CHAPTER 14 Disorders of puberty
Introduction
Puberty and adolescence are recognized as periods involving marked endocrine changes which regulate growth and sexual development. Normal pubertal development is known to be centrally driven and dependent upon appropriate gonadotrophin and growth hormone (GH) secretion, and normal functioning of the hypothalamic–pituitary–gonadal axis. The mechanisms which control the precise timing of the onset of puberty, however, are still not clearly understood but are influenced by many factors including general health, nutrition, exercise, genetic influences and socio-economic conditions (Rees 1993). Most of the changes during puberty are gradual, although menarche is a single event that can be dated in girls. Normal puberty involves a fairly regular sequence of events between the ages of 10 and 16 years, and abnormal puberty can be defined as any disturbance in this. This chapter will provide an overview of the endocrine changes observed during normal pubertal development, and will describe the factors believed to influence the tempo of puberty and ovarian maturation before discussing some of the conditions that result in disordered pubertal development.
This chapter will deal largely with the latter group, apart from when there is overlap with other chapters (see Chapters 13, 16 and 31).
Pubertal Development
Puberty represents a period of significant growth, hormonal change and the attainment of reproductive capacity. Its onset is marked by a significant increase in the amplitude of pulsatile release of gonadotrophin-releasing hormone (GnRH) by the hypothalamus (Mauras et al 1996). This usually occurs between the ages of 8 and 13.5 years in girls, and stimulates an increase in pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which initially occurs as high-amplitude nocturnal pulses, although eventually both daytime and nocturnal pulsatile release are established (Delemarre et al 1991). FSH and LH, in turn, act upon the ovary to promote follicular development and sex steroid synthesis. As endocrine activity is initially nocturnal, there is no point in measuring gonadotrophin or sex steroid levels during the day.
In the female, this period is characterized clinically by accelerated linear growth, the development of breasts and pubic hair, and the eventual onset of menstruation (menarche) which occurs between the ages of 11 and 16 years in most women in the UK (Stark et al 1989). Menarche is often used as a marker of pubertal development as it is an easily identifiable event which can usually be dated with some accuracy. Adrenarche, the growth of pubic hair, is due to the secretion of adrenal androgens and precedes gonadarche by about 3 years. Thus, prepubertal children often have pubic hair, although, if pronounced, this should be investigated to exclude pathological causes (see below). Whilst androgen secretion is essential, oestrogen secretion facilitates pubic hair growth.
In tandem with the onset of pulsatile GnRH secretion, there is an increase in the amplitude of GH pulses released by the pituitary. Evidence suggests that this amplification of GH secretion may be regulated by the pubertal increase in levels of both androgenic and oestrogenic hormones (Mauras et al 1996, Caufriez 1997). In addition to this action, sex steroids have been shown to stimulate skeletal growth directly and thus augment the role of GH in promoting somatic growth and development (Giustina et al 1997). During the adolescent growth spurt, there is a greater gain in sitting height (mean 13.5 cm for girls, 15.4 cm for boys) than leg length (mean 11.5 cm for girls, 12.1 cm for boys) as the spurt for sitting height lasts longer (and is longer in boys). At this time, there is a minimum rate of fat gain and maximum attainment of muscle bulk, with differential and opposite changes in boys and girls. Ossification of the skeleton, as measured in terms of ‘bone age’, is helpful in determining a child’s progress through puberty and as a determinant of final stature.
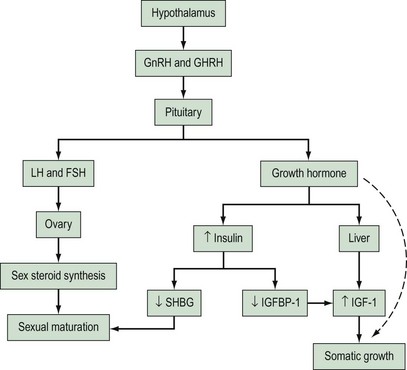
Rising concentrations of GH are also believed to exert some effects on circulating insulin levels, although precise mechanisms are unclear. Some authors believe that GH induces peripheral insulin resistance which leads to compensatory increases in insulin secretion (Smith et al 1988, Nobels and Dewailly 1992). Increased levels of insulin during puberty may directly stimulate protein anabolism (Amiel et al 1991). Insulin also acts as a regulator of insulin-like growth factor-1 (IGF-1) through its effects on insulin-like growth factor binding protein-1 (IGFBP-1). IGF-1 is produced by hepatic cells under the influence of GH, and has actions which stimulate cellular growth and maturation. IGFBP-1 competes with IGF receptors to bind IGF-1, thus inhibiting cellular action of IGF-1. Studies have indicated that insulin acts to suppress production of IGFBP-1 and therefore increases circulating IGF-1 bioavailability (Holly et al 1989, Nobels and Dewailly 1992). Insulin has also been shown to be a regulator of free sex steroids through the control of sex-hormone-binding globulin (SHBG) production by the liver. Studies have shown that levels of SHBG decrease during puberty and that this fall parallels the rising levels of insulin (Holly et al 1989, Nobels and Dewailly 1992). The endocrine interactions involved in normal pubertal development are represented in a simplified diagram in Figure 14.1.
Tempo of pubertal development
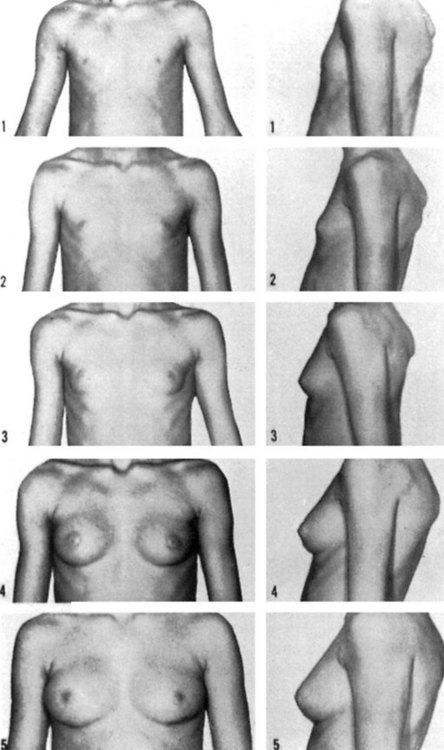
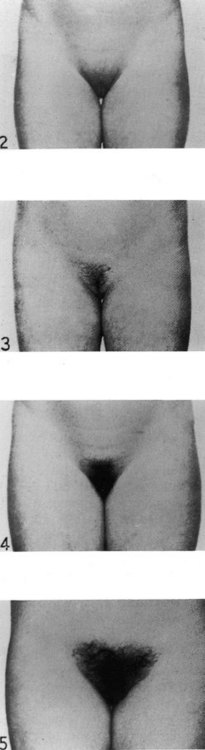
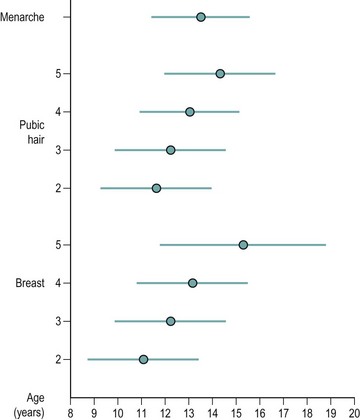
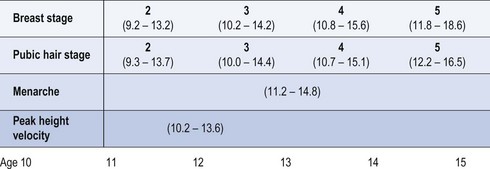
The first physical sign of puberty in girls is breast development, which occurs at an average age of 10–12.5 years (Figure 14.2). Age at onset of puberty is influenced by family history, race (earlier in Afro-Caribbeans) and nutrition (earlier in obese girls, with a secular trend to younger ages). Fifty percent of girls show signs of breast development by the age of 11 years, and investigations should be performed if there is no sign of breast development by the age of 14 years. In girls, the adolescent growth spurt occurs around 1 year after pubertal onset, while menarche occurs in the later stages of puberty at an average age of 12–15 years, at breast and pubic hair stage 4 (Figures 14.3 and 14.4; Marshall and Tanner 1969). Oestradiol concentration affects both the uterus and skeletal maturity. Most girls begin to menstruate when their bone age is between 13 and 14 years, and fewer than 20% are outside these limits. The appearance of axillary hair may antedate the rest of puberty by a few years and does not bear a constant relationship to the development of breasts as, again, it is androgens and not oestrogens that are most significant. The apocrine glands of the axilla and vulva begin to function at the time when axillary and pubic hair is appearing.
It has long been recognized that early maturing women have a tendency to be heavier for their height than their later maturing counterparts and, in contrast, that malnutrition and anorexia nervosa are associated with delayed menarche and amenorrhoea. These observed relationships between body weight and menarche led to the ‘critical weight hypothesis’ of Frisch and Revelle (1970), which hypothesized that attainment of a critical body weight led to metabolic changes which, in turn, triggered menarche. In further work, the investigators demonstrated that the ratio of lean body weight to fat decreased during the adolescent growth spurt in females from 5 : 1 to 3 : 1 at menarche, when the proportion of body fat was approximately 22% (Frisch 1990). They suggested that adipose tissue specifically was responsible for the development and maintenance of reproductive function, through its action as an extragonadal site for the conversion of androgens to oestrogens.
While few authors will dispute that nutrition and body weight play a role in pubertal development, the ‘critical weight hypothesis’ for menarche has not been supported by other studies. Cameron (1976) studied 36 British girls longitudinally, measuring weight and skinfold thickness at 3-month intervals for the 2 years before menarche to 2 years post menarche. He postulated that if a ‘critical body fatness’ was the true explanation for onset of menarche, he should expect to detect a reduction in the variability of his measurements at menarche compared with measurements taken before and after menarche, and this was not demonstrated in the study. In a much larger study, Garn et al (1983) analysed the triceps skinfold distributions of 2251 girls collected during three national surveys in the USA. Comparing premenarcheal and postmenarcheal girls, they confirmed that those who had attained menarche were, on average, fatter than premenarcheal girls. However, there was a marked overlap in skinfold thickness for both groups, and there was no evidence of a threshold level of fatness below which menarche did not occur. In a more recent study, de Ridder et al (1992) performed a longitudinal assessment of 68 premenarcheal school girls, including pubertal staging, skinfold measurements, waist–hip ratios and blood samples for the monitoring of gonadotrophins. They did not demonstrate a relationship between body fat mass and the age at onset of puberty or age at menarche, but did find that a greater body fat mass was related to a faster rate of pubertal development.
Although the studies above dispute the association of age at menarche with the achievement of a threshold weight or body fat percentage, the association of earlier maturation in heavier girls still exists. Stark et al (1989) reported on data from 4427 girls who were part of the National Child Development Study cohort. Data relating to birth weight, age at menarche, and weights and heights measured at 7, 11 and 16 years were available. The authors reported that a larger proportion of girls with early menarche (before the age of 11 years) were heavier for their height at all ages when compared with those with late menarche (after 14 years). Interestingly, however, changes in relative weight in the years preceding menarche (ages 7–11 years) were not strongly associated with age at menarche, whereas being overweight at the age of 7 years was much more strongly associated with an early age at menarche. Birth weight was not related to age at menarche. The authors concluded that the increase in weight for height associated with early maturation actually begins well before the onset of puberty. These findings were replicated by Cooper et al (1996) who examined 1471 girls in the Medical Research Council National Survey of Health and Development. They found that girls who were heavier at 7 years of age experienced an earlier menarche; however, contrary to Stark et al, they found that birth weight was related to age at menarche and that girls with heavier birth weights experienced menarche at a later age.
Intense exercise, such as long-distance running, ballet, rowing, long-distance cycling and gymnastics, is associated with delayed menarche in young girls and with amenorrhoea in older women (Malina et al 1978, 1979, Frisch et al 1981, Baxter-Jones et al 1994). These ‘endurance’ sports are associated with lower body weight and percentage fat. The extent to which menarche is ‘delayed’ has been shown to be related to the age at which participation in the sport begins and to the intensity of training (Frisch et al 1981). In view of the association between body weight and menarche described above, it is perhaps not surprising that girls participating in intense sporting activity experience a later age of menarche.
The influence of genetic factors on age at menarche is evident in the correlation demonstrated between mother–daughter and sister–sister pairs. Garn and Bailey (1978) analysed data from 550 mother–daughter pairs and reported a correlation of 0.25, which was adjusted to 0.23 once a correction was made for fatness. Similar correlation coefficients have been reported in other studies of mother–daughter pairs from European, African and American populations (Flug et al 1984, Benso et al 1989, Malina et al 1994, Cameron and Nagdee 1996). The strength of this ‘genetic effect’, however, must be interpreted carefully as the mothers’ age at menarche was based on recall in most of these studies, and the accuracy of the estimation is therefore questionable. In addition, one must consider the effect of similarity in socio-economic status (and hence nutrition and fatness) that is likely to exist between mothers and daughters, and this in itself may contribute to similarity in menarcheal age.
The association between body weight and age at menarche has formed part of the basis for the explanation of the secular trend towards earlier menarche noted in the UK and other industrialized countries over the last century (Roberts et al 1971, Ostersehlt and Danker Hopf 1991). It has been generally accepted that this trend has reflected improvements in nutrition, health and environmental conditions. However, a recent plateau in this trend has been observed, and even reversal in some countries, which is at present unexplained (Dann and Roberts 1993, Tryggvadottir et al 1994, Veronesi and Gueresi 1994). It has been suggested that the recent decrease in menarcheal age is related to changes in social conditioning in developed countries. Promotion of very thin women as ‘ideal’ role models through media and fashion has contributed to an increase in dieting in adolescent girls. Increasing participation of women in endurance sports which have intense training regimens may also be a factor related to lower body weight in young girls.
These studies all indicate that pubertal development and body weight are intrinsically linked. However, the mechanism for this relationship has not been defined conclusively. Insulin has been suggested as a modulator of the tempo of pubertal development through the regulation of IGFBP-1 and SHBG (Smith et al 1988, Holly et al 1989, Nobels and Dewailly 1992). States of overnutrition and obesity are associated with increased serum concentrations of insulin (Parra et al 1971, Rosenbaum et al 1997). Therefore, if excessive nutritional intake persists during childhood, it is possible that hyperinsulinaemia may lead to lower levels of IGFBP-1 and reduced SHBG concentrations, thus enhancing IGF-1 and sex steroid bioavailability. The converse would be true in states of malnutrition, where low levels of insulin would allow for the development of increased IGFBP-1 and SHBG levels.
However, it is still unclear whether hyperinsulinaemia in childhood is a result of obesity or if it is the cause of obesity. The roles of genetic factors, which may determine insulin production and obesity risk in childhood, are also yet to be clearly explained. Recently, there has been much interest in the actions of the newly identified hormone leptin which is produced by adipose tissue (Sorensen et al 1996). Serum concentrations of leptin, a 167 amino acid product of white fat, have been shown to be related to body fat mass, and it is believed that leptin exerts its action on the hypothalamus to control calorie intake, decrease thermogenesis, increase levels of serum insulin and increase pulsatility of GnRH (Considine et al 1996, Sorensen et al 1996). With these actions, leptin may potentially have a role in the hormonal control of pubertal development, and some studies have shown that leptin levels do increase before the onset of puberty (Clayton et al 1997, Mantzoros et al 1997). It has been postulated that changes in circulating leptin levels may act as an initiator for the onset of puberty, and that this may explain the relationships between body fat and maturation observed by Frisch and Revelle (1970). This hypothesis is supported by the recent work of Clement et al (1998), who identified a homozygous mutation of the leptin receptor gene which results in early-onset morbid obesity and the absence of pubertal development in association with reduced GH secretion. These findings suggest that increased leptin levels associated with gains in fat mass may signal the hypothalamus to act as an important regulator of sexual maturation. It is possible that future research may clarify the role of leptin and leptin receptors in pubertal development, and may explain the variation in the timing of onset of puberty between individuals.
Ovarian and uterine development
There are relatively few normal data on the maturation of the internal genitalia in girls, and a paper by Holm et al (1995) provides cross-sectional data of a large cohort of 166 school girls and medical students. It was demonstrated that uterine and ovarian volumes increase in size prepubertally, from as early as 6 years of age. Griffin et al (1995) demonstrated similar changes from the age of 4 years and, in their study of 153 girls, included subjects as young as 3 days. It was found that uterine shape changes constantly throughout childhood from a pear shape to a cylindrical shape and, finally, to the adult heart shape.
As girls go through puberty, the ovaries have been described as characteristically becoming multicystic due to low levels of FSH stimulating only partial folliculogenesis (Stanhope et al 1985). The multicystic appearance is also seen during resumption of ovarian activity after periods of quiescence; for example, in women who are recovering from weight-related amenorrhoea or those with hypogonadotrophic hypogonadism who are treated with pulsatile GnRH. The multicystic ovary differs from the polycystic ovary in that the cysts are larger (6–10 mm) and the stroma is of normal echogenicity. The morphological appearance of the polycystic ovary, on the other hand, requires the presence of at least 10 cysts (2–8 mm) per ovary in the presence of an echodense stroma (see Chapter 18).
Precocious Puberty
Precocious onset of puberty is defined as occurring younger than 2 standard deviations before the average age, i.e. <8 years old in females (compared with <9 years in males). Thus, in many girls, early onset of puberty merely represents one end of the normal distribution. However, a number of pathological conditions may prematurely activate the GnRH–LH/FSH axis, resulting in the precocious onset of puberty. Furthermore, certain physical secondary sexual features (e.g. virilization without breast development) may occur in the absence of ‘true puberty’ (i.e. absent hypothalamic–pituitary activation) due to abnormal peripheral secretion of sex steroids (Box 14.1).
Box 14.1 Causes of precocious puberty
Thelarche variants
There appears to be a whole spectrum of presentations between premature thelarche and true precocious puberty. Thus, some girls with early breast development also demonstrate increased height velocity and bone maturation, and ovarian ultrasound reveals a multicystic appearance (as distinct from polycystic, see Chapter 18).
Congenital adrenal hyperplasia
CAH due to deficiency of the enzyme 21-hydroxylase leads to cortisol deficiency, oversecretion of adrenocorticotrophic hormone (ACTH) due to loss of feedback inhibition, and excessive production of adrenal androgens (see Chapter 11). Inheritance is autosomal recessive. Classic CAH usually presents in the neonate with ambiguous genitalia and a salt-losing crisis (see Chapter 13), whilst non-classic or late-onset CAH presents in adolescence with moderate to severe virilization (pubic hair, hirsutism, acne and clitoromegaly) and rapid growth.
Delayed Puberty
Delayed puberty is defined as the absence of onset of puberty by more than 2 standard deviations later than the average age, i.e. >14 years in females (compared with >16 years in males). Delayed puberty may be idiopathic/familial or due to a number of general conditions resulting in undernutrition. Absence of puberty may also be due to gonadal failure (elevated gonadotrophin levels) or impairment of gonadotrophin secretion. Occasionally, some girls enter puberty spontaneously but then fail to progress normally through puberty (Figure 14.5 and Box 14.2).
Box 14.2 Causes of delayed puberty
Turner’s syndrome
Management includes low-dose oestrogen therapy to promote breast development without further disturbing linear growth (Figure 14.6). Treatment with GH has also benefited some individuals. Cyclical oestrogen plus progestogen may be used as maintenance therapy. A regular withdrawal bleed is essential in order to prevent endometrial hyperplasia. Spontaneous conception has been reported in patients with Turner’s syndrome, but is rare. However, the possibility of assisted conception and oocyte donation should be discussed at an early age.
Other causes of premature ovarian failure
POF, by definition, is the cessation of periods accompanied by raised gonadotrophin concentration prior to the age of 40 years. It may occur at any age. The exact incidence of this condition is unknown as many cases go unrecognized, but estimates vary between 1% and 5% of the female population (Coulam et al 1986). In approximately two-thirds of cases, the cause of ovarian failure cannot be identified (Conway et al 1996). It is unknown whether these cases are truly ‘idiopathic’ or due to as yet undiscovered genetic, immunological or environmental factors. A series of 323 women with POF attending an endocrinology clinic in London identified 23% with Turner’s syndrome, 6% after chemotherapy, 4% with familial POF and 2% each who had pelvic surgery, pelvic irradiation, galactosaemia and 46XY gonadal dysgenesis (Conway et al 1996). Viral and bacterial infection may also lead to ovarian failure; thus, infections such as mumps, cytomegalovirus or human immunodeficiency virus in adult life can adversely affect long-term ovarian function, as can severe pelvic inflammatory disease.
Adolescents who lose ovarian function soon after menarche are often found to have a Turner’s mosaic (46XX/45X) or an X-chromosome trisomy (47XXX). There are many genes on the X chromosome that are essential for normal ovarian function. It would appear that two active X chromosomes are required during fetal life in order to lay down a normal follicle store. In fetuses with Turner’s syndrome, normal numbers of oocytes appear on the genital ridge but accelerated atresia takes place during late fetal life. Thus, streak gonads occur and it is only the mosaic form of Turner’s syndrome that permits any possibility of ovarian function. X mosaicisms are the most common chromosomal abnormality in reported series of POF, ranging from 5% to 40% (Anasti 1998). Other X chromosome anomalies may result in ovarian failure; for example, balanced translocations in the long arm of chromosome X between Xq13 and q26, which is a critical region for ovarian function.
Management of delayed puberty
Following exclusion of other diagnoses, many patients are happy to await spontaneous pubertal development. However, severe delay in pubertal onset may be a risk factor for decreased bone mineral density and osteoporosis. In subjects with hypergonadotrophic hypogonadism, puberty may be induced from any age; however, in Turner’s syndrome, delay in induction to around 14 years of age possibly permits maximal response to GH therapy (see Box 14.3).
Presentation of Congenital Anomalies of the Genital Tract (see Chapter 13)
Congenital absence of the vagina
Müllerian/uterine anomalies
Gynaecological Complaints
Menorrhagia
In a young woman with intractable menorrhagia, an assessment of blood clotting may be beneficial. Disorders of haemostasis such as von Willebrand’s disease, deficiencies of factors V, VII, X and XI, and idiopathic thrombocytopenic purpura are thought to increase menstrual loss. Young girls with heavy periods in the years after the menarche are very unlikely to have any pelvic pathology. A very small number of young girls have persistent heavy irregular periods associated with anovular cycles, particularly those with polycystic ovary syndrome (see Chapter 18). In these cases, sustained unopposed oestrogen levels lead to endometrial hyperplasia which may ultimately progress to carcinoma.
Summary
KEY POINTS
Amiel SA, Caprio S, Sherwin RS, Plewe G, Hymond MW, Tamborlane WV. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. Journal of Clinical Endocrinology and Metabolism. 1991;72:277-282.
Anasti JN. Premature ovarian failure: an update. Fertility and Sterility. 1998;70:1-15.
Baxter Jones AD, Helms P, Baines Preece J, Preece M. Menarche in intensively trained gymnasts, swimmers and tennis players. Annals of Human Biology. 1994;21:407-415.
Benso L, Lorenzino C, Pastorin L, Barotto M, Signorile F, Mostert M. The distribution of age at menarche in a random series of Turin girls followed longitudinally. Annals of Human Biology. 1989;16:549-552.
Cameron N, Nagdee I. Menarcheal age in two generations of South African Indians. Annals of Human Biology. 1996;23:113-119.
Cameron N. Weight and skinfold variation at menarche and the critical body weight hypothesis. Annals of Human Biology. 1976;3:279-282.
Caufriez A. The pubertal spurt: effects of sex steroids on growth hormone and insulin-like growth factor I. European Journal of Obstetrics, Gynecology and Reproductive Biology. 1997;71:215-217.
Clayton PE, Gill MS, Hall CM, Tillmann V, Whatmore AJ, Price DA. Serum leptin through childhood and adolescence. Clinical Endocrinology. 1997;46:727-733.
Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398-401.
Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292-295.
Conway GS, Kaltas G, Patel A, Davies MC, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertility and Sterility. 1996;65:337-341.
Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. British Journal of Obstetrics and Gynaecology. 1996;103:814-817.
Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstetrics and Gynecology. 1986;67:604-606.
Dann TC, Roberts DF. Menarcheal age in University of Warwick young women. Journal of Biosocial Science. 1993;25:531-538.
Delemarre HA, Wennink JM, Odink RJ. Gonadotrophin and growth hormone secretion throughout puberty. Acta Paediatrica Scandinavica. 1991;372(Suppl):26-31.
de Ridder CM, Thijssen JH, Bruning PF, van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. Journal of Clinical Endocrinology and Metabolism. 1992;75:442-446.
Flug D, Largo RH, Prader A. Menstrual patterns in adolescent Swiss girls: a longitudinal study. Annals of Human Biology. 1984;11:495-508.
Frisch RE. The right weight: body fat, menarche and ovulation. Baillière’s Clinical Obstetrics and Gynaecology. 1990;4:419-439.
Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169:397-399.
Frisch RE, Gotz Welbergen AV, McArthur JW, et al. Delayed menarche and amenorrhea of college athletes in relation to age of onset of training. JAMA: the Journal of the Anerican Medical Association. 1981;246:1559-1563.
Garn SM, LaVelle M, Pilkington JJ. Comparisons of fatness in premenarcheal and postmenarcheal girls of the same age. Journal of Pediatrics. 1983;103:328-331.
Garn SM, Bailey SM. Genetics of maturational processes. In: Falkner F, Tanner J, editors. Human Growth. London: Baillière Tindall, 1978.
Giustina A, Scalvini T, Tassi C, et al. Maturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosterone. Journal of Clinical Endocrinology and Metabolism. 1997;82:1210-1219.
Griffin IJ, Cole TJ, Duncan KA, Hollman AS, Donaldson MDC. Pelvic ultrasound measurements in normal girls. Acta Paediatrica. 1995;84:536-543.
Holly JM, Smith CP, Dunger DB, et al. Relationship between the pubertal fall in sex hormone binding globulin and insulin-like growth factor binding protein-I. A synchronized approach to pubertal development? Clinical Endocrinology. 1989;31:277-284.
Holm K, Mosfeldt Laursen E, Brocks V, Muller J. Pubertal maturation of the internal genitalia: an ultrasound evaluation of 166 healthy girls. Ultrasound in Obstetrics and Gynecology. 1995;6:175-181.
Malina RM, Spirduso WW, Tate C, Baylor AM. Age at menarche and selected menstrual characteristics in athletes at different competitive levels and in different sports. Medicine and Science in Sports. 1978;10:218-222.
Malina RM, Bouchard C, Shoup RF, Demirjian A, Lariviere G. Age at menarche, family size, and birth order in athletes at the Montreal Olympic Games, 1976. Medicine and Science in Sports. 1979;11:354-358.
Malina RM, Ryan RC, Bonci CM. Age at menarche in athletes and their mothers and sisters. Annals of Human Biology. 1994;21:417-422.
Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. Journal of Clinical Endocrinology and Metabolism. 1997;82:1066-1070.
Mauras N, Rogol AD, Haymond MW, Veldhuis JD. Sex steroids, growth hormone, insulin-like growth factor-1: neuroendocrine and metabolic regulation in puberty. Hormone Research. 1996;45:74-80.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291-303.
Nobels F, Dewailly D. Puberty and polycystic ovarian syndrome: the insulin/insulin-like growth factor I hypothesis. Fertility and Sterility. 1992;58:655-666.
Ostersehlt D, Danker Hopf E. Changes in age at menarche in Germany: evidence for a continuing decline. American Journal of Human Biology. 1991;3:647-654.
Parra A, Schultz RB, Graystone JE, Cheek DB. Correlative studies in obese children and adolescents concerning body composition and plasma insulin and growth hormone levels. Pediatric Research. 1971;5:603-613.
Rees M. Menarche when and why? The Lancet. 1993;342:1375-1376.
Roberts DF, Rozner LM, Swan AV. Age at menarche, physique and environment in industrial north east England. Acta Paediatrica Scandinavica. 1971;60:158-164.
Rosenbaum M, Leibel RL, Hirsch J. Obesity. New England Journal of Medicine. 1997;337:396-407.
Stanhope R, Adams J, Jacobs HS, Brook CGD. Ovarian ultrasound assessment in normal children, idiopathic precocious puberty and during low dose pulsatile GnRH therapy of hypogonadotrophic hypogonadism. Archives of Diseases in Childhood. 1985;60:116-119.
Stark O, Peckham CS, Moynihan C. Weight and age at menarche. Archives of Diseases in Childhood. 1989;64:383-387.
Smith CP, Archibald HR, Thomas JM, et al. Basal and stimulated insulin levels rise with advancing puberty. Clinical Endocrinology. 1988;28:7-14.
Sorensen TI, Echwald S, Holm JC. Leptin in obesity. British Medical Journal. 1996;313:953-954.
Tryggvadottir L, Tulinius H, Larusdottir M. A decline and a halt in mean age at menarche in Iceland. Annals of Human Biology. 1994;21:179-186.
Veronesi FM, Gueresi P. Trend in menarcheal age and socioeconomic influence in Bologna (northern Italy). Annals of Human Biology. 1994;21:187-196.