Solomon, L, Schnitzler, C, Browett, O. Osteoarthritis of the hip, the patient behind the disease. In: Peyron J, ed. Epidemiology of Osteoarthritis. Paris: CIBA-Geigy; 1981:40–52.
37. Dequeker, J, Goris, F, Uytterhoeven, R, Osteoporosis–osteoarthrosis: anthropometric distinctions. JAMA 1983; 249:1448–1451. ![]()
38. Janda, V. Muscle Function Testing. London: Butterworth; 1983.
39. Lewit, K. Manipulative Therapy in Rehabilitation of the Locomotor System, 2nd ed. Oxford: Butterworth Heinemann; 1991.
40. Kendall, F, McCreary, E. Muscles Testing and Function, 3rd ed. Baltimore: Williams & Wilkins; 1983.
41. Sims, K, The development of hip osteoarthritis: implications for conservative management. Manual Therapy. 1999;4(3):127–135. ![]()
42. Kisner, C, Colby, L. Therapeutic Exercise: Foundations and Techniques, 3rd ed. Philadelphia: FA Davis; 1996.
43. Ishikawa, K. A study of deleterious effects of intra-articular corticosteroid on knee joints: a clinical investigation on primary gonarthrosis. J Jpn Orthop Assoc. 1978; 52(3):359–374.
44. Migliore, A, Tormenta, S, Martin Martin, LS, et al, The symptomatic effects of intraarticular administration of hylan G-F 20 on osteoarthritis of the hip: clinical data of 6 months follow-up. Clin Rheumatol. 2006;25(3):389–393. ![]()
45. Tikiz, C, Unlü, Z, Sener, A, et al, Comparison of the efficacy of lower and higher molecular weight viscosupplementation in the treatment of hip osteoarthritis. Clin Rheumatol. 2005;24(3):244–250. ![]()
46. Caglar-Yagci, H, Unsal, S, Yagci, I, et al, Safety and efficacy of ultrasound-guided intra-articular hylan G-F 20 injection in osteoarthritis of the hip: a pilot study. Rheumatol Int. 2005;25(5):341–344. ![]()
47. Rasanen, P, Paavolainen, P, Sintonen, H, et al, Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop 2007; 78:108–115. ![]()
48. Ethgen, O, Bruyere, O, Richy, F, et al, Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 2004; 86:963–974. ![]()
49. Barrack, RL, Early failure of modern cemented stems. J Arthroplasty. 2000;15(8):1036–1050. ![]()
50. Herberts, P, Malchau, H, Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand. 2000;71(2):111–121. ![]()
51. Breusch, SJ, Aldinger, PR, Thomsen, M, et al, Anchoring principles in hip prosthesis implantation. II: Acetabulum components. Unfallchirurg. 2000;103(12):1017–1031. ![]()
52. Bozic, KJ, Pui, CM, Ludeman, MJ, et al, Do the potential benefits of metal-on-metal hip resurfacing justify the increased cost and risk of complications? Clin Orthop Relat Res. 2010;468(9):2301–2312. ![]()
53. Pollard, TC, Baker, RP, Eastaugh-Waring, SJ, Bannister, GC, Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br 2006; 88:592–600. ![]()
54. Greenwood, MJ, Erhard, RE, Jones, DL, Differential diagnosis of the hip vs. lumbar spine: five case reports. J Orthop Sports Phys Ther. 1998;27(4):308–315. ![]()
55. Erhard, RE, Egloff, BP, Patient with metastatic adenocarcinoma imitating lumbar herniated nucleus pulposis. J Manipulative Physiol Ther. 2004;27(9):569–573. ![]()
56. VanWye, WR, Patient screening by a physical therapist for nonmusculoskeletal hip pain. Phys Ther. 2009;89(3):248–256. ![]()
57. François, F. Une Nouvelle Entité clinique: la bursite ischiatique. Rev Rhum Mal Ostéoartic. 1966; 33:255–256.
58. Parks, AG, Gordon, PH, Hardcastle, JD, A classification of fistula-in-ano. Br J Surg 1976; 63:1. ![]()
59. Smereck, J, Ybarra, M, Acute hip pain and inability to ambulate: a rare presentation for perirectal abscess. Am J Emerg Med. 2011;29(3):356.e1–356.e3. ![]()
60. Golighter, JC, Ellis, M, Pissidis, AG, A critique of anal glandular infection in the aetiology and treatment of idiopathic ano-rectal abscesses and fistulas. Br J Surg 1967; 54:977. ![]()
61. Denis, F, Davis, S, Comfort, T, Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop 1988; 227:67–81. ![]()
62. Leroux, JL, Denat, B, Thomas, E, et al, Sacral insufficiency fractures presenting as acute low back pain. Spine. 1993;8(16):2502–2506. ![]()
63. Weber, M, Hasler, P, Gerber, H, Insufficiency fractures of the sacrum. Spine. 1993;19(16):2507–2512. ![]()
64. Schmidek, R, Smith, DA, Kristiansen, TK, Sacral fractures. Neurosurgery 1984; 15:735–746. ![]()
65. Kristiansen, TK. Fractures of the sacrum and coccygodynia. In: Fryomer JW, ed. The Adult Spine. New York: Raven Press; 1991:2145–2148.
66. Mirra, JM. Bone Tumours: Diagnosis and Treatment. Philadelphia: Lippincott; 1980.
67. Shamahan, MDG, Ackroyd, CE, Pyogenic infections of the sacro-iliac joint. J Bone Joint Surg 1985; 64B:605–608. ![]()
68. Dunn, EJ, Bryan, DM, Nugent, JT, Robinson, A, Pyogenic infections of the sacro-iliac joint. Clin Orthop 1976; 118:113–117. ![]()
69. Feldman, JC, Menkes, CJ, Weill, B, et al, Les Sacro-iliites infectieuses. Etude multicentrique sur 214 observations. Rev Rhum Mal Ostéoartic 1981; 48:83–91. ![]()
70. Kerr, R, Pyogenic sacro-iliitis. Orthopedics. 1985;8(8):1028–1034. ![]()
71. Hamdam, J, Ashra, M, Mallouch, A, et al, Early diagnosis of septic arthritis of the sacroiliac joint by use of computed tomography. J Rheumatol 1981; 8:979–982. ![]()
72. Masri, BA, Masterson, EL, Duncan, CP, The classification and radiographic evaluation of bone loss in revision hip arthroplasty. Orthop Clin North Am 1998; 2:219–227. ![]()
73. Haenisch, H. Arthritis dissecans de Hüfte. Zentralbl Chir. 1925; 52:999.
74. Jacobs, B, Epidemiology of traumatic and non-traumatic osteonecrosis of the head of the femur. Clin Orthop 1978; 130:5. ![]()
75. Streda, A, Participation of osteonecrosis in the development of severe coxarthrosis. Acta Univ Coal (Med) (Praha) Monogr 46 1971; 103–142. ![]()
76. Hungerford, DS, Zizic, TM, Pathogenesis of ischemic necrosis of the femoral head. Hip 1983; 5:249–262. ![]()
77. Vreden, SG, Hermus, AR, van Liessum, PA, et al, Aseptic bone necrosis in patients on glucocorticoid replacement therapy. Netherlands J Med 1991; 39:153–157. ![]()
78. Matsuo, K, Horohata, T, Sugioka, Y, et al, Influence of alcohol intake, cigarette smoking and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop 1988; 234:115–123. ![]()
79. Warner, JJP, Philip, JH, Brodsky, GL, Thornhill, TS, Studies of nontraumatic osteonecrosis; manometric and histologic studies of the femoral head after chronic steroid treatment: an experimental study in rabbits. Clin Orthop 1987; 225:128–140. ![]()
80. Cruess, RL, Osteonecrosis of bone. Current concepts as to etiology and pathogenesis. Clin Orthop 1986; 208:31–39. ![]()
81. Conklin, J, Alderson, PO, Zizic, TM, et al, Comparison of bone scan and radiographic sensitivity in the detection of steroid-induced ischemic necrosis of bone. Radiology 1983; 147:221–226. ![]()
82. Mitchell, DG, Kressel, HY, Arger, PH, et al, Avascular necrosis of the femoral head: morphologic assessment by MR imaging, with CT correlation. Radiology 1986; 161:739–742. ![]()
83. Robinson, HJ, Hartleben, PD, Lund, G, et al, Evaluation of magnetic resonance imaging in the diagnosis of the osteonecrosis of the femoral head. J Bone Joint Surg 1989; 71A:650–663. ![]()
84. Kukobo, T, Takatori, Y, Ninpmiya, S, et al, Magnetic resonance imaging and scintigraphy of avascular necrosis of the femoral head. MR Imag Scintig ANFH 1992; 27:54–60. ![]()
85. Halland, AM, Klemp, P, Botes, D, et al, Avascular necrosis of the hip in systemic lupus erythematosus: the role of magnetic resonance imaging. Br J Rheum 1993; 32:972–976. ![]()
86. Ficat, RP, Arlet, J, Ischemia and Necrosis of Bone. Williams & Wilkins, Baltimore, 1980.. ![]()
87. Mitchell, DG, Rao, VM, Dalinka, MK, et al, Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology 1987; 162:709–715. ![]()
88. Ficat, RP, Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg 1985; 67B:3. ![]()
89. Griffiths, HJ, Etiology, pathogenesis and early diagnosis of ischemic necrosis of the hip. JAMA 1981; 246:2615–2617. ![]()
90. Mandell, BF, Avascular necrosis of the femoral head presenting as trochanteric bursitis. Ann Rheum Dis. 1990;49(9):730–732. ![]()
91. Webber, MM, Wagner, J, Craign, MD, Radionuclide patterns of femoral head disease. Int J Nucl Med Biol 1977; 4:167. ![]()
92. Steinberg, ME, Brighton, CT, Hayken, GD, et al, Early results in the treatment of avascular necrosis of the femoral head with electrical stimulation. Orthop Clin North Am 1984; 15:163. ![]()
93. Sen, RK, Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop. 2009;43(1):6–16. ![]()
94. Glueck, CJ, Freiberg, RA, Sieve, L, Wang, P, Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res 2005; 435:164–170. ![]()
95. Pritchett, JW, Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res 2001; 386:173–178. ![]()
96. Lai, KA, Shen, WJ, Yang, CY, et al, The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. J Bone Joint Surg Am 2005; 87:2155–2159. ![]()
97. Hungerford, DS, Treatment of ischemic necrosis of the femoral head. Surgery of the musculoskeletal system. Evarts, CD, eds. Surgery of the musculoskeletal system; vol. 3. Churchill Livingstone, New York, 1983:5029–5043.
98. Mont, MA, Carbone, JJ, Fairbank, AC, Core decompression vs. non-operative management for avascular necrosis of the femoral head. Clin Orthop Relat Res 1996; 324:169–178. ![]()
99. Sakano, S, Hasegawa, Y, Torii, Y, et al, Curved intertrochanteric varus osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Br 2002; 84:817–824. ![]()
100. Batt, ME, Timmerman, LA, Hip pains in a recreational runner. Int J Sports Med. 1995;16(8):563–567. ![]()
101. Cline, AD, Jansen, GR, Melby, CL, Stress fractures in female army recruits: implications of bone density, calcium intake, and exercise. J Am Coll Nutr. 1998;17(2):128–135. ![]()
102. Stoneham, MD, Morgan, NV, Stress fractures of the hip in Royal Marine recruits under training: retrospective analysis. Br J Sports Med. 1991;25(3):145–148. ![]()
103. Tountas, AA, Waddell, JP, Stress fractures of the femoral neck. A report of seven cases. Clin Orthop 1986; 210:160–165. ![]()
104. Jones, DL, Erhard, RE, Diagnosis of trochanteric bursitis versus femoral neck stress fracture. Phys Ther. 1997;77(1):58–67. ![]()
105. Dorne, HL, Lander, PH, Spontaneous stress fractures of the femoral neck. Am J Roentgenol. 1985;144(2):343–347. ![]()
106. Shin, AY, Morin, WD, Gorman, JD, et al, The superiority of magnetic resonance imaging in differentiating the cause of hip pain in endurance athletes. Am J Sports Med 1996; 24:168–176. ![]()
107. Dorfmann, H, Boyer, T, Arthroscopy of the hip: 12 years of experience. Arthroscopy. 1999;15(1):67–76. ![]()
108. Allen, WC, Cope, R, Coxa saltans. The snapping hip revisited. J Am Acad Orthop Surg 1995; 3:303–308. ![]()
109. Beck, M, Kalhor, M, Leunig, M, Ganz, R, Hip morphology influences the pattern of damage to the acetabular cartilage. J Bone Joint Surg Br 2005; 87-B:1012–1018. ![]()
110. Lage, LA, Patel, JV, Villar, RN, The acetabular labral tear: an arthroscopic classification. Arthroscopy 1996; 12:269–272. ![]()
111. Kim, YT, Azuma, H, The nerve endings of the acetabular labrum. Clin Orthop 1995; 320:176–181. ![]()
112. Philippon, MJ, Maxwell, RB, Johnston, TL, et al, Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc 2007; 15:1041–1047. ![]()
113. Fitzgerald, RH, Jr., Acetabular labrum tears. Diagnosis and treatment. Clin Orthop 1995; 311:60–68. ![]()
114. Braly, BA, Beall, DP, Martin, HD, Clinical examination of the athletic hip. Clin Sports Med 2006; 25:199–210. ![]()
115. Farjo, LA, Glick, JM, Sampson, TG, Hip arthroscopy for acetabular labral tears. Arthroscopy. 1999;15(2):132–137. ![]()
116. Pfirrmann, CW, Mengiardi, B, Dora, C, et al, Cam and pincer femoroacetabular impingement: characteristic MR arthrographic findings in 50 patients. Radiology. 2006;240(3):778–785. ![]()
117. Chandler, SB. The iliopsoas bursa in man. Anat Rec. 1934; 58:235.
118. Harper, MC, Schaberg, JE, Allen, WC, Primary iliopsoas bursography in the diagnosis of disorders of the hip. Clin Orthop Rel Res 1987; 221:238–241. ![]()
119. Toohey, AK, LaSalle, TL, Martinez, S, Polisson, RP, Iliopsoas bursitis: clinical features, radiographic findings, and disease associations. Semin Arthritis Rheum. 1990;20(1):41–47. ![]()
120. Fortin, L, Bélanger, R, Bursitis of the iliopsoas: four cases with pain as the only clinical indicator. J Rheumatol 1995; 10:1971–1973. ![]()
121. O’Connor, DS. Early recognition of iliopectineal bursitis. Surg Gynecol Obstet. 1933; 57:674–684.
122. Samuelson, C, Ward, JR, Albo, D, Rheumatoid synovial cyst of the hip. Arthritis Rheum 1971; 14:105. ![]()
123. Letourneau, L, Dessureault, M, Carette, S, Rheumatoid iliopsoas bursitis presenting as unilateral femoral nerve palsy. J Rheumatol. 1991;18(3):462–463. ![]()
124. Ginesty, E, Dromer, C, Galy-Fourcade, D, et al, Iliopsoas bursopathies. A review of twelve cases. Rev Rhum Engl. 1998;65(3):181–186. ![]()
125. Mallant, MPJH, Mastboom, WJB, De Backer, GPM, Liesklachten door bursitis iliopectinea. Ned Tijdschr Geneeskd. 1998;142(23):1328–1331. ![]()
126. Generini, S, Matucci-Cerinic, M, Iliopsoas bursitis in rheumatoid arthritis. Clin Exp Rheumatol. 1993;11(5):549–551. ![]()
127. Weiner, JR, Robinson, DW, Pigmented villonodular synovitis of iliopectineal bursa. J Bone Joint Surg 1951; 33A:988. ![]()
128. Binek, R, Levinsohn, M, Enlarged iliopsoas bursa; an unusual cause of thigh mass and hip pain. Clin Orthop 1987; 224:158–163. ![]()
129. Swezey, RL, Pseudo-radiculopathy in subacute trochanteric bursitis of the subgluteus maximus bursa. Arch Phys Med Rehabil 1976; 57:387–390. ![]()
130. Segal, NA, Felson, DT, Torner, JC, et al, Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007; 88:988–992. ![]()
131. Spear, IM, Lipscomb, PR, Noninfectious trochanteric bursitis and peritendinitis. Surg Clin North Am 1952; 32:1217. ![]()
132. Williams, BS, Cohen, SP, Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg. 2009;108(5):1662–1670. ![]()
133. Collée, G, Dijkmans, BAC, Vandenbroucke, JP, Cats, A, Greater trochanteric pain syndrome in low back pain. Scand J Rheumatol 1991; 20:262–266. ![]()
134. Little, H, Trochanteric bursitis: a common cause of pelvic girdle pain. Can Med Assoc J 1979; 120:456–458. ![]()
135. Duthie, RB, Bentley, C. Trochanteric bursitis (subgluteal bursa. In Mercer’s Orthopaedic Surgery, 8th ed, London: Edward Arnold; 1983:710.
136. Bird, PA, Oakley, SP, Shnier, R, Kirkham, BW, Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum. 2001;44(9):2138–2145. ![]()
137. Traycoff, RB, ‘Pseudotrochanteric bursitis’: the differential diagnosis of lateral hip pain. J Rheumatol. 1991;18(12):1810–1812. ![]()
138. Fearon, AM, Scarvell, JM, Cook, JL, Smith, PN, Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthop Relat Res. 2010;468(7):1838–1844. ![]()
139. Samson, M, Lequesne, M, Tendinites de la région de la hanche. Rev Prat. 1991;14(18):1667–1671. ![]()
140. Woodley, SJ, Nicholson, HD, Livingstone, V, et al, Lateral hip pain: findings from magnetic resonance imaging and clinical examination. J Orthop Sports Phys Ther. 2008;38(6):313–328. ![]()
141. De Maeseneer, M, Gosselin, R, De Ridder, F, et al, MR imaging changes in the trochanteric area of asymptomatic individuals: a potential for misdiagnosis of pain in the trochanteric region. Eur J Radiol. 2009;72(3):480–482. ![]()
142. Kong, A, Van der Vliet, A, Zadow, S, MRI and US of gluteal tendinopathy in greater trochanteric pain syndrome. Eur Radiol. 2007;17(7):1772–1783. ![]()
143. Blankenbaker, DG, Ullrick, SR, Davis, KW, et al, Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skeletal Radiol. 2008;37(10):903–909. ![]()
144. Haliloglu, N, Inceoglu, D, Sahin, G, Assessment of peritrochanteric high T2 signal depending on the age and gender of the patients. Eur J Radiol. 2010;75(1):64–66. ![]()
145. Schipira, D, Menachem, N, Scharf, Y, Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil 1986; 67:815–917. ![]()
146. Raman, D, Haslock, I, Trochanteric bursitis: a frequent cause of ‘hip’ pain in rheumatoid arthritis. Ann Rheum Dis 1982; 41:602–603. ![]()
147. De Windt, VE, Verhaar, J, Vanderlinden, A, Duysings, J. De operative behandeling van de bursitis trochanterica. Ned Tijdschr Geneeskd. 1990; 134:2167.
148. Anderson, TP, Trochanteric bursitis: diagnostic criteria and clinical significance. Arch Phys Med Rehabil 1958; 39:617–622. ![]()
149. Karpinsky, MRK, Pigott, H, Greater trochanteric pain syndrome. J Bone Joint Surg. 1985;67B(5):762–763. ![]()
150. Shbeeb, MI, Matteson, EL, Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc. 1996;71(6):565–569. ![]()
151. Traycoff, RB, ‘Pseudotrochanteric bursitis’: the differential diagnosis of lateral hip pain. J Rheumatol. 1991;18(12):1810–1812. ![]()
152. Schein, AJ, Lehrmann, O. Acute trochanteric bursitis with calcification. Surgery. 1941; 9:771–779.
153. Hitora, T, Kawaguchi, Y, Mori, M, et al, Ischiogluteal bursitis: a report of three cases with MR findings. Rheumatol Int. 2009;29(4):455–458. ![]()