Disorders of the contractile structures
Introduction
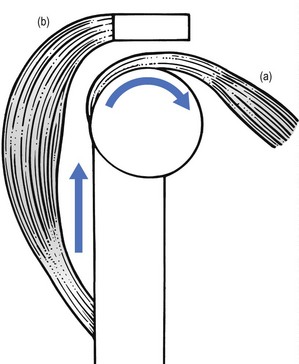
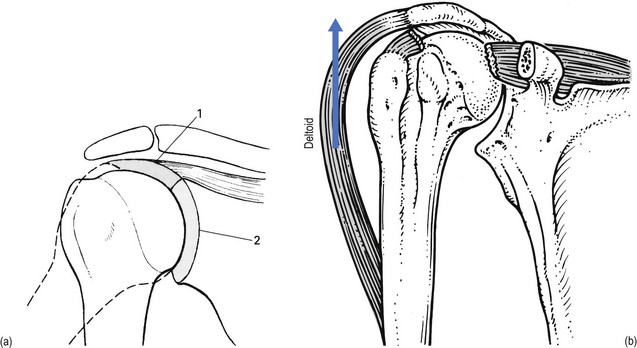
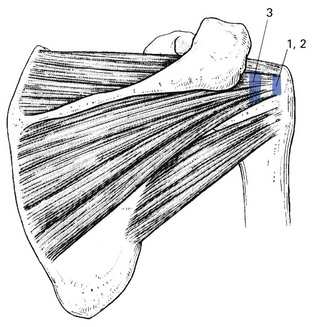
Functionally, shoulder muscles are of two types: stabilizing muscles and effector muscles. Stabilizing muscles (A, Fig. 15.1) are relatively small, with insertion tendons that lie close to, or even in, the substance of the fibrous capsule. Therefore they are not capable of causing significant shoulder movement but rather maintain the humeral head in the glenoid fossa. These stabilizing muscles are called the rotator cuff and include supra- and infraspinatus, teres minor and subscapularis. They all originate from the scapula, run partly under the acromial roof and insert on the humeral tubercles.
Effector muscles (B, Fig. 15.1) are much larger, with tendon insertions at a greater distance from the joint. Consequently, they produce powerful movements and are not primarily involved in stabilization. They are the deltoid complex, the pectoralis major, the latissimus dorsi and the teres major.
Rotator cuff
Rotator cuff disorder is one of the commonest afflictions of the shoulder and is a major cause of impairment of health in the young as well as in older individuals.1
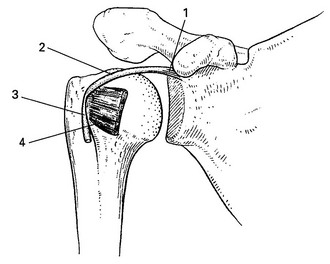
Lesions of the rotator cuff should be recognized as different from those of other tendons in the body for a variety of reasons. The tendons of the rotator cuff blend intimately with each other and with the capsule. The insertion of the tendons, as a continuous cuff around the humeral head, permits the cuff muscles to provide an almost infinite variety of movements to rotate the head and to oppose unwanted movements generated by the larger effector muscles.2 In addition, the long head of the biceps may be considered a functional part of the rotator cuff because tension in the tendon helps to compress the humeral head into the glenoid (Fig. 15.2).
Apart from their primary function (to rotate the humerus with respect to the scapula), the cuff muscles have two other actions: they compress the head of the humerus into the glenoid fossa and provide muscular balance (Box 15.1). The latter is mainly performed through eccentric contraction of the muscle. Both functions are extensively discussed in the online chapter Excessive range of movement: instability of the shoulder.
Pathology
Pathological changes associated with rotator cuff tendinopathy features are variable.
Inflammatory tendinitis is a reversible process, associated with an inflammatory infiltrate, increased vascularity and hyperaemic changes within the rotator cuff tendon.3
Partial rotator cuff tears may develop within the substance of the tendon on either the acromial (bursal) or articular surface of the tendon.4 Most of the lesions occur near to the tendon insertion side. Full thickness tears are often initiated by a partial tear. They can be associated with a traumatic event or can progress with normal daily use of the arm.
The primary cause of tendon degeneration is age.5 Changes in the rotator cuff include diminution of fibrocartilage at the cuff insertion, diminution of vascularity, fragmentation of the tendon and disruptions of the attachment to the bone.6,7
Changes in the coracoacromial arch have been described in association with cuff disease and it is quite clear from both cadaver and clinical data that individuals with full thickness rotator cuff tears have changes in the acromial shape, with spur formation on the undersurface of the acromion and/or hypertrophy of the acromioclavicular joint.8–11 Although these data indicate a strong association between the presence of cuff tears and alterations of acromial contour,12–14 it is still unclear whether the change in acromial shape is the cause or the result of the cuff defect or if both are consequences of ageing.15 Recent studies suggest that acromial deformity is usually developmental. Most acromial ‘hooks’ develop within the acromial ligament as traction spurs (analogous to the traction spur in the plantar ligament at its attachment to the calcaneus – see Fig. 15.11). The traction results from loading of the ligament by the cuff, which is increased when superior instability and cuff degeneration are present.16,17
Changes in the microvascular supply to the rotator cuff also have a possible role in the pathogenesis of rotator cuff lesions.18 A hypovascular region exists at the ‘critical zone’ of the supraspinatus (the deep surface of the anterior insertion).19,20 Microangiographic studies demonstrate that inadequate vascular supply to this critical zone is present in the adducted position of the arm.21 Furthermore, microvascular supply changes within the thickness of the tendon: the acromial part has much better vascularity than the articular part.22–24
It is likely that a combination of age, anatomical changes and vascular insufficiency is responsible for rotator cuff ‘failure’.25 Throughout life the cuff is subjected to various adverse factors such as traction, compression, contusion, subacromial abrasion, inflammation and age-related degeneration. A lesion may start where the loads are the greatest and the vascular supply the lowest, i.e. at the deep surface of the anterior insertion of the supraspinatus.26 Each fibre rupture then generates other adverse effects: it increases the load on the neighbouring fibres, it compromises the blood supply of the tendon fibres by distorting the microcirculation and it exposes increasing amounts of the tendon to joint fluid that contains lytic enzymes. The cuff is gradually weakened and at increased risk of further failure. With subsequent loading episodes the pattern repeats itself, rendering the cuff weaker and progressively more susceptible to additional failure (Fig. 15.3).27
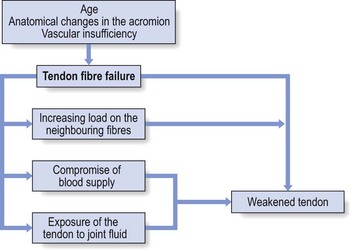
Fig 15.3 Tendon fibre failure.
Incidence
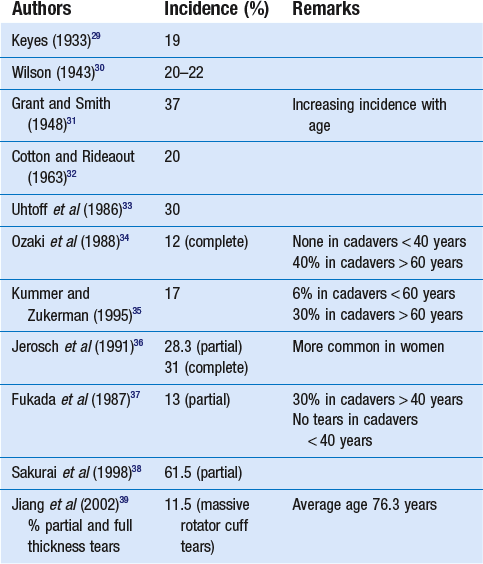
The incidence of rotator cuff tears has been studied both in cadaveric studies and in living subjects and found to range from 5 to 80%. All the studies show a strong relationship with age: rotator cuff tears are rare before age 40 and common after age 60.28 However, almost all of the reported cadaver studies failed to correlate cuff disorder with a history of clinical symptoms (Table 15.1). Some of the most important studies in living subjects have concerned the prevalence of cuff lesions in asymptomatic patients (Table 15.2). They all demonstrated a high prevalence of tears of the rotator cuff in asymptomatic individuals, an increasing frequency with advancing age and compatibility with normal, painless functional activity.
Table 15.2
Incidence of rotator cuff tears in asymptomatic patients
| Authors | Incidence (%) | Remarks |
| Petterson (1942)40 | 20 (partial and full) | 50% in subjects 55–85 years of age |
| Milgrom et al (1995)41 | 50 (tears after age 50 years) | Rotator cuff lesions are a normal correlate of age; they often present without clinical symptoms |
| Sher et al (1995)42 | 15 (full), 20 (partial) | Significant increase of tears with age; defects are compatible with normal, painless, functional activity |
| Yamaguchi (2006)43 | 35.5% prevalence of a full thickness tear on the painless side | Sonography of 588 consecutive patients with unilateral shoulder pain |
| Tempelhof (1999)44 | Rotator cuff tears in 23% 13% age 50–59 years 20% age 60–69 years 31% age 70–79 years 51% age > 80 years |
Ultrasonographic study of 411 asymptomatic volunteers |
| Shibany (2004)45 | Complete rupture of the supraspinatus tendon in 6% | Ultrasound examination of 212 asymptomatic shoulders at 56–83 years (mean: 67 years) |
| Kim (2009)46 | 0% age 40–49 years 10% age 50–59 years 20% age 60–69 years 40.7% > 70 years |
Ultrasound and MRI investigation of asymptomatic individuals |
| Moosmayer (2009)47 | Full thickness tears in 7.6% 15% age 70–79 years |
420 asymptomatic volunteers aged 50–79 years |
| Yamamoto (2010)48 | 16.9% asymptomatic 20.7% total group |
Prevalence of tears among the 683 residents of a mountain village in Japan |
Diagnosis and treatment
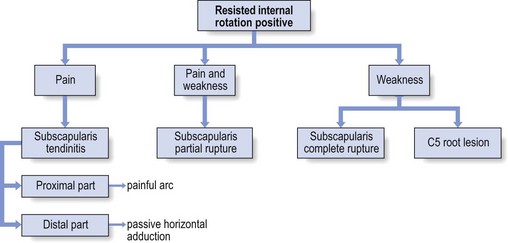
Given the high prevalence rate of asymptomatic macroscopic rotator cuff lesions, it is unwise to rely solely on imaging techniques (sonography, computed tomography (CT), MRI) for a diagnosis.49 The examiner should be wary of the common belief that what is found on technical investigation is always relevant to the cause of the patient’s pain.50 Diagnosis should first of all be made functionally; paraclinical and technical investigations have a secondary function only. During resisted movements, pain and weakness, separately or in combination with each other, are sought. If pain only is found, this points to an uncomplicated tendinitis or to a partial thickness lesion. A combination of pain and weakness brings a partial (full thickness) tendinous rupture to mind. Painless weakness is usually the outcome of a massive tear of a rotator cuff tendon or of a neurological problem. It is worth emphasizing once again that, when performing resisted movements at the shoulder, it is very important to use the correct technique, as has been explained earlier (see p. 214). If this is neglected, misdiagnosis can easily result.51
Treatment options are also determined only by the outcome of the clinical examination and not by the extensiveness of the (anatomical) lesion. Asymptomatic cuff lesions, for instance, in which the shoulder does not bother the patient but imaging studies document a partial or even full thickness lesion in the cuff tendon, should not receive treatment. However, if the rotator cuff lesion is symptomatic, it will usually not heal by itself because rotator cuff tendinitis is not self-limiting.52
Initial treatment for a symptomatic cuff lesion should always be conservative, no matter what the result of imaging may be. Non-operative treatment consists of either deep friction transversely to the affected tendon fibres or local infiltration with small amounts of triamcinolone at the tenoperiosteal junction of the affected tendon. In recurrent cases it is wise to add functional exercises for strength and proprioception (see online chapter Excessive range of movement: instability of the shoulder).53
The effectiveness and safety of steroids for the treatment rotator cuff disease remains the subject of much controversy. Repeated injections with steroids are believed to produce tendon atrophy or to reduce the ability of damaged tendon to repair itself. Animal studies suggest that corticosteroids damage the ultrastructure of collagen molecules54 and reduce collagen density, as well as inhibiting the reparative properties of tendon by inhibiting tendon cell migration and synovial fibroblast proliferation.55 This has been shown experimentally to weaken collagen fibres and precipitate tendon ruptures.56–58 In human subjects, repeated injections have been correlated with softening of the rotator cuff substance59 and an inferior result of surgical repair.60 Other studies, however, failed to find a deleterious long-term effect of corticosteroid injections in animal tendons,61,62 and a recent case-controlled study suggests that corticosteroid use in patients with ‘subacromial impingement’ should not be considered a causative factor in rotator cuff tears.63 Although rotator cuff disorders are generally believed to benefit from steroid injections, evidence for the efficacy of the injections is difficult to demonstrate. Most reviews have found conflicting results,64 which can be explained mainly by the fact that very heterogenous populations with poorly designed subgroups were used.65 In other studies that used a better anatomical classification, local triamcinolone infiltrations were shown to be superior to placebo66–70 and to methylprednisolone71 in reducing pain, improving active abduction and reducing functional limitation. The success rate of the infiltrations is further increased if precise diagnostic and infiltration techniques are used.72 In a randomly allocated double-blind study, Hollingworth et al compared two different methods of corticosteroid injection. The method of anatomical injection after diagnosis by the technique of selective tissue tension gave a 60% success rate, compared with the method using tender or trigger point localization, which produced only 20% success.73 Also, a recent meta-analysis concluded that injections of corticosteroids are effective for improvement for rotator cuff tendinitis for up to a 9-month period.74
• The infiltration is always on the tenoperiosteal junction, never in the body of the tendon.
• The arm should be rested for 2 weeks following the infiltration.
• No more than three consecutive infiltrations should be given.
Calcifying tendinitis
Calcium deposits may form in the tendons of the rotator cuff. The aetiology is still a matter of speculation but it is generally accepted that degeneration precedes calcification. The incidence of calcification ranges from 3 to 20%75,76 and in most instances the lesion is completely asymptomatic.77 The highest incidence occurs in those aged between 31 and 50,78,79 and calcification is absent in elderly patients. The disease is usually self-limiting with a variable natural course: 80% of calcific lesions show spontaneous resorption over a period of 3 years.79 If the lesion causes symptoms, these are treated in the same way as uncomplicated tendinitis of the rotator cuff. The treatment of choice is infiltration with triamcinolone. If the pain reappears after initially successful treatment, the calcium deposits can be dissolved with weekly infiltrations of procaine 0.5%.80 Surgical intervention is seldom necessary but if it is indicated, most calcifications can easily be removed by arthroscopic procedures.81 In recent years extracorporeal shock wave therapy has been proposed as an alternative to operative treatment.82,83
Resisted abduction
Pain
Deltoid muscle
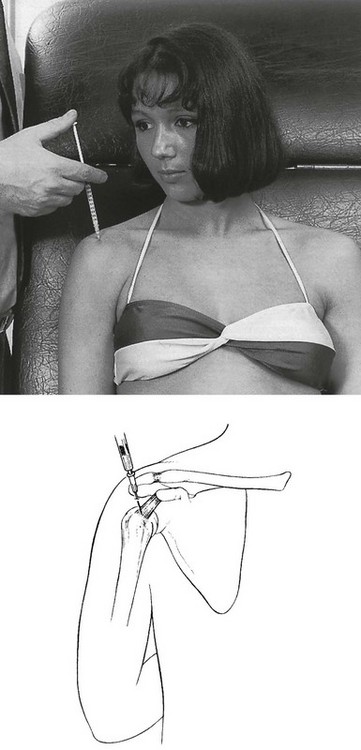
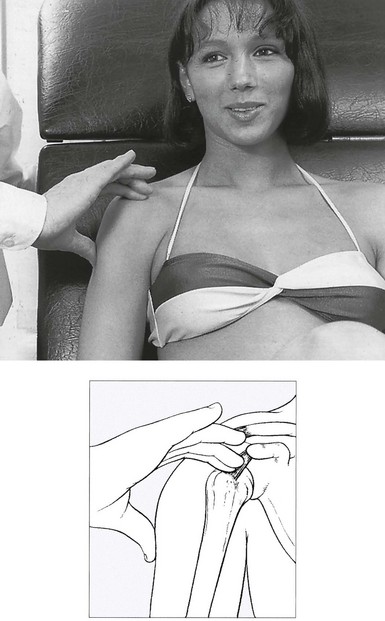
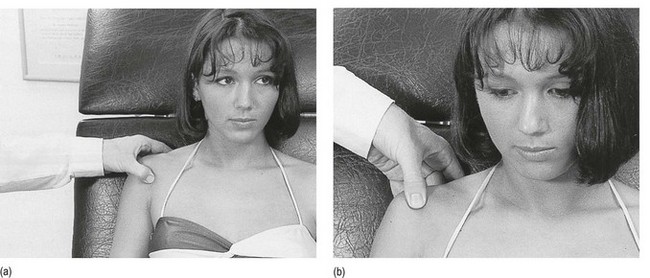
• Resisted horizontal adduction (Fig. 15.4): the examiner stands at the painful side and stabilizes this shoulder with one hand. With the other hand, he grasps the patient’s arm just above the elbow and brings it into horizontal abduction. The patient is now asked to push the arm forwards against the examiner’s hand. This tests the anterior portion of the deltoid.

Fig 15.4 Resisted horizontal adduction.
• Resisted horizontal extension (Fig. 15.5): this is the exact opposite of the previous test. The patient is asked to push backwards, the examiner applying counterpressure in an anterior direction. The posterior fibres of the deltoid are tested in this movement.

Fig 15.5 Resisted horizontal extension.
Supraspinatus
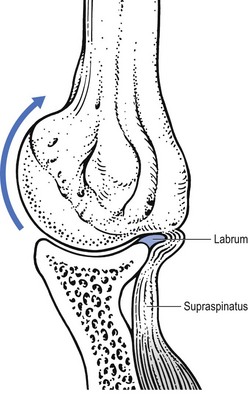
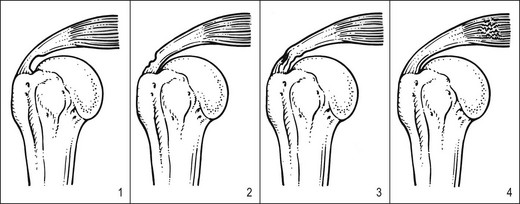
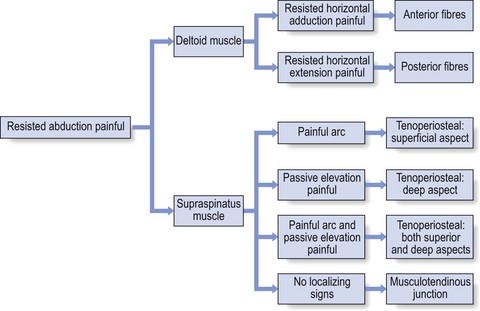
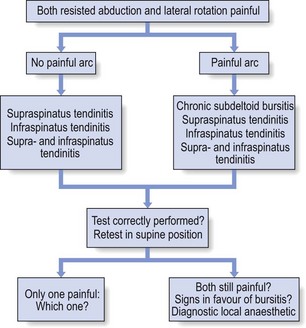
Most of the lesions occur at the tenoperiosteal insertion into the greater tuberosity. Inflammation and partial tears may develop within the substance of the tendon on the acromial surface (bursal side) or the deep surface (articular side of the tendon).84 If situated on the deep part, pain on full passive elevation is also present. This is believed to be caused by the abutment of the deep surface of cuff insertion against the glenoid rim at the extremes of motion (Fig. 15.6).85 If situated on the bursal part of the tendon, a painful arc is found. Cyriax regarded this as the most common cause of a painful arc on shoulder elevation.86 A third possibility is a lesion that involves both superficial and deep aspects tenoperiosteally. In this case, pain on full passive elevation is present, together with a painful arc (Fig. 15.7).
Figure 15.8 summarizes the differential diagnosis of painful resisted abduction.
Treatment of tenoperiosteal lesions
Localization by palpation
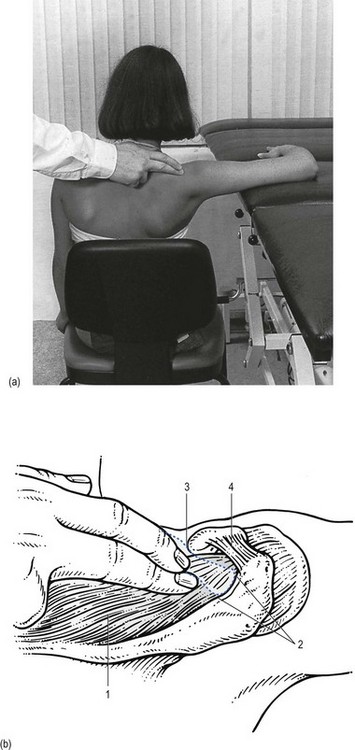
The main problem in treatment is finding the structure involved. Although the tendon lies quite superficially, many have difficulty in localizing it. It cannot be easily localized with the arm in the neutral position at the side. Although the greater tuberosity points laterally in this position, part of the insertion can be covered by the outer rim of the acromion. Moreover, all structures here feel the same on palpation. Therefore, it is better to bring the upper arm into full medial rotation by asking the patient to put the lower arm behind the back, with the elbow bent to 90° (Fig. 15.9). In this position the tenoperiosteal insertion lies anterior to the acromion.87,88 The fibres at the insertion are now situated in a sagittal plane because the tendon curves around the base of the coracoid process as a result of the medial rotation.
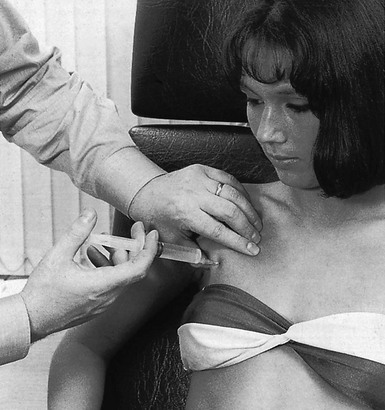
Technique: infiltration of the supraspinatus
A tuberculin syringe, filled with 1 mL of triamcinolone, is fitted to a 2.5 cm needle. The patient sits in the same position as for palpation, the arm behind the back. After the insertion has been precisely located, the needle is thrust in vertically downwards at its centre (Fig. 15.10). The needle glides in smoothly initially, until it encounters the tenoperiosteal junction, at which point the typical tendinous resistance is felt. When the needle is thrust in a little further, it is arrested against the bone. Then 1 mL of triamcinolone is infiltrated at 5–10 different places over an area of 1 cm2, in close bony contact. During the whole infiltration, a typical counter-pressure is felt.
Treatment that leads to good but temporary results is a common experience in supraspinatus tendinitis. After one or two infiltrations the pain has disappeared but recurs a few months later. The patient should then be sent for standard radiography of the shoulder. If some intratendinous calcification is confirmed, 4–5 weekly infiltrations with 5 mL of procaine 2% are administered. In most cases this is sufficient to dissolve the calcium deposits and to alleviate the pain. If no calcification is visible, the patient must be referred to the therapist for deep transverse friction because it is unwise to infiltrate the tendon repeatedly, even with small doses such as 10 mg of triamcinolone. In cases of recurrent tendinitis it is also good practice to look for an underlying cause. This may be a small multidirectional or superior instability or an anatomical divergence in the acromial roof that causes recurrent impingement. The diagnosis and treatment of the former have been discussed earlier (see online chapter Excessive range of movement: instability of the shoulder). The diagnosis of the latter is through a lateral X-ray that visualizes the so-called ‘supraspinatus outlet’ – the space between the coracoacromial roof and head of the humerus (Fig. 15.11). If there is an anatomical divergence in the supraspinatus outlet, and the tendinitis tends to recur despite proper treatment, surgical decompression (deletion of anterior and inferior parts of the roof) is highly recommended.89
The treatment of supraspinatus tendinitis is summarized in Figure 15.12.
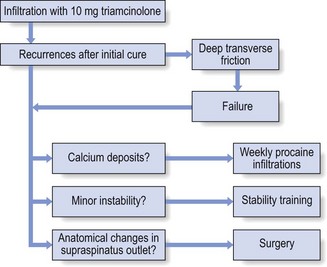
Fig 15.12 Treatment of supraspinatus tendinitis.
Technique: deep friction to the supraspinatus
The patient adopts the same position as for infiltration: seated with the back against the couch, the arm behind the back. The therapist stands laterally on the patient’s painful side. The index finger of the ipsilateral hand, reinforced by the middle finger, is placed at the medial edge of the insertion. The hand is meanwhile stabilized by the thumb placed against the lateral aspect of the upper arm, almost vertically under the index finger (Fig. 15.13). When the index finger is pulled outwards over the tendon, pressure is applied. This is the active phase of the friction. The pressure is directed caudally, not towards the clavicle – easily obtained if the stabilizing thumb is placed rather low on the upper arm, the nail of the finger that applies friction thus pointing upwards.
Treatment of musculotendinous lesions
Technique: deep friction to musculotendinous lesions
The patient sits on a chair with the arm abducted sideways to the horizontal, the elbow and forearm resting on a couch. In this position the musculotendinous junction lies in the supraspinous fossa at the angle between the scapular spine and the acromion, just posterior to the clavicle. The therapist stands at the pain-free side facing the shoulder. The ipsilateral middle finger reinforced by the index finger is placed deeply into the scapuloacromial angle, holding the slightly bent finger parallel to the muscle (Fig. 15.14). Friction is given by pronation–supination movements of the lower arm, the active moment being in supination.
Friction is applied for about 15 minutes. Cure is expected after about 10 sessions.
Painful weakness
If resisted abduction is found to be both weak and painful, a partial rupture of the supraspinatus tendon is most likely.90
Ruptures of the rotator cuff occur most frequently at the supraspinatus tendon. A full-thickness defect usually starts at the critical zone (articular side of the anterior part near to the bicipital groove) and may propagate either in the direction of the infraspinatus or towards the subscapularis tendons.91,92
Treatment
Conservative treatment is reasonable for most partial ruptures of the supraspinatus tendon.93 Treatment is relief of pain, achieved by infiltration of 1 mL of triamcinolone at the tenoperiosteal insertion and into the most distal part of the tendon. The same position and technique are used as for an uncomplicated tendinitis.
Painless weakness
Total rupture of the supraspinatus tendon
The supraspinatus muscle initiates active elevation of the arm and is active during the entire arc of abduction. It is responsible for about 50% of the torque and can abduct the joint without action of the deltoid.94 Therefore a total rupture of the supraspinatus tendon presents as painless weakness on resisted abduction.
Previously it was believed that the arm could not be abducted actively by the deltoid muscle alone if some contraction of the supraspinatus did not initiate the movement.95 However, studies in which the suprascapular nerve and the axillary nerve were blocked have shown that both the supraspinatus and the deltoid muscles are capable of initiating elevation of the arm, in both sagittal and coronal planes.96–98 This is not in accord with what is found clinically. When the supraspinatus tendon is massively ruptured, the patient is unable to initiate active scapulohumeral abduction. Starting from a position of 0° (the arm hanging by the side), the deltoid muscle only pulls the humerus upwards. Elevation is then only produced by rotation of the scapula in relation to the thorax and not by any movement between scapula and humerus. This is explained by the superior displacement of the humerus that occurs during active contraction of the deltoid in the absence of an intact supraspinatus tendon.99,100 In a normal situation the rotator cuff muscles form a supplementary musculotendinous glenoid which, in conjunction with the osseous glenoid, holds the humeral head stable. Experimental sections of the supraspinatus tendon also show the humeral head to move in a cranial direction until the superior humeral load is applied directly to the acromion (Fig. 15.15).101 This phenomenon has been referred to as ‘the spacer effect’102 and is one of the most significant plain radiographic signs of massive cuff deficiency.103
Symptoms and signs
The lesion usually affects middle-aged and elderly people. In patients under 40 years of age a rupture is usually acute and results from indirect trauma, such as a fall on the outstretched hand.104,105 In most cases the rupture is due to a chronic failure of the tendon: repeated failure of small groups of fibres leads to progressive weakness of the supraspinatus, making it increasingly susceptible to damage from lesser loads.106,107 The observation that major cuff defects may occur without recognized injury has led to the concept of ‘creeping tendon failure’.108
Frequently, because the patient is unable to move the arm actively, an immobilizational arthritis will set in after a few months and a capsular pattern emerges (see p. 228).
Treatment
Because most total ruptures occur insidiously in the elderly, surgical correction to restore normal function will not be the first option.109 Some defects cannot be repaired simply because they only offer ‘rotten cloth to sew’ (McLaughlin).110 Many defects do not need to be repaired because they exist without causing much in the way of clinical symptoms.111 However, surgery should always be considered in a younger patient with a significant acute tear in a previously normal shoulder,112,113 and in the patient with a chronic tear associated with significant symptoms and not responding to conservative treatment. Fair functional results have been observed using open114,115 and arthroscopic techniques,116 but it is important to bear in mind the fact that repair does not restore the quality of the tendinous tissue. Reported recurrence rates after rotator cuff repair range between 15% and 90%.117,118 Because rotator cuff integrity is important to its function,119 long-term results of repair are better in younger patients with acute tears.120,121
For most supraspinatus ruptures the treatment of choice is conservative. The primary aim is to get rid of the pain. To that end, an infiltration with 10 mg of triamcinolone should be given at the remnants of the insertion into the humeral tuberosity to abolish the painful arc, following the same procedure as for uncomplicated supraspinatus tendinitis. The therapist should try to reach the inflamed tissue because infiltrating the gap is useless. Therefore the typical counterpressure on syringe and needle should be felt during the entire procedure. Once the painful arc has gone, patients should be encouraged to use the arm normally. To lift the arm up, they should initiate elevation via a swinging movement of the trunk so that the arm is thrown laterally until it reaches the point where the deltoid muscle becomes effective. It is also wise to prescribe functional exercises for the antagonists of the deltoid and the remaining rotator cuff muscles. In spite of the slight disability, the function of the joint is usually good and the patient should be capable of doing light work.122–124 Outcome studies report good to fair results in 60–90% of conservatively treated supraspinatus ruptures.125–127
Neurological lesions
Several neurological disorders may provoke painless weakness on resisted abduction.
Axillary nerve palsy
This may follow a subluxation of the humeral head. The axillary nerve innervates the deltoid muscle. Differential diagnosis is based on testing the strength of both the deltoid and infraspinatus muscles. Isolated painless weakness of abduction is found. A painful arc is not present, which differentiates this disorder from a supraspinatus rupture. Active elevation is difficult but possible (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
Suprascapular nerve palsy
This is most often the result of idiopathic neuritis, and seldom of an injury. The suprascapular nerve innervates both supra- and infraspinatus muscles. Weakness of abduction and lateral rotation is present. This disorder can be differentiated from a combined supra- and infraspinatus rupture by the absence of a painful arc (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
Fifth cervical root palsy
The C5 root innervates both spinatus muscles, the deltoid and the biceps brachii. Weakness on abduction and lateral rotation of the arm and on flexion of the elbow is present (see p. 155).
See (Fig. 15.16) for a summary of resisted abduction.
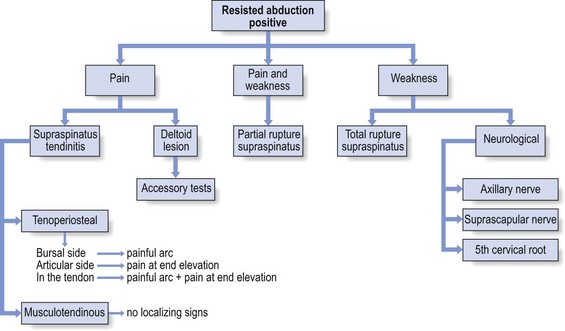
Fig 15.16 Resisted abduction positive.
Resisted adduction
Pain
It may happen that isometric movements indirectly provoke pain in structures other than the ones that are supposed to be tested. Cyriax called this phenomenon ‘transmitted stress’. During strong resisted adduction with the arm hanging alongside the body, the strong actions of the latissimus dorsi and pectoralis major pull indirectly on the acromioclavicular joint. Resisted adduction may therefore also provoke local shoulder pain in a chronic strain of the acromioclavicular joint. The pain will then be localized within the C4 dermatome and other passive tests will point to the acromioclavicular joint (see p. 241).
Pain on resisted adduction of the arm may rarely be caused by a tendinitis of the long head of the biceps at the glenoid origin. Cyriax arrived at this conclusion after finding that resisted adduction proved to be painful with the elbow in extension but not if it was kept in flexion. As a consequence, the structure at fault must overlie both shoulder and elbow (see p. 269). The pain is mainly felt under the acromioclavicular joint.
More often, however, a positive test will indicate a lesion of one of the adductors: pectoralis major, latissimus dorsi, teres major and teres minor.128 An important element in distinguishing between them is the localization of the pain. Pain in the thorax anteriorly suggests the pectoralis major and posterolaterally the other three muscles. Axillary pain may be the result of a problem in the pectoralis major, teres major or latissimus dorsi.
The pectoralis major muscle can be tested on its own by resisted horizontal adduction with the arm forwards (Fig. 15.17a). The examiner stands on the painful side. One hand is placed on the patient’s shoulder. With the other hand, the patient’s arm is grasped just above the elbow and brought anteriorly to the horizontal. The patient is now asked to exert further medial force against the examiner’s hand. The same can be achieved, but with loss of assessment of muscle strength, when the patient pushes both hands against each other holding both arms horizontally in front of the body.
Further differentiation is done by a resisted extension movement (Fig. 15.17b), which involves the latissimus dorsi, teres major and teres minor muscles. The patient tries to move the arm backwards with the arm hanging down and the examiner applying anteriorly directed counterpressure.
Pectoralis major
Lesions of the pectoralis major may be the result of overuse or of direct injury.
The lesion usually lies in the muscle belly, either in its lateral lower portion or in the fibres just below the outer portion of the clavicle (Cyriax:86 p. 145). In rare instances, the pain is felt at the shoulder and radiates down the inner aspect of the arm. The problem must then be sought at the insertion at the crest of the greater tubercle.
Treatment
Technique: infiltration of the pectoralis major
The patient adopts the half-lying position and puts the hand on the affected side on the iliac crest. A 4 cm needle is fitted to a syringe filled with 10–20 mL of procaine 0.5%. After accurate palpation, the lesion is held between thumb and index finger and the needle is introduced between them obliquely and to its full length. The finger and thumb are kept in this position during the infiltration, to feel whether the product is deposited at exactly the right place (Fig. 15.18). The injection is given while withdrawing the needle. The syringe is emptied gradually, over about five withdrawals and reinsertions.
Technique: deep transverse friction to the pectoralis major
• To the muscle belly: massage is best given with the patient in the half-lying position and the hand resting on the iliac crest, so that the upper arm is somewhat abducted. This brings the pectoralis major more into prominence. The therapist sits facing the patient at the painful side. With the ipsilateral hand, the painful spot is taken between fingers and thumb and pulled laterally while pressure is applied (Fig. 15.19). This is the active moment of the friction. Massage is given for 20 minutes. Cure is normally obtained after 10 sessions.
• To the insertion: if palpation shows the lesion to lie at the insertion, deep friction is the only treatment. First the bicipital groove is localized, which is best done by putting the finger proximally between the minor and major humeral tubercles and in the long axis of the arm. By rotating the arm alternately medially and laterally, the groove can be identified. The therapist sits facing the patient at the painful side and places the contralateral thumb between the fibres of the deltoid muscle at the outer aspect of the bicipital groove. Counterpressure is applied over the dorsal aspect of the arm by the other fingers. Friction is given by pronation–supination movements.
Latissimus dorsi
Technique: deep transverse friction to the latissimus dorsi
The patient lies prone, the hand on the iliac crest. The therapist faces the patient’s painful side. The tender area is taken between thumb and fingers of the ipsilateral hand, and friction is performed by pulling the hand laterally (Fig. 15.20).
Painful weakness
Ruptures of the pectoralis major follow extreme muscle tension or direct trauma, or a combination of both. They have been reported in gymnastics or after a fall.129,130 The lesion is more common in weightlifting and is caused in particular by a bench press.131 The rupture usually occurs at the lateral fibres when the bar is at its lowest (loading of the fibres that are maximally stretched).132,133
In an acute injury there is sharp and burning pain, sometimes accompanied by significant swelling and ecchymosis. Passive elevation of the arm is very painful and somewhat limited. Resisted adduction and medial rotation of the arm are painful134 and weak.
The functional tests are followed by palpation for tenderness and for a gap, and are best performed with the patient’s arm slightly abducted and the hand resting on the iliac crest (see Fig. 15.19).
Treatment consists of surgical repair. Results are usually good to excellent.135
Painless weakness
A severe C7 root palsy can provoke painless weakness of adduction (see p. 156). Resisted extension of the elbow is also remarkably weak. The triceps reflex is sluggish or absent, together with some weakness on flexion of the wrist, more rarely on extension or on both. Numbness of the second and third fingers is usually present.
See Fig. 15.21 for a summary of resisted adduction.
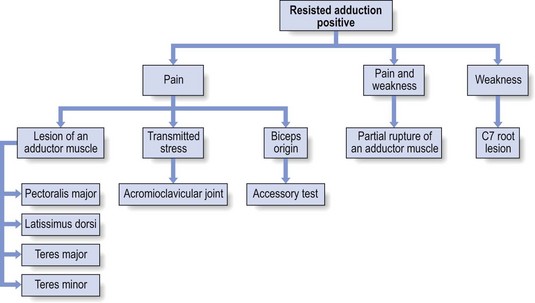
Fig 15.21 Resisted adduction positive.
Resisted lateral rotation
Pain
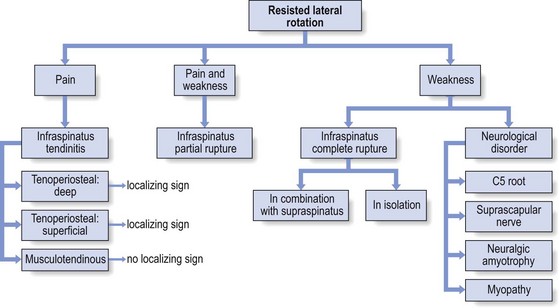
Infraspinatus
There are some localizing signs of lesions of the infraspinatus. A painful arc indicates that the lesion lies at the superficial part of the tenoperiosteal insertion. If full passive elevation causes pain, the deep part of the tenoperiosteal insertion is affected (Fig. 15.22). If, apart from resisted lateral rotation, no other tests are painful, the lesion is in the body of the tendon or at the musculotendinous junction.136,137 Palpation will disclose the exact location of the lesion.
Treatment
Technique: infiltration of the infraspinatus tenoperiosteally
A 1 mL syringe is filled with 10 mg of triamcinolone and fitted to a 3 cm long needle. After the landmarks have been located, the needle is introduced in an anterocaudal direction, aiming at the tenoperiosteal insertion, which is reached after passing through soft tissue for about 2 cm (Fig. 15.23). Just before bone is reached, the tough tendinous resistance of the insertion is felt. The total amount is now injected in 5–10 slightly different sites. At each partial injection, strong counterpressure is felt.

Fig 15.23 Infiltration of the infraspinatus.
Technique: deep friction to the infraspinatus
The patient lies in the same position as for infiltration. The therapist stands at the painful side. The thumb of the ipsilateral hand is placed on the lesion, while counterpressure is applied with the other fingers at the front of the shoulder (Fig. 15.24). The body of the tendon feels like a cord, whereas the tenoperiosteal insertion feels more like a flat resistance just covering the underlying bone.
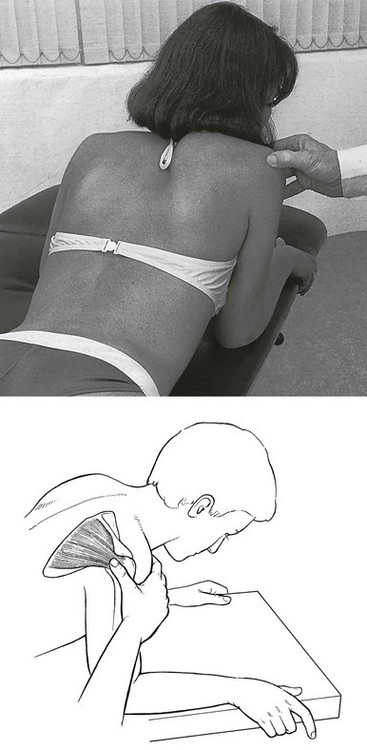
Fig 15.24 Deep friction to the infraspinatus.
Friction is given for 20 minutes, three times a week, and is usually curative after 10–15 sessions.
A specific case: abduction and lateral rotation against resistance are both painful
Both resisted abduction and lateral rotation are frequently painful on clinical examination and often there is a painful arc. This clinical pattern usually causes differential diagnostic difficulties that are not always easy to solve (Fig. 15.25).
Painful arc
If a painful arc is also found, then the following lesions may be present:
If a painful arc is present, the tests should be repeated standing and lying.
Signs in favour of chronic bursitis
Presence of these signs makes it more likely that chronic bursitis is present:
• All passive movements painful at full range
• Tests are less painful in a supine position
• Resisted movements become less painful with axial traction, which prevents the humeral head approaching the coracoacromial roof
• Tenderness of the superficial part of the subdeltoid bursa on palpation
• Infiltration of the deep part of the bursa with local anaesthetic abolishes the pain.
Painless weakness
Muscular disorder: total rupture of the infraspinatus tendon
Infraspinatus tears may present in isolation or in combination with a partial or total rupture of the supraspinatus tendon. The infraspinatus defect then propagates in a posterior direction from an established full thickness tear in the supraspinatus.138
As in supraspinatus tears, the lesion usually develops in patients over 50 years of age and is often asymptomatic (see p. 145). The lesion results from a combination of wear and tear and repetitive minitrauma. Sometimes the tear occurs suddenly during an acute overload or a fall.
Chronic tears are characterized by marked and visible atrophy in the infraspinatus fossa. Secondary to the permanent loss of the infraspinatus, the patient can no longer bring the arm into full lateral rotation. This reduces the elasticity of the anterior capsule of the shoulder and results in a permanent isolated limitation of external rotation. The impaired external rotation leads to the typical ‘hornblower’s sign’: bringing the hand to the mouth is only possible in internal rotation of the arm with accompanying excessive elevation of the elbow. Hornblower’s sign has a 100% sensitivity and 93% specificity for irreparable degeneration of the infraspinatus.139,140
Neurological disorders
Suprascapular nerve palsy (see online chapter Nerve lesions and entrapment neuropathies of the upper limb)
Suprascapular nerve entrapment is usually an acquired neuropathy secondary to compression of the nerve in the bony suprascapular notch. Chronic mechanical irritation may be caused by overhead activities such as raquet sports, lifting and volleyball. Direct trauma, compression (e.g. backpacking) or a fracture of the scapula may also cause the lesion.141 Sometimes it is idiopathic.
Spontaneous cure is the rule in an idiopathic ‘neuritis’. The pain disappears within 3 weeks, weakness within 6–12 months.142
During the last few decades, there have been several reports of isolated infraspinatus muscle paralysis in volleyball players. The reported incidence in high-level volleyball players is between 25 and 45%.143,144. Usually the finding is accidental and the disorder without any complaint: no pain, no loss of function, and even no reduced efficiency in playing volleyball.145 The most plausible pathomechanism for this suprascapular neuropathy is traction or stretching of the nerve.146 This traction injury may occur when repetitive overhead activities result in local nerve strain that exceeds the passive tolerance of the nerve.147,148 In one study the players with suprascapular nerve entrapment displayed significantly greater shoulder mobility than those without.149
Neuralgic amyotrophy
This usually affects young males and starts with central neck pain, later spreading towards both shoulders and arms. Finally it leaves one arm and remains only in the other one. The pain is very severe and may last for about 6 months. Right from the onset, muscular involvement is visible. Often wasting is present together with a total palsy, not just weakness. It affects muscles of both sides, irrespective of segmental origin. Paralysis of the infraspinatus and the triceps, extensors of the fingers or extensors of the thumb is a common pattern (Cyriax:86 p. 90) (see p. online chapter Nerve lesions and entrapment neuropathies of the upper limb).
Resisted medial rotation
Pain
Subscapularis
The upper portion of the subscapularis inserts through a collagen-rich tendon into the lesser tuberosity. The rest (about 40%) of the tendon inserts into the humerus below the lesser tubercle and medial to the bicipital groove. Here the insertion is directly from muscle to bone.150
Tendinitis of the subscapularis can be localized tenoperiosteally in the cranial or caudal part of the tendon. Lesions in the muscle belly or in the tendon itself do not seem to occur. A lesion in the cranial part causes a painful arc because of impingement under the coracoacromial arch.151 If the caudal portion is affected, the accessory test of passive horizontal adduction is painful as a result of pinching of the lesion between the lesser tuberosity and the coracoid process. Full active and passive elevation and full lateral rotation may be painful as well, because they stretch the subscapularis. Passive lateral rotation can even be so painful that it seems limited. To exclude arthritis, the examiner must gently try to bring the arm to full range, so as to confirm that limitation does not exist.
Treatment
Technique: infiltration of the subscapularis
A 3 cm needle fitted to a syringe filled with 1 mL of triamcinolone is inserted at the subscapularis insertion in the middle of the most painful part, and directed towards the bone (Fig. 15.27). Just before the humerus is reached, tendinous resistance is felt. As always, the infiltration is given in droplets. This time the needle is moved along a line in a craniocaudal direction over about 1.5 cm so as to include the whole lesion. Counterpressure is experienced during the infiltration. Care must be taken not to move the needle too far laterally, so as to avoid infiltrating the tendon of the long head of biceps, which could lead to its early degeneration.
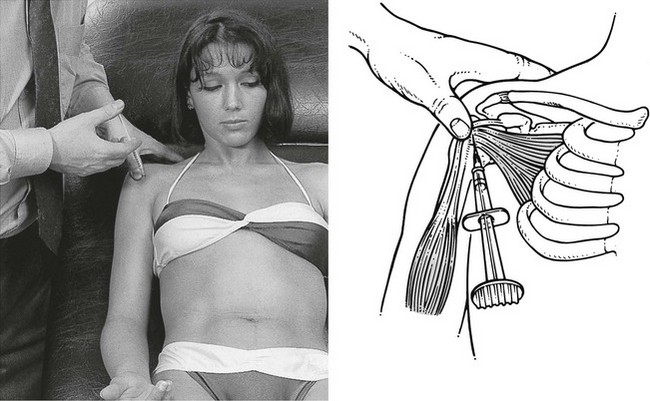
Fig 15.27 Infiltration of the subscapularis.
Technique: deep friction to the subscapularis
This form of treatment is mainly used in recurrences or in patients who refuse steroids.
The therapist stands on the affected side and faces the patient. The contralateral hand is used. Beginning at the coracoid process, the thumb is brought just underneath it and the other fingers curved laterally around the arm, on to the back of the shoulder. The thumb is now pulled laterally while pressure is applied in a posterior direction. During this movement two tendinous structures are momentarily felt slipping under the thumb; these are the coracobrachialis muscle and the short head of the biceps. By moving the thumb yet further laterally, while maintaining some dorsal pressure, the anteromedial edge of the deltoid is reached and is pulled to the side. Finally the lesser tuberosity is contacted (Fig. 15.28a). The hand is now turned into a vertical position (Fig. 15.28b), still keeping the deltoid to the side.
Painless weakness
Rupture of the subscapularis tendon
Tears of the subscapularis tendon occur either in isolation or as a propagation of a supraspinatus tear.152 The former usually is the result of a hyperextension–external rotation trauma or follows an anterior dislocation of the shoulder.153 The lower part of the muscle is mostly affected, where the insertion is more or less directly from muscle to bone. In the latter, the lesion is more proximal at the lesser tubercle, the distal part of the tendon remaining unaffected.154,155 In traumatic rupture there may also be an excessive range of passive lateral rotation. Weakness of internal rotation can also be demonstrated by the ‘lift-off’ signs described by Gerber and Krushell: the patient places the arm in internal rotation with the dorsum of the hand on the sacroiliac joint. If the patient is unable to rotate the arm internally any further and lift the hand off the pelvis, incompetence of the subscapularis is suspected.156
Treatment
Isolated traumatic tears of the subscapularis are best treated with surgery.157 The surgical outcomes are less favorable when other tendons are involved.158 If the condition remains painful, an infiltration with corticosteroid into the remnants at the tenoperiosteal insertion can be helpful.
Resisted elbow flexion
Pain
The classification of biceps lesions can be divided into tendinosis of the biceps and biceps instability. The former may be an impingement lesion or occurs in isolation as a primary lesion of the biceps tendon.159
Lesions of the biceps can lie at different levels. Only those that can provoke pain at the shoulder are discussed here. These are tendinitis of the long tendon at the glenoid insertion, in its intra-articular course and at the bicipital sulcus (Fig. 15.30). Lesions of the muscle belly and further down are discussed in Chapter 19.
Lesion of the long bicipital tendon at the glenoid origin
Strain on the glenoid from the working biceps is greatest when the arm is in overhead abduction and lesions are usually caused in throwing athletes by traction injuries.160,161 The pain is usually felt underneath the acromioclavicular joint but may radiate in the C5 dermatome. Besides pain on resisted flexion and supination of the elbow, resisted adduction of the arm will also show positive. When the resisted adduction is tested again, this time with the elbow flexed, it becomes painless. This phenomenon is explained by the constant-length phenomenon on a structure spanning two joints (see Ch. 4): the degree of stress on the painful tissue in one joint depends on the position in which the adjacent joint is held.
Treatment
Technique: infiltration of the long bicipital tendon
A 2 mL syringe is filled with 20 mg of triamcinolone and a 4 cm needle fitted to it. One thumb is placed just beyond the acromion at the groove. The needle is inserted just distally to the thumb parallel to the bicipital sulcus and aiming at the glenoid (Fig. 15.31). It is then moved further in between the humeral tuberosities until it strikes the glenoid. A tough resistance is felt just before touching the bone. The steroid is infiltrated here in drops so that the whole insertion is treated. During the infiltration some counterpressure is felt.
The patient is re-examined after 2 weeks and, if necessary, the infiltration is repeated.
Lesion in the sulcus of the long head of biceps
Technique: deep friction to the long head
With the other hand the therapist grasps the patient’s lower arm, the elbow bent to 90°, and brings the arm into medial rotation until the radial side of the frictioning thumb comes in contact with the outer edge of the sulcus (Fig. 15.32). During this movement the tendon glides under the thumb and friction is achieved. The arm is now brought back into lateral rotation while releasing the pressure on the tendon (passive moment).
Snapping the long head of biceps
Biceps instability is seen most commonly in throwing athletes. Motion is often accompanied by a palpable snap or pop at a certain position in the arc of rotation.162
According to Slätis and Aalto, a rupture of the intertubercular transverse ligament allows for no appreciable medial or lateral movement of the tendon.159 The key structure which guides the tendon in its groove is the medial portion of the coracohumeral ligament, which inserts at the lesser tubercle. It fills the space between the upper margin of the subscapularis and the anterior border of the supraspinatus. If it is transected, the long tendon of the biceps can easily be displaced medially.
Instability test
Dislocation of the biceps is tested with the biceps instability test of Abbott and Saunders.163 The arm, abducted to 90° and fully rotated externally, is slowly brought to the side and rotated slightly internally. A palpable and even audible and sometimes painful click is noted as the biceps tendon, now forced against the lesser tuberosity, becomes subluxated or dislocated from the groove.
Resisted flexion of the arm
The patient is asked to push the arm forwards against the examiner’s hand, the latter applying counterpressure in the opposite direction (Fig. 15.33). Pain on this movement alone is the outcome of a lesion of the coracobrachialis muscle, which is rare. Although resisted adduction should also be painful, in practice it is not.

Fig 15.33 Resisted flexion.
References
1. Bjelle, A, Epidemiology of shoulder problems. Baillière’s Clin Rheumatol 1989; 3:437–451. ![]()
2. Clark, JM, Sidles, JA, Matsen, FA, III., The relationship of the glenohumeral joint capsule to the rotator cuff. Clin Orthop 1990; 254:29–34. ![]()
3. Ulthoff, HK, Sarkar, K, Lohr, J. Repair in rotator cuff tendons. In: Post M, Morrey BF, Hawkins RJ, eds. Surgery of the Shoulder. St Louis: Mosby-Year Book; 1990:216–219.
4. Fukuda, H, Hamada, K, Yamanaka, K, Pathology and pathogenesis of bursal side rotator cuff tears viewed from en-bloc histologic sections. Clin Orthop 1990; 254:75–80. ![]()
5. Ulthoff, HK, Sarkar, K. The effect of ageing on the soft tissues of the shoulder. In: Matsen FA, III., Fu FH, Hawkins RJ, eds. The Shoulder: A Balance of Mobility and Stability. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1993:269–278.
6. Satoshi, O, Uhthoff, H, Acromial enthesopathy and rotator cuff tear. Clin Orthov Rel Res 1990; 254:39–48. ![]()
7. Riley, GP, Harrall, RL, Constant, CR, et al, Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 1994; 35:359–366. ![]()
8. Bigliani, LU, Morrison, DS, April, EW. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986; 10:228.
9. Morrison, DS, Bigliani, LU. The clinical significance of variations in acromial morphology. Orthop Trans. 1987; 11:234.
10. Cotton, RE, Rideout, D, Tears of the humeral rotator cuff: a radiological and pathological necropsy survey. J Bone Joint Surg 1964; 46B:314–328. ![]()
11. Aoki, M, Ishii, S, Usui, M. Clinical application for measuring the slope of the acromion. In: Post M, Morrey BF, Hawkins RJ, eds. Surgery of the Shoulder. St Louis: Mosby-Year Book; 1990:200–203.
12. Chung, SM, Nissenbaum, MM, Congenital and developmental defects of the shoulder. Orthop Clin North Am 1975; 6:381–392. ![]()
13. Saupe, N, Pfirrmann, CW, Schmid, MR, et al, Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376–382. ![]()
14. Worland, RL, Lee, D, Orozco, CG, et al, Correlation of age, acromial morphology, and rotator cuff tear pathology diagnosed by ultrasound in asymptomatic patients. J South Orthop Assoc. 2003;12(1):23–26. ![]()
15. Ogata, S, Uhthoff, HK, Acromial enthesopathy and rotator cuff tear: a radiologic and histologic postmortem investigation of the coracoacromial arch. Clin Orthop 1990; 254:39–48. ![]()
16. Flatow, EL, Soslowsky, LJ, Ticker, JB, et al, Excursions of the rotator cuff under the acromion. Patterns of subacromial contact. Am J Sports Med 1994; 22:779–788. ![]()
17. Putz, R, Reichelt, A, Structural findings of the coraco-acromial ligament in rotator rupture, tendinosis calcarea and supraspinatus syndrome. Z Orthop Ihre Grenzgeb 1990; 128:46–50. ![]()
18. Rothman, RH, Parke, WW, The vascular anatomy of the rotator cuff. Clin Orthop 1965; 41:176–186. ![]()
19. Lindblom, K. On pathogenesis of ruptures of the tendon aponeurosis of the shoulder joint. Acta Radiol. 1939; 20:563–577.
20. Moseley, HF, Goldie, L, The arterial pattern of the rotator cuff of the shoulder. J Bone Joint Surg 1963; 45B:780. ![]()
21. Rathbun, JB, Macnab, L, The microvascular pattern of the rotator cuff. J Bone Joint Surg 1970; 52B:540–553. ![]()
22. Fukuda, H, Hamada, K, Yamanaka, K, Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop 1990; 254:75–80. ![]()
23. Lohr, J, Uhthoff, H, The microvascular pattern of the supraspinatus tendon. Clin Orthop Res 1990; 254:35–38. ![]()
24. Worland, R, Treatment of rotator cuff impingement. Orthop Rev. 1993;22(1):76–79. ![]()
25. Neviaser, R, Observations on impingement. Clin Orthop Rel Res 1990; 254:60–63. ![]()
26. Codman, EA. The Shoulder. Rupture of the Supraspinatus Tendon and Other Lesions in or about the Subacromial Bursa. Boston: Thomas Todd; 1934.
27. Matsen, FA, III., Arnitz, CT, Lippitt, SB. Rotator cuff. In Rockwood CA, Matsen FA, III., eds.: The Shoulder, 2nd ed, Philadelphia: Saunders, 1998.
28. Reilly, P, Macleod, I, Macfarlane, R, et al, Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116–121. ![]()
29. Keyes, EL. Anatomical observations of rupture of the supraspinatus tendon. Based upon a cadaveric study of 73 cadavers. Ann Surg. 1933; 97:849–856.
30. Wilson, CL. Lesions of the supraspinatus tendon. Degeneration, rupture and calcification. Arch Surg. 1943; 46:307.
31. Grant, JCB, Smith, CG. Age incidence of rupture of the supraspinatus tendon, abstract. Anat Rec. 1948; 100:666.
32. Cotton, RE, Rideaout, D, Tears of the humeral rotator cuff: a radiological and pathological necropsy survey. J Bone Joint Surg 1963; 46B:314–328. ![]()
33. Uhtoff, J, Loehr, J, Sarkar, K, et al. The pathogenesis of rotator cuff tears. In: Proceedings of the Third International Conference on Surgery of the Shoulder. Japan: Fukuora; 1986.
34. Ozaki, J, Fujimoto, S, Nakagawa, Y, et al, Tears of the rotator cuff of the shoulder associated with pathologic changes in the acromion: a study in cadavera. J Bone Joint Surg 1988; 70A:1224–1230. ![]()
35. Kummer, FJ, Zuckerman, JD, The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis 1995; 54:30–31. ![]()
36. Jerosch, J, Muller, T, Castro, WH, The incidence of rotator cuff rupture. An anatomic study. Acta Orthop Belg. 1991;57(2):124–129. ![]()
37. Fukada, H, Mikasa, M, Yamanaka, K, et al, Incomplete rotator cuff tears diagnosed by subacromial bursography. Clin Orthop 1987; 223:51–58. ![]()
38. Sakurai, G, Ozaki, J, Tomita, Y, et al, Incomplete tears of the subscapularis tendon associated with tears of the supraspinatus tendon: cadaveric and clinical studies. J Shoulder Elbow Surg. 1998;7(5):510–515. ![]()
39. Jiang, Y, Zhao, J, van Holsbeeck, MT, et al, Trabecular microstructure and surface changes in the greater tuberosity in rotator cuff tears. Skeletal Radiol 2002; 31:522–528. ![]()
40. Petterson, G. Rupture of the tendon aponeurosis of the shoulder joint in antero-inferior dislocation. Acta Chirur Scand. 1942; 77(suppl):1–187.
41. Milgrom, C, Schaffler, M, Gilbert, S, van Holsbeeck, M, Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg. 1995;77B(2):296–298. ![]()
42. Sher, JS, Uribe, JW, Posada, A, et al, Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg 1995; 77:933–936. ![]()
43. Yamaguchi, K, Ditsios, K, Middleton, WD, et al, The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am 2006; 88:1699–1704. ![]()
44. Tempelhof, S, Rupp, S, Seil, R, Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg 1999; 8:296–299. ![]()
45. Schibany, N, Zehetgruber, H, Kainberger, F, et al, Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study. Eur J Radiol 2004; 51:263–268. ![]()
46. Kim, HM, Teefey, SA, Zelig, A, et al, Shoulder strength in asymptomatic individuals with intact compared with torn rotator cuffs. J Bone Joint Surg Am. 2009;91(2):289–296. ![]()
47. Moosmayer, S, Smith, HJ, Tariq, R, Larmo, A, Prevalence and characteristics of asymptomatic tears of the rotator cuff: an ultrasonographic and clinical study. J Bone Joint Surg Br. 2009;91(2):196–200. ![]()
48. Yamamoto, A, Takagishi, K, Osawa, T, et al, Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. ![]()
49. Krief, OP, Huguet, D, Shoulder pain and disability: comparison with MR findings. AJR Am J Roentgenol. 2006;186(5):1234–1239. ![]()
50. Ellman, H, Diagnosis and treatment of incomplete rotator cuff tears. Clin Orthop Rel Res 1990; 254:64–74. ![]()
51. Pellecchia, GL, Paolino, J, Connell, J, Intertester reliability of the Cyriax evaluation in assessing patients with shoulder pain. JOSPT 1996; 23:34–38. ![]()
52. Chard, MID, Sattelle, LM, Hazleman, BL, The long-term outcome of rotator cuff tendinitis – a review study. Br J Rheumatol 1988; 27:385–389. ![]()
53. Kamkar, A, Irrgan, JJ, Whitney, SL, Nonoperative management of secondary shoulder impingement syndrome. J Orthop Sports Physical Therapy 1993; 17:212–224. ![]()
54. Shibata, Y, Midorikawa, K, Emoto, G, Naito, M, Clinical evaluation of sodium hyaluronate for the treatment of patients with rotator cuff tear. J Shoulder Elbow Surg 2001; 10:209–216. ![]()
55. Hamilton, JH, Bootes, A, Phillips, PE, Slywka, J, Human synovial fibroblast plasminogen activator. Modulation of enzyme activity by anti-inflammatory steroids. Arthritis Rheum 1981; 24:1296–1303. ![]()
56. Akpinar, S, Hersekli, M, Demirors, H, et al, Effects of methylprednisolone and betamethasone injections on the rotator cuff: an experimental study in rats. Adv Ther 2002; 19:194–201. ![]()
57. Gottlieb, NL, Riskin, WG, Complications of local corticosteroid injections. JAMA 1980; 243:1547–1548. ![]()
58. Schneeberger, AG, Nyffeler, RW, Gerber, C, Structural changes of the rotator cuff caused by experimental subacromial impingement in the rat. J Shoulder Elbow Surg 1998; 7:375–380. ![]()
59. Wei, AS, Callaci, JJ, Juknelis, D, et al, The effect of corticosteroid on collagen expression in injured rotator cuff tendon. J Bone Joint Surg Am 2006; 88:1331–1338. ![]()
60. Watson, M, Major ruptures of the rotator cuff. The results of surgical repair in 89 patients. J Bone Joint Surg Br 1985; 67:618–624. ![]()
61. Matthews, LS, Sontesgard, DA, Phelps, DB, A biomechanical study of rabbit patellar tendon: effects of steroid injection. J Sports Med 1974; 2:9. ![]()
62. Kennedy, JC, Willis, RB, The effects of local steroid injections on tendons: a biomechanical and microscopic correlative study. Am J Sports Med 1976; 4:11–21. ![]()
63. Bhatia, M, Singh, B, Nicolaou, N, Ravikumar, KJ, Correlation between rotator cuff tears and repeated subacromial steroid injections: a case-controlled study. Ann R Coll Surg Engl. 2009;91(5):414–416. ![]()
64. Green, S, Buchbinder, R, Glazier, R, Forbes, A. Interventions for shoulder pain: systematic review. Cochrane Musculoskeletal Group Cochrane Database of Systematic Reviews. 2002; 3.
65. Van der Heyden, GJ, Van der Windt, DA, Kleijnen, J, et al, Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract 1996; 46:309–316. ![]()
66. Adebajo, AO, Nash, P, Hazleman, BL, A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis. J Rheumatol. 1990;17(9):1207–1210. ![]()
67. Goupille, P, Sibilia, J, Local corticosteroid injections in the treatment of rotator cuff tendinitis (except for frozen shoulder and calcific tendinitis). Groupe Rheumatologique Français de l’Epaule (G.R.E.P.). Clin Exp Rheumatol. 1996;14(5):561–566. ![]()
68. Blair, B, Rokito, AS, Cuomo, F, et al, Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg. 1996;78A(11):1685–1689. ![]()
69. Plafki, C, Steffen, R, Willburger, RE, Wittenberg, RH, Local anaesthetic injection with and without corticosteroids for subacromial impingement syndrome. Int Orthop. 2000;24(1):40–42. ![]()
70. White, RH, Paull, DM, Fleming, KW, Rotator cuff tendinitis: comparison of subacromial injection of a long acting corticosteroid versus oral indomethacin therapy. J Rheumatol 1986; 13:608–613. ![]()
71. Valtonen, EJ, Subacromial triamcinolone mexacetonide and methylprednisolone injections in treatment of supra spinam tendinitis. A comparative trial. Scand J Rheumatol. 1976;16(suppl):1–13. ![]()
72. Symonds, G. Accurate diagnosis and treatment in painful shoulder conditions. J Int Med Res. 1975; 3:261–266.
73. Hollingworth, GR, Ellis, RM, Hattersley, TS, Comparison of injection techniques for shoulder pain: results of a double blind, randomised study. BMJ (Clin Res edn). 1983;287(6402):1339–1341. ![]()
74. Arroll, B, Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract. 2005;55(512):224–228. ![]()
75. Bosworth, BM. Calcium deposits in the shoulder and subacromial bursitis. A survey of 12,122 shoulders. JAMA. 1941; 116:2427–2428.
76. Rupp, S, Seil, R, Kohn, D, Tendinosis calcarea of the rotator cuff. Orthopade 2000; 29:852–867. ![]()
77. Rüttiman, G. Ueber die Hüfigkeit röntgenologischer Veränderungen bei Patienten mit typischer Periarthritis humeroscapularis und Schultergesunden. Zürich: Inaugural dissertation; 1959.
78. De Palma, AF, Kruper, JS, Long term study of shoulder joints affected with and treated for calcific tendinitis. Clin Orthop 1961; 20:61–72. ![]()
79. Welfling, J, Kahn, MF, Desroy, M, et al, Les Calcifications de l’épaule II La maladie des calcifications tendineuses multiples. Rev Rheum 1965; 32:325–334. ![]()
80. Gärtner, J, Tendinosis calcarea – results of treatment with needling. Z Orthop Ihre Grenzgeb 1993; 131:461–469. ![]()
81. Ark, JW, Flock, TJ, Flatow, EL, Bigliani, LU, Arthroscopic treatment of calcific tendinitis of the shoulder. Arthroscopy 1992; 8:183–188. ![]()
82. Loew, M, Daecke, W, Kusnierczak, D, et al, Shock-wave therapy is effective for chronic calcifying tendinitis of the shoulder. J Bone Joint Surg Br 1999; 81:863–867. ![]()
83. Gerdesmeyer, L, Wagenpfeil, S, Haake, M, et al, Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA. 2003;290(19):2573–2580. ![]()
84. Neer, CS, III., Impingement lesions. Clin Orthop 1983; 173:70–77. ![]()
85. Matsen, FA, III., Lippitt, SB, Sidles, JA, Harryman, DT. Practical evaluation and management of the shoulder. Philadelphia: Saunders; 1994.
86. Cyriax, JH. Textbook of Orthopaedic Medicine, vol I, Diagnosis and Treatment of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
87. Vaes, P, Annaert, JM, Claes, PH, Opdecam, P. Anatomische en kinesiologische studie van de rotatorcuffpezen. Ned Tijdschr Manuele Ther. 1992; 11(1):2–11.
88. Mattingley, GE, Mackarey, PJ, Optimal methods for shoulder tendon palpation. A cadaver study. Phys Ther 1996; 76:166–174. ![]()
89. Stuart, M, Azevedo, A, Cofield, R, Anterior acromioplasty for treatment of the shoulder. Clin Orthop Rel Res 1990; 260:195–200. ![]()
90. Kirschenbaum, D, Coyle, M, Leddy, J, et al, Shoulder strength with rotator cuff tears. Clin Orthop Rel Res 1993; 288:174–178. ![]()
91. Codman, E, Akerson, I, The pathology associated with rupture of the supraspinatus tendon. Ann Surg 1931; 93:348. ![]()
92. Yamanaka, K, Matsumoto, T, The joint side tear of the rotator cuff: a followup study by arthrography. Clin Orthop 1994; 304:68–73. ![]()
93. Wright, SA, Cofield, RH, Management of partial-thickness rotator cuff tears. J Shoulder Elbow Surg. 1996;5(6):458–466. ![]()
94. Markhede, G, Monastyrski, J, Stener, B, Shoulder function after deltoid muscle removal. Acta Orthop Scand 1985; 56:242–244. ![]()
95. Post, M, Silver, R, Singh, M, Rotator cuff tear. Clin Orthop Rel Res 1983; 173:78–91. ![]()
96. Howell, SM, Imobergsteg, AM, Seger, DH, et al, Clarification of the role of the supraspinatus muscle in shoulder function. J Bone Joint Surg 1986; 68A:398–404. ![]()
97. Colachis, S, Strohm, B, Effect of suprascapular and axillary nerve blocks on muscle force in upper extremity. Arch Physical Med Rehabil. 1971;52(1):22–29. ![]()
98. Kronberg, M, Nemeth, G, Brostrom, LA, Muscle activity and coordination in the normal shoulder: an electromyographic study. Clin Orthop 1990; 257:76–85. ![]()
99. Keener, JD, Wei, AS, Kim, HM, et al, Proximal humeral migration in shoulders with symptomatic and asymptomatic rotator cuff tears. J Bone Joint Surg Am. 2009;91(6):1405–1413. ![]()
100. Ozaki, J, Fujimoto, K, Nakagawa, Y, et al, Reconstruction of chronic massive rotator cuff tears with synthetic materials. Clin Orthop Rel Res 1986; 202:173–183. ![]()
101. Lazarus, MID, Harryman, DT, Yung, SW, et al. Anteriosuperior humeral displacement: limitation by the coraco-acromial arch. Orlando, FL: AAOS Annual Meeting; 1995.
102. Flatow, EL, Soslowsky, U, Ticker, JB, et al, Excursions of the rotator cuff under the acromion. Patterns of subacromial contact. Am J Sports Med 1994; 22:779–788. ![]()
103. Kaneko, K, DeMoy, EH, Brunet, ME, Massive rotator cuff tears. Screening by routine radiographs. Clin Imaging 1995; 19:8–11. ![]()
104. Itoi, E, Tabata, S, Rotator cuff tears in the adolescent. Orthop. 1993;16(1):78–81. ![]()
105. SooHoo, NF, Rosen, P, Diagnosis and treatment of rotator cuff tears in the emergency department. J Emerg Med. 1996;14(3):309–317. ![]()
106. Neviaser, R, Ruptures of the rotator cuff. Management of shoulder problems. Orthop Clin North Am. 1987;18(3):387–394. ![]()
107. Bokor, D, Hawkins, R, Uckell, G, et al. Results of non-operative management of full-thickness tears of the rotator cuff. Clin Orthop Rel Res. 1993; 294:103–110.
108. Petterson, G. Rupture of the tendon aponeurosis of the shoulder joint in antero-inferior dislocation. Acta Chir Scand. 1942; 77(suppl):1–187.
109. Hattrup, SJ, Rotator cuff repair: relevance of patient age. J Shoulder Elbow Surg. 1995;4(2):95–100. ![]()
110. McLaughlin, HL, Repair of major cuff ruptures. Surg Clin North Am 1963; 43:1535–1540. ![]()
111. Harryman, DT, Mack, LA, Wang, KY, et al, Repairs of the rotator cuff. J Bone Joint Surg 1991; 73A:982–989. ![]()
112. Gerber, C, Fuchs, B, Hodler, J, The results of repair of massive tears of the rotator cuff. J Bone Joint Surg. 2000;82A(4):505–515. ![]()
113. Ma, HL, Wu, JJ, Lin, CF, Lo, WH, Surgical treatment of full thickness rotator cuff tear in patients younger than 40 years. Chung Hua I Hsueh Tsa Chih (Taipei). 2000;63(6):452–458. ![]()
114. Fealy, S, Kingham, TP, Altchek, DW, Mini-open rotator cuff repair using a two-row fixation technique: outcomes analysis in patients with small, moderate, and large rotator cuff tears. Arthroscopy 2002; 18:665–670. ![]()
115. Galatz, LM, Griggs, S, Cameron, BD, Iannotti, JP, Prospective longitudinal analysis of postoperative shoulder function: a ten-year follow-up study of full-thickness rotator cuff tears. J Bone Joint Surg Am 2001; 83:1052–1056. ![]()
116. Wolf, EM, Pennington, WT, Agrawal, V, Arthroscopic rotator cuff repair: 4- to 10-year results. Arthroscopy 2004; 20:5–12. ![]()
117. Boileau, P, Brassart, N, Watkinson, DJ, et al, Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am 2005; 87:1229–1240. ![]()
118. Jost, B, Zumstein, M, Pfirrmann, CW, Gerber, C, Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am 2006; 88:472–479. ![]()
119. Teefey, SA, Hasan, SA, Middleton, WD, et al, Ultrasonography of the rotator cuff: a comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000; 82:498–504. ![]()
120. Watson, M, Major ruptures of the rotator cuff. The results of surgical repair in 89 patients. J Bone Joint Surg. 1985;67B(4):618–624. ![]()
121. Nich, C, Mütschler, C, Vandenbussche, E, Augereau, B, Long-term clinical and MRI results of open repair of the supraspinatus tendon. Clin Orthop Relat Res. 2009;467(10):2613–2622. ![]()
122. Wirth, MA, Basamania, C, Rockwood, CA, Jr., Nonoperative management of full-thickness tears of the rotator cuff. Orthop Clin North Am. 1997;28(1):59–67. ![]()
123. Mantone, JK, Burkhead, WZ, Jr., Noonan, J, Jr., Nonoperative treatment of rotator cuff tears. Orthop Clin North Am. 2000;31(2):295–311. ![]()
124. Itoi, E, Tabata, S, Conservative treatment of rotator cuff tears. Clin Orthop 1992; 275:165–173. ![]()
125. Koubaa, S, Ben Salah, FZ, Lebib, S, et al, Conservative management of full-thickness rotator cuff tears. A prospective study of 24 patients. Ann Readapt Med Phys 2006; 49:62–67. ![]()
126. Goldberg, BA, Nowinski, RJ, Matsen, FA, 3rd., Outcome of nonoperative management of full-thickness rotator cuff tears. Clin Orthop Relat Res 2001; 382:99–107. ![]()
127. Baydar, M, Akalin, E, El, O, Gulbahar, S, et al, The efficacy of conservative treatment in patients with full-thickness rotator cuff tears. Rheumatol Int. 2009;29(6):623–628. ![]()
128. Ackland, DC, Pak, P, Richardson, M, Pandy, MG, Moment arms of the muscles crossing the anatomical shoulder. J Anat. 2008;213(4):383–390. ![]()
129. Rask, M. Pectoralis major muscle rupture: report of five patients. J Neurol Orthop Med Surg. 1992; 13:272–274.
130. Scott, B, Wallace, W, Barton, M, Diagnosis and assessment of pectoralis major rupture by dynamometry. J Bone Joint Surg 1992; 74B:111–114. ![]()
131. Zeman, SC, Rosenfeld, RT, Lipscomb, PR, Tears of the pectoralis major muscle. Am J Sports Med 1979; 7:343–347. ![]()
132. Wolfe, SW, Wickiewicz, TL, Cavanaugh, JT, Ruptures of the pectoralis major muscle. An anatomic and clinical analysis. Am J Sports Med 1992; 20:587–593. ![]()
133. Pochini, AC, Ejnisman, B, Andreoli, CV, et al, Exact moment of tendon of pectoralis major muscle rupture captured on video. Br J Sports Med. 2007;41(9):618–619. ![]()
134. Kretzler, HH, Jr., Richardson, AB, Rupture of the pectoralis major muscle. Am J Sports Med 1989; 17:453–458. ![]()
135. Zhu, J, Jiang, Y, Hu, Y, et al, Evaluating the long-term effect of ultrasound-guided needle puncture without aspiration on calcifying supraspinatus tendinitis. Adv Ther. 2008;25(11):1229–1234. ![]()
136. Tavernier, T, Walch, G, Barthelemy, R, et al, Lésion isolée de l’infra-épineux à la jonction myotendineuse : une nouvelle lésion. J Radiol. 2006;87(12 Pt 1):1875–1882. ![]()
137. Walch, G, Nové-Josserand, L, Liotard, JP, Noël, E, Musculotendinous infraspinatus ruptures: an overview. Orthop Traumatol Surg Res. 2009;95(7):463–470. ![]()
138. Yamanaka, K, Matsumoto, T, The joint side tear of the rotator cuff: a followup study by arthrography. Clin Orthop 1994; 304:68–93. ![]()
139. Nobuhara, K, Hata, Y, Komai, M, Surgical procedure and results of repair of massive tears of the rotator cuff. Clin Orthop 1994; 394:54–59. ![]()
140. Walch, G, Boulahia, A, Calderone, S, Robinson, AH, The ‘dropping’ and ‘hornblower’s’ signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br. 1998;80(4):624–628. ![]()
141. Berry, H, Kong, K, Hudson, AR, Moulton, RJ, Isolated suprascapular nerve palsy: a review of nine cases. Can J Neurol Sci. 1995;22(4):301–304. ![]()
142. Black, KP, Lombardo, JA, Suprascapular nerve injuries with isolated paralysis of the infraspinatus. Am J Sports Med. 1990;18(3):225–228. ![]()
143. Eggert, S, Holzgraefe, M, Compression neuropathy of the suprascapular nerve in high performance volleyball players. Sportverletz Sportschaden. 1993;7(3):136–142. ![]()
144. Holzgraefe, M, Kukowski, B, Eggert, S, Prevalence of latent and manifest suprascapular neuropathy in high-performance volleyball players. Br J Sports Med. 1994;28(3):177–179. ![]()
145. Ferretti, A, De Carli, A, Fontana, M, Entrapment of suprascapular nerve at spinoglenoid notch. Book of Abstracts and Outlines, 1997.
146. Ferretti, AG, Cerullo, G, Russo, G, Suprascapular neuropathy in volleyball players. J Bone Joint Surg [Am] 1987; 77:1061–1063. ![]()
147. Montagna, P, Colonna, S, Suprascapular neuropathy restricted to the infraspinatus muscle in volleyball players. Acta Neurol Scand. 1993;87(3):248–250. ![]()
148. Ringel, SP, Treifhaft, M, Carry, M, Suprascapular neuropathy in pitchers. Am J Sports Med 1990; 18:80–86. ![]()
149. Witvrouw, E, Cools, A, Lysens, R, Cambier, D, et al, Suprascapular neuropathy in volleyball players. Br J Sports Med. 2000;34(3):174–180. ![]()
150. Hinton, MA, Parker, AW, Drez, D, Altchek, D, An anatomic study of the subscapularis tendon and the myotendinous junction. J Shoulder Elbow Surg 1994; 3:224–229. ![]()
151. Okoro, T, Reddy, VR, Pimpelnarkar, A, Coracoid impingement syndrome: a literature review. Curr Rev Musculoskelet Med. 2009;2(1):51–55. ![]()
152. Sakurai, G, Ozaki, J, Tomita, Y, et al, Incomplete tears of the subscapularis tendon associated with tears of the supraspinatus tendon: cadaveric and clinical studies. J Shoulder Elbow Surg. 1998;7(5):510–515. ![]()
153. Deutsch, A, Altchek, DW, Veltri, DM, et al, Traumatic tears of the subscapularis tendon. Clinical diagnosis, magnetic resonance imaging findings, and operative treatment. Am J Sports Med. 1997;25(1):13–22. ![]()
154. Li, XX, Schweitzer, ME, Bifano, JA, et al, MR evaluation of subscapularis tears. J Comput Assist Tomogr. 1999;23(5):713–717. ![]()
155. Nove Josserand, L, Levigne, C, Noel, E, Walch, G, Isolated lesions of the subscapularis muscle: à propos of 21 cases. Rev Chir Orthop Reparatrice Appar Mot 1994; 80:595–601. ![]()
156. Gerber, C, Krushell, RJ, Isolated ruptures of the tendon of the subscapularis muscle. J Bone Joint Surg. 1991;73B(3):389–394. ![]()
157. Kreuz, PC, Remiger, A, Erggelet, C, et al, Isolated and combined tears of the subscapularis tendon. Am J Sports Med. 2005;33(12):1831–1837. ![]()
158. Mansat, P, Frankle, MA, Cofield, RH, Tears in the subscapularis tendon: descriptive analysis and results of surgical repair. Joint Bone Spine. 2003;70(5):342–347. ![]()
159. Slatis, P, Aalto, K, Medial dislocation of the tendon of the long head of the biceps brachii. Acta Orthop Scand 1979; 50:73–77. ![]()
160. Grauer, JD, Paulos, LE, Smutz, WP, Biceps tendon and superior labral injuries. Arthroscopy 1992; 8:488–497. ![]()
161. Andrews, J, Carson, W, McLeod, W, Glenoid labrum tears related to the long head of the biceps. Am J Sports Med 1985; 13:337–341. ![]()
162. Petersson, CJ, Spontaneous medial dislocation of the tendon of the long biceps brachii. Clin Orthop 1986; 211:224–227. ![]()
163. Abbott, LC, Saunders, LB. Acute traumatic dislocations of the tendon of the long head of the biceps brachii; report of 6 cases with operative findings. Surgery. 1939; 6:817–840.
164. Van Laarhoven, C, Van Der Werken, C, Bicepspeersrupturen. Ned Tijdschr Geneeskd. 1990;134(21):1048–1053. ![]()