Chapter 75 Disorders of Nerve Roots and Plexuses
Disorders of Nerve Roots
Anatomical Features
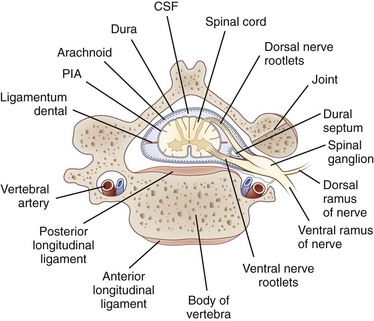
Each nerve root is attached to the spinal cord by four to eight rootlets that are splayed out in a longitudinal direction (Rankine, 2004). The dorsal roots are attached to the spinal cord at a well-defined posterolateral sulcus. The ventral rootlets are more widely separated and emerge over a greater area. At each spinal cord segment, a pair of dorsal and ventral roots unite just beyond the DRG to form a short mixed spinal nerve that divides into a thin dorsal ramus and a thicker ventral ramus (Fig. 75.1). The dorsal ramus innervates the deep posterior muscles of the neck and trunk (the paraspinal muscles) and the skin overlying these areas. The ventral ramus (the large anterior branch) contributes to the intercostal nerve or the cervical, brachial, or lumbosacral plexus and thereby supplies the trunk and limb muscles.
The nerve roots lie freely in the subarachnoid space, covered by a thin root sheath which is a layer of flattened cells continuous with the pial and arachnoidal coverings of the spinal cord. They lack the epineurial and perineurial coverings found in peripheral nerves. Compared with spinal nerves, the roots have many fewer connective tissue cells in the endoneurium and considerably less collagen. A capillary network derived from the radicular arteries provides the blood supply to the spinal nerve roots (Levin, 2002).
Where the nerve roots form the mixed spinal nerve, the pial covering of the root becomes continuous with spinal nerve perineurium, and the nerve takes the dural nerve root sheath through the intervertebral foramen to become continuous with the epineurium of the mixed nerve. At the intervertebral foramen, the root-DRG-spinal nerve complex is securely attached by a fibrous sheath to the transverse process of the vertebral body. In general, the DRG is located in a protected position within the intervertebral foramina, but at the lumbar and sacral levels, the DRG resides proximal to the neural foramina in an intraspinal location (Levin, 2002). There they may be vulnerable to disk herniation and the complications of osteoarthritis and lumbosacral spondylosis.
The dorsal roots contain sensory fibers that are central processes of the unipolar neurons of the DRG. On reaching the spinal cord, these fibers either synapse with other neurons in the posterior horn or pass directly into the posterior columns. In the ventral root, most fibers are essentially direct extensions of anterior horn motor neurons (alpha, beta, and gamma fibers) or of neurons in the intermediolateral horn (preganglionic sympathetic neurons found in lower cervical and thoracic segments). In addition, ventral roots contain a population of unmyelinated and thinly myelinated axons that come from sensory and sympathetic ganglia (Hildebrand et al., 1997).
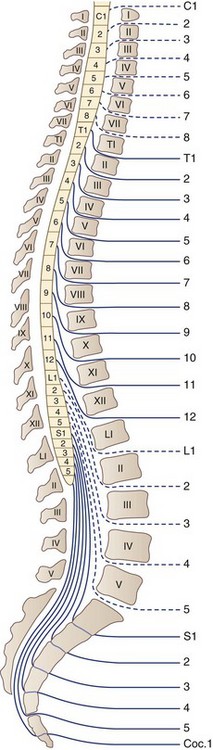
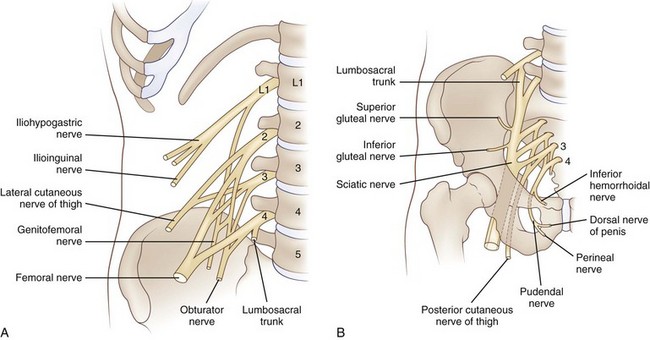
There are 31 pairs of spinal nerves that run through the intervertebral foramina of the vertebral column: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal (Fig. 75.2). A feature of clinical relevance is the pattern formed by the lumbar and sacral roots as they leave the spinal cord and make their way to their respective DRG to form spinal nerves. In the adult, the spinal cord is shorter than the spinal column, ending usually between L1 and L2. Therefore, the lumbar and sacral roots descend caudally from the spinal cord to reach the individual intervertebral foramina, forming the cauda equina. The concentration of so many nerve roots in a confined area makes this structure vulnerable to a range of pathological processes.
Traumatic Radiculopathies
Nerve Root Avulsion
The spinal roots have approximately one-tenth the tensile strength of the peripheral nerves because of lesser amounts of collagen and the absence of epineurial and perineurial sheaths in the roots. Therefore, the nerve roots are the weak link in the nerve root–spinal nerve–plexus complex, and nerve root avulsion from the spinal cord typically results from a severe traction injury affecting the upper limb. Ventral roots are more vulnerable to avulsion than dorsal roots, a consequence of the dorsal roots having the interposed DRG and a thicker dural sheath. In most cases, root avulsion occurs in the cervical region. Lumbosacral nerve root avulsions are rare, with only 35 cases reported between 1955 and 1996, and when they occur are generally associated with fractures of the sacroiliac joint with diastasis of the symphysis pubis or fractures of the pubic rami (Chin and Chew, 1997).
Avulsion at the level of the cervical roots can be total, as for a motorcyclist injury, or can result in two clinical syndromes of partial avulsion. One is Erb-Duchenne palsy, in which the arm hangs at the side internally rotated and extended at the elbow because of paralysis of C5- and C6-innervated muscles (the supraspinatus and infraspinatus, deltoid, biceps). The second is Dejerine-Klumpke palsy, in which there is weakness and wasting of the intrinsic hand muscles, with a characteristic clawhand deformity due to paralysis of C8- and T1-innervated muscles. Injuries responsible for Erb-Duchenne palsy are those that cause a sudden and severe increase in the angle between the neck and shoulder, generating stresses that are readily transmitted in the direct line along the upper portion of the brachial plexus to the C5 and C6 roots. Today, motorcycle accidents are the most common causes of this injury, but the C5 and C6 root avulsions classically occurred in the newborn during obstetrical procedures. Brachial plexus injuries in the newborn are discussed in Chapter 80. Dejerine-Klumpke palsy occurs when the limb is elevated beyond 90 degrees and tension falls directly on the lower trunk of the plexus, C8, and T1 roots. Such an injury may occur in a fall from a height in which the outstretched arm grasps an object to arrest the fall, leading to severe stretching of the C7, C8, and T1 roots, or during obstetrical traction on the extended arm when delivering the baby arm first.
Clinical Features and Diagnosis
Electrophysiological tests include the measurement of a sensory nerve action potential (SNAP) and needle EMG examination of the cervical paraspinal muscles. In the setting of an isolated C5 root avulsion, the SNAP should be preserved despite complete anesthesia in the dermatome, because the peripheral axons and the DRG cell bodies remain intact. Needle EMG of the cervical paraspinal muscles permits separation of damage of the plexus and of ventral root fibers because the posterior primary ramus, which arises just beyond the DRG and proximal to the plexus as the first branch of the spinal nerve, innervates these muscles (see Fig. 75.1). Thus, cervical paraspinal fibrillation potentials support the diagnosis of root avulsion. Paraspinal muscles have also been evaluated radiologically in the setting of root avulsion. Contrast-enhanced MRI studies of the cervical paraspinal muscles showing severe atrophy were accurate in indicating root avulsion injuries, and abnormal enhancement in the multifidus muscle was the most accurate among paraspinal muscle findings (Hayashi et al., 2002). Intraspinal neuroimaging using postmyelographic CT or MRI usually demonstrates an outpouching of the dura filled with contrast or CSF at the level of the avulsed root (Hayashi et al., 1998). This posttraumatic meningocele results from tears in the dura and arachnoid sustained during root avulsion. Advances in MRI technology now provide high-resolution images that can demonstrate root avulsion and obviate the need for CT myelography (Rankine, 2004).
Treatment
Root avulsion produces a severe neurological deficit that was considered an untreatable injury up until a few decades ago. In the past 30 years, microsurgical techniques and intraoperative electrophysiological studies have improved prospects for recovery for many patients with severe injury to peripheral nerves. The procedures of neurolysis (freeing intact nerve from scar tissue), nerve grafting (bridging ruptured nerves), and neurotization, or nerve transfer (attaching a donor nerve to the ruptured distal stump), have all been employed in the management of root avulsion injuries (Rankine, 2004). After C5 and C6 root avulsion injuries, for example, the plegic elbow flexors may be restored by several procedures that provide for neurotization of the musculocutaneous nerve, including reinnervating the biceps with an ulnar nerve fascicle (Tung et al., 2003) or employing a branch of the accessory nerve using a sural nerve graft (Samil et al., 2003); intercostal and phrenic nerves have also been used (Rankine, 2004). Carlstedt and colleagues (1995) pioneered another approach—nerve root repair and reimplantation. They reported on a patient who had an avulsion injury involving C6-T1 in whom they were able to successfully implant two ventral roots (C6 directly and C7 via sural nerve grafts) into the spinal cord through slits in the pia mater. The surgical treatment of patients with avulsion injuries is an area of active ongoing investigation with the promise that if continuity between spinal cord and nerve roots can be restored, subsequent recovery of function may be possible (Fournier et al., 2001). The sometimes intractable pain of cervical root avulsion injuries may be successfully treated with dorsal root entry zone coagulation procedures (Samii et al., 2001).
Disk Herniation
Beginning in the third or fourth decade of life, cervical and lumbar intervertebral disks are liable to herniate into the spinal canal or intervertebral foramina and impinge on the spinal cord (in the case of cervical disk herniations), nerve roots (in both cervical and lumbosacral regions), or both (at the cervical level where on occasion large central and paracentral disk herniations may produce a myeloradiculopathy) (see Chapter 73).
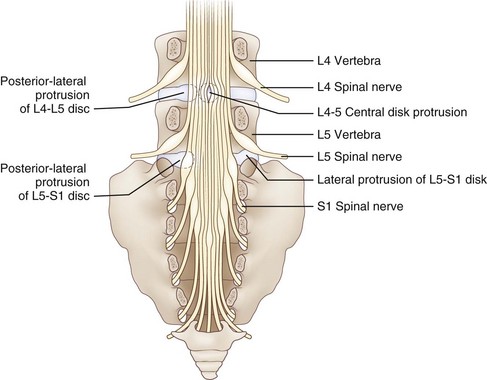
Reinforcing the annulus fibrosus posteriorly is the posterior longitudinal ligament, which in the lumbar region is dense and strong centrally and less well developed in its lateral portion. Because of this anatomical feature, the direction of lumbar disk herniations tends to be posterolateral, compressing the nerve roots in the lateral recess of the spinal canal. Less commonly, more lateral (foraminal) herniations compress the nerve root against the vertebral pedicle in the intervertebral foramen (Fig. 75.3). On occasion, the degenerative process may be particularly severe. This leads to large rents in the annulus and posterior longitudinal ligament, thereby permitting disk material to herniate into the spinal canal as a free fragment with the potentially damaging capacity to migrate superiorly or inferiorly and compress two or more nerve roots of the cauda equina. Most cervical disk herniations are posterolateral or foraminal.
In the cervical and lumbar regions, alteration in the integrity of the disk space is a component of a degenerative condition termed spondylosis, characterized by osteoarthritic changes in the joints of the spine, the disk per se (desiccation and shrinkage of the normally semisolid, gelatinous nucleus pulposus), and the facet joints. Immunohistochemical examination of herniated cervical disks points to an inflammatory process associated with neovascularization and increased expression of matrix metalloproteinase and inducible nitric oxide (NO) synthetase (Furusawa et al., 2001). The release of NO by disk cells may contribute to the process of disk degeneration by inducing apoptosis (Kohyama et al., 2000). Because it spawns osteophyte formation, spondylosis leads to compromise of the spinal cord in the spinal canal and the nerve roots in the intervertebral foramina. Restriction in the dimensions of these bony canals may be exacerbated by thickening and hypertrophy of the ligamentum flavum, which is especially detrimental in patients with congenital cervical or lumbar canal stenosis.
Clinical Features
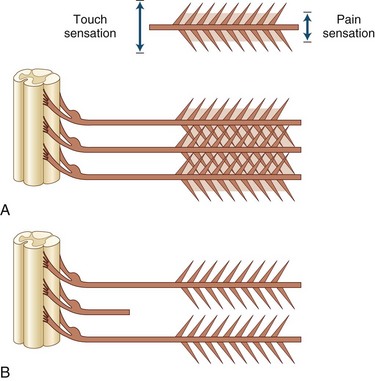
Root compression from disk herniation gives rise to a distinctive clinical syndrome that in its fully developed form comprises radicular pain, dermatomal sensory loss, weakness in the myotome, and reduction or loss of the deep tendon reflex subserved by the affected root (Carette and Fehlings, 2005). Nerve root pain is variably described as knifelike or aching and is widely distributed, projecting to the sclerotome (defined as deep structures such as muscles and bones innervated by the root). Typically, root pain is aggravated by coughing, sneezing, and straining at stool (actions that require a Valsalva maneuver and raise intraspinal pressure). Accompanying the pain are paresthesias referred to the specific dermatome, especially to the distal regions of the dermatomes; indeed, these sensations strongly suggest that the pain has its origins in compressed nerve roots rather than spondylotic facet joints. Sensory loss caused by the compromise of a single root may be difficult to ascertain because of the overlapping territories of adjacent roots, although loss of pain is usually more easily demonstrated than loss of light touch sensation (Fig. 75.4).
In the lumbosacral region, 95% of disk herniations occur at the L4-L5 or L5-S1 levels; L3-L4 and higher lumbar disk herniations are uncommon (Deyo and Weinstein, 2001). Knowing that the L4 root exits beneath the pedicle of L4 through the L4-L5 foramen and that L5 exits through the L5-S1 foramen, one might predict that disk herniation at these levels would generally compress the L4 and L5 roots, respectively (see Fig. 75.3). In perhaps only 10% of cases of the disk herniating far laterally into the foramen is there compression of the exiting nerve root. More commonly, the posterolateral disk herniation compresses the nerve root passing through the foramen below that disk, so L4-L5 and L5-S1 herniations usually produce L5 and S1 radiculopathies, respectively.
In the cervical region, it is likely that the greater mobility at levels C5-C6 and C6-C7 promotes the development of cervical disk degeneration with annulus fraying and subsequent disk protrusion. Cervical nerve roots emerge above the vertebra that shares the same numerical designation. Therefore C7 exits between C6 and C7, and spondylotic changes with or without additional acute disk herniation would be expected to compress the C7 nerve root. Similarly, disk protrusion at C5-C6 and C7-T1 would compress the C6 and C8 roots, respectively. In the classic study of Yoss and associates in 1957, clinical and radiological evidence of radiculopathy was found to occur most often at C7 (70%), less frequently at C6 (19%-25%), uncommonly at C8 (4%-10%) and C5 (2%). Radiculopathy involving the T1 root is a clinical rarity (Levin, 1999).
Diagnosis
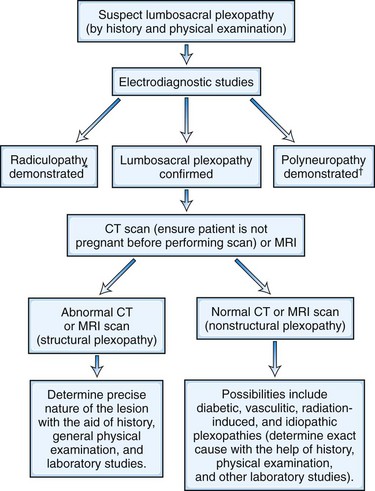
Diagnosis is aided by a variety of imaging techniques (e.g., plain radiography, myelography, CT myelography, MRI) and EMG testing (Carette and Fehlings, 2005) (see Chapter 73). Both diagnostic modalities—the imaging approach that reveals anatomical details and the EMG techniques that disclose neurophysiological function—agree in the majority of patients (60%) with a clinical history compatible with cervical or lumbosacral radiculopathy, although only the results of one study will be positive in a significant minority of patients (40%) (Nardin et al., 1999). Although plain radiography is unhelpful in the identification of a herniated disk per se, in both the cervical and the lumbar area, it reveals spondylotic changes when present. It also may be useful for identifying less common disorders that produce radicular symptoms and signs: bony metastases, infection, fracture, and spondylolisthesis, for example.
In the cervical region, the best methods for assessing the relationship between neural structures (spinal cord and nerve root) and their fibro-osseous surroundings (disk, spinal canal, and foramen) are postmyelography CT (unenhanced CT reveals little more than the presence of bony changes) and MRI. MRI is equivalent in diagnostic capacity to postmyelography CT and therefore is preferred. In the lumbosacral region, CT is an effective method for evaluating disk disease, but when available, MRI is considered the superior imaging study. Its excellent resolution, multiplanar imaging, the ability to see the entire lumbar spine including the conus, and the absence of ionizing radiation make it highly sensitive in detecting structural radicular disorders (Ashkan et al., 2002).
A variety of neurophysiological tests are used to assess patients with disk herniation: motor and sensory nerve conduction studies, late responses, somatosensory evoked potentials, nerve root stimulation, and needle electrode examination. Sensory conduction studies are useful in the evaluation of a patient suspected of radiculopathy because SNAPs are typically normal (because the lesion is rostral to the DRG in the intervertebral foramina) even in the face of clinical sensory loss, in contrast to the situation in plexopathy and peripheral nerve trunk lesions, where SNAPs are attenuated or absent. In the specific instance of L5 radiculopathy, however, because the L5 DRG may reside proximal to the neural foramen, if intraspinal pathology is severe enough, compression of the L5 DRG may lead to loss of the superficial peroneal nerve SNAP (Levin, 1998).
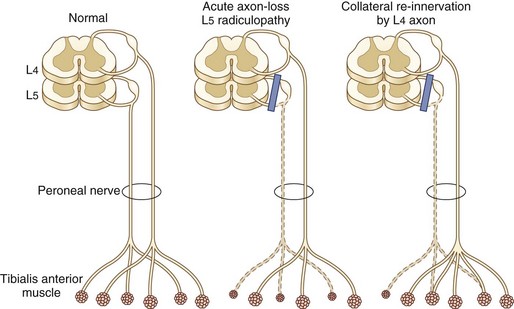
Needle EMG is the most useful electrodiagnostic procedure in the diagnosis of suspected radiculopathy (Wilbourn and Aminoff, 1998). A study is considered positive if abnormalities—especially acute changes of denervation including fibrillation potentials and positive sharp waves—are present in two or more muscles that receive innervation from the same root, preferably via different peripheral nerves. No abnormalities should be detected in muscles innervated by the affected root’s rostral and caudal neighbors. Reduced motor unit potential (MUP) recruitment (manifested by decreased numbers of MUPs firing at an increased rate) and MUP abnormalities of reinnervation (high-amplitude, increased duration, polyphasic MUPs) are also sought by the needle electrode but are not as reliable as fibrillation potentials in establishing a definitive diagnosis of radiculopathy. Absence of fibrillation potentials does not, however, exclude the diagnosis of radiculopathy. Two main reasons for this exist. First, examination in the first 1 to 3 weeks after onset of nerve root compromise may be negative because it takes approximately 2 weeks for these potentials to appear. At the early stages in the process of nerve root compression, the only needle electrode examination manifestation of radiculopathy might be reduced MUP recruitment resulting from either axon loss, conduction block, or both. Second, fibrillation potentials disappear as denervated fibers are reinnervated by axons of the same or an adjacent myotome beginning 2 to 3 months after nerve root compression (Fig. 75.5). Thus in the later phases of nerve root compression, the only needle EMG changes indicative of radiculopathy might be chronic neurogenic changes of reduced recruitment and MUP remodeling. The distribution of fibrillation potentials is relatively stereotyped for C5, C7, and C8 radiculopathies, whereas C6 radiculopathy has the most variable presentation. In about half of patients, the findings are similar to C5 radiculopathy, whereas in the other half, findings are identical to C7 radiculopathy (Levin et al., 1996). A patient with the uncommon disk compression of T1 was found to have isolated fibrillation potential activity of the abductor pollicis brevis (Levin, 1999).
Treatment
For cervical disk protrusion and spondylotic radiculopathy, the mainstay of treatment is conservative management—a combination of a period of reduced physical activity with use of a soft cervical collar, physiotherapy, and antiinflammatory and analgesic agents. Most patients improve, even those with mild to moderate motor deficits. Indeed, in some cases, herniated cervical disks have been observed to regress on MRI images, a circumstance that appears more likely to occur if disk material has extruded and becomes exposed to the epidural space (Mochida et al., 1998). Although there appears to be a short-term benefit to surgical decompression of an affected nerve root with regard to pain, weakness, or sensory loss, at 1 year, there is no significant difference between the outcome of surgical or conservative management (physical therapy or hard cervical collar immobilization) (Fouyas et al., 2002). A surgical approach may be warranted, however, in selected cases: (1) if there is unremitting pain despite an adequate trial of conservative management, (2) if there is progressive weakness in the territory of the compromised nerve root, or (3) if there are clinical and radiological signs of an accompanying new onset of myelopathy, although in a group of patients with mild or moderate myelopathy, those managed surgically had the same outcome (degree of functional disability) as those allocated to conservative treatment (Fouyas et al., 2002).
In the lumbosacral region, disk herniation and spondylotic changes respond to conservative management in more than 90% of patients. Bed rest had been recommended as the centerpiece of patient care, but controlled trials have demonstrated that back-strengthening exercises under the direction of a physical therapist, performed within the limits of the patient’s pain, result in more rapid resolution of pain and return to normal function (Vroomen et al., 1999). Follow-up MRI studies in conservatively managed patients indicate reduction in size or disappearance of herniated nucleus pulposus corresponding to improvement in clinical findings (Komori et al., 1996). Epidural corticosteroid injection may help relieve pain but does not improve neurological function or reduce the need for surgery (Carette et al., 1997). A single intravenous (IV) bolus of methylprednisolone (500 mg) given to patients with acute diskogenic sciatica of less than 6 weeks’ duration provided short-term improvement in leg pain, but the effect was relatively small, with no effect on functional disability, and transitory (3 days) (Finckh et al., 2006). When a patient population with sciatica due to a herniated lumbar disk is followed at regular intervals for more than 10 years, surgically treated patients report more complete relief of leg pain and improved function and satisfaction compared with the nonsurgically treated group. However, improvement in the patient’s predominant symptom and work or disability outcomes were similar in the two groups (Atlas et al., 2005). Three situations occur in which surgical referral is indicated: (1) in patients presenting with cauda equina syndrome, for which surgery may be required urgently, (2) if the neurological deficit is severe or progressing, or (3) if severe radicular pain continues after 4 to 6 weeks of conservative management.
Diabetic Polyradiculoneuropathy
When there is predominant involvement of the thoracic roots, the presenting symptoms are generally pain and paresthesias of rapid onset in the abdominal and chest wall. The trunk pain may be severe, described variably as burning, sharp, aching, and throbbing. It may mimic the pain of acute cardiac or intraabdominal medical emergencies and may simulate disk disease, but the rarity of thoracic disk protrusions and the usual development of a myelopathy help exclude this diagnosis. Findings of diabetic thoracoabdominal polyradiculoneuropathy include heightened sensitivity to light touch over affected regions; patches of sensory loss on the anterior, lateral, or posterior aspects of the trunk; and unilateral abdominal swelling due to localized weakness of the abdominal wall muscles (Longstreth, 1997).
Diabetic lumbosacral polyradiculoneuropathy involves the legs, especially the anterior thighs, with pain, dysesthesia, and weakness, reflecting the major involvement of upper lumbar roots. A variety of names have been used to describe it, including diabetic amyotrophy, proximal diabetic neuropathy, diabetic lumbosacral plexopathy, diabetic femoral neuropathy, and Bruns-Garland syndrome. Because it is likely that the brunt of nerve pathology falls on the nerve roots, it can be designated as diabetic polyradiculoneuropathy. Motor, sensory, and autonomic fibers are all affected by the disease process (Dyck et al., 1999).
In most patients, onset is fairly abrupt, with symptoms developing over days to a couple of weeks. Early in the course of the condition, the clinical findings are usually unilateral and include weakness of muscles supplied by L2-L4 roots (iliopsoas, quadriceps, and hip adductors), reduced or absent patellar reflex, and mild impairment of sensation over the anterior thigh. As time passes, there may be territorial spread, a term used by Bastron and Thomas in 1981 to describe proximal, distal, or contralateral involvement as the polyradiculoneuropathy evolves. Worsening may occur in a steady or a stepwise fashion, and it may take several weeks to progress from onset to peak of the disease. At its peak, weakness varies in severity and extent from a mildly affected patient with slight unilateral thigh weakness to a profound degree of bilateral leg weakness in the territory of the L2-S2 nerve roots. Upper-extremity involvement appears to occur in approximately 15% of patients with diabetic lumbosacral radicular plexopathy as a unilateral or asymmetrical sensorimotor neuropathy that primarily affects hands and forearms. Like the lumbosacral syndrome, EMG findings suggest a multifocal axon-loss process localized to roots, plexus, or peripheral nerve (Katz et al., 2001). Rarely, the process of territorial spread is so extensive that it involves a multiplicity of nerve roots along the entire spinal cord and leads to profound generalized weakness, a condition designated diabetic cachexia.
Laboratory studies disclose elevated fasting blood glucose in the vast majority of patients; when values are normal, they are found in treated diabetics. The erythrocyte sedimentation rate is usually normal, but in a subgroup of patients with diabetic lumbosacral polyradiculoneuropathy, it is elevated, a clue perhaps to an immune-mediated pathogenesis. The typical electrodiagnostic findings comprise features of a sensorimotor axon-loss polyneuropathy (diminished sensory and motor action potentials, normal or slightly prolonged distal latencies, and normal or mildly slowed conduction velocities) with additional needle electrode examination findings of active and chronic denervation changes in paraspinal, pelvic-girdle, and thigh muscles. Taken together, the findings reflect multifocal axonal damage to the nerve roots and lumbosacral plexus (Amato and Barohn, 2001). Although clinical findings may point to unilateral involvement, the electrodiagnostic examination generally discloses bilateral signs. Imaging studies of the thoracic and lumbosacral spinal canal with CT, myelography, and MRI are typically normal but are almost always necessary to exclude a structural abnormality of the nerve roots that may simulate diabetic polyradiculopathy. The CSF protein level is usually increased to an average of 120 mg/dL, but in some patients values exceed 350 mg/dL; pleocytosis is not a feature of this condition. Biopsy of proximal nerve sensory branches reveals axon loss and demyelination; in more severely affected patients, inflammatory cell infiltration and vasculitis is found (Said et al., 1997). Further studies of nerve biopsy specimens indicate that a microscopic vasculitis (involvement of small arterioles, venules, and capillaries) leads to ischemic injury, which in turn causes axonal degeneration and secondary segmental demyelination (Dyck et al., 1999). The presence of a small-vessel vasculitis with distinctive pathological features including transmural polymorphonuclear leukocyte infiltration of postcapillary venules and endothelial deposits of immunoglobulin (Ig)M and activated complement supports an immune-mediated inflammatory pathogenesis for this disorder (Kelkar et al., 2000).
Electrophysiological studies have suggested that a demyelinating polyneuropathy indistinguishable from chronic inflammatory demyelinating polyneuropathy (CIDP) occurs frequently in diabetes and may be the cause of a severe motor sensory polyneuropathy, sometimes with features of a plexopathy (Sharma et al., 2002a, 2002b).
Therapy is usually directed toward ameliorating the severe pain of this condition. The tricyclics, especially nortriptyline (with a better side-effect profile than amitriptyline), selective serotonin reuptake inhibitors (e.g., sertraline, nefazodone hydrochloride), anticonvulsants (e.g., gabapentin, carbamazepine), clonazepam, baclofen, clonidine, mexiletine, IV lidocaine, and topical capsaicin may have a role separately or in combination. Histopathological findings indicative of an immune-mediated pathogenesis have led to treatment of selected patients with intravenous immunoglobulin (IVIG) or immunosuppressive treatment or both (Krendel et al., 1995; Younger et al., 1998). Although immunotherapy may be beneficial, spontaneous improvement in some patients with painful proximal diabetic neuropathy with different patterns of inflammatory nerve lesions has been described (Said et al., 1997). A comprehensive and critical review of the literature on the role of immunotherapy of diabetic polyradiculopathy concludes that treatment remains controversial because the natural history is for spontaneous improvement, the side effects of immunotherapy may be significant, and information on efficacy is lacking from controlled clinical trials (Amato and Barohn, 2001). Prospective studies have suggested a role for immunotherapy in the treatment plan of diabetic polyradiculoneuropathy where electrophysiological findings are those of CIDP (Sharma et al., 2002a, 2002b), although the degree of improvement has been shown not to be as robust as in the immunotherapy of idiopathic CIDP (Gorson et al., 2000).
Neoplastic Polyradiculoneuropathy (Neoplastic Meningitis)
A wide variety of neoplasms are known to spread to the leptomeninges. These include solid tumors (carcinoma of the breast and lung and melanoma), non-Hodgkin lymphomas, leukemias, and intravascular lymphomatosis (Viali et al., 2000). Although neoplastic polyradiculoneuropathy usually occurs in patients known to have an underlying neoplasm, meningeal symptoms may be the first manifestation of malignancy. Neoplastic meningitis occurs in approximately 5% of all patients with cancer (Chamberlain, 2006). The clinical features of neoplastic polyradiculoneuropathy include radicular pain, dermatomal sensory loss, areflexia, weakness of the lower motor neuron type and bowel/bladder dysfunction (Balm and Hammack, 1996; Mammoser and Groves, 2010). Often the distribution of the sensory and motor deficits is widespread and simulates a severe sensorimotor polyneuropathy. Associated clinical manifestations (e.g., nuchal rigidity, confusion, cranial polyneuropathies) result from infiltration of the meninges.
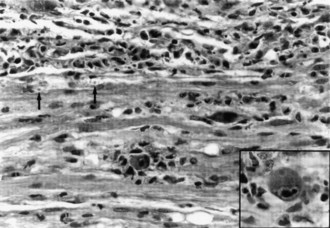
At postmortem examination, the cauda equina shows discrete nodules or focal granularity (Fig. 75.6). Microscopy discloses spinal roots encased by tumor cells, which appear to infiltrate the root. Malignant cells have the capacity to penetrate the pial membrane and invade the spinal nerves (Mammoser and Groves, 2010). It is presumed that disturbed nerve root function results from several mechanisms including nerve fiber compression and ischemia.
The most revealing diagnostic procedure is lumbar puncture, which is almost always abnormal, disclosing one or more or the following: mononuclear pleocytosis, reduced CSF glucose, elevated protein, and neoplastic cells (Grossman and Krabak, 1999). Spinal fluid cytological analysis is, however, persistently negative in up to a third of patients who have compelling evidence of leptomeningeal carcinomatosis (Kim and Glantz, 2001). A sensitive electrophysiological indicator of nerve root involvement is a change in the F wave. In the symptomatic patient with cancer, prolonged F-wave latencies or absent F responses should raise suspicion of leptomeningeal metastases. Postmyelography CT adds strong evidence in support of the diagnosis if it demonstrates multiple nodular defects on the nerve roots, but spinal MRI, especially with gadolinium enhancement, is the initial test of choice in the cancer patient in whom leptomeningeal involvement of the spine is suspected (Gleissner and Chamberlain, 2006). Approximately 50% of patients with neoplastic meningitis and spinal symptoms have abnormalities on these studies. Gadolinium-enhanced MRI of the brain discloses abnormalities, including contrast enhancement of the basilar cisterns or cortical convexities and hydrocephalus.
Standard therapy for neoplastic meningitis is essentially palliative; it does, however, afford stabilization and protection from further neurological deterioration (Chamberlain, 2006). A multidisciplinary approach is recommended, with input from medical oncology, neuro-oncology, radiation oncology, and neurosurgery (Mammoser and Groves, 2010). With treatment that includes radiotherapy to sites of symptomatic disease, intrathecal or intraventricular chemotherapy (methotrexate, thiotepa, and cytosine arabinoside), and optimal management of the underlying malignancy, median survivals of 3 to 6 months may be achieved (Chamberlain, 2006; Kim and Glantz, 2001). On occasion, longer-term survival is observed in patients with neoplastic meningitis accompanying breast cancer (13% survival rate at 1 year and 6% at 2 years), melanoma, and lymphoma (Jaeckle, 2006). A complication of aggressive treatment is a necrotizing leukoencephalopathy that becomes symptomatic months after treatment with radiation and intrathecal methotrexate (Grossman and Krabak, 1999).
Infectious Radiculopathy
Tabes Dorsalis
Tabes dorsalis, the most common form of late neurosyphilis, begins as a spirochetal (Treponema pallidum) meningitis (see Chapter 53C). After 10 to 20 years of persistent infection, damage to the dorsal roots is severe and extensive, producing a set of characteristic symptoms and signs. Symptoms are lightning pains, ataxia, and bladder disturbance; signs are Argyll Robertson pupils, areflexia, loss of proprioceptive sense, Charcot joints, and trophic ulcers. Lancinating or lightning pains are brief, sharp, and stabbing; they are more apt to occur in the legs than elsewhere. Sensory disturbances such as coldness, numbness, and tingling also occur and are associated with impairment of light touch, pain, and thermal sensation. Sudden visceral crises, characterized by the abrupt onset of epigastric pain that spreads around the body or up over the chest, occur in some 20% of patients.
Most of the features of tabes dorsalis can be explained by lesions of the dorsal roots, dorsal root ganglia, and posterior columns (Gilad et al., 2007). Ataxia is due to the destruction of proprioceptive fibers, insensitivity to pain follows partial loss of small myelinated and unmyelinated fibers, and bladder hypotonia with overflow incontinence, constipation, and impotence is the result of sacral root damage. Pathological study discloses thinning and grayness of the posterior roots, especially in the lumbosacral region, and the spinal cord shows degeneration of the posterior columns. A mild reduction of neurons in the DRG occurs, and there is little change in the peripheral nerves. Inflammation may occur all along the posterior root.
Polyradiculoneuropathy in Human Immunodeficiency Virus–Infected Patients
Cytomegalovirus (CMV) polyradiculoneuropathy is a rapidly progressive opportunistic infection that usually occurs late in the course of human immunodeficiency virus (HIV) infection when the CD4 count is very low (<200 cells/mL) and acquired immunodeficiency syndrome (AIDS)-defining infections are present. Uncommonly, it is the initial manifestation of AIDS (Anders and Goebel, 1998). Patients often have evidence of systemic CMV infection (retinitis, gastroenteritis). The presentation is marked by rapid onset of pain and paresthesias in the legs and perineal region, associated with urinary retention and progressive ascending weakness of the lower extremities (Robinson-Papp and Simpson, 2009). Examination discloses a severe cauda equina syndrome, the combination of flaccid paraparesis, absent lower-limb deep tendon reflexes, reduced or absent sphincter tone, and variable loss of light touch, vibration, and joint position sense. The upper extremities and cranial nerves may be involved in advanced cases (Robinson-Papp and Simpson, 2009).
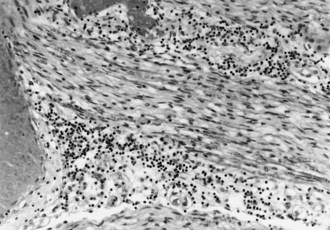
A gadolinium-enhanced MRI of the lumbosacral spine is necessary to exclude a compressive lesion of the cauda equina and is generally the first study performed (Robinson-Papp and Simpson, 2009). The CSF has an elevated protein level, depressed glucose level, polymorphonuclear pleocytosis, and a positive CMV polymerase chain reaction (PCR) (Anders and Goebel, 1998). CMV may be isolated from CSF cultures. The needle EMG discloses widespread fibrillation potentials in lower-extremity muscles, and sensory conduction studies may reveal an associated distal sensory neuropathy that is common in the late stages of HIV infection. MRI of the lumbosacral region is usually normal, but adhesive arachnoiditis has been described. The pathological features are marked inflammation and extensive necrosis of dorsal and ventral roots. Cytomegalic inclusions may be found in the nucleus and cytoplasm of endothelial and Schwann cells (Fig. 75.7).
Other causes of rapidly progressive lumbosacral polyradiculoneuropathy in the HIV-infected patient are meningeal lymphomatosis, Mycobacterium tuberculosis, and axonal polyradiculoneuritis associated with HIV infection per se (Corral et al., 1997). Additionally, one must consider acute inflammatory demyelinating polyradiculoneuropathy. Syphilis has an accelerated course in the patient with AIDS, and syphilitic polyradiculoneuropathy may present with rapidly progressive pain, paraparesis, muscle wasting, and hyporeflexia. In addition to markedly elevated CSF protein level, hypoglycorrhachia, and brisk pleocytosis, the CSF and serum Venereal Disease Research Laboratories (VDRL) serology results are positive. Intravenous penicillin leads to prompt improvement. Other considerations include herpes simplex virus type 2 and varicella-zoster virus infections that involve the lumbosacral nerve roots and the spinal cord, producing a radiculomyelitis. Toxoplasma gondii may also cause myelitis, presenting as a subacute conus medullaris syndrome that simulates the clinical features produced by CMV polyradiculoneuropathy. In the case of T. gondii, MRI may reveal abscess formation.
Lyme Radiculoneuropathy
Lyme disease is caused by the spirochete, Borrelia burgdorferi, transmitted by the deer tick, Ixodes dammini, and is most prevalent in the American northeast and upper Midwest. It is a multisystem disease affecting the skin, peripheral nervous system, central nervous system (CNS; referred to as neuroborreliosis), musculoskeletal system, and heart. To help bring order to the understanding of this illness, it may be divided into three clinical stages (Bratton et al, 2008). Stage 1 follows within 1 month of the tick bite and is marked by a characteristic rash in 60% to 80% of patients, designated erythema chronica migrans (oval or annular shape with a clear center in the area of the bite), and influenza-like symptoms of fatigue, fever, headache, stiff neck, myalgias, and arthralgias. In stage 2, the stage of dissemination of the spirochete from the initial lesion and occurring within weeks of the rash, peripheral nerve, joint, and cardiac abnormalities may appear. Stage 3, caused by late or persistent infection, may occur up to 2 years after the tick bite and is characterized by chronic neurological syndromes, among them neuropathy, encephalopathy, myelopathy, and psychiatric disturbances and migratory oligoarthritis.
Nerve root and peripheral nerve abnormalities that characterize stage 2 develop in about 15% of untreated patients in the United States (Steere, 2001). Possible manifestations occurring within weeks after the onset of erythema chronica migrans include headache with lymphocytic (aseptic) meningitis, cranial neuropathy (especially facial mononeuropathies, bilateral in 25% of cases), multifocal radiculoneuropathy, radiculoplexopathy, mononeuritis multiplex, myelitis, subtle encephalopathy, and cerebellar ataxia (Steere, 2001). The clinical features of nerve root involvement include burning radicular pain with sensory loss and hyporeflexia in the territory of the involved roots. Nerve conduction studies provide evidence for an associated primarily axon-loss polyneuropathy. Chronic neuroborreliosis, seen in stage 3, occurs in some 5% of untreated patients and is characterized by an axon-loss polyneuropathy that manifests as radicular pain or distal paresthesias (Steere, 2001). In a nonhuman primate model of neuroborreliosis, spread of B. burgdorferi within the nervous system—leptomeninges, motor and sensory roots, DRG, but not the brain parenchyma—has been demonstrated. In peripheral nerves from such animals, spirochetes were seen in the perineurium (Steere, 2001). Treatment of Lyme radiculoneuropathy with IV ceftriaxone (cefotaxime and penicillin G are acceptable alternates) for 2 to 4 weeks is associated with resolution of symptoms and signs in most patients.
Herpes Zoster
Herpes zoster, also known as shingles, is a common painful vesicular eruption occurring in a segmental or radicular (dermatomal) distribution and due to reactivation of latent varicella-zoster virus in DRG (see Chapter 53B). Primary infection presents as varicella (chickenpox) earlier in life, usually in epidemics among susceptible children (Gnann and Whitley, 2002). Involvement may occur at any level of the neuraxis but is most commonly seen in the thoracic dermatomes, followed by the face. Zoster may present in a division of the trigeminal nerve (e.g., herpes zoster ophthalmicus), where it is often accompanied by keratitis (a potential cause of blindness requiring immediate treatment), in the maxillary and mandibular nerves, and in the seventh nerve, where it is associated with a facial palsy and ipsilateral external ear or hard palate vesicles known as Ramsay Hunt syndrome (Gilden et al., 2000). Rarely, the viral episode can present as dermatomal pain without a rash, known as herpes sine herpete.
Zoster occurs during the lifetime of 10% to 20% of all people, with an incidence in the general population of approximately 3 to 5 per 1000 per year. The incidence is low in young people and increases with age—among persons older than 75 years exceeds 10 cases per 1000 person-years—and when immunocompetence is compromised. For example, the incidence among HIV-positive individuals was 15-fold greater than that of a control group (Gnann and Whitley, 2002).
Herpes zoster is characterized by sharp or burning radicular pain associated with itching and dysesthesias (altered sensation), sometimes accompanied by fever, malaise, and rash. In affected dermatomes, sensation is decreased, yet there is allodynia (painful response to normally non-noxious stimulation) (Gilden et al., 2000). The cutaneous eruption, unilateral and respecting the midline, begins as an erythematous maculopapular rash and progresses to grouped clear vesicles that continue to form for 3 to 5 days (Gnann and Whitley, 2002). These become pustules by 3 to 4 days and form crusts by 10 days. In the normal immunocompetent host, lesions resolve in 2 to 4 weeks, often leaving a region of reduced sensation, scarring, and pigmentation. Pain usually disappears as vesicles fade, but 8% to 70% of patients experience persisting severe pain termed postherpetic neuralgia (PHN), defined as “pain that persists more than 30 days after the onset of rash or after cutaneous healing” (Gnann and Whitley, 2002). This complication is more likely to develop in the elderly, occurring in 50% of patients older than 60 years. In half of patients affected with PHN, the pain resolves within 2 months, and 70% to 80% of patients are pain free by 1 year. Rarely, pain persists for years.
A complication of cutaneous herpes zoster is segmental motor weakness, which occurs in up to 30% of patients with zoster reactivation (Bahadir et al, 2008; Merchut and Gruener, 1996; Yaszay et al., 2000). Segmental zoster paresis is about equally divided between the arms and legs, with predominantly proximal muscle weakness reflecting weakness in cervical and lumbar—C5, C6, and C7 or L2, L3, and L4—myotomes, respectively. The diaphragm and abdominal muscles may be affected, and bladder and bowel dysfunction may occur in the setting of lumbosacral zoster (Gilden et al., 2000). The interval between skin eruption and paralysis is approximately 2 weeks, with a range of 1 day to 5 weeks and a rare instance reported of delayed (4.5 months) onset of diaphragmatic paralysis. Weakness peaks within hours or days and generally follows the dermatomal distribution of zoster eruptions (Yaszay et al., 2000); spread to muscles served by unaffected segments is very uncommon (<3% of cases). The prognosis for recovery is good, with nearly complete return of function in two-thirds of patients over the course of 1 to 2 years, 55% showing full recovery, and another 30% showing significant improvement. One in five patients is left with severe and permanent residua.
Prognosis for recovery in patients with diaphragmatic paralysis is not as good as it is with segmental paresis involving the limb muscles, probably owing to the challenge of axonal regeneration along the long course of the phrenic nerve (Bahadir et al, 2008). The histopathological correlate of herpes zoster is inflammation and neuronal loss in the DRG that correspond to the affected segmental levels. In the case of segmental zoster paresis, there is lymphocytic inflammation and vasculitis involving adjacent motor roots and the spinal cord gray matter, with resulting motor fiber degeneration (Gilden et al., 2000). A low-grade viral ganglionitis may contribute to PHN (Quan et al., 2005).
The major goals of treatment are to relieve local discomfort, prevent dissemination, and reduce the severity of PHN (Sandy, 2005). Acyclovir, valacyclovir, and famciclovir are indicated for the immunocompetent patient older than 50 years with herpes zoster and should be started within 48 hours of the viral episode to receive the most benefit from therapy. These drugs reduce the duration of viral shedding, limit the duration of new lesion formation, and accelerate healing and pain resolution. They are all safe and well-tolerated, but because of superior pharmacokinetic profiles and simpler dosing regimen, the latter two are preferred to acyclovir (Gnann and Whitley, 2002). Treatment with acyclovir speeds recovery in HIV-positive patients (Robinson-Papp and Simpson, 2009). The U.S. Food and Drug Administration (FDA) has approved a vaccine for use in the United States to reduce the risk for herpes zoster in older adults (≥60 years) without compromised immune systems (Mitka, 2006). The vaccine is effective in preventing herpes zoster and decreasing the incidence of complications (Adams et al., 2010) and is well tolerated (Simberkoff et al., 2010).
The pain of PHN—described variably as continuous deep aching, burning, sharp, stabbing, and shooting, and triggered by light touch over the affected dermatomes—is often debilitating and difficult to treat (Watson, 2000). Singly or in combination, tricyclics (amitriptyline or desipramine), selective serotonin reuptake inhibitors (sertraline or nefazodone hydrochloride), anticonvulsants (carbamazepine and gabapentin), oral opioids (oxycodone), and topical capsaicin cream or lidocaine patches are helpful for about 50% of patients. For intractable cases, intrathecally administered methylprednisolone and lidocaine has been shown to provide relief without adverse effects of arachnoiditis or neurotoxicity in more than 90% of patients treated (Kotani et al., 2000). Intravenous acyclovir followed by oral valacyclovir was found to reduce the pain of PHN in more than 50% of treated patients (Quan et al., 2005).
Acquired Demyelinating Polyradiculoneuropathy
Acquired demyelinating polyradiculoneuropathy has two major clinical forms. One develops acutely and is known as Guillain-Barré syndrome (GBS); the other is chronic, progressive, or relapsing and remitting and is designated CIDP. These disorders are described in detail in Chapter 76 but are mentioned here briefly because pathological changes may be pronounced in the spinal nerve roots, especially the ventral roots. There is a dense mononuclear inflammatory infiltrate of lymphocytes, monocytes, and plasma cells (Fig. 75.8), and segmental demyelination with relative sparing of axons. Neuroimaging with MRI discloses contrast enhancement of lumbosacral roots in both GBS and CIDP (Bertorini et al., 1995; Koller et al., 2005). The predilection for nerve root involvement in these conditions helps explain certain features including CSF and some neurophysiological findings, as well as disturbances in autonomic function that may be especially problematic in patients with GBS.
A CSF profile of albuminocytological dissociation is characteristic of this syndrome. A high lumbar CSF protein concentration in the face of a normal cisternal protein level supports the hypothesis that increased CSF protein derives largely from capillaries of the spinal roots. Nerve conduction studies usually disclose slowed motor conduction velocities, dispersed motor responses, and partial conduction block, but additional abnormalities include delayed or unobtainable F-wave responses or H-reflexes, reflecting demyelination in nerve roots (Koller et al., 2005). Indeed, abnormalities of these late responses may be the sole finding in 10% to 20% of patients with GBS in the first few weeks of the illness. The autonomic disturbances that occur in GBS may be due to involvement of preganglionic sympathetic fibers, which travel in the ventral roots en route to the paravertebral sympathetic ganglia.
Acquired Disorders of the Dorsal Root Ganglia
DRG may be selectively vulnerable to a variety of malignant and nonmalignant conditions. The resulting neurological disorder is a sensory neuronopathy syndrome whose clinical features are explained by the loss of large- and small-diameter DRG neurons (Bryer and Chad, 1999). Large-cell dropout leads to kinesthetic sensory impairment, poor coordination, loss of manual dexterity, ataxia, and areflexia, whereas small-cell depletion contributes to a hyperalgesic state marked by burning pains and painful paresthesias. The sensory neuronopathies are characterized by non–length-dependent abnormalities of SNAPs, a global decrease in SNAP amplitudes, and hyperintensities on T2-weighted MRI images of the dorsal spinal cord (Kuntzer et al., 2004; Lin and Chiu, 2008). Clinical and electrophysiological criteria have been proposed to help separate patients with sensory neuronopathy from those with predominantly sensory polyneuropathy (Camdessanche et al., 2009). Favoring the diagnosis of neuronopathy are the following findings: the presence of limb ataxia early in the disease, asymmetrical sensory loss at onset, upper-limb sensory loss, lost or attenuated upper-limb sensory action potentials, and relatively normal motor conduction studies.
Perhaps the best known of these uncommon conditions is paraneoplastic subacute sensory neuropathy (or neuronopathy), a disorder developing over weeks to months and characterized by ataxia and hyperalgesia while muscle strength is well preserved (Posner and Dalmau, 1997). Some patients have clinical signs of brainstem and cerebral dysfunction, reflecting a more widespread encephalomyelitis. The neuronopathy may antedate the diagnosis of cancer, usually small-cell lung carcinoma, by months to years. The CSF profile discloses elevated protein concentration and a mild mononuclear cell pleocytosis. Nerve conduction studies reveal widespread loss of sensory potentials. Neuropathological features include inflammation and phagocytosis of the sensory neurons in the DRG. This condition is associated with the presence of specific antineuronal (anti-Hu) antibodies that are complement-fixing polyclonal IgG antibodies that react with the nuclei of the neurons of the CNS and sensory ganglia but not with non-neuronal nuclei. The antigens recognized by the anti-Hu antibodies have been characterized as proteins with molecular weights of 35 to 40 kD. The presence of identical protein antigens in small-cell lung cancer cells and neuronal nuclei supports the view that the pathogenesis of paraneoplastic subacute sensory neuropathy is immunologically mediated, with tumor antigens triggering the production of cross-reactive antibodies. Morphological studies provide evidence for both cytotoxic T cell–mediated attack and humoral mechanisms in the pathogenesis of this condition. Response to immunotherapy is disappointing; early diagnosis of cancer, however, gives the best chance of helping the neurological disorder before it becomes devastating (Kuntzer et al., 2004).
Other causes of DRG neuronopathy include hereditary, toxic, and autoimmune disorders (Kuntzer et al., 2004). Hereditary sensory neuropathies are usually marked by their chronicity, acrodystrophic ulcerations, fractures, bouts of osteomyelitis, and lack of paresthesias. Pyridoxine abuse and cisplatin neurotoxicity are generally easily recognized. Sjögren syndrome may be accompanied by ataxia and kinesthetic sensory loss very similar to subacute sensory neuropathy. The presence of antibodies to extractable nuclear antigens such as anti-Ro (SS-A) and anti-La (SS-B) are suggestive of the diagnosis, but their absence does not exclude Sjögren syndrome. Diagnosis may be established with the demonstration of clusters of inflammatory cells in minor salivary glands (focal sialadenitis) from a biopsy of clinically normal lip mucosa. In some cases of Sjögren sensory neuronopathy, cervical MRI shows cervical cord atrophy with increased T2 signal intensity at the dorsal column, which probably stems from wallerian degeneration of neurons in the dorsal root ganglia (Lin and Chiu, 2008). Improvement from courses of IVIG may occur in some patients when treated early in the course of the illness. Other autoimmune acute ataxic syndromes range from ataxic Guillain-Barré syndrome to Fisher syndrome and Bickerstaff brainstem encephalitis. In these syndromes, serum anti-GQ1B IgG antibody levels are frequently elevated. The disorders may respond to immunotherapy with IVIG and plasma exchange (Kuntzer et al., 2004).
Radiculopathies Simulating Motor Neuron Disease
Disorders of the motor roots may lead to clinical features that resemble those encountered in motor neuron disease. Detailed study of such motor neuron syndromes is important because it might provide clues to the pathogenesis of the most common form of motor neuron disease, ALS. Clinicians should consider the possibility of an ALS-mimic syndrome when a patient with clinical features of lower motor neuron involvement is found to have a monoclonal gammopathy (Chad and Harris, 1999). In that instance, investigations must vigorously pursue the possibility that physical findings stem from ventral root involvement rather than anterior horn cell degeneration. An elevated CSF protein level, the presence of a monoclonal gammopathy, along with a demyelinating process identified by nerve conduction studies suggest a potentially treatable lymphoproliferative disease manifesting as motor polyradiculoneuropathy. In some instances of IgM monoclonal gammopathy, there is antibody specificity for the gangliosides GM1, asialo-GM1, and GD1b, among others. In rare instances, immunotherapy that reduces the serum concentrations of IgM gangliosides is associated with improvement in the lower motor neuron syndrome, thereby suggesting a possible pathogenic role of antiganglioside antibodies.
A postradiation lower motor neuron syndrome affecting the lumbosacral region, probably a polyradiculopathy, has been described occurring 4 months to 25 years after radiation therapy for testicular cancer, vertebral metastases, and lymphoma (Hsia et al., 2003). In some patients, MRI shows gadolinium enhancement of the conus medullaris and cauda equina that may mimic a leptomeningeal tumor (Hsia et al., 2003). Neuropathological study in a case of testicular cancer disclosed radiation-induced vasculopathy of proximal spinal roots with preserved motor neurons (Bowen et al., 1996). The course of the disorder is typically one of progression for 1 to 2 years followed by eventual stabilization.
Disorders of the Brachial Plexus
Anatomical Features
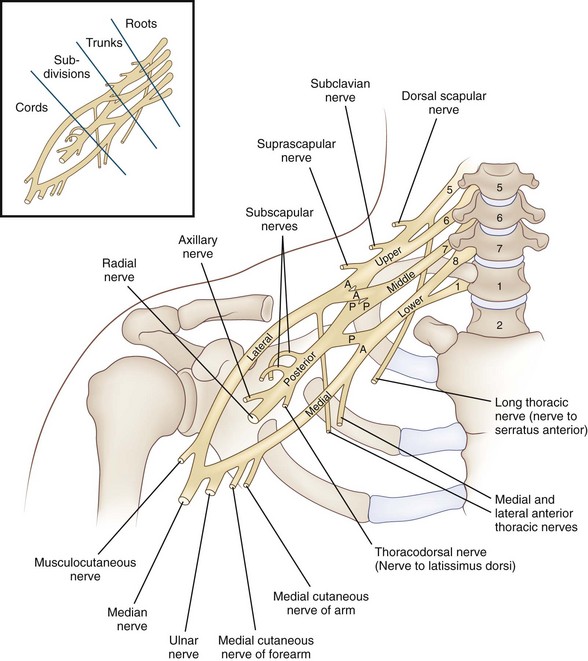
The brachial plexus is formed by five spinal nerve ventral rami (C5-T1), each of which carries motor, sensory, and postganglionic sympathetic fibers to the upper limb. It is a large and complex peripheral nervous system structure that contains 100,000 to 160,000 individual nerve fibers (Ferrante and Wilbourn, 2002). These five rami unite above the level of the clavicle to form the three trunks of the brachial plexus (Fig. 75.9): C5 and C6 join to form the upper trunk; T1 and C8 unite to form the lower trunk; and C7, the largest of the five rami, continues as the middle trunk. (To review, a ventral ramus—anterior primary ramus—derives from a mixed spinal nerve that is formed in turn by the fusion of the posterior dorsal and ventral roots in the intervertebral foramen.) Beneath the clavicle, each trunk divides into an anterior and posterior branch leading to six divisions, which become the three cords of the brachial plexus, the lateral, medial, and posterior. The cords, which lie behind the pectoralis minor, take their names from their relationship to the subclavian artery. The lateral and medial cords carry motor fibers to the ventral muscles of the limb. The lateral cord is formed from anterior divisions of the upper and middle trunks, the medial cord from anterior division of the lower trunk. The posterior cord carries motor fibers to the dorsal muscles of the limb; it is formed from posterior divisions of the upper, middle, and lower trunks.
Clinical Features and Diagnosis
Not surprisingly, disorders of the brachial plexus are determined in large part by its anatomical relationships (Ferrante and Wilbourn, 2002). Because of its location between two highly mobile structures, the neck and the shoulder, it is vulnerable to trauma. And because neighboring tissues such as lymph nodes, blood vessels, and lung parenchyma may themselves be targets of a variety of disease processes, the brachial plexus itself may be secondarily affected as an innocent bystander.
Electrodiagnostic Studies
Nerve conduction studies and needle EMG provide helpful information for confirming the clinical diagnosis of brachial plexopathy, determining the character of the lesion—predominantly axon loss, demyelinating, or both—and arriving at a judgment with respect to prognosis for recovery of function. In axon-loss brachial plexopathies, SNAPs and compound motor action potentials (CMAPs) are attenuated or lost depending on the severity of the disease process because the amplitude of these responses correlates directly with the number of conducting fibers. (In preganglionic lesions [at a root level], sensory responses are expected to be spared, and only motor responses will be affected.) As long as at least some fast-conducting fibers are spared, the conduction velocities and distal latencies are unaffected. In the case of demyelinating lesions, however, nerve conduction velocities are typically slowed, motor evoked responses dispersed, and distal latencies prolonged. Most brachial plexus lesions are axon-loss in nature (Ferrante and Wilbourn, 2002). The needle examination is very sensitive for detecting even mild motor fiber loss because fibrillation potentials develop in affected muscles by 3 weeks after the onset of a disease process.
Ferrante and Wilbourn (2002) note that axon-loss brachial plexus neuropathies fall along a spectrum of severity that may be determined by the results of the electrodiagnostic study. In the context of a minimal lesion affecting both sensory and motor fibers, SNAPs and CMAPs will typically be unaffected, but needle examination will disclose fibrillation potentials because the loss of one motor fiber will result in denervation of hundreds of muscle fibers. With an increase in lesion severity, SNAPs become attenuated while CMAPs are still spared. The most severe lesions compromise sensory responses and affect motor responses. Ferrante and Wilbourn also observed that it is the CMAPs that are most useful for quantifying the amount of loss suffered by a nerve. In contrast, SNAPs may be attenuated, even absent, with only partial lesions; and needle electrode examination, as we have seen, may reveal prominent fibrillation potentials with only mild motor axon loss. The needle examination is helpful in evaluating whether or not recovery from axon-loss lesions is ongoing because features of MUP remodeling (increased duration and complexity of MUPs) indicate the ongoing process of collateral reinnervation, distal-to-proximal reinnervation, or both.
Radiological Studies
Plain films of the neck and chest are often very helpful in evaluating arm weakness that is thought to be caused by a disorder of the brachial plexus. The presence of a cervical rib or long transverse process of C7 may provide an explanation for hand weakness and numbness, as seen in thoracic outlet syndrome. A lesion in the pulmonary apex, erosion of the head of the first and second rib, or the transverse processes of C7 and T1 may reveal the cause of a lower brachial plexopathy, as found in cases of Pancoast tumor. High-resolution CT and MRI scanning are also useful in detecting mass lesions of the plexus and may allow early diagnosis and specific therapy (Amrami and Port, 2005). There is an emerging role for magnetic resonance neurography to evaluate brachial plexus lesions (Zhou et al., 2004). CT-guided biopsy can be used to obtain cytological or histological material for precise diagnosis.
Traumatic Plexopathy
Three general categories of brachial plexus injury exist: (1) direct trauma, (2) secondary injury from damage to structures around the shoulder and neck, such as fractures of the clavicle and first rib, and (3) iatrogenic injury, most commonly seen as a complication of the administration of nerve blocks. The most pronounced of the lesions affecting the brachial plexus are preganglionic, characterized by a nerve root avulsion when rootlets are torn from the spinal cord. Lesions affecting the postganglionic portion of the plexus may be severe because of nerve rupture or of lesser severity if caused by nerve stretch (Giuffre et al., 2010). Direct injury may be either open (gunshot wounds and lacerations) or closed (stretch or traction). The main causes of brachial plexus palsies are traction and heavy impact. Injuries are usually secondary to motorcycle and snowmobile accidents, but sporting accidents in football, bicycling, skiing, and equestrian events are also important. Supraclavicular injuries are more common and more severe and have a worse prognosis than infraclavicular injuries (Midha, 1997). Another form of brachial plexus traction is seen in rucksack paralysis. The straps of a rucksack or backpack pressed to the shoulders may exert heavy pressure in the region of the upper trunk of the brachial plexus and thus lead to weakness in the muscles supplied by the suprascapular and axillary nerves and sensory loss in the C5-C6 distributions.
Long-Term Management
Often, surgery must be performed to provide an exact intraoperative definition of the lesion’s extent (see Chapter 50D). Intraoperative motor evoked potentials are helpful in assessing the functional state of anterior motor roots and motor fibers. Depending on the findings, innovative microsurgical techniques are available to provide an array of options: direct nerve repair, nerve grafting, nerve transfers, and free-functioning muscle transfers (Giuffre et al., 2010). Primary nerve reconstruction combined with joint fusion and tendon transfers provides a worthwhile return of function to many patients. The joint and tendon surgeries are best performed as secondary operations after a period of physiotherapy. Intensive physiotherapy and use of orthoses are often necessary to help restore maximum function. In general, the outcome after nerve grafting is relatively good for recovery of elbow flexors and extensors and for those of the shoulder girdle, but it is very poor for forearm and hand intrinsic muscles. Quality-of-life surveys after brachial plexus surgery indicate that 78% of patients report at least moderate satisfaction (Choi et al., 1997). In a large series of more than 1000 patients treated over a 30-year period (Kim et al., 2003), results of repair by suture and grafts were best for injuries located at the C5, C6, and C7 levels, the upper and middle trunk, the lateral cord to the musculocutaneous nerve, and the median and posterior cords to the axillary and radial nerves. Results were poor for injuries at the C8 and T1 levels and for lower trunk and medial cord lesions, and the chance of recovery was reduced with delays of more than 6 months in undertaking repair.
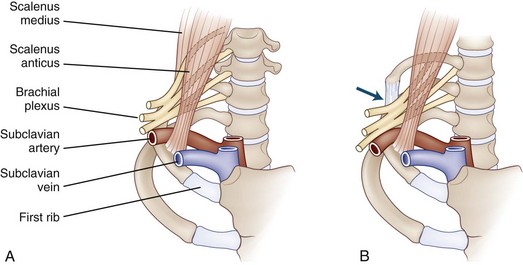
Neurogenic Thoracic Outlet Syndrome
In many cases, cervical spine roentgenograms disclose small bilateral cervical ribs or enlarged down-curving C7 transverse processes. When not visualized in anteroposterior radiographs of the cervical spine, they can be seen on oblique views. MRI of the thoracic outlet is a useful diagnostic method, revealing deviation or distortion of nerves or blood vessels and suggesting the presence of vasculonervous compressions (Demondion et al., 2003). Electrodiagnostic studies on the affected side disclose a reduced median motor response with normal median sensory amplitudes, along with a mildly reduced ulnar motor response and reduced ulnar sensory amplitude. The needle electrode examination typically discloses features of chronic axon loss with mild fibrillation potential activity in C8- and T1-innervated muscles. The clinical and electrophysiological findings point to a lesion of the lower trunk of the brachial plexus. Levin and colleagues (1998) have refined our understanding of the precise lesion localization of the neurogenic thoracic syndrome. They compared electrophysiological results between a group of patients with true neurogenic thoracic outlet syndrome and a group with “brachial plexopathy” stemming from median sternotomy. In the former group, the findings pointed to severe axon loss in the medial antebrachial cutaneous nerve and the abductor pollicis brevis, both sharing T1 root innervation. In the latter, an iatrogenic disorder resulting from rib retraction, the findings indicated axon loss in the ulnar sensory and motor nerves, conforming most to involvement predominantly of C8. These findings suggest that thoracic outlet syndrome and median sternotomy brachial plexopathy are due to damage to the mixed spinal nerve fibers at the level of the anterior primary rami (Levin, 2002)—distal to the C8 or T1 nerve roots but proximal to the lower trunk of the brachial plexus.
In most patients, a fibrous band extending from the tip of a rudimentary cervical rib to the scalene tubercle of the first rib causes angulation of either the C8 and T1 roots or the lower trunk of the brachial plexus (Fig. 75.10). Surgical division of the fibrous band can be expected to relieve pain and paresthesias and arrest muscle wasting and weakness in the majority of patients; return of muscle bulk and strength, however, is unlikely.
Metastatic and Radiation-Induced Brachial Plexopathy in Patients with Cancer
Metastatic Plexopathy
Damage to the brachial plexus in patients with cancer is usually secondary to either metastatic plexopathy or radiation-induced injury (Jaeckle, 2010). Lung and breast carcinoma are the tumors that most commonly metastasize to the brachial plexus; lymphoma, sarcoma, melanoma, and a variety of other types are less common. Tumor metastases spread via lymphatics, and the area most commonly involved is adjacent to the lateral group of axillary lymph nodes.
An important syndrome first described by Pancoast in 1932 is a superior pulmonary sulcus tumor, the vast majority of which are non–small-cell bronchogenic carcinomas (Arcasoy and Jett, 1997). The tumor arises near the pleural surface of the apex of the lung and grows into the paravertebral space and posterior chest wall, invading the C8 and T1 extraspinal nerves, the sympathetic chain and stellate ganglion, the necks of the first three ribs, and the transverse processes and borders of the vertebral bodies of C7 through T3. The tumor may eventually invade the spinal canal and compress the spinal cord. Clinical features include a number of symptoms and signs: severe shoulder pain radiating to the head and neck, axilla, chest, and arm; pain and paresthesias of the medial aspect of the arm and the fourth and fifth digits; and weakness with atrophy of intrinsic hand muscles.
On occasion, metastatic brachial plexopathy may be difficult to distinguish from radiation plexopathy (see the following section on Radiation-Induced Plexopathy). Imaging studies are usually informative. In patients with metastases, MRI can identify a mass adjacent to the brachial plexus and reveal whether the tumor has encroached on the epidural space. Magnetic resonance neurography is a novel noninvasive means of helping to exclude tumor in patients presenting with brachial plexopathy who have undergone radiation therapy to the brachial plexus (Du et al., 2010). Results of the treatment of metastatic plexopathy are disappointing. Radiotherapy to the involved field and chemotherapy of the underlying tumor are the mainstays of treatment. Radiotherapy may relieve pain in 50% of patients but has little effect on return of muscle strength. A variety of procedures have been implemented to ameliorate the severe pain of this condition, including opioid analgesics and nonopioid adjuvant analgesics such as antidepressants and antiepileptic drugs, including an emerging role for levetiracetam (Dunteman, 2005), transcutaneous stimulation, paravertebral sympathetic blockade, and dorsal rhizotomies.
In the patient with Pancoast tumor, preoperative radiotherapy followed by extended surgical resection is the most common treatment, with an overall 5-year survival rate of 20% to 35% (Arcasoy and Jett, 1997).
Radiation-Induced Plexopathy
Radiation-induced plexopathy is unlikely to occur if the dose is less than 6000 cGy. If more than 6000 cGy is given, the interval between the end of radiation therapy and the onset of symptoms and signs of radiation plexopathy ranges from 3 months to 26 years, with a mean interval of approximately 6 years. The brachial plexus is more vulnerable to large fraction size, and thus small doses per fraction are recommended (Johansson et al., 2000); cytotoxic therapy also adds to the damaging effect of radiotherapy. Limb paresthesias and swelling are common complaints. Although the pain of radiation plexopathy is usually less intense than that of metastatic plexopathy, it may nonetheless be problematic (severe and persistent), requiring opioids and chemical sympathectomy (Fathers et al., 2002). Weakness is usually most prominent in muscles innervated by branches of the upper trunk, but involvement of the entire limb from damage to the upper and lower portions of the plexus has also been described. Indeed, in a group of women with radiation plexopathy following treatment for carcinoma of the breast, progressive weakness resulted in loss of hand function in 90% of patients (Fathers et al., 2002). Dropcho (2010) points out that breast carcinoma is the tumor most often associated with radiation plexopathy, accounting for 40% to 75% of patients, followed by lung carcinoma and then by lymphoma.
The relative resistance of the lower trunk of the brachial plexus to radiation injury is perhaps explained by the protective effect of the clavicle and the relatively shorter course of the lower trunk and its divisions through the radiation port. The pathogenesis of radiation damage is thought to involve two factors: (1) radiation-induced endoneurial and perineurial fibrosis with obliteration of blood vessels, triggered by small-vessel or microvascular endothelial injury, and (2) direct radiation-induced damage to myelin sheaths and axons. Radiation-induced arteritis of large vessels was found in a patient with delayed onset (21 years) of brachial plexopathy following radiation therapy for breast carcinoma, who underwent arteriography for acrocyanosis in the affected limb (Rubin et al., 2001). The natural history of radiation-induced plexopathy is that of steadily increasing deterioration, although at times a plateau may be reached after 4 to 9 years of progression.
Unfortunately, treatment options are not very satisfactory. Surgical treatment using neurolysis has been reported to relieve pain in some patients, but there is little information on long-term outcome, and surgery may cause significant deterioration in motor and sensory function (Dropcho, 2010). A diagnostic dilemma arises when symptoms and signs of brachial plexopathy develop in a patient who is known to have had cancer and radiation in the region of the brachial plexus. A painful lower-trunk lesion with Horner syndrome strongly suggests metastatic plexopathy, whereas a relatively painless upper-trunk lesion with lymphedema favors radiation-induced plexopathy. MRI does not always discriminate between metastatic and radiation because it may reveal an appearance of high signal intensity on T2-weighted images and contrast enhancement in cases of both radiation fibrosis and tumor infiltration (Wouter van Es et al., 1997), although radiation fibrosis is favored by finding thickening and diffuse enlargement of the brachial plexus without a focal mass (Wittenberg and Adkins, 2000). Magnetic resonance neurography may be useful to exclude tumor (Du et al., 2010) and fluorodeoxyglucose positron emission tomography (FDG-PET) scanning may be useful for identifying metastatic breast cancer in or near the brachial plexus, not clearly imaged by CT or MRI (Dropcho, 2010). In the early and middle stages of radiation plexopathy, nerve conduction studies disclose features of demyelinating conduction block, but as time passes, there is conversion to axon loss (Ferrante and Wilbourn, 2002). Needle EMG is helpful in separating radiation-induced plexopathy from neoplastic plexopathy by the presence of myokymic discharges in the former. These are spontaneously occurring grouped action potentials (triplets or multiplets) followed by a period of silence, with subsequent repetition of a grouped discharge of identical potentials in a semirhythmic manner. They appear to result from spontaneous activity in single axons induced by local membrane abnormalities. They have not been reported in cases of tumor plexopathy.
Idiopathic Brachial Plexopathy
Arm pain and weakness are the cardinal manifestations of idiopathic brachial plexopathy. They occur in all age groups, particularly between the third and seventh decades of life. Men are affected two to three times more often than women; there appears to be a higher incidence among men engaged in vigorous athletic activities such as weight lifting, wrestling, and gymnastics. Although half the cases seem unrelated to any precipitating event, in others the plexopathy follows an upper respiratory tract infection, a flulike illness, an immunization, surgery, or psychological stress, or it occurs postpartum (van Alfen and van Engelen, 2006). A familial form of brachial plexus neuropathy, so-called hereditary neuralgic amyotrophy, is an autosomal dominant disorder causing repeated episodes of intense pain, paralysis, and sensory disturbances in an affected limb (Chance, 2006). Like the idiopathic disorder, there may be similar antecedent triggering events. Onset is at birth or early childhood, with a good prognosis for recovery after each attack. Three point mutations have been found in the gene SEPT9, encoding the septin-9 protein, in 49 pedigrees with hereditary neuralgic amyotrophy linked to chromosome 17q25 (Hannibal et al., 2009; Kuhlenbaumer et al., 2005). In some individuals, associated findings include relative hypertelorism, occasional cleft palate, and skin folds or creases on the neck or forearm (Hannibal et al., 2009).
Clinical Features
The illness begins with abrupt onset of intense pain, described as sharp, stabbing, throbbing, or aching and located in a variety of sites that include the shoulder, scapular area, trapezius ridge, upper arm, forearm, and hand. The pain may last from hours to many weeks, and then it gradually abates. Lessening of pain is associated with the appearance of weakness. This may have been present during the painful period but was not appreciated because the pain prevented the patient from moving the limb. Weakness may progress for 2 to 3 weeks after the onset of pain. Although pain subsides in most patients, it may continue for several weeks after weakness has reached its peak, and rarely, it recurs episodically for a year or more (van Alfen and van Engelen, 2006).
Recognition is growing that the typical syndrome of brachial plexopathy need not always be associated with lesions of trunks or cords but can be caused by discrete lesions of individual peripheral nerves, including the suprascapular, axillary, musculocutaneous, long thoracic, median, and anterior interosseous. It can also involve cranial nerves VII and X, and the phrenic nerves (Cruz-Martinez et al., 2002). Individual fascicular involvement of the musculocutaneous nerve, causing isolated brachialis wasting, has also been reported (Watson et al., 2001). Thus, the term brachial plexus neuropathy may be appropriate. Sensory loss, found in two-thirds of patients and most commonly over the outer surface of the upper arm and the radial surface of the forearm, is usually less marked than the motor deficit, although the spectrum of brachial plexus neuropathy includes patients with isolated clinical and electrophysiological sensory deficits (Seror, 2004). One-third of cases are bilateral, but many fewer are symmetrical. In a small number of patients, unilateral or bilateral diaphragmatic paralysis occurs (Lahrmann et al., 1999), and the combination of acute shoulder pain with respiratory symptoms should suggest the diagnosis of brachial plexus neuropathy. In a small subset of patients, neuralgic amyotrophy presents with isolated phrenic neuropathy (sometimes bilateral) with no abnormalities on clinical or electrodiagnostic examinations of the limbs (Tsao et al., 2006).
Diagnosis
Electrodiagnostic testing is helpful in confirming the diagnosis and ruling out other conditions. Findings suggest axonal lesions of peripheral nerves occurring singly (mononeuritis) or in various combinations (mononeuritis multiplex) (Cruz-Martinez et al., 2002). Sensory studies are abnormal in one-third of patients; the most common abnormality is reduced amplitude of one or more sensory action potentials of the median, ulnar, and radial nerves and the lateral and medial antebrachial cutaneous nerves. Van Alfen and colleagues (2009) found sensory abnormalities in less than 20% of nerves studied, even when the nerve was clinically affected, suggesting that the pathology may be in the nerve roots. Needle EMG is helpful because it shows absence of fibrillation potentials in the cervical paraspinal muscles, thereby pointing to a pathological process distal to the DRG. Needle EMG is also helpful in sorting out the problems of localization, identifying lesions localized to the brachial plexus, individual peripheral nerves, or peripheral nerve branches. Finally, in a small number of patients, needle EMG is abnormal on the asymptomatic side as well as the symptomatic side, indicating that brachial plexus neuropathy can sometimes be subclinical. MRI of the brachial plexus is important in excluding structural lesions that might simulate this disorder and should be performed where there is failure to recover function (van Alfen and van Engelen, 2006). The MRI appearance in idiopathic brachial plexopathy reveals findings of diffuse high T2 signal intensity abnormality and fatty atrophy of involved muscles (Gaskin and Helms, 2006). There are additional laboratory findings of interest (van Alfen and van Engelen, 2006). Among the group of patients with severe bilateral brachial plexus neuropathy with phrenic nerve involvement, elevated liver enzymes are found, possibly reflecting an antecedent subclinical hepatitis. In 25% of patients, antiganglioside antibodies are found, and CSF protein elevations with oligoclonal bands are also noted in some patients, reflecting the likelihood of an immune pathogenesis for this condition.
Pathophysiology and Etiology
The pathophysiology and pathogenesis of the disorder are unclear. An abrupt onset might suggest an ischemic mechanism, and prior history of a viral syndrome or an immunization raises the possibility of an immune-mediated disorder. Complement-dependent antibody-mediated demyelination may have participated in the peripheral nerve damage and nerve biopsy findings in four cases of brachial plexus neuropathy that revealed florid multifocal mononuclear infiltrates, suggesting a cell-mediated component as well (Suarez et al., 1996). In some cases, rapid recovery bespeaks demyelination and remyelination; in others, a long recovery period is more in keeping with axonal degeneration followed by axonal regeneration. Indeed, a biopsy of a cutaneous radial branch in a severe case of plexopathy showed profound axonal degeneration. In most patients, electrophysiological abnormalities are restricted to the affected limb, while in a small number of cases there is evidence of a more generalized polyneuropathy. Nerve biopsy studies of patients with autosomal dominant attacks of brachial plexus neuropathy during symptomatic phases disclosed prominent perivascular inflammatory infiltrates with vessel wall disruption, suggesting that the hereditary disorder has an immune pathogenesis possibly caused by genetic abnormalities of immune regulation (Klein et al., 2002).
Treatment and Prognosis
In the acute stage of the disorder, long-acting nonsteroidal antiinflammatory drugs (NSAIDs) and opioid analgesics are required to control pain (van Alfen, 2007). Evidence from one open-label retrospective series suggests that oral prednisone given in the first month after the onset can shorten the duration of the initial pain and leads to earlier recovery in some patients (van Alfen et al., 2009). Arm and neck movements often aggravate pain, so immobilization of the arm in a sling is helpful. With the onset of paralysis, range-of-motion exercises help prevent contractures. Following the phase of acute pain, van Alfen (2007) reported two additional categories of pain. The first, experienced by nearly 80% of patients, is a shooting or radiating neuropathic pain, believed to originate from the heightened mechanical sensitivity of damaged nerves of the plexus and lasting for weeks to months. This pain may respond to gabapentin and tricyclic medications. A second type of pain that develops in many is a musculoskeletal-type pain localized to the origin or insertion of the paretic or compensating muscles, especially in the periscapular, cervical, and occipital regions. This pain requires physical therapy modalities. Up to 30% of patients who have experienced neuralgic amyotrophy will have long-standing pain for an average follow-up of 6 years (van Alfen, 2007). Accordingly, pain management becomes the mainstay of therapy for these individuals and requires a multidisciplinary approach that blends pharmacotherapy with physical and occupational modalities.
Disorders of the Lumbosacral Plexus
Anatomical Features
The lumbar plexus is formed within the psoas major muscle by the anterior primary rami of lumbar spinal nerves L1, L2, L3, and L4. It is connected to the sacral plexus in the true pelvis by the anterior division of L4 (Fig. 75.11, A). Branches of the lumbar plexus include the iliohypogastric and ilioinguinal nerves arising from L1 (with a contribution from T12), the lateral femoral cutaneous nerve of the thigh originating from the posterior divisions of L2 and L3, and the genitofemoral nerve arising from the anterior division of L1 and L2. Other branches are the femoral nerve, formed from the posterior divisions of L2, L3, and L4 within the substance of the psoas muscle, and the obturator nerve, formed by the anterior divisions of L2, L3, and L4.
The lumbar plexus communicates with the sacral plexus via the anterior division of L4, which joins with L5 to form the lumbosacral trunk at the medial border of the psoas at the ala of the sacrum. The trunk enters the pelvis and joins the sacral plexus in the piriformis fossa. The sacral plexus, derived from the anterior rami of spinal nerves L4, L5, S1, S2, and S3, forms in front of the sacroiliac joint (see Fig. 75.11, B). Like the lumbar plexus, the sacral plexus has anterior and posterior divisions. The anterior division contributes to the tibial portion, and the posterior division contributes to the peroneal portion of the sciatic nerve, which leaves the pelvis through the greater sciatic notch. A number of important branches come from the sacral plexus in the pelvis; the superior and inferior gluteal nerves arise from posterior divisions of the sacral plexus and supply the gluteus medius and minimus muscles and the gluteus maximus, respectively. The posterior cutaneous nerve of the thigh is formed by the anterior divisions of S1, S2, and S3. It passes through the greater sciatic foramen into the buttock. The pudendal nerve originates from the undivided anterior primary rami of spinal nerves S2, S3, and S4 and extends into the gluteal region via the greater sciatic foramen.
Clinical Features
Neuroimaging Studies
Bone destruction found in plain radiographs of lumbar and sacral vertebrae and the pelvis provides evidence for a structural plexopathy. Intravenous pyelography (IVP) may demonstrate distortion of a ureter or the bladder. Barium enema may disclose displacement of the bowel. CT scanning of the abdomen and pelvis from a rostral point at the level of L1-L2 to a caudal point below the level of the symphysis pubis allows the regional anatomy of the entire lumbosacral plexus to be scrutinized (see Chapter 33A).
The resolution of modern CT and MRI scanners allows identification of individual plexus components. The administration of contrast is usually required to demonstrate the extent of structural abnormalities of the lumbosacral plexus, but it may not differentiate benign and malignant neoplasms, inflammatory masses, and hematoma. A normal MRI makes a structural plexopathy very unlikely. Clues to the nature of a plexopathy are given in Box 75.1. An approach to evaluation of a plexopathy is summarized in (Fig. 75.12).
Box 75-1 Clues to the Nature of a Plexopathy
Structural Lumbosacral Plexopathy
Hematoma
Pain, often severe, is usually the first manifestation of a retroperitoneal hematoma. The pain is present in the groin and radiates to the anterior thigh and lumbar region. It is associated with gradually increasing paresthesias and weakness. When the femoral nerve is involved, weakness and sensory loss occur in its territory; when other components of the plexus are involved, changes are more extensive and conform to the territories supplied by the involved branches of the plexus. If the hemorrhage is large, a mass may develop in the lower abdominal quadrant and be associated with systemic signs like tachycardia, hypotension, and a falling hematocrit (Parmer et al., 2006). It typically arises from the lateral wall of the pelvis and can be seen in a CT scan to obscure the normal concavity of the inner aspect of the wing of the ilium (Fig. 75.13). Because of iliacus muscle spasm, the patient usually lies in a characteristic posture with the hip flexed and laterally rotated because hip extension aggravates the pain. Several days after the onset of the hematoma, a bruise may appear in the inguinal area or anterior thigh. In some patients, especially those with relatively small hematomas and mild neurological deficits, recovery may be satisfactory with conservative measures including reversing anticoagulation, although 10% to 15% of patients show no improvement. Accordingly, some centers explore the abdomen through a limited retroperitoneal incision after reversing the coagulopathy; a complete iliacus fasciotomy is performed and the hematoma is evacuated, thus relieving compression on the femoral nerve and enhancing the prospects for full recovery (Parmer et al., 2006).
Abscess
Psoas abscess was more common when tuberculosis was prevalent, but neurological complications such as lumbar plexopathy and femoral neuropathy were rare. This phenomenon was explained by the slow distention of the psoas sheath and by the fact that the abscess ruptured through the psoas fascia before the femoral nerve could be damaged by raised intrapsoas compartment pressure. Similarly, acute nontuberculous psoas abscess rarely produces nerve compression, presumably because the psoas fascia is distensible. Femoral neuropathy, however, does occur with iliacus muscle abscess because the fascia iliaca is relatively indistensible. Rarely, lumbar plexopathy is a complication of pelvic hydatidosis caused by the tapeworm, Echinococcus granulosus (Serradilla et al., 2002).
Aneurysm
Back and abdominal pain are often early manifestations of abdominal aortic aneurysms. Knowledge of abdominal and pelvic regional anatomy helps explain the radiating characteristics of these pains. An expanding abdominal aortic aneurysm may compress the iliohypogastric or ilioinguinal nerve, leading to pain radiating into the lower abdomen and inguinal areas. Pressure on the genitofemoral nerve produces pain in the inguinal area, testicle, and anterior thigh. Compression of nerve trunks L5 through S2, which lie directly posterior to the hypogastric artery, may give rise to sciatica (Shields et al., 1997); 13% of patients with aneurysms of the iliac artery will present with features of sciatica (Delgado-Garcia et al., 1999).
Trauma
Because of the relatively protected position of the lumbosacral plexus, traumatic lesions are uncommon. However, fracture of the pelvis, acetabulum, or femur or surgery on the proximal femur and hip joint may injure the lumbosacral plexus. Sacral fractures or sacroiliac joint separation accounts for most cases (68%) of traumatic lumbosacral plexopathy, while acetabular and femoral fractures are much less frequently implicated (14% and 9%, respectively) (Kutsy et al., 2000). The latter, however, are more likely to cause injury to proximal nerves originating from the plexus. The mechanism of posttraumatic paresis in lumbosacral plexopathies may involve a number of factors, including nerve crush caused by fractured bone fragments; retroperitoneal hemorrhage; and traction as a result of hyperextension, hyperflexion, or rotation around the hip joint. Conservative measures appear to be the most appropriate way to manage posttraumatic injuries. More than two-thirds of patients show good or moderate recovery of paresis after 18 months of follow-up after injury.
Pregnancy
The lumbosacral trunk may be compressed by the fetal head during the second stage of labor. This tends to occur in prolonged labor with midforceps rotation in a small primigravida mother carrying a relatively large baby. A day or so after delivery when the patient gets out of bed, she notes difficulty walking because of foot dorsiflexor weakness. Examination discloses weakness in dorsiflexion and inversion, with reduced sensation over the lateral aspect of the leg and dorsal surface of the foot. Nerve conduction studies disclose attenuation or absence of the superficial peroneal SNAP on the affected side, and needle EMG reveals denervation in muscles innervated by L5 below the knee (Katirji et al., 2002). The primary pathology is predominantly demyelination, and the prognosis for complete recovery within 5 months is very good. In subsequent pregnancies, a trial of labor can be allowed so long as there is no evidence of disproportion or malpresentation. If labor proceeds, forceps should be used with great caution. Midforceps use in a woman with a previous obstetrical lumbosacral trunk palsy invites danger. It is prudent to perform cesarean section if the trial of labor is unsuccessful or if the infant is large.
Femoral neuropathy may occur in a thin patient during cesarean section in cases managed with self-retaining retractors (Alsever, 1996). In the thin abdominal wall, a deep lateral insertion of retractor blades exerts pressure on the psoas and may injure the femoral nerve. After surgery, the patient notes weakness and numbness in the territory of the femoral nerve. Recovery is usually rapid and full. The obturator nerve may be compressed by the fetal head or forceps near the pelvic brim. Patients note pain in the groin and anterior thigh as well as weakness and sensory loss in the territory of this nerve.
Neoplasia
The lumbosacral plexus may be damaged by tumors that invade the plexus either by direct extension from intraabdominal neoplasm or by metastases. Most tumors involve the plexus by direct extension (73%), whereas metastases account for only a fourth of cases. The primary tumors most frequently encountered are colorectal, cervical, and breast, as well as sarcoma and lymphoma (Jaeckle, 2010). Three clinical syndromes occur: upper plexopathy with findings referable to the L1-L4 segments (31%); lower plexopathy with changes in the L4-S1 segments (51%); and panplexopathy with abnormalities in the L1-S3 distribution (18%). Neoplastic plexopathy typically has an insidious onset over weeks to months. Severe and unrelenting pain is a prominent early manifestation and is aching or cramping in quality; it typically radiates from the low back to the lower extremities. Weeks to months after pain begins, numbness, paresthesias, weakness, and leg edema develop. Incontinence or impotence occurs in fewer than 10% of patients. The most commonly encountered tumors are colorectal in upper plexopathy, sarcomas in lower plexopathy, and genitourinary in panplexopathies. The majority of neoplastic plexopathies are unilateral, although bilateral plexopathies, caused usually by breast cancer, occur in approximately 25% of patients. The prognosis in lumbosacral plexopathy due to neoplasm is poor, with a median survival of 5.5 months.
Neuroimaging with CT or MRI usually establishes the diagnosis of neoplastic plexopathy, but MRI is probably more sensitive (Taylor et al., 1997). Because pelvic neoplasms may extend into the epidural space, most often below the conus medullaris, MRI of the lumbosacral spine is indicated in most patients. On occasion, a plexus neoplasm is difficult to discern by the best neuroimaging procedures. Two main explanations for this phenomenon exist. First, patients who have received previous radiotherapy may have developed tissue fibrosis that cannot be distinguished from recurrent tumor. Second, some tumors track along the plexus roots and do not produce an identifiable mass. In these instances, ancillary imaging tests (high-resolution MRI, bone scan, plain films, IVP), a biopsy of the plexus, or both may be required to determine the etiology. Prostate cancer may cause a lumbosacral radiculoplexopathy when tumor advances into the lumbosacral plexus by perineurial spread, a process that may evolve for up to 8 years, is associated with prominent urinary dysfunction, and leads to an MRI appearance of asymmetrical nerve enlargement but otherwise normal pelvic and abdominal imaging (Ladha et al., 2006).
Nonstructural Lumbosacral Plexopathy
Radiation Plexopathy
Radiation plexopathy usually produces slowly progressive painless weakness (Jaeckle, 2010). Dropcho (2010) points out that the radiation injury to the plexus most commonly occurs after treatment of pelvic tumors, testicular tumors, or tumors involving paraaortic lymph nodes. Pain develops in approximately half of patients with radiation plexopathy but is usually not early or severe (Dropcho, 2010). Most patients with radiation plexopathy eventually develop bilateral weakness, which is often asymmetrical and affects any muscles innervated by L2 through S1 but typically has an L5-S1 predominance (Dropcho, 2010). In most patients, leg reflexes are absent, and superficial sensation is impaired. Symptoms referable to bowel or urinary tract are usually the result of proctitis or bladder fibrosis. The latent interval between radiation and the onset of neurological manifestations is between 1 and 31 years (median 5 years), although very short latencies of less than 6 months have also been reported. An acute presentation of lumbosacral plexopathy 10 weeks following completion of radiation therapy for cervical cancer has also been observed (Abu-Rustum et al., 1999). No consistent relationship is evident between the duration of the symptom-free interval and the amount of radiation.
In most patients, radiation plexopathy is gradually progressive and results in significant or severe disability. CT and MRI of the abdomen and pelvis are typically normal and are useful in detecting lumbosacral plexus metastases (Dropcho, 2010). EMG discloses paraspinal fibrillation potentials in 50% of patients, suggesting that radiation damages the nerve roots in addition to the plexus; hence, a more appropriate designation is radiation radiculoplexopathy. In almost 60% of patients, the EMG discloses myokymic discharges, a feature that is only rarely seen in neoplastic plexopathy.
Idiopathic Lumbosacral Plexopathy
Because lumbosacral plexopathy may occur in the absence of a recognizable underlying disorder, it can be considered a counterpart of idiopathic brachial plexus neuropathy (van Alfen and van Engelen, 1997). It may begin suddenly with pain, followed by weakness, which progresses for days or sometimes many weeks. In many patients, the condition stabilizes, but in some, the course is chronic progressive or relapsing/remitting. Weakness is found in the distribution of the upper and lower portions of the lumbosacral plexus in 50% of cases; major involvement occurs in the territory of the upper portion in 40% and in the lower portion in only 10% of patients. Most patients recover over a period of months to 2 years, although recovery is often incomplete. The EMG discloses a patchy pattern of denervation in the distribution of part or all of the lumbosacral plexus, but the paraspinal muscles are spared, indicating that the process does not affect the lumbosacral roots. Dyck and colleagues (2001) designated idiopathic lumbosacral plexopathy as nondiabetic lumbosacral radiculoplexus neuropathy and found that it resembles diabetic polyradiculoplexopathy in terms of its clinical presentation (subacute, asymmetrical, and painful with delayed and incomplete recovery) and pathological findings (ischemic injury and microvasculitis) and suggested that it probably has an immune pathogenesis. MRI has been reported to show gadolinium enhancement in the lumbar plexus that disappeared in association with resolution of symptoms and signs of plexopathy following IV gamma globulin treatment (Ishii et al., 2004). Immune-modulating therapy may be beneficial for a subgroup of patients with idiopathic lumbosacral plexopathy (Dyck and Windebank, 2002).
Abu-Rustum N.R., Rajbhandari D., Glusman S., et al. Acute lower extremity paralysis following radiation therapy for cervical cancer. Gynecol Oncol. 1999;75:152-154.
Adams E.N., Pamapy S., Bautista P. Herpes zoster and vaccination: a clinical review. Am J Health Syst Pharm. 2010;67:724-727.
Alsever J.D. Lumbosacral plexopathy after gynecologic surgery: case report and review of the literature. Am J Obstet Gynecol. 1996;174:1769-1778.
Amato A.A., Barohn R.J. Diabetic lumbosacral polyradiculopathies. Curr Treat Options Neurol. 2001;3:139-146.
Amrami K.K., Port J.D. Imaging the brachial plexus. Hand Clin. 2005;21:25-27.
Anders H.J., Goebel F.D. Cytomegalovirus polyradiculopathy in patients with AIDS. Clin Infect Dis. 1998;27:345-352.
Arcasoy S.M., Jett J.R. Superior pulmonary sulcus tumors and Pancoast’s syndrome. N Engl J Med. 1997;337:1370-1376.
Ashkan K., Johnston P., Moore A.J. A comparison of magnetic resonance imaging and neurophysiological studies in the assessment of cervical radiculopathy. Br J Neurosurg. 2002;16:146-148.
Atlas S.J., Keller R.B., Wu Y.A., et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10-year results from the Maine Lumbar Spine Study. Spine. 2005;30:847-849.
Bahadir C., Kalpakcioglu A.B., Kurtulus D. Unilateral diaphragmatic paralysis and segmental motor paresis following herpes zoster. Muscle Nerve. 2008;38:1070-1073.
Balm M., Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626-632.
Bertorini T., Halford H., Lawrence J., et al. Contrast-enhanced magnetic resonance imaging of the lumbosacral roots in the dysimmune inflammatory polyneuropathies. J Neuroimaging. 1995;5:9-15.
Bowen J., Gregory R., Squier M., et al. The post-irradiation lower motor neuron syndrome: neuronopathy or radiculopathy. Brain. 1996;119:1429-1439.
Bratton R.L., Whiteside J.W., Hovan M., et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571.
Bryer M.A., Chad D.A. Sensory neuronopathies. Neurologist. 1999;5:90-100.
Camdessanche J.P., Jousserand G., Ferraud K., et al. The pattern and diagnostic criteria of sensory neuronopathy: a case-control study. Brain. 2009;132:1723-1733.
Carette S., Fehlings M.G. Cervical radiculopathy. N Engl J Med. 2005;353:392-399.
Carette S., Leclaire R., Marcoux S., et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997;336:1634-1640.
Carlstedt T., Grane P., Hallin R.G., et al. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323-1325.
Chad D.A., Harris N.L. Case 16-1999—A 71-year-old man with progressive weakness and a gammopathy. N Engl J Med. 1999;340:1661-1669.
Chamberlain M.C. Neoplastic meningitis. Neurologist. 2006;12:179-187.
Chance P.F. Hereditary focal, episodic neuropathies: hereditary neuropathy with liability to pressure palsies and hereditary neurologic amyotrophy. Neuromolecular Med. 2006;8:159-174.
Chin C.H., Chew K.C. Lumbosacral nerve root avulsion. Injury. 1997;28:674-678.
Choi P.D., Novak C.B., Mackinnon S.E., et al. Quality of life and functional outcome following brachial plexus injury. J Hand Surg Am. 1997;22:605-612.
Corral I., Quereda C., Casado J.L., et al. Acute polyradiculopathies in HIV-infected patients. J Neurol. 1997;244:499-504.
Cruz-Martinez A., Barrio M., Arpa J. Neuralgic amyotrophy: variable expression in 40 patients. J Peripher Nerv Syst. 2002;40:198-204.
Delgado-Garcia F., Lopez-Dominguez J.M., Casado-Chocan J.L., et al. Lumbosacral plexopathy as a form of presentation of an aneurysm of the iliac artery. Rev Neurol. 1999;28:1072-1074.
Demondion X., Bacqueville E., Paul C., et al. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227:461-468.
Deyo R.A., Weinstein J.N. Low back pain. N Engl J Med. 2001;344:363-370.
Dropcho E.J. Neurotoxicity of radiation therapy. Neurol Clin. 2010;28:217-234.
Du R., Auguste K.I., Chin C.T., et al. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112:362-371.
Dunteman E.D. Levetiracetam as an adjunctive analgesic in neoplastic plexopathies: case series and commentary. J Pain Palliat Care Pharmacother. 2005;19:35-43.
Dyck P.J., Norell J.E., Dyck P.J. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 1999;53:2113.
Dyck P.J., Norell J.E., Dyck P.J. Non-diabetic lumbosacral radiculoplexus neuropathy. Brain. 2001;124:1197-1207.
Dyck P.J., Windebank A.J. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25:477-491.
Fathers E., Thrush D., Huson S.M., et al. Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin Rehabil. 2002;16:160-165.
Ferrante M.A., Wilbourn A.J. Electrodiagnostic approach to the patient with suspected brachial plexopathy. Neurol Clin. 2002;20:423-450.
Finckh A., Zufferey P., Schurch M.A., et al. Short-term efficacy of intravenous pulse glucocorticoids in acute discogenic sciatica. A randomized controlled trial. Spine. 2006;15:377-381.
Fournier H.D., Menei P., Khalifa R., et al. Ideal intraspinal implantation site for the repair of ventral root avulsion after brachial plexus injury in humans. A preliminary anatomical study. Surg Radiol Anat. 2001;23:191-195.
Fouyas I.P., Statham P.F.X., Sandercock P.A. Cochrane review on the role of surgery in cervical spondylotic radiculomyelopathy: surgery for radiculomyelopathy. Spine. 2002;2:736-747.
Furusawa N., Baba H., Miyoshi N., et al. Herniation of cervical intervertebral disc: immunohistochemical examination and measurement of nitric oxide production. Spine. 2001;26:1110-1116.
Gaskin C.M., Helms C.A. Parsonage-Turner syndrome: MR imaging findings and clinical information of 27 patients. Radiology. 2006;240:501-507.
Gilad R., Lampl Y., Blumstein G., et al. Neurosyphilis: the reemergence of an historical disease. Isr Med Assoc J. 2007;9:117-118.
Gilden D.H., Kleinschmidt-DeMasters B.K., LaGuardia J.J., et al. Medical progress. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635-645.
Giuffre J.L., Kakar S., Bishop A.T., et al. Current concepts of the treatment of adult brachial plexus injuries. J Hand Surg AM. 2010;35A:678-688.
Gleissner B., Chamberlain M.C. Neoplastic meningitis. Lancet Neurol. 2006;5:443-452.
Gnann J.W., Whitley R.J. Herpes zoster. N Engl J Med. 2002;347:340-346.
Gorson K.C., Ropper A.H., Adelman L.S., et al. Influence of diabetes mellitus on chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2000;23:37-43.
Grossman S.A., Krabak M.J. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;2:103-119.
Hannibal M.C., Ruzzo E.K., Miller L.R., et al. SEPT9 gene sequencing analysis reveals recurrent mutations in hereditary neuralgic amyotrophy. Neurology. 2009;72:1755-1759.
Hayashi N., Masumoto T., Abe O., et al. Accuracy of abnormal paraspinal muscle findings on contrast-enhanced MR images as indirect signs of unilateral cervical root-avulsion injury. Radiology. 2002;223:397-402.
Hayashi N., Yamamoto S., Okubo T., et al. Avulsion injury of cervical nerve roots: enhanced intradural nerve roots at MR imaging. Radiology. 1998;206:817-822.
Hildebrand C., Karlsson M., Risling M. Ganglionic axons in motor roots and pia mater. Prog Neurobiol. 1997;51:89-128.
Hsia A.W., Katz J.S., Hancock S.L., et al. Post-irradiation polyradiculopathy mimics leptomeningeal tumor on MRI. Neurology. 2003;60:1694-1696.
Ishii K., Tamaoka A., Shoji S. MRI of idiopathic lumbosacral plexopathy. Neurology. 2004;27:63.
Jaeckle K.A. Neoplastic meningitis from systemic malignancies: diagnosis, prognosis and treatment. Semin Oncol. 2006;33:312-323.
Jaeckle K.A. Neurological manifestations of neoplastic- and radiation-induced plexopathies. Semin Neurol. 2010;30:254-262.
Johansson S., Svensson H., Denkeamp J. Timescale of evolution of late radiation injury after postoperative radiotherapy of breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;48:745-750.
Katirji B., Wilbourn A.J., Scarberry S.L., et al. Intrapartum maternal lumbosacral plexopathy. Muscle Nerve. 2002;26:340-347.
Katz J.S., Saperstein D.S., Wolfe G., et al. Cervicobrachial involvement in diabetic radiculoplexopathy. Muscle Nerve. 2001;24:794-798.
Kelkar P., Masood M., Parry G. Distinctive pathologic findings in proximal diabetic neuropathy (diabetic amyotrophy). Neurology. 2000;55:83-88.
Kim D.H., Cho Y-J., Tiel R.L., et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
Kim L., Glantz M.L. Neoplastic meningitis. Curr Treat Options Oncol. 2001;2:517-527.
Klein C.J., Dyck P.J., Friedenberg S.M., et al. Inflammation and neuropathic attacks in hereditary brachial plexus neuropathy. J Neurol Neurosurg Psychiatry. 2002;73:45-50.
Kohyama K., Saura R., Doita M., et al. Intervertebral disc cell apoptosis by nitric oxide: biological understanding of intervertebral disc degeneration. Kobe J Med Sci. 2000;46:283-295.
Koller H., Kieseier B.C., Jander S., et al. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343-1356.
Komori H., Shinomiya K., Nakai O., et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 1996;21:225-229.
Kotani N., Kushikata T., Hashimoto H., et al. Intrathecal methyl-prednisolone for intractable post herpetic neuralgia. N Engl J Med. 2000;343:1514-1519.
Krendel D.A., Costigan D.A., Hopkins L.C. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol. 1995;52:1053-1061.
Kuhlenbaumer G., Hannibal M.C., Nelis E., et al. Mutations in SEPT 9 cause hereditary neuralgic amyotrophy. Nat Genet. 2005;37:1044-1046.
Kuntzer T., Antoine J.C., Steck A. Clinical features and pathophysiological basis of sensory neuronopathies (ganglionopathies). Muscle Nerve. 2004;30:255-268.
Kutsy R.L., Robinson L.R., Routt M.L. Lumbosacral plexopathy in pelvic trauma. Muscle Nerve. 2000;23:1757-1760.
Ladha S.S., Spinner R.J., Suarez G.A., et al. Neoplastic lumbosacral radiculoplexopathy in prostate cancer by direct perineurial spread: an unusual entity. Muscle Nerve. 2006;34:659-665.
Lahrmann H., Grisold W., Authier F.J., et al. Neuralgic amyotrophy with phrenic nerve involvement. Muscle Nerve. 1999;22:437-442.
Levin K.H. L5 radiculopathy with reduced superficial peroneal sensory responses: intraspinal and extraspinal causes. Muscle Nerve. 1998;21:3-7.
Levin K.H. Neurologic manifestations of compressive radiculopathy of the first thoracic root. Neurology. 1999;53:1149.
Levin K.H. Electrodiagnostic approach to the patient with suspected radiculopathy. Neurol Clin. 2002;20:397-421.
Levin K.H., Maggiano H.J., Wilbourn A.J. Cervical radiculopathies: comparison of surgical and EMG localization of single root lesions. Neurology. 1996;46:1022-1025.
Lin C.C., Chiu M.J. Teaching neuroimage: cervical cord atrophy with dorsal root ganglionpathy in Sjogren syndrome. Neurology. 2008;70:e27.
Longstreth G.F. Diabetic thoracic polyradiculopathy: ten patients with abdominal pain. Am J Gastroenterol. 1997;92:502-505.
Mammoser A.G., Groves M.D. Biology and therapy of neoplastic meningitis. Curr Oncol Rep. 2010;12:41-49.
Merchut M.P., Gruener G. Segmental zoster paresis of limbs. Electromyogr Clin Neurophysiol. 1996;36:369-375.
Midha R. Epidemiology of brachial plexus injuries in a multitrauma population. Neurosurgery. 1997;40:1182-1188.
Mitka M. FDA approves shingles vaccine. Herpes zoster vaccine targets older adults. JAMA. 2006;296:157-158.
Mochida K., Komori H., Okawa A., et al. Regression of cervical disc herniation observed on magnetic resonance images. Spine. 1998;23:990-995.
Nardin R.A., Patel M.R., Gudas T.F., et al. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151-155.
Parmer S.S., Carpenter J.P., Fairman R.M., et al. Femoral neuropathy following retroperitoneal hemorrhage: case series and review of the literature. Ann Vasc Surg. 2006;20:536-540.
Posner J.B., Dalmau J.O. Paraneoplastic syndromes affecting the central nervous system. Annu Rev Med. 1997;48:157-166.
Quan D., Hammack B., Kittelson J., et al. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2005;63:940-942.
Rankine J.J. Adult traumatic brachial plexopathy. Clin Radiol. 2004;59:767-774.
Robinson-Papp J., Simpson D. Neuromuscular diseases associated with HIV-1 infection. Muscle Nerve. 2009;40:1043-1053.
Rubin D., Schomberg P.J., Shepherd R.F., et al. Arteritis and brachial plexus neuropathy as delayed complications of radiation therapy. Mayo Clin Proc. 2001;76:849-852.
Said G., Elgrably F., Lacroix C., et al. Painful proximal diabetic neuropathy: Inflammatory nerve lesions and spontaneous favorable outcome. Ann Neurol. 1997;41:762-770.
Sandy M.C. Herpes zoster: medical and nursing management. Clin J Oncol Nursing. 2005;9:443-446.
Samii M., Bear-Henney S., Ludemann W., et al. Treatment of refractory pain after brachial plexus avulsion with dorsal root entry zone lesions. Neurosurgery. 2001;48:1269-1275.
Samil A., Carvalho G.A., Samil M. Brachial plexus injury: factors affecting functional outcome in spinal accessory nerve transfer for the restoration of elbow flexion. J Neurosurg. 2003;98:307-312.
Seror P. Isolated sensory manifestations in neuralgic amyotrophy: report of 8 cases. Muscle Nerve. 2004;29:134-138.
Serradilla M., Guerrero P.A.L., Ivarez M., et al. Lumbar plexopathy secondary to pelvic hydatid cyst. Rev Neurol. 2002;34:944-949.
Sharma K.R., Cross J., Farronary O., et al. Diabetic demyelinating polyneuropathy responsive to intravenous immunoglobulin therapy. Arch Neurol. 2002;59:751-757.
Sharma K.R., Cross J., Farronary O., et al. Demyelinating neuropathy in diabetes mellitus. Arch Neurol. 2002;59:758-765.
Shields R.E., Aaron J.O., Postel G., et al. A fatal illness presenting as an S1 radiculopathy. Vascular causes of lumbar radicular pain. J Ky Med Assoc. 1997;95:268-270.
Simberkoff M.S., Arbeit R.D., Johnson G.R., et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:609-611.
Steere A.C. Lyme disease. N Engl J Med. 2001;345:115-125.
Suarez G.A., Giannini C., Bosch E.P., et al. Immune brachial plexus neuropathy: suggestive evidence for an inflammatory-immune pathogenesis. Neurology. 1996;46:559-561.
Taylor B.V., Kimmel D.W., Krecke K.N., et al. Magnetic resonance imaging in cancer-related lumbosacral plexopathy. Mayo Clin Proc. 1997;72:823-829.
Tsao B.E., Ostrovskiy D.A., Wilbourn A.J., et al. Phrenic neuropathy due to neuralgic amyotrophy. Neurology. 2006;66:1582-1584.
Tung T.H., Novak C.B., Mackinnon S.E. Nerve transfers to the biceps and brachialis branches to improve elbow flexion strength after brachial plexus injuries. J Neurosurg. 2003;98:313-318.
van Alfen N. The neuralgic amyotrophy consultation. J Neurol. 2007;254:695-704.
van Alfen N., Huisman W.J., Overeem S., et al. Sensory nerve conduction studies in neuralgic amyotrophy. Am J Phys Med Rehabil. 2009;88:941-946.
van Alfen N., van Engelen B.G.M. Lumbosacral plexus neuropathy: a case report and review of the literature. Clin Neurol Neurosurg. 1997;99:138-141.
van Alfen N., van Engelen B.G.M. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129:438-450.
van Alfen, N., van Engelen, B.G., Hughes, R.A., 2009. Treatment for idiopathic and hereditary neuralgic amyotrophy (brachial neuritis). Cochrane Database Syst Rev; CD006976.
Viali S., Hutchinson D.O., Hawkins T.E., et al. Presentation of intravascular lymphomatosis as lumbosacral polyradiculopathy. Muscle Nerve. 2000;23:1295-1300.
Vroomen P.C.A.J., de Krom M., Wilmink J.T., et al. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340:418-423.
Watson B.V., Rose-Innes A., Engstrom J.W., et al. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24:1699-1702.
Watson C.P.N. A new treatment for post herpetic neuralgia. N Engl J Med. 2000;343:1563-1565.
Wilbourn A.J., Aminoff M.J. The electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612-1631.
Wittenberg K.H., Adkins M.C. MR imaging of nontraumatic brachial plexopathies: frequency and spectrum of findings. Radiographics. 2000;20:1023-1032.
Wouter van Es H., Engelen A.M., Witkamp T.D., et al. Radiation-induced brachial plexopathy: MR imaging. Skeletal Radiol. 1997;26:284-288.
Yaszay B., Jablecki C.K., Safran M.R. Zoster paresis of the shoulder. Clin Orthop Relat Res. 2000;377:112-118.
Younger D.S., Rosoklija G., Hays A.P. Diabetic peripheral neuropathy. Semin Neurol. 1998;18:95-104.
Zhou L., Yousem D.M., Chaudhry V. Role of magnetic resonance neurography in brachial plexus lesions. Muscle Nerve. 2004;30:305-309.