32 Diseases of the Stomach
• Dyspepsia, gastritis, peptic ulcer disease, and gastric carcinoma represent a progressive spectrum of illness.
• Helicobacter pylori is the causative agent of 90% of duodenal ulcers and 60% of gastric ulcers; it is a precursor to gastric carcinoma and is the only bacterium listed as a class I carcinogen.
• In the absence of H. pylori, use of nonsteroidal antiinflammatory drugs is the cause of peptic ulcer disease in up to 60% of patients.
• The findings associated with acute dyspepsia and acute coronary syndrome are often indistinguishable in the emergency department. The primary diagnostic goal of the emergency physician is to exclude acute coronary syndrome through risk stratification and appropriate testing.
• In a hemodynamically stable patient with peptic ulcer disease without perforation, first-line treatment consists of a 6-week trial of a proton pump inhibitor.
• Serologic testing for H. pylori is more cost-effective than empiric treatment without testing.
• All emergency department patients with dyspepsia require referral for outpatient evaluation for serologic testing or endoscopy (or both) to exclude malignant disease.
Epidemiology
Dyspepsia refers to chronic or recurrent pain of gastroduodenal origin centered in the upper part of the abdomen. Dyspepsia affects 25% to 40% of people in industrialized nations, who regularly experience pain accompanied by any or all of the following associated symptoms: bloating, fullness, early satiety, nausea, anorexia, heartburn, regurgitation, and belching.1 Patients experiencing dyspepsia with endoscopic evidence of gastric mucosal inflammation are said to have gastritis, and those with ulcerations of the stomach or duodenal lining have peptic ulcer disease (PUD). Dyspepsia, gastritis, PUD, and the long-term complication gastric carcinoma represent a progressive spectrum of illness.
Dyspepsia accounts for 2% to 5% of annual visits to primary care physicians in the United States, and more than 1 billion health care dollars is spent on prescription medications for this disorder each year.1 PUD chronically affects large portions of the U.S. population and leads to impressive health expenditures because many affected patients leave the workforce. More than 7000 American deaths are attributed to PUD each year, largely as a result of perforation and gastrointestinal (GI) bleeding.
In up to 60% of patients in whom dyspepsia is diagnosed, the results of endoscopic evaluation are nondiagnostic.1 The remaining 40% generally have one of three causative diagnoses: PUD, gastroesophageal reflux disease, or gastric cancer.2 The broad differential diagnosis of recognized causes of PUD is summarized in Table 32.1.
| Cardiovascular |
From Ferri FF. Ferri’s clinical advisor 2007: instant diagnosis and treatment. St. Louis: Mosby; 2006.
Duodenal ulcers occur three to four times more frequently than gastric ulcers worldwide, with men being affected more commonly than women for both ulcer types. The male-to-female ratio for duodenal ulcer (4 : 1) is higher than that for gastric ulcer (2 : 1). Such distributions are observed mainly in the developing countries of Africa and Asia. In westernized societies, the distribution of both ulcer types between the sexes has become more equal over the past few decades, although the mechanisms underlying these epidemiologic changes are unknown.3
PUD is the most common cause of upper GI bleeding in the United States and accounts for 27% to 40% of all cases.4 Patients with PUD who are at highest risk for upper GI bleeding include alcoholics, chronic users of nonsteroidal antiinflammatory drugs (NSAIDs), and patients with renal dysfunction.
Pathophysiology
The stomach is anatomically subdivided into three parts: the fundus, the corpus (body), and the antrum (Fig. 32.1).5 This subdivision reflects differences in function. The stomach functions as a reservoir for ingested meals, as well as the site of food agitation with gastric secretions—the first step in digestion (see Fig. 32.1). The acidic fluid is secreted by parietal cells in the fundus and body of the stomach under the control of hormones secreted by endocrine cells in the antrum. Acid secretion is controlled by the complex interaction of gastrin from G cells and histamine from enterochromaffin-like cells. Somatostatin further regulates these cells by inhibition (Fig. 32.2).
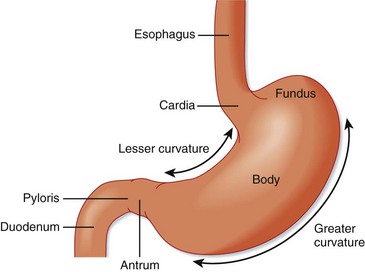
Fig. 32.1 Divisions of the stomach.
(From Zuidema G. Shackelford’s surgery of the alimentary tract. 4th ed. Philadelphia: Saunders; 1995.)
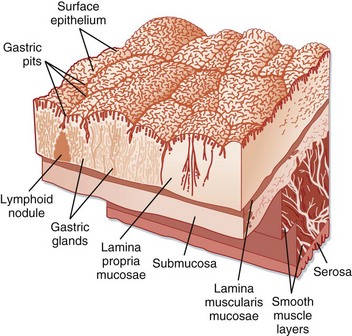
Fig. 32.2 Surface of the gastric mucosa.
(From Zuidema G. Shackelford’s surgery of the alimentary tract. 4th ed. Philadelphia: Saunders; 1995.)
PUD results from a complex interplay of factors that disrupt the delicate balance between the production of protective barrier agents, such as bicarbonate-rich mucus, and the hypersecretion of acid from parietal cells. Recognition of Helicobacter pylori as the causative agent of 90% of duodenal ulcers and 60% of gastric ulcers has led to a paradigm shift in the understanding and management of PUD.6
Infectious Causes
H. pylori, a gram-negative, helix-shaped bacterium, infects at least 50% of the population worldwide and is recognized as an important risk factor for the development of PUD and ultimately gastric adenocarcinoma and lymphoma. Infection is probably acquired in childhood and, in the absence of antimicrobial therapy, persists for life with an estimated lifetime risk for PUD of 15%.7–9 Risk factors for the acquisition of H. pylori include sharing of a bed in childhood, large number of siblings, lower socioeconomic status, and lack of a fixed hot water supply.6,19 Although it is still unclear whether transmission occurs person to person, three different mechanisms for such transmission have been postulated: fecal-oral, through saliva, and by vomitus.6,9
H. pylori causes acute dyspepsia characterized by epigastric pain, nausea, vomiting, and halitosis. Symptoms usually last several weeks and then disappear, although the organism loiters within the stomach. Gastritis persists throughout life in various locations; it shifts from an antrum-predominant pattern to a corpus-predominant pattern or pangastritis.10 The chronic phase of the disease is usually benign, but in susceptible individuals, complications such as gastric or duodenal ulcers may arise.
If sufficiently virulent, H. pylori gastritis leads to destruction of the gastric mucosa, which can ultimately result in the development of intestinal metaplasia or eventually carcinoma. The rate of gastric acid secretion declines, thereby fostering conditions beneficial to colonization of the gastric lumen by fecal organisms. H. pylori eventually disappears because the organism is ill-equipped to compete with a growing number of other organisms and is unable to thrive on metaplastic cells in the absence of hyperacidic conditions. Despite its eventual demise, the initial invasion of the stomach lumen by H. pylori incites conditions that favor the evolution of non-cardia–associated gastric cancer.6
Though most common, H. pylori is not the sole infectious cause of PUD. Helicobacter heilmannii, another spiral nonspirochetal bacterium transmitted via domestic animals and monkeys, has been associated with H. pylori–negative duodenal ulcers. It is much rarer than H. pylori, is less pathogenic, and can easily be differentiated by routine histology. Treatment strategies similar to those for H. pylori are successful in eradicating this organism.10
Additional infectious agents include cytomegalovirus (CMV), herpes simplex virus type 1, (HSV-1) and syphilis. CMV was first associated with peptic ulcers in renal transplant recipients and is the only organism to be significantly associated with peptic ulceration in persons positive for human immunodeficiency virus. The ulcers in these patients are usually gastric and numerous. The diagnosis is confirmed by the finding of intranuclear inclusion bodies or CMV DNA (or both) in the gastric mucosa on biopsy specimens taken from the base of the ulcer. HSV-1–induced ulcers are generally located in the antrum and often occur in immunocompetent individuals.10
Drug-Related Causes
In the absence of H. pylori, use of NSAIDs is the cause of PUD in up to 60% of patients.10 Because gastroduodenal damage does not occur in all patients taking NSAIDs, it is important to identify those at increased risk (Box 32.1). Factors associated with higher rates of NSAID-related GI complications are a previous history of PUD or hemorrhage, age older than 65 years, prolonged use of high-dose NSAIDs, use of more than one NSAID, concomitant use of corticosteroids or anticoagulants, and serious comorbid illness such as cardiovascular, renal, or hepatic impairment, diabetes, or hypertension.11 Many other drugs are associated with PUD and are listed in Box 32.2.
Box 32.1
Nonsteroidal Antiinflammatory Drug–Induced Risk Factors for Peptic Ulcer Disease
History of complications of peptic ulcer disease
Concurrent use of other anticoagulants
High dose of nonsteroidal antiinflammatory drugs or prolonged use
Significant comorbid diseases (coronary artery disease, chronic kidney disease)
From Dincer D, Duman A, Dikici H, et al. NSAID-related upper gastrointestinal bleeding: are risk factors considered during prophylaxis? Int J Clin Pract 2006;60:546-8.
Crack cocaine and methamphetamines have been linked to PUD, as well as a higher rate of gastric perforation thought to be secondary to vasoconstriction and resulting local tissue ischemia. Additionally, tobacco and alcohol can further precipitate gastritis and PUD.11
Zollinger-Ellison Syndrome
Zollinger-Ellison syndrome (ZES) is an uncommon cause of PUD but should be considered in patients with refractory, atypically located, or numerous ulcers, especially in the absence of H. pylori or NSAID use.10,12 The ulcers in patients with ZES are caused by a gastrin-secreting tumor, or gastrinoma.
The incidence of ZES in the United States ranges from 0.1% to 1% in patients with PUD. The most common functional pancreatic tumors in patients with multiple endocrine neoplasia type 1 (MEN-1) are gastrinomas, and approximately 20% of patients with ZES also have MEN-1. The mean age at diagnosis is 50 years, but patients with ZES range in age from 7 to 90 years. Patients with both MEN-1 and ZES become symptomatic at an earlier age, with PUD usually developing in the third decade of life. The male-to-female ratio of patients with ZES ranges from 2 : 1 to 3 : 2.10,12
Patients with ZES commonly have symptoms of PUD such as epigastric pain, nausea, and vomiting. Alternatively, severe diarrhea may be the only symptom. Perforated ulcer remains a common complication; approximately 7% of patients with ZES are initially seen with perforation of the jejunum.12
Evaluation for ZES includes measurement of fasting serum gastrin and basal gastric acid secretion with a secretin stimulation test. Imaging fails to demonstrate the tumor in up to 50% of cases; nonetheless, computed tomography, magnetic resonance imaging, radionuclide octreotide scanning, endoscopic ultrasonography, and the selective arterial secretin injection test are recommended for potential localization.12
Idiopathic Ulcers
Once all known causes are excluded, a group of peptic ulcers remain that are then considered idiopathic. The etiology of idiopathic PUD is poorly understood but is thought to include a genetic predisposition, altered acid secretion, rapid gastric emptying, defective mucosal defense mechanisms, psychologic stress, and smoking.10 Management of idiopathic peptic ulcers is challenging because these ulcers are more resistant to standard therapy and are associated with more frequent complications. Patients who relapse may require long-term maintenance proton pump inhibitor (PPI) therapy.10
Presenting Signs and Symptoms
Dyspepsia refers to a group of symptoms attributed to various pathologic conditions of the upper GI tract, including epigastric pain, bloating, nausea, anorexia, fullness, early satiety, regurgitation, and belching.1,13,14
Several red flags should be considered when taking a history from patients with dyspepsia. These red flags increase the risk for gastric carcinoma as the cause of the patient’s symptoms and include age older than 45 years, unintentional weight loss, persistent vomiting, progressive dysphagia, odynophagia, unexplained anemia or iron deficiency, hematemesis, palpable abdominal mass, family history of GI cancer, and previous gastric surgery or jaundice (see the Red Flags box).14 Several ethnic groups also have a higher disease prevalence, including Afro-Caribbean, Hispanic, Asian, and Native American populations.14,16,17
Abdominal findings in the setting of uncomplicated PUD are generally normal or may be significant only for mild epigastric pain. Peritoneal findings suggest perforation.18
Differential Diagnosis and Medical Decision Making
Other organ-specific conditions in the differential diagnosis include pulmonary disease (lower lobe pneumonia, embolism), pancreatitis, biliary disease, inflammatory bowel disease, irritable bowel syndrome, gastroparesis, mesenteric ischemia, and abdominal wall pathology, including abscess, rectus hematoma, or musculoskeletal pain. Systemic and metabolic diseases that should be considered include sarcoidosis, thyroid disease, parathyroid disease, hyperkalemia, and hypercalcemia. The broad differential diagnosis of dyspepsia is summarized in Table 32.1.
![]() Priority Actions
Priority Actions
Keep the differential diagnosis of dyspepsia broad. Investigate potential life threats, especially atypical acute coronary syndrome.
Search for red flags suggestive of disease progression or gastric carcinoma by findings on the history, physical examination, or laboratory testing.
Hemodynamically unstable patients mandate prompt resuscitation, intensive care unit monitoring, and gastroenterology consultation.
All patients with acute, severe epigastric pain should have an upright chest radiograph to exclude free air secondary to perforation.
Perform a digital rectal examination with Hemoccult testing to assess for occult or fresh blood in the stool.
Perform serial examinations of patients with dyspepsia to exclude the possibility of the development of perforation and to assess the benefit of therapeutic interventions.
Medical Treatment
In hemodynamically stable patients whose symptoms are not severe enough to warrant admission, three options are available for the management of dyspepsia not related to NSAIDs. The first option is a single, short-term trial of empiric antiulcer therapy with a PPI in a setting in which the patient has reliable follow-up.19 Cytoprotective agents such as sucralfate suspension may prevent further injury to the gastric mucosa and provide immediate relief of symptoms. Regardless of response, all such trials should be stopped after about 6 weeks and an evaluation performed if symptoms recur; further investigation is warranted earlier if the symptoms do not respond in 2 weeks. The second option is a definitive endoscopic evaluation, especially in patients with red flags by history, physical examination, or laboratory evaluation. The urgency for definitive diagnosis stems from a risk that gastric ulcers harbor malignancy in this population. The third option is noninvasive testing for H. pylori via serum antibody screening followed by outpatient prescription of antibiotics in H. pylori–positive subjects. For most low-risk patients, referral to a primary care physician for H. pylori testing plus initiation of symptomatic PPI therapy in the ED is the preferred option.13,20
Patients with known H. pylori disease who are either treatment naïve or were inadequately treated should start a regimen of either a bismuth-metronidazole-tetracycline combination plus a PPI or a PPI plus clarithromycin and either metronidazole or amoxicillin.21 When H. pylori is a consideration, endoscopy should be delayed for 4 to 8 weeks after discontinuation of antibiotics, PPI, and bismuth so that infection status can be more reliably assessed at biopsy.
Communicate with the patient’s primary provider if serologic testing was performed or empiric therapy for H. pylori was started so that the outpatient provider can arrange further diagnostic evaluation. Additionally, confirm with patients that they understand the treatment plan, the seriousness of the diagnosis, and which lifestyle modifications would aid in healing. Specifically, patients should be educated about the negative effects of smoking, excessive alcohol use, failure to follow up, and noncompliance with medication. Finally, patients should decrease or avoid the use of NSAIDs.19 If NSAID therapy must continue, the addition of a PPI will allow most duodenal and gastric ulcers to heal in timely fashion.10,19 Patients with healed NSAID-induced ulcers are among those at highest risk for further gastroduodenal injury and serious complications such as perforation and bleeding; strict return precautions should be provided for these patients.11
![]() Patient Teaching Tips
Patient Teaching Tips
Refer to a primary care provider and gastroenterologist to ensure follow-up and to tailor a cohesive treatment plan to fit the individual patient.
Educate patients about the dangers of smoking, excessive use of alcohol and caffeine, and medication noncompliance.
Provide information about various smoking cessation aids and programs.
Stress the importance of abstaining from the use of nonsteroidal antiinflammatory drugs.
Provide well-written, clear, concise anticipatory discharge instructions. Every patient should leave the emergency department understanding the dangers of worsening pain, hematemesis, melena, or hematochezia.
Surgical Treatment
Surgery is rarely necessary given that medical therapies for PUD are so effective. Indications for elective surgical treatment of PUD are (1) protracted and failed medical therapy and (2) suspicion of malignancy.22 For the rare nonhealing benign gastric ulcer, resection is indicated for the management of symptoms and prevention of malignancy.
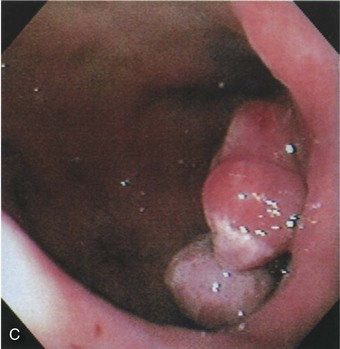
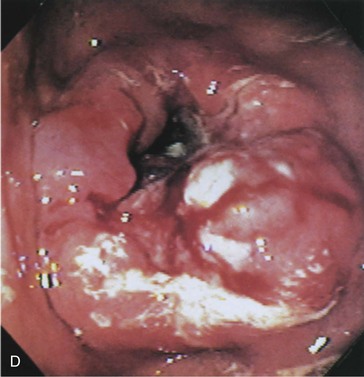
Profuse bleeding was a previous indication for surgery, but endoscopic therapy (whether by electrocautery, heater probe, or injection sclerotherapy) controls bleeding in the majority of cases (Fig. 32.3).22 Surgery is indicated for cases in which bleeding is not controlled with endoscopy or is recurrent.
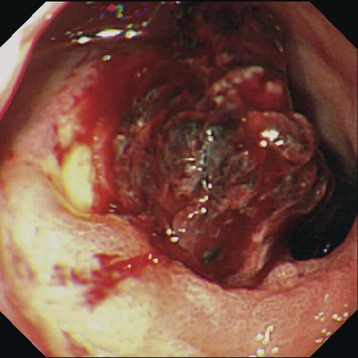
Fig. 32.3 Endoscopic appearance of a duodenal bulb ulcer with a fresh adherent clot.
(From Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran’s gastrointestinal and liver disease: pathophysiology/diagnosis/management. 8th ed. Philadelphia: Saunders; 2006.)
When surgery is being considered, some well-established risk factors increase the likelihood of a fatal outcome, including the presence of severe comorbid conditions, perforation longer than 24 hours in duration, and the presence of hypotension on initial evaluation. Conservative management consisting of nasogastric suction, circulatory support, and antibiotics should be used in elderly patients with these risk factors.22
Sequelae of Peptic Ulcer Disease
Gastric Cancer
Gastric cancer is the second most common cause of cancer-related death worldwide. It is the third most common cancer in southeastern Europe and South America, and globally it is the fourth most common form of cancer.23 In comparison with the rest of the world, western Europe and the United States have a relatively low incidence of gastric cancer; the highest incidence of gastric cancer is reported in Japan, China, and South America.4 Gastric cancer rates remain quite high in some subgroups of African Americans, Hispanics, and Native Americans. In the United States, the mean age at diagnosis is 48 years, and the male-to-female ratio is 1 : 1.23
Although certain well-established genetic factors predispose one to gastric cancer, such as blood type A, the main causative agents are environmental.24 H. pylori infection promotes apoptosis in infected cells, thereby starting a metaplastic path of destruction. Inhibition of E cadherin synthesis by H. pylori is also associated with the development of gastric cancer. The International Agency for Research on Cancer has classified H. pylori as a class I carcinogen, the only bacterial agent with this distinction.23
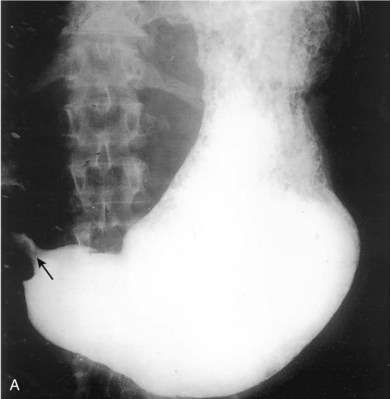
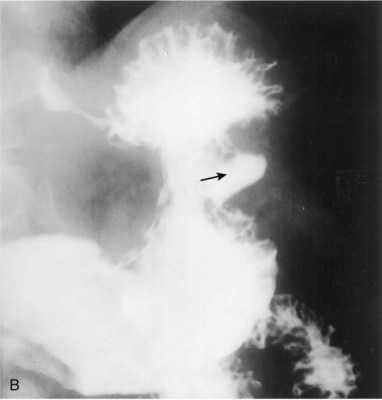
The prognosis of patients with gastric cancer is poor, with 5-year survival rates of less than 25%.23 The improved survival seen in Japanese populations has been attributed to identification of earlier-stage lesions by aggressive screening programs. Such screening programs are less common in the United States. ED patients with suspected dyspepsia and red flags must undergo urgent outpatient follow-up for endoscopic screening for gastric malignancy (Fig. 32.4).
Gastric Outlet Obstruction
Chronic peptic ulcers in either the stomach or duodenum may cause scarring and impair gastric emptying, a condition known as gastric outlet obstruction (Fig. 32.5). In adults, PUD is the major cause of benign gastric outlet obstruction—more than 95% of cases are associated with duodenal or pyloric channel ulceration. Resolution of gastric outlet obstruction after the eradication of H. pylori has been demonstrated in several studies. Symptomatic improvement can be seen a few weeks after the start of antimicrobial therapy for H. pylori, and the benefits seem to persist on long-term follow-up.25
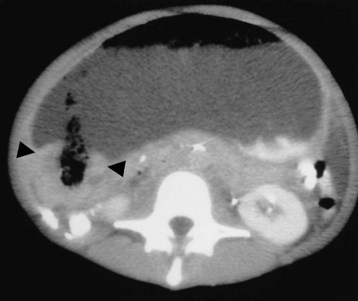
Fig. 32.5 Gastric outlet obstruction—cancer of the antrum.
(From Grainger RG, Allison DJ, Dixon AK, editors. Grainger & Allison’s diagnostic radiology: a textbook of medical imaging. 4th ed. St. Louis: Churchill Livingstone; 2001.)
Treatment of gastric outlet obstruction should start with pharmacologic eradication of H. pylori, even when the stenosis is considered to be fibrotic or when some gastric stasis is present. If such noninvasive treatment is unsuccessful, NSAID use may be the underlying cause.25 Dilation or surgery should be reserved for patients who show no response to medical therapy.
1 Friedman LS. Helicobacter pylori and nonulcer dyspepsia. N Engl J Med. 1998;339:1928–1930.
2 Fisher RS, Parkman HP. Management of nonulcer dyspepsia. N Engl J Med. 1998;339:1376–1381.
3 Sonnenberg A, Everhart JE. The prevalence of self-reported peptic ulcer in the United States. Am J Public Health. 1996;86:200–205.
4 Stabile BE, Stamos MJ. Surgical management of gastrointestinal bleeding. Gastroenterol Clin North Am. 2000;29:189–222.
5 Ford AC, Delaney BC, Forman D, et al. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99:1833–1855.
6 Axon A. Helicobacter pylori: what do we still need to know? J Clin Gastroenterol. 2006;40:15–19.
7 Pinto-Santini D, Salama NR. The biology of Helicobacter pylori infection, a major risk factor for gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:1853–1858.
8 Peterson WL, Fendrick AM, Cave DR, et al. Helicobacter pylori–related disease: guidelines for testing and treatment. Arch Intern Med. 2000;160:1285–1291.
9 Calam J, Baron JH. ABC of the upper gastrointestinal tract: pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ. 2001;323:980–982.
10 Quan C, Talley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol. 2002;97:2950–2961.
11 Singh G, Triadafilopoulos G. Appropriate choice of proton pump inhibitor therapy in the prevention and management of NSAID-related gastrointestinal damage. Int J Clin Pract. 2005;59:1210–1217.
12 Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395–411.
13 Kurata JH, Nogawa AN, Everhart JE. A prospective study of dyspepsia in primary care. Dig Dis Sci. 2002;47:797–803.
14 Talley NJ. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129:1753–1755.
15 Vakil N, Moavvedi P, Fennerty MB, et al. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131:390–401. quiz 659-60
16 Goh KL, Cheah PL, Md N, et al. Ethnicity and H. pylori as risk factors for gastric cancer in Malaysia: a prospective case control study. Am J Gastroenterol. 2007;102:40–45.
17 Williams B, Luckas M, Ellingham JH, et al. Do young patients with dyspepsia need investigation? Lancet. 1988;2:1349–1351.
18 Suleiman S, Johnston DE. The abdominal wall: an overlooked source of pain. Am Fam Physician. 2001;64:431–438.
19 Soll AH. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA. 1996;275:622–629.
20 Logan RP, Walker MM. ABC of the upper gastrointestinal tract: epidemiology and diagnosis of Helicobacter pylori infection. BMJ. 2001;323:920–922.
21 Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev. Gastroenterol Hepatol. 2011;8:79–88.
22 Jamieson GG. Current status of indications for surgery in peptic ulcer disease. World J Surg. 2000;24:256–258.
23 Zivny J, Wang TC, Yantiss R, et al. Role of therapy or monitoring in preventing progression to gastric cancer. J Clin Gastroenterol. 2003;36(5 Suppl):S50–S60. discussion S61-62
24 Chan AO, Kuk JM, Hui WN, et al. Molecular biology of gastric carcinoma: from laboratory to bedside. J Gastroenterol Hepatol. 1999;14:1150–1160.
25 Gisbert JP, Pajares JM. Review article: Helicobacter pylori infection and gastric outlet obstruction—prevalence of the infection and role of antimicrobial treatment. Aliment Pharmacol Ther. 2002;16:1203–1208.