32 Discontinuing Cardiopulmonary Bypass
Cardiopulmonary bypass (CPB) has been used since the 1950s to facilitate surgery on the heart and great vessels, and even with the increased interest in off-pump coronary artery bypass grafting, CPB remains a critical part of most cardiac operations. Managing patients with CPB remains one of the defining characteristics of cardiac surgery and cardiac anesthesiology (see Chapters 28 to 31). Discontinuing CPB is a necessary part of every operation involving extracorporeal circulation. Through this process, the support of the circulation by the bypass pump and oxygenator is transferred back to the patient’s heart and lungs. This chapter reviews important considerations involved with discontinuing CPB and presents an approach to managing this critical component of a cardiac operation, which may be routine and easy or extremely complex and difficult. The key to success in discontinuing CPB is proper preparation. The period during and immediately after weaning from CPB usually is busy for the anesthesiologist, and having to do things that could have been accomplished earlier in the operation is not helpful. The preparations for bringing a patient off CPB may be organized into several parts: general preparations, preparing the lungs, preparing the heart, and final preparations.
General preparations
Temperature
Because at least moderate hypothermia is used during CPB in most cardiac surgery cases, it is important that the patient is sufficiently rewarmed before attempting to wean from CPB (Table 32-1).1 Initiation of rewarming is a good time to consider whether additional drugs need to be given to keep the patient anesthetized. Anesthetic vaporizers need to be off for 20 to 30 minutes before coming off CPB to clear the agent from the patient if so desired.2 However, currently, the patient is frequently removed from CPB with low doses of the inhalation agent still on to continue the anesthetic preconditioning of the heart (see Chapter 9). Monitoring the temperature of a highly perfused tissue such as the nasopharynx is useful to help prevent overheating the brain during rewarming, but these temperatures may increase more rapidly than others, such as bladder, rectum, or axilla temperatures, leading to inadequate rewarming and temperature drop-off after CPB as the heat continues to distribute throughout the body.3 Different institutions have various protocols for rewarming, but the important point is to warm gradually, avoiding hyperthermia of the central nervous system while getting enough heat into the patient to prevent significant drop-off after CPB4 (see Chapters 28 and 29). After CPB, there is a tendency for the patient to lose heat, and measures to keep the patient warm such as fluid warmers, a circuit heater-humidifier, and forced-air warmers should be set up and turned on before weaning from CPB. The temperature of the operating room may need to be increased as well; this is probably an effective measure to keep a patient warm after CPB, but it may make the scrubbed and gowned personnel uncomfortable.
TABLE 32-1 General Preparations for Discontinuing Cardiopulmonary Bypass
| Temperature | Laboratory Results |
|---|---|
| Adequately rewarm before weaning from CPB | Correct metabolic acidosis |
| Avoid overheating the brain | Optimize hematocrit |
| Start measures to keep patient warm after CPB | Normalize K+ |
| Use fluid warmer, forced air warmer | Consider giving Mg2+ or checking Mg2+ level |
| Warm operating room | Check Ca2+ level and correct deficiencies |
CPB, cardiopulmonary bypass.
Laboratory Results
An arterial blood gas should be measured before weaning from CPB and any abnormalities corrected. Severe metabolic acidosis depresses the myocardium and should be treated with NaHCO3 or THAM.5–8 The optimal hematocrit for weaning from CPB is controversial and probably varies from patient to patient.9,10 It makes sense that sicker patients with lower cardiovascular reserve may benefit from a higher hematocrit (optimal is considered to be 30%), but the risks and adverse consequences of transfusion need to be considered as well. Suffice it to say that the hematocrit should be measured and optimized before weaning from CPB (see Chapters 30 and 31). Serum potassium level should be measured before weaning from CPB and may be high because of cardioplegia or low, especially in patients receiving loop diuretics. Hyperkalemia may make establishing an effective cardiac rhythm difficult and can be treated with NaHCO3, CaCl2, or insulin, but the levels usually decrease quickly after cardioplegia has been stopped. Low serum potassium levels probably should be corrected before coming off CPB, especially if arrhythmias are present. Administration of magnesium (Mg2+) to patients on CPB decreases postoperative arrhythmias and may improve cardiac function, and many centers routinely give all CPB patients magnesium sulfate.11 Theoretic disadvantages include aggravation of vasodilation and inhibition of platelet function.12 If Mg2+ is not given routinely, the level should be checked before weaning from CPB and deficiencies corrected. The ionized calcium level should be measured and significant deficiencies corrected before discontinuing CPB. Many centers give all patients a bolus of calcium chloride just before coming off CPB because it transiently increases contractility and systemic vascular resistance (SVR).13 However, it has been argued that this practice is to be avoided because Ca2+ may interfere with catecholamine action and aggravate reperfusion injury.14
Preparing the lungs
As the patient is weaned from CPB and the patient’s heart starts to support the circulation, the lungs again become the site of gas exchange, delivering oxygen and eliminating carbon dioxide. Before weaning from CPB, the lung function must be restored (Table 32-2). The trachea should be suctioned and, if necessary, lavaged with saline to clear secretions. If the abdomen appears to be distended, the stomach should be suctioned so that gastric distention does not impair ventilation after CPB. The lungs are reinflated by hand gently and gradually, with sighs using up to 30 cm H2O pressure, and then mechanically ventilated with 100% oxygen. Care should be taken not to allow the left lung to injure an in situ internal mammary artery graft as the lung is reinflated. The compliance of the lungs can be judged by their feel with hand ventilation, with stiff lungs suggesting more difficulty with oxygenation or ventilation after CPB. If visible, both lungs should be inspected for residual atelectasis, and they should be rising and falling with each breath. Ventilation alarms and monitors should be activated. If prolonged expiration or wheezing is detected, bronchodilators should be given. The surgeon should inspect both pleural spaces for pneumothorax, which should be treated with opening the pleural space. Any fluid present in the pleural spaces should be removed before attempting to wean the patient from CPB. In its most severe form, pulmonary dysfunction after CPB may require positive end-expiratory pressure, an intensive care unit–type ventilator, or nitric oxide (see Chapters 33, 35, and 37). If needed, this equipment should be obtained before attempting to wean the patient from CPB.
TABLE 32-2 Preparing the Lungs for Discontinuing Cardiopulmonary Bypass
| Suction trachea and endotracheal tube. |
| Inflate lungs gently by hand. |
| Ventilate with 100% oxygen. |
| Treat bronchospasm with bronchodilators. |
| Check for pneumothorax and pleural fluid. |
| Consider need for positive end-expiratory pressure, intensive care unit ventilator, and nitric oxide. |
Preparing the heart
Preparing the heart to resume its function of pumping blood involves optimizing the five hemodynamic parameters that can be controlled: rhythm, rate, contractility, afterload, and preload (Table 32-3).
TABLE 32-3 Preparing the Heart for Discontinuing Cardiopulmonary Bypass
| Hemodynamic Parameters | Preparation |
|---|---|
| Rhythm | Normal sinus rhythm is ideal. |
| Defibrillate if necessary when temperature > 30° C. | |
| Consider antiarrhythmic drugs if ventricular fibrillation persists more than a few minutes. | |
| Try synchronized cardioversion for atrial fibrillation or flutter. | |
| Look at the heart to diagnose atrial rhythm. | |
| Try atrial pacing if AV conduction exists. | |
| Try AV pacing for heart block. | |
| Heart rate | Rate should be between 75 and 95 beats/min in most cases. |
| Treat slow rates with electrical pacing. | |
| Treat underlying causes of fast heart rates. | |
| Heart rate may decrease as the heart fills. | |
| Control fast supraventricular rates with drugs and then pace as needed. | |
| Always have pacing immediately available during heart surgery. | |
| Contractility | Inotropic support is more likely needed with depressed cardiac function before CPB, advanced age, long bypass or clamp time, poor preservation, or incomplete revascularization. |
| Look for the vigorous “snap” of a heartbeat with good contractility. | |
| If depressed contractility is likely, begin inotropic drugs before weaning from CPB. | |
| Severely impaired function may require mechanical support. | |
| Afterload | Systemic vascular resistance is a major component of afterload. |
| Keep MAP between 60 and 80 mm Hg at full CPB flow. | |
| Consider a vasoconstrictor if the MAP is low and a vasodilator if the MAP is high. | |
| Preload | End-diastolic volume is the best measure of preload and can be seen with TEE. |
| Filling pressures provide a less direct measure of preload. | |
| Consider baseline filling pressures. | |
| Assess RV volume and function with direct inspection. | |
| Assess LV volume and function with TEE. | |
| Cardiac distention may cause MR and TR. |
AV, atrioventricular; CPB, cardiopulmonary bypass; LV, left ventricular; MAP, mean arterial pressure; MR, mitral regurgitation; RV, right ventricular; TEE, transesophageal echocardiography; TR, tricuspid regurgitation.
Rhythm
There must be an organized, effective, and stable cardiac rhythm before attempting to wean from CPB. This can occur spontaneously after removal of the aortic cross clamp, but the heart may resume electrical activity with ventricular fibrillation. If the blood temperature is greater than 30° C, the heart may be defibrillated with internal paddles applied directly to the heart using 10 to 20 J. Defibrillation at lower temperatures may be unsuccessful because extreme hypothermia can cause ventricular fibrillation.15–17 If ventricular fibrillation persists or recurs repeatedly, antiarrhythmic drugs such as lidocaine or amiodarone may be administered to help achieve a stable rhythm. It is not unusual for the rhythm to remain unstable for several minutes immediately after cross clamp removal, but persistent or recurrent ventricular fibrillation should prompt concern about impaired coronary blood flow. Because it provides an atrial contribution to ventricular filling and a normal, synchronized contraction of the ventricles, normal sinus rhythm is the ideal cardiac rhythm for weaning from CPB.18,19 Atrial flutter or fibrillation, even if present before CPB, often can be converted to normal sinus rhythm with synchronized cardioversion, especially if antiarrhythmic drugs are administered. It often is helpful to look directly at the heart when there is any question about the cardiac rhythm. Atrial contraction, flutter, and fibrillation are easily seen on CPB when the heart is visible. Ventricular arrhythmias should be treated by correcting underlying causes such as potassium or magnesium deficits and, if necessary, with antiarrhythmic drugs such as amiodarone.20 If asystole or complete heart block occurs after cross clamp removal, electrical pacing with temporary epicardial pacing wires may be needed to achieve an effective rhythm before weaning from CPB. If atrioventricular conduction is present, atrial pacing should be attempted because, as with normal sinus rhythm, it provides atrial augmentation to filling and synchronized ventricular contraction. Atrioventricular sequential pacing is used when there is heart block, which frequently is present for 30 to 60 minutes as the myocardium recovers after cross clamp removal. Ventricular pacing remains the only option if no organized atrial rhythm is present, but this sacrifices the atrial “kick” to ventricular filling and the more efficient synchronized ventricular contraction of the normal conduction system21,22 (see Table 32-3).
Contractility
The contractile state of the myocardium should be considered before attempting to wean from CPB. The likelihood of decreased contractility requiring inotropic support after CPB is greater with preexisting ventricular impairment (e.g., low ejection fraction, high left ventricular end-diastolic pressure before surgery or before CPB), advanced age, long CPB time, long aortic cross clamp time, inadequate myocardial preservation, and incomplete revascularization.23 A heart with good contractility often has a vigorous snap with contraction that can be seen while on CPB, in contrast with the weak contractions of a heart with impaired contractility, but it may be difficult to assess global ventricular function while the heart is empty and on CPB. If significant depression of contractility is likely, inotropic support can be started before attempting to wean the patient from CPB. If depressed myocardial contractility becomes evident during weaning, the safest approach is to prevent cardiac distention by resuming CPB and resting the heart for 10 to 20 minutes while inotropic therapy with a catecholamine or phosphodiesterase (PDE) inhibitor drug is started. Extreme depression of contractile function of the myocardium may require mechanical support with an intra-aortic balloon pump (IABP) or ventricular assist device (see Pharmacologic Management of Ventricular Dysfunction section later in this chapter; see also Chapters 27 and 34).
Afterload
Afterload is the tension developed within the ventricular muscle during contraction. An important component of afterload in patients is the SVR (see Chapters 5, 14, and 34).24 While on CPB at full flow, usually about 2.2 L/min/m2, mean arterial pressure (MAP) is directly related to SVR and indicates whether the SVR is appropriate, too high, or too low. Low SVR after CPB can cause inadequate systemic arterial perfusion pressure, and high SVR can significantly impair cardiac performance, especially in patients with poor ventricular function. SVR usually is within a reasonable range when the arterial pressure is between 60 and 80 mm Hg at full pump flow. If below that range, infusion of a vasopressor may be needed to increase SVR before attempting to wean from CPB. If the MAP is high while on CPB, vasodilator therapy may be needed.
Preload
Preload is the amount of stretch on the myocardial muscle fibers just before contraction. In the intact heart, the best measure of preload is end-diastolic volume. Less direct clinical measures of preload include left atrial pressure (LAP), pulmonary artery occlusion pressure, and pulmonary artery diastolic pressure, but there may be a poor relation between end-diastolic pressure and volume during cardiac surgery25,26 (see Chapters 5, 14, and 34). Transesophageal echocardiography (TEE) is a useful tool for weaning from CPB because it provides direct visualization of the end-diastolic volume and contractility of the left ventricle27–29 (see Chapters 11 to 13). The process of weaning a patient from CPB involves increasing the preload (i.e., filling the heart from its empty state on CPB) until an appropriate end-diastolic volume is achieved. When preparing to discontinue CPB, some thought should be given to the appropriate range of preload for the particular patient at hand. The filling pressures before CPB may indicate what they need to be after CPB; a heart with high filling pressures before CPB may require high filling pressures after CPB to achieve an adequate preload.
Final preparations
The final preparations before discontinuing CPB include leveling the operating table, rezeroing the pressure transducers, ensuring the proper function of all monitoring devices, confirming that the patient is receiving only intended drug infusions, ensuring the immediate availability of resuscitation drugs and appropriate fluid volume, and verifying that the lungs are being ventilated with 100% oxygen (Table 32-4).
TABLE 32-4 Final Preparations for Discontinuing Cardiopulmonary Bypass
| Anesthesiologist’s Preparations | Surgeon’s Preparations |
|---|---|
| Level operating table. | Remove macroscopic collections of air from the heart. |
| Rezero transducers. | Control major sites of bleeding. |
| Activate monitors. | Ensure CABG is lying nicely without kinks. |
| Check drug infusions. | Turn off or remove cardiac vents. |
| Have resuscitation drugs and fluid volume at hand. | Take clamps off the heart and great vessels. |
| Reestablish TEE/PAC monitoring. | Loosen tourniquets around caval cannulas. |
CABG, coronary artery bypass graft; PAC, pulmonary artery catheter; TEE, transesophageal echocardiography.
The surgeon must confirm that he or she has completed the necessary preparations in the surgical field before discontinuing CPB. Macroscopic collections of air in the heart should be evacuated before starting to wean from CPB. These are detected most easily with TEE, which also can be helpful in monitoring and directing the deairing process.30 Major sites of bleeding should be controlled, cardiac vent suction should be off, all clamps on the heart and great vessels should be removed, coronary artery bypass grafts should be checked for kinks and bleeding, and tourniquets around the caval cannulas should be loosened or removed before starting to wean a patient from CPB.
Routine weaning from cardiopulmonary bypass
The actual process of weaning from CPB begins with partially occluding the venous return cannula with a clamp (Figure 32-1). This may be done in the field by the surgeon or at the pump by the perfusionist. This causes blood to flow into the right ventricle. As the right ventricle fills and begins to pump blood through the lungs, the left heart will begin to fill. When this occurs, the left ventricle will begin to eject and the arterial waveform will become pulsatile. Next, the perfusionist will gradually decrease the pump flow rate. As more of the venous return goes through the heart and less to the pump reservoir, it becomes necessary to gradually decrease the pump flow to avoid emptying the pump reservoir. One approach to weaning from CPB is to bring the filling pressure being monitored (e.g., central venous pressure, pulmonary artery occlusion pressure, LAP) to a specific, predetermined level somewhat lower than may be necessary and then assess the hemodynamics. Volume (preload) of the heart also may be judged by direct observation of its size or with TEE. Further filling is done in small increments (50 to 100 mL) while closely monitoring the preload until the hemodynamics appear satisfactory as judged by the arterial pressure, the appearance of the heart, and the trend of the Svo2. It typically is easy to see the right-heart volume and function directly in the surgical field and the left heart with TEE, and combining the two observations is a useful approach for weaning from CPB. Overfilling and distention of the heart should be avoided because it may stretch the myofibrils beyond the most efficient length and dilate the annuli of the mitral and tricuspid valves, rendering them incompetent, which easily is detected with TEE. If the patient has two venous cannulas, the smaller of the two may be removed when the pump flow is half of the full flow rate to improve movement of blood from the great veins into the right atrium. When the pump flow has been decreased to 1 L/min or less in an adult and the hemodynamics are satisfactory, the venous cannula may be completely clamped and the pump flow turned off. At this point, the patient is “off bypass” (Figure 32-2).
After discontinuing CPB, the anticoagulation by heparin is reversed with protamine. Depending on institutional preference, protamine may be administered before or after removal of the arterial cannula. Giving it before removal allows for continued transfusion from the pump and easier return to CPB if there is a severe protamine reaction (see Chapter 31). Giving protamine after removal of the arterial cannula may decrease the risk for thrombus formation and systemic embolization. After the infusion of protamine is started, pump suction return to the reservoir should be stopped to keep protamine out of the pump circuit in case subsequent return to CPB becomes necessary. Protamine should be given slowly through a peripheral intravenous catheter over 5 to 15 minutes while watching for systemic hypotension and pulmonary hypertension, which may indicate that an untoward (allergic) reaction to protamine is occurring.31–33 Technically flawed coronary artery bypass grafts may thrombose after protamine administration, causing acute ischemia mimicking a protamine reaction.
When transfusion of the pump reservoir blood is completed, a thorough assessment of the patient’s condition should be made before removing the arterial cannula, because after this is done, returning to CPB becomes much more difficult. The cardiac rhythm should be stable. Cardiac function is assessed by evaluating pressures, cardiac output, and TEE. Hemodynamics should be satisfactory and stable. Adequate oxygenation and ventilation should be confirmed by arterial blood gas or pulse oximetry and capnography. Bleeding from the heart should be at a manageable level before removal of the arterial cannula. The perfusionist should not have to transfuse significant amounts of blood through the arterial cannula before removing it, because it may be difficult to keep up with the blood loss through intravenous infusions alone. Bleeding sites behind the heart may have to be repaired on CPB if the patient cannot tolerate lifting the heart to expose the problem area. At the time of arterial decannulation, the systolic pressure should be between 85 and 100 mm Hg to minimize the risk for dissection or tearing of the aorta.34 The head of the bed may be raised, or small boluses of a short-acting vasodilator (e.g., nitroglycerin, nitroprusside) may be given to lower the systemic blood pressure as necessary. Tight control of the arterial blood pressure may be needed for a few minutes until the cannulation site is secure.
Pharmacologic management of ventricular dysfunction
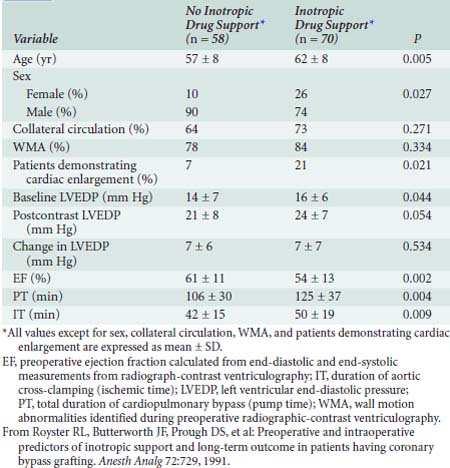
Severe ventricular dysfunction, specifically the low cardiac output syndrome (LCOS), occurring after CPB and cardiac surgery differs from chronic congestive heart failure (CHF) (Box 32-1). Patients emerging from CPB have hemodilution, moderate hypocalcemia, hypomagnesemia, and altered potassium levels. Depending on temperature and depth of anesthesia, these individuals may demonstrate low, normal, or high SVR. Increasing age, female sex, decreased LVEF, and increased duration of CPB are associated with a greater likelihood that inotropic support will be needed after CABG surgery23 (Table 32-5).
Contractile dysfunction during or after cardiac surgery can result from preexisting impairment in contractility or be a new-onset condition. Abnormal contraction, especially in the setting of coronary artery disease (CAD), usually is caused by myocardial injury resulting in ischemia or infarction. The magnitude of contractile dysfunction corresponds to the extent and duration of injury. Brief periods of myocardial oxygen deprivation (< 10 minutes) produce regional contractile dysfunction, which can be rapidly reversed by reperfusion. Extension of the ischemia to 15 to 20 minutes also is associated with restoration of cardiac function with reperfusion; however, this process is very slow and can take hours to days. This condition of postischemic reversible myocardial dysfunction in the presence of normal flow is referred to as myocardial stunning.35–37 Irreversible cell injury will occur with longer periods of ischemia, producing a myocardial infarction characterized by release of intracellular enzymes, disruption of cell membranes, influx of calcium, persistent contractile dysfunction, and eventual cellular swelling and necrosis.38
In addition to the previously described factors, right ventricular (RV) dysfunction and failure are potential sources of morbidity and mortality after cardiac surgery. Numerous factors may predispose patients to the development of perioperative RV dysfunction, including CAD, RV hypertrophy, previous cardiac surgery, and operative considerations such as inadequate revascularization or hypothermic protection. Technical and operative difficulties are associated with various cardiac surgical procedures (e.g., right ventriculotomy), RV trauma, rhythm and conduction abnormalities, injury to the right ventricle during cessation of CPB, or protamine reaction (see Chapter 31).
The following discussion provides an overview of the pharmacologic approach to management of perioperative ventricular dysfunction in the setting of cardiac surgery. Management goals are described in Table 32-6.39 These are extensions of the routine preparations made for discontinuing CPB shown in Table 32-3.
| Variable | Physiologic Management |
|---|---|
| Heart rate and rhythm | Maintain normal sinus rhythm, avoid tachycardia; for tachycardia or bradycardia, consider pacing or chronotropic agents (atropine, isoproterenol, epinephrine), correct acid-base, electrolytes, and review of current medications |
| Preload | Reduce increased preload with diuretics or venodilators (nitroglycerin or sodium nitroprusside); monitor CVP, PCWP, and SV; obtain echocardiogram to rule out ischemia, valvular lesions, tamponade, and intracardiac shunts; consider using inotropes, IABP, or both |
| Afterload | Avoid increased afterload (increased wall tension), use vasodilators (sodium nitroprusside); avoid hypotension; maintain coronary perfusion pressure; consider IABP, inotropes devoid of α1-adrenergic effects (dobutamine or milrinone), or both IABP and inotropes |
| Contractility | Assess hemodynamics, rule out ischemia/infarction, assess rate/rhythm, preload, and afterload, use inotropes; if uncertain, obtain echocardiogram to assess cardiac function; consider combination therapy with inotropes and vasodilators and/or assist devices (IABP/LVAD/RVAD) |
| Oxygen delivery | Increase Fio2 and CO; check ABGs and chest radiograph; mechanical ventilation if indicated; correct acid-base disturbances |
ABG, arterial blood gas; CO, cardiac output; CVP, central venous pressure; Fio2, inspired oxygen concentration; IABP, intraaortic balloon pump; LVAD, left ventricular assist device; PCWP, pulmonary capillary wedge pressure; RVAD, right ventricular assist device; SV, stroke volume.
Sympathomimetic Amines
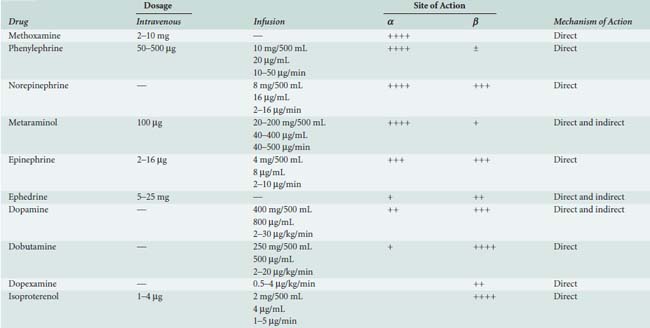
Sympathomimetic drugs (i.e., catecholamines) are pharmacologic agents capable of providing inotropic and vasoactive effects (Box 32-2). Catecholamines exert positive inotropic action by stimulation of the β1 receptor. The predominant hemodynamic effect of a specific catecholamine depends on the degree to which the various α, β, and dopaminergic receptors are stimulated (Tables 32-7 and 32-8).
The physiologic effect of an adrenergic agent is determined by the sum of its actions on α, β, and dopaminergic receptors. The effectiveness of any adrenergic agent will be influenced by the availability and responsiveness of adrenergic receptors. Chronically increased levels of plasma catecholamines (e.g., chronic CHF and long CPB time) cause downregulation of the number and sensitivity of β receptors.40 Maintenance of normal acid-base status, normothermia, and electrolytes also improve the responsiveness to adrenergic-receptor stimulation.
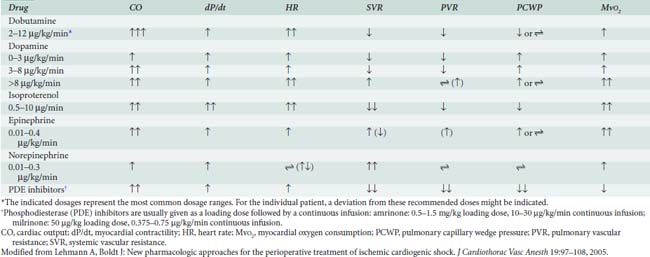
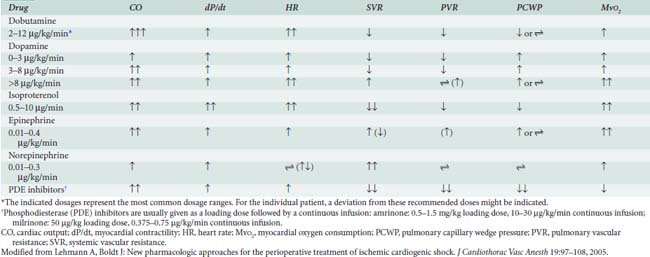
Catecholamines also are effective for treating primary RV contractile dysfunction, with all of the β1-adrenergic agonists augmenting RV contractility. Studies have documented the efficacy of epinephrine, norepinephrine, dobutamine, isoproterenol, dopamine, and PDE-III inhibitors in managing RV contractile dysfunction. When decreased RV contractility is combined with increased afterload, agents that exert vasodilator and positive inotropic effects should be used, including epinephrine, isoproterenol, dobutamine, and the PDE-III inhibitors (see Chapters 10, 24, and 34).41–44
Epinephrine
Epinephrine stimulates α- and β-adrenergic receptors in a dose-dependent fashion. It frequently is the inotrope of choice after CPB (Box 32-3). Dosages of 10, 20, and 40 ng/kg/min increased stroke volume by 2%, 12%, and 22%, respectively, and increased cardiac index by 0.1, 0.7, and 1.2 L/min/m2. The HR also increased, but by no more than 10 beats/min at any dose.45 Epinephrine frequently is used after cardiac surgery to support the function of the “stunned” reperfused heart. During emergence from CPB, Butterworth et al46 showed epinephrine (30 ng/kg/min) increased cardiac index and stroke volume by 14% without increasing HR. In cardiac surgical patients, epinephrine infusion (0.01 to 0.4 μg/kg/min) effectively increases cardiac output, minimally increases HR, and has acceptable side effects (see Table 32-8; see Chapters 10 and 34).
Dobutamine
Dobutamine is a synthetic catecholamine that generally produces dose-dependent increases in cardiac output and reductions in diastolic filling pressures. The effects of epinephrine (30 ng/kg/min) were compared with dobutamine (5 μg/kg/min) in 52 patients recovering from CABG surgery.46 Both drugs significantly and similarly increased stroke volume index, but epinephrine increased the HR by only 2 beats/min, whereas dobutamine increased the HR by 16 beats/min.
In addition to increasing contractility, dobutamine may have favorable metabolic effects on ischemic myocardium. Intravenous and intracoronary injections of dobutamine increase coronary blood flow in animal studies.47 In paced cardiac surgical patients, dopamine increased oxygen demand without increasing oxygen supply, whereas dobutamine increased myocardial oxygen uptake and coronary blood flow. However, because increases in HR are a major determinant of Mvo2, these favorable effects of dobutamine could be lost if dobutamine induces tachycardia. During dobutamine stress echocardiography, segmental wall motion abnormalities suggestive of myocardial ischemia can occur as a result of tachycardia and increases in Mvo248 (see Chapters 2 and 12).
Dopamine
Dopamine is an endogenous catecholamine and an immediate precursor of norepinephrine and epinephrine. Its actions are mediated by stimulation of adrenergic receptors and specific postjunctional dopaminergic receptors (D1 receptors) in the renal, mesenteric, and coronary arterial beds.49 In low doses (0.5 to 3.0 μg/kg/min), dopamine predominantly stimulates the dopaminergic receptors; at doses ranging from 3 to 7 μg/kg/min, it activates most adrenergic receptors in a nonselective fashion; and at higher doses (> 10 μg/kg/min), dopamine behaves as a vasoconstrictor. The dose-dependent effects of dopamine are not very specific and can be influenced by multiple factors such as receptor regulation, concomitant drug use, and interindividual and intraindividual variability.
Dopamine is unique in comparison with other endogenous catecholamines because of its effects on the kidneys. It has been shown to increase renal artery blood flow by 20% to 40% by causing direct vasodilation of the afferent arteries and indirect vasoconstriction of the efferent arteries.50 This results in increases in glomerular filtration rate and in oxygen delivery to the juxtamedullary nephrons.
Despite favorable effects, dopamine has several undesirable features that may limit its use. Its propensity to increase HR and cause tachyarrhythmias can result in demand-related myocardial ischemia. After cardiac surgery, dopamine causes more frequent and less predictable degrees of tachycardia than dobutamine or epinephrine at doses that produce comparable improvement in contractile function.51
Norepinephrine
Norepinephrine is used primarily to treat vasodilated patients after CPB. Meadows et al52 treated 10 patients with severe sepsis and hypotension unresponsive to volume expansion, dopamine, and dobutamine. Norepinephrine infusion (0.03 to 0.89 μg/kg/min) alone improved arterial blood pressure, left ventricular stroke work index, urine output, and, in most cases, cardiac index. Desjars et al53 studied the renal effects of prolonged norepinephrine infusion in hypotensive patients with sepsis. Norepinephrine (0.5 to 1.0 μg/kg/min) plus low-dose dopamine improved urine flow and renal function compared with dopamine alone.53 The α-adrenergic agonists benefit certain patients with circulatory failure refractory to inotropic and fluid therapy. Phenylephrine, norepinephrine, or vasopressin may be used to restore MAP in patients with a low SVR after CPB (i.e., vasoplegia syndrome).54 When RV dysfunction is primarily a result of decreased CPP, vasoconstrictors can be used to optimize RV performance.55
Isoproterenol
Isoproterenol is a potent, nonselective β-adrenergic agonist, devoid of α-adrenergic agonist activity. Isoproterenol dilates skeletal, renal, and mesenteric vascular beds and decreases diastolic blood pressure. The potent chronotropic action of isoproterenol, combined with its propensity to decrease CPP, limit its usefulness in patients with CAD. Applications include treatment of bradycardia (especially after orthotopic heart transplantation), pulmonary hypertension, and heart failure after congenital cardiac surgery.55 Isoproterenol remains the inotrope of choice for stimulation of cardiac pacemaker cells in the management of acute bradyarrhythmias or atrioventricular heart block. Its use for this purpose during cardiac surgery is limited because artificial pacing is usually easily accomplished in this setting. It reduces refractoriness to conduction and increases automaticity in myocardial tissues. The tachycardia seen with isoproterenol is a result of direct effects of the drug on the sinoatrial and atrioventricular nodes and reflex effects caused by peripheral vasodilation. It is routinely used in the setting of cardiac transplantation for increasing automaticity and inotropy, as well as for its vasodilatory effect on the pulmonary arteries (see Chapter 23).
Phosphodiesterase Inhibitors
The PDE-III inhibitors amrinone (inamrinone) and milrinone increase cyclic adenosine monophosphate, calcium flux, and calcium sensitivity of contractile proteins. These drugs have a similar mode of action because they are noncatecholamine and nonadrenergic agents. They do not rely on β-receptor stimulation for their positive inotropic activity. As a result, the effectiveness of the PDE-III inhibitors is not altered by previous β-blockade, nor is it reduced in patients who may experience β-receptor downregulation.40 In addition to their positive inotropic effects, these agents produce systemic and pulmonary vasodilation. As a result of this combination of hemodynamic effects (i.e., positive inotropic support and vasodilation), the term inodilator has been used to describe these drugs (Box 32-4).
Amrinone is more effective than dobutamine for weaning from CPB, with increases in stroke volume and cardiac output, and decreases in SVR and pulmonary vascular resistance (PVR).56,57 Because these agents exert their hemodynamic effects by a nonadrenergic mechanism of action, when used in combination with β-agonists, they have an additive effect on myocardial performance. Gage et al58 reported that when amrinone was used in combination with dobutamine, cardiac output was significantly increased compared with therapy with dobutamine alone. Since this initial report, other investigators have demonstrated the clinical application of combination therapy using PDE-III inhibitors and dopamine, phenylephrine, epinephrine, and nitroglycerin.59
A second-generation PDE-III inhibitor milrinone has a similar hemodynamic profile to amrinone; however, its positive inotropic action is approximately 15 to 30 times that of amrinone. Thrombocytopenia has been a potential clinical concern with the administration of PDE-III inhibitors, particularly amrinone. However, George et al60 were unable to demonstrate any significant reduction in platelet count after 48 hours of milrinone infusion in cardiac surgical patients. Intravenous milrinone has been studied extensively and demonstrates a favorable short-term effect in CHF and ventricular dysfunction after CPB61 (see Chapters 10 and 34).
Milrinone, like other PDE-III inhibitors, appears to increase cardiac output without increasing overall Mvo2. Monrad et al62 administered milrinone to patients with CHF, increasing cardiac index by 45%, but overall Mvo2 did not change. Data also suggest that milrinone may improve myocardial diastolic relaxation (i.e., positive “lusitropic” effect) and augment coronary perfusion. The proposed mechanism for this effect on diastolic performance is that by decreasing LV wall tension, ventricular filling is enhanced, and myocardial blood flow and oxygen delivery are optimized (see Table 32-8).
The ability of short-term administration of milrinone to augment ventricular performance in patients undergoing cardiac surgery was shown in the results from the European Milrinone Multicentre Trial Group.63 In this prospective study, intravenous milrinone was studied in patients after CPB. All patients received a bolus infusion of milrinone at 50 μg/kg over 10 minutes, followed by a maintenance infusion of 0.375, 0.5, or 0.75 μg/kg/min for 12 hours. Significant increases in stroke volume and cardiac index were observed. In addition, significant decreases in pulmonary capillary wedge pressure, central venous pressure, pulmonary artery pressure, MAP, and SVR were seen. Eighteen patients (14%) had arrhythmias; most occurred in the group receiving 0.75 μg/kg/min. Two arrhythmic events were deemed serious; both were bouts of rapid atrial fibrillation occurring with the greater dose.
Bailey et al64 showed that after CPB, a loading dose of milrinone at 50 μg/kg, followed by a continuous infusion of 0.5 μg/kg/min, resulted in a significant increase in cardiac output. Butterworth et al65 also studied the pharmacokinetics and pharmacodynamics of milrinone in adult patients undergoing cardiac surgery; milrinone (25, 50, or 75 μg/kg) was given if the cardiac index was less than 3.0 L/min/m2 after separation from CPB. All three doses of milrinone significantly increased cardiac index. The 50- and 75-μg/kg doses produced significantly greater increases in cardiac index than the 25-μg/kg dose. The 75-μg/kg dose produced increases in cardiac index comparable with the 50-μg/kg dose, but it was associated with more hypotension, despite administration of intravenous fluid, blood, and a phenylephrine infusion. The initial redistribution half-lives were 4.6, 4.3, and 6.9 minutes, and the terminal elimination half-lives were 63, 82, and 99 minutes for the 25-, 50-, and 75-μg/kg doses, respectively. The results of these investigations suggest that for optimizing hemodynamic performance (while minimizing any potential for arrhythmias), the middle dose range (i.e., loading dose of 50 μg/kg) of milrinone may be most efficacious with a continuous infusion of 0.5 μg/kg/min, leading to a plasma concentration of more than 100 ng/mL. In patients with poor LV function, the loading dose should be given during CPB to avoid a decrease in MAP and to minimize the need for other inotropes on discontinuing CPB.66
Vasodilators
The indications for using vasodilators such as nitroglycerin or nitroprusside in cardiac surgery include management of perioperative systemic or pulmonary hypertension, myocardial ischemia, and ventricular dysfunction complicated by excessive pressure or volume overload67 (Box 32-5). In most conditions, nitroglycerin or nitroprusside may be used. Both share common features such as rapid onset, ultra-short half-lives (several minutes), and easy titratability. Nevertheless, there are important pharmacologic differences between nitroglycerin and nitroprusside. In the setting of ischemia, nitroglycerin is preferred because it selectively vasodilates coronary arteries without producing a coronary “steal” (see Chapters 6, 10, and 18). Likewise, in the management of ventricular volume overload or RV pressure overload, nitroglycerin may offer some advantage over nitroprusside. It has a predominant influence on the venous bed such that preload can be reduced without significantly compromising systemic arterial pressure. The benefits of nitroglycerin are improvement in stroke volume, reduction in wall tension and Mvo2, increased perfusion to the subendocardium as a result of a lower left ventricular end-diastolic pressure, and maintenance of CPP. Nitroprusside is a more potent arterial vasodilator and may potentiate myocardial ischemia because of a coronary steal phenomenon or a reduction in CPP. Its greater potency, however, makes nitroprusside the vasodilator of choice for management of perioperative hypertensive disorders and for afterload reduction during or after surgery for regurgitant valvular lesions (see Chapter 19).
Additional uses of vasodilators include management of RV dysfunction. Sodium nitroprusside can augment cardiac output by decreasing RV afterload and PVR.68 Similarly, nitroglycerin has been shown to decrease PVR, transpulmonary pressure, and mean pulmonary artery pressure and to increase cardiac output in patients with increased PVR resulting from mitral valve disease.69 Although nitroglycerin and nitroprusside decrease the impedance to RV ejection and increase the RV ejection fraction by reducing afterload, they are nonspecific pulmonary vasodilators. As a result, newer studies have focused on the ability of agents such as prostaglandins (particularly prostaglandin E1), nitric oxide, and the PDE-III inhibitors to more specifically decrease PVR (see Chapters 10, 24, and 34).
Despite proven benefits of vasodilator therapy in the management of CHF, they can be difficult drugs to use in treatment of perioperative ventricular dysfunction. This is most evident in cases of the LCOS when impaired pump function is complicated by inadequate perfusion pressure. In these situations, multidrug therapy with vasoactive and cardioactive agents is warranted (i.e., nitroglycerin or nitroprusside plus epinephrine or milrinone and norepinephrine). Combination therapy enables greater selectivity of effect. The unwanted side effects of one drug can be avoided while supplementing the desired effects with another agent.70,71 To maximize the desired effects of any particular combination of agents, frequent assessment of cardiac performance with a PAC and TEE is needed. This allows the Starling curve and the pressure-volume loops to be visualized as they are shifted up and to the left with therapy (see Chapters 5, 14, and 34).
Vasoplegic Syndrome and Cardiopulmonary Bypass
The concept of the vasoplegic syndrome, hypotension associated with profound vasodilation unresponsive to conventional catecholamines or vasopressors, was introduced in association with CPB in the late 1990s.72 It has been linked with preoperative use of vasodilators and shown to be a risk factor for increased morbidity and mortality after cardiac surgery.73 Two pharmacologic agents have been reported to be used to treat vasoplegic syndrome after CPB: vasopressin and methylene blue.
Vasopressin
Arginine vasopressin (antidiuretic hormone) is a peptide hormone normally produced in the posterior pituitary that plays a crucial role in water homeostasis by controlling water resorption in the renal collecting ducts.74 Administered as an intravenous infusion, vasopressin was initially used as a potent vasoconstrictor for vasodilatory shock associated with sepsis75 and ventricular assist device implantation.76 Because its vasopressor effect is mediated through a different mechanism (VP1 receptors) from the catecholamines, vasopressin can be infused at a constant rate as a strategy to decrease high doses of catecholamines such as norepinephrine and has been used in this way to treat vasodilation occurring after CPB.77 The vasoconstricting effects of vasopressin may spare the pulmonary vasculature, making it an attractive choice to treat hypotension associated with right-heart dysfunction, but this effect has not been clearly demonstrated in intact humans.78 Reported infusion doses vary widely from 0.01 to 0.6 IU/min.79 Use of vasopressin has been associated with necrotic lesions of the skin and should be used with caution and in the lowest possible effective dose.80
Methylene Blue
Methylene blue, a substance commonly used intravenously during surgery for its ability to dye certain tissues, inhibits guanylate cyclase and hence the production of cyclic guanosine monophosphate, a substance known to increase vascular smooth muscle relaxation.81 It has been used as a rescue treatment for profound vasodilatory shock in a number of settings, including cardiac surgery.82,83 Methylene blue in a dose of 3 mg/kg given while on CPB was shown to increase SVR and MAP without adverse effects in a randomized trial of patients taking angiotensin-converting enzyme inhibitors, as well as decrease pressor requirements and serum lactate levels after CPB.84 In another randomized trial, methylene blue, 2 mg/kg, was given 1 hour before surgery to patients at risk for CPB-associated vasoplegic syndrome. None of the treatment group developed vasoplegic syndrome, while 26% of the control group did.85 Methylene blue causes transient discoloration of the urine and the skin and interferes with pulse oximetry measurements of arterial oxygen saturation. Although a number of transient adverse side effects of methylene blue have been reported in other contexts, no serious side effects have been reported in its use for vasoplegia associated with CPB.86
Additional Pharmacologic Therapy
Following the steps outlined in Tables 32-3 and 32-6, most patients can be weaned off of CPB. However, a small percentage will be difficult to remove safely from CPB because of their chronic end-stage CHF or an acute insult during cardiac surgery producing cardiogenic shock. These patients probably will require mechanical circulatory support (e.g., IABP), as discussed subsequently or in Chapter 27 (e.g., ventricular assist device). However, while instituting these further steps, some clinicians try additional pharmacologic therapy.
Controversial Older Treatments
Some studies suggest that a reduction in plasma thyroid hormone concentration may be the cause of decreased myocardial function after CPB.87,88 Some patients exhibit signs of hypothyroidism, including decreases in HR, cardiac index, and myocardial and systemic oxygen consumption, and increases in arteriovenous oxygen difference and SVR. Multiple investigators have documented declines in the circulating triiodothyronine (T3) concentration during and after CPB, and the most dramatic decreases in T3 are seen at the end of CPB and during the first few hours after CPB.87,88 The reduced thyroid hormone concentrations after CPB may exacerbate myocardial stunning and the LCOS encountered in the post-CPB period. Thyroid hormone in the form of an intravenous T3 infusion (2 μg/hr to a total dose of 0.5 μg/kg) has been used during cardiac surgery. This therapy has resulted in increases in the MAP and HR, as well as reductions in LAP and central venous pressure in patients who initially could not be weaned from CPB. Some of these patients have been successfully weaned from CPB and have required lower doses of dobutamine and other cardiac drug support after the treatment with thyroid hormone.89–91
The administration of glucose-insulin-potassium (GIK) or just glucose and insulin has been found to be useful for metabolic support of the heart after CPB. The trauma of cardiac surgery produces insulin resistance, which restricts the availability of carbohydrates to the heart. The increased level of catecholamines during CPB also may put further strain on the energy metabolism of the heart, whereas insulin may improve this situation.92–94 The administration of high-dose insulin has been compared with dopamine in patients undergoing CABG surgery. The infusion of dopamine (7 μg/kg/min) alone induced metabolic changes unfavorable to the myocardium, whereas dopamine plus insulin increased carbohydrate use with cessation of cardiac uptake of free fatty acids.95 Gradinac et al96 studied GIK in patients with refractory CHF after CPB. All of the patients started with a low cardiac index and were receiving cardiac support with an inotropic drug and an IABP. There was a significant increase in cardiac index in the GIK patients at 12 and 24 hours, the need for inotropic and IABP support was decreased, and the 30-day survival rate was increased.81 A recent study using high-dose insulin therapy during CABG surgery found significant hypolipidemia with a large lowering of the plasma free fatty acid concentration in the treatment group.97 The importance of this finding requires further investigation before high-dose insulin therapy becomes part of the everyday armamentarium in dealing with poor ventricular function.
New Treatments for Heart Failure and Cardiogenic Shock
Levosimendan is a new positive inotropic drug belonging to the class of calcium sensitizers. The drug stabilizes the calcium-induced conformational change in cardiac troponin C and prolongs the effective cross-bridging time. In contrast with other positive inotropic drugs, levosimendan does not increase intracellular calcium. The drug has vasodilating and anti-ischemic properties produced by opening K+-ATP channels98–100 (Table 32-9; see Chapters 10 and 34).
Levosimendan is recommended by the European Society of Cardiology for treatment of acute worsening of heart failure and for acute heart failure after myocardial infarction.101 It also has been found to enhance contractile function of stunned myocardium in patients with acute coronary syndromes.102 It is available clinically in Europe and is undergoing evaluation in the United States. The use of levosimendan has been reported in cardiac surgical patients with high perioperative risk, compromised LV function, difficulties in weaning from CPB, and severe RV failure after mitral valve replacement.103,104 The doses used were 12 μg/kg as a 10-minute loading dose, followed by an infusion of 0.1 μg/kg/min. It has been used before surgery, during emergence from CPB, and in the postoperative period for up to 28 days. The potential for levosimendan to produce increased contractility, decreased resistance, minimal metabolic cost, and no arrhythmias makes it a potentially useful addition to the treatments for patients with the LCOS or RV failure. Randomized trials have demonstrated that levosimendan can facilitate weaning from CPB and decrease the need for additional inotropic support during CABG surgery.105,106
Nesiritide is a recombinant human brain-type natriuretic peptide with vasodilatory and diuretic effects. In patients with heart failure, intravenous nesiritide acts as a vasodilator and reduces preload; SVR is decreased, and cardiac index subsequently increases.107–109 The drug has no positive inotropic effects (see Chapter 10). Compared with nitroglycerin and dobutamine, nesiritide had a greater effect on decreasing preload than nitroglycerin, and it did not cause as many arrhythmias as dobutamine.110,111 Its ultimate role in the treatment of acute heart failure is uncertain, but it may augment the vasodilators or diuretics. There also is some evidence suggesting nesiritide may have a role in preserving renal function during cardiac surgery.112,113
Numerous other drugs are being studied for their uses in patients with acute decompensated heart failure and cardiogenic shock. These drugs include positive inotropic agents such as toborinone (a PDE-III inhibitor), vasodilators such as tezosentan (a specific and potent dual endothelin-receptor antagonist), and vasopressors such as l-NAME (a nitric oxide inhibitor; see Table 32-9).98 These various drugs may prove useful for certain types of cardiovascular problems in the future.
Intra-aortic balloon pump counterpulsation
Indications and Contraindications
Since its introduction, the indications for the IABP have grown (Table 32-10). The most common use of the IABP is for treatment of cardiogenic shock. This may occur after CPB or after cardiac surgery in patients with preoperative shock, with acute postinfarction ventricular septal defects or mitral regurgitation, those who require stabilization before surgery, or patients who decompensate hemodynamically during cardiac catheterization. Patients with myocardial ischemia refractory to coronary vasodilation and afterload reduction are stabilized with an IABP before cardiac catheterization, and some patients with severe CAD will prophylactically have an IABP inserted before undergoing CABG or off-pump CABG surgery.114–118
TABLE 32-10 Intra-aortic Balloon Pump Counterpulsation Indications and Contraindications
| Indications | Contraindications |
|---|---|
Contraindications to IABP use are relatively few (see Table 32-10). The presence of severe aortic regurgitation (AR) or aortic dissection is listed as an absolute contraindication for the IABP, although successful reports of its use in patients with aortic insufficiency or acute trauma to the descending thoracic aorta have appeared. Other relative contraindications are listed; use of the IABP in these instances is at the discretion of the physician. Because the hemodynamic changes caused by an IABP theoretically would tend to worsen dynamic outflow tract obstruction caused by systolic anterior motion (SAM) of the mitral valve, it should be used with caution, if at all, in these patients.
Insertion Techniques
The femoral artery with the greater pulse is sought by careful palpation. The length of the balloon to be inserted is estimated by laying the balloon tip on the patient’s chest at Louis’ angle and appropriately marking the distal point corresponding to the femoral artery. Care must be taken when removing the balloon from its package to follow the manufacturer’s procedures exactly so as not to cause perforation of the balloon before insertion. Available balloons come wrapped and need only be appropriately deflated before removal from the package. The femoral artery is entered with the supplied needle, a J-tipped guidewire is inserted to the level of the aortic arch, and the needle is removed. The arterial puncture site is enlarged with the successive placement of an 8-Fr dilator and then a 10.5- or 12-Fr dilator and sheath combination (Figure 32-3). In the adult-sized (30- to 50-mL) balloons, only the dilator needs to be removed, leaving the sheath and guidewire in the artery. The balloon is threaded over the guidewire into the central aorta and into the previously estimated correct position in the proximal segment of the descending aorta. The sheath is gently pulled back to connect with the leak-proof cuff on the balloon hub, ideally so that the entire sheath is out of the arterial lumen to minimize risk for ischemic complications to the distal extremity. Alternatively, the sheath may be stripped off the balloon shaft much like a peel-away pacemaker lead introducer, thereby entirely removing the sheath from the insertion site. At least one manufacturer offers a “sheathless” balloon for insertion.
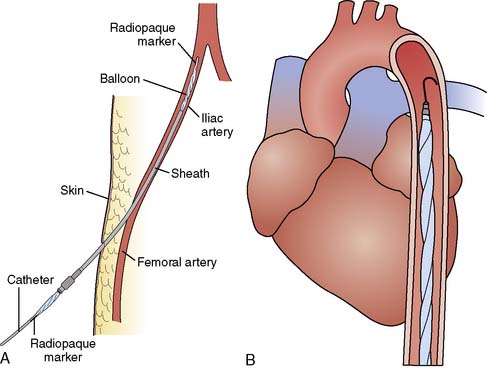
Figure 32-3 Diagram of intra-aortic balloon pump (IABP) insertion.
(A, Courtesy of Datascope Corporation; B, Courtesy of Kontron, Inc.)
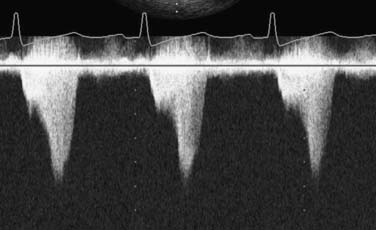
If fluoroscopy is available during the procedure, correct placement is verified before fixing the balloon securely to the skin. Position also may be checked by radiography or echocardiography after insertion. If an indwelling left radial arterial catheter is functioning at the time of insertion, a reasonable estimate of position may be made by watching balloon-mediated alteration of the arterial pulse waveform (Figure 32-4). After appropriate positioning and timing of the balloon, 1:1 counterpulsation may be initiated. The entire external balloon assembly should be covered in sterile dressings.
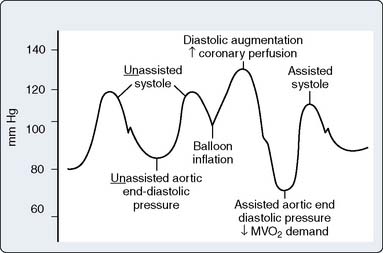
Timing and Weaning
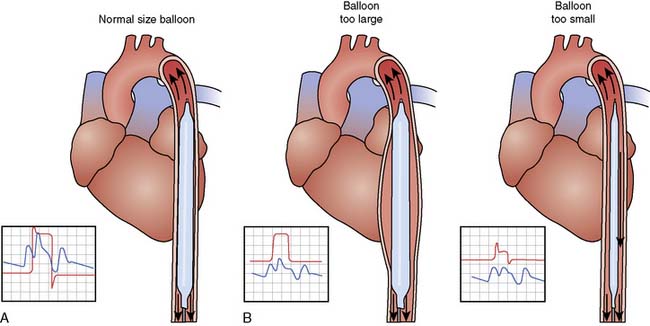
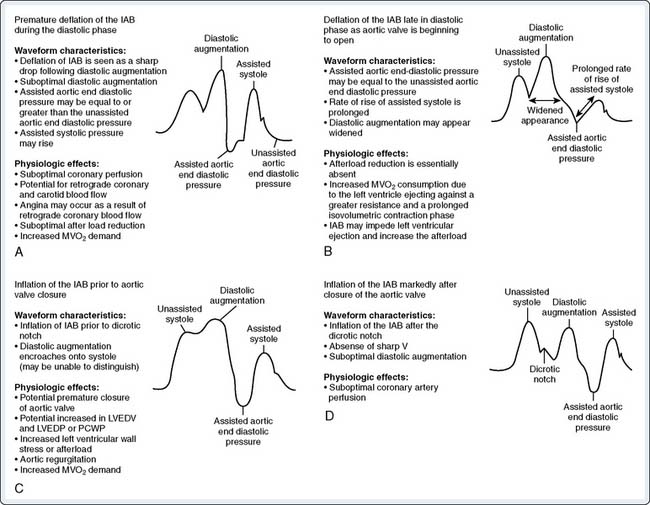
For optimal effect of the IABP, inflation and deflation need to be correctly timed to the cardiac cycle. Although a number of variables, including positioning of the balloon within the aorta, balloon volume (Figure 32-5), and the patient’s cardiac rhythm, can affect the performance of the IABP, basic principles regarding the function of the balloon must be followed. Balloon inflation should be timed to coincide with aortic valve (AV) closure, or aortic insufficiency and LV strain will result. Similarly, late inflation will result in a diminished perfusion pressure to the coronary arteries. Early deflation will cause inappropriate loss of afterload reduction, and late deflation will increase LV work by causing increased afterload, if only transiently. These errors and correct timing diagrams are shown in Figures 32-4 and 32-6.
Complications
Several complications have been associated with IABP use (Table 32-11). The most frequently seen complications are vascular injuries, balloon malfunction, and infection.114–118 Treatment for these respective problems is straightforward. Flaps, dissections, perforations, embolic events, and pseudoaneurysms should be dealt with directly by surgical intervention and repair. Steal syndromes or ischemia, if not severe, may be dealt with expectantly, but if severe extremity compromise is observed, the balloon should be moved to another site. An alternative means of treatment is a femoral-to-femoral crossover graft placed surgically to help alleviate the affected extremity.
TABLE 32-11 Intra-aortic Balloon Pump Counterpulsation Complications
| Vascular | Miscellaneous | Balloon |
|---|---|---|
| Arterial injury (perforation, dissection) | Hemolysis | Perforation (preinsertion) |
| Aortic perforation | Thrombocytopenia | Tear (during insertion) |
| Aortic dissection | Infection | Incorrect positioning |
| Femoral artery thrombosis | Claudication (postremoval) | Gas embolization |
| Peripheral embolization | Hemorrhage | Inadvertent removal |
| Femoral vein cannulation | Paraplegia | |
| Pseudoaneurysm of femoral vessels | Entrapment | |
| Lower extremity ischemia | Spinal cord necrosis | |
| Compartment syndrome | Left internal mammary artery occlusion | |
| Visceral ischemia | Aggravation of dynamic outflow tract obstruction |
Because of multiple improvements in medical and anesthetic management, myocardial preservation (see Chapters 28 and 29), and surgical techniques, most patients can be safely weaned off CPB after successful surgery. However, perioperative heart failure and the LCOS still occur in high-risk patients who require complex pharmacologic support to discontinue CPB. Other patients may require treatment of arrhythmias with drugs or pacemakers. Patients with the most severe ventricular dysfunction will require mechanical support (e.g., IABP, left ventricular assist device, RV assist device) and possibly an artificial heart (e.g., AbioCor; Abiomed, Danvers, MA) or heart transplantation (see Chapters 23 and 27).
Decision making with transesophageal echocardiography while discontinuing cardiopulmonary bypass
Aortic Regurgitation on Cardiopulmonary Bypass
Data Collection
Before CPB, the AV is examined using the midesophageal AV short-axis and long-axis TEE views with 2-D imaging and color-flow Doppler to detect abnormalities of the valve structure and the presence and severity of AR. Transgastric long-axis and deep transgastric long-axis TEE views are used to display with continuous-wave Doppler the velocity profile of any AR present, and the AR pressure half-time is measured to provide a rough index of severity (see Chapters 12 and 13). Pulsed-wave Doppler is used to detect pan-diastolic flow reversal in the distal descending thoracic aorta, a somewhat insensitive but specific sign of severe AR. The same TEE views are used to check for AR while on CPB, which may occur with a normal AV that is distorted by manipulation of the heart or partial clamping of the aorta. Midesophageal and transgastric views of the LV are used to monitor its size before and after aortic cross-clamp removal while on CPB. The presence of arterial pulsatility on CPB may be an indication of AR. Distention of the LV may cause increased pressure to back up across the mitral valve, through the pulmonary veins and lungs to the pulmonary artery, causing an increasing pressure that can be detected with a pulmonary artery catheter. On CPB, excessive left-heart vent return may be an indication of AR when the aorta is not cross-clamped.
Discussion
The anesthesiologist and the surgeon both need to be aware when patients have AR on CPB to avoid harmful distention of the left ventricle. With AR, as soon as the ventricle is unable to keep itself empty with effective contractions, it becomes progressively fuller. Unchecked, this leads to equalization of the pressures between the LV and the aorta, which on CPB is typically at a systemic level. This high pressure can impair myocardial perfusion and stretch the myofibrils, resulting in subsequent poor contractility. As the ventricle distends, the mitral valve becomes incompetent and the increased pressure can back up into the pulmonary vessels, causing injury at the level of the pulmonary capillaries. This dangerous sequence of events may occur even if the AR is trivial before CPB because, given enough time, it will persist until the aortic and ventricular pressures are equal (see AR Video 1, available online). Normal AVs without AR before CPB may be rendered incompetent if distorted by surgical manipulation of the heart or partial clamping of the aorta, leading to ventricular distention in a few minutes. When AR is present on CPB, the LV must eject the regurgitant volume or it will distend. This ejection provides a clue to the presence of AR by causing persistent arterial pulsatility despite adequate venous drainage to the pump (Figure 32-7).
Mitral Systolic Anterior Motion after Cardiopulmonary Bypass
Data Collection
Mitral SAM should be suspected when encountering unexpected hemodynamic instability while weaning from CPB, especially in situations known to be associated with this physiology: hypertrophic obstructive cardiomyopathy, mitral valve repair for myxomatous disease, and AV replacement for aortic stenosis. Standard TEE views of the mitral valve are used to detect the characteristic, abnormal motion of the mitral leaflets during systole (see SAM Video 1, available online). Color-flow Doppler will demonstrate high-velocity, turbulent flow in the LVOT from the level of the abnormally anterior mitral leaflets through the AV and the mitral regurgitation (see SAM Video 2, available online). Continuous-wave Doppler is directed through the LVOT from the transgastric long-axis or deep transgastric long-axis TEE view to reveal the typical dagger-shaped, late-peaking systolic velocity profile caused by SAM (Figure 32-8). The severity of the dynamic LVOT obstruction is calculated from the continuous-wave Doppler peak velocity by the Bernoulli equation (peak LVOT gradient in mm Hg = 4 V2, where V = peak velocity in m/sec) and is considered severe if more than 50 mm Hg. Pulsed-wave Doppler is used to localize the level of the outflow obstruction by moving the sample volume from the midventricular level into the LVOT toward the AV until the high velocity of the obstruction is detected. Mitral SAM causes increase of the left heart filling pressures and decline of the cardiac output that may be detected with a pulmonary artery catheter, but these findings also are consistent with ventricular dysfunction. Because the outflow tract obstruction is dynamic, the arterial blood pressure may be extremely labile, depending mainly on the volume status of the left heart (see SAM Videos 3 through 6 on the website).
Discussion
Although classically associated with hypertrophic obstructive cardiomyopathy, mitral SAM has been reported in a number of other situations involving patients having cardiac surgery. SAM after mitral valve repair for myxomatous degeneration is due to excessively redundant residual leaflet tissue and is a well-recognized complication of this surgery. It also has been reported after AV replacement for aortic stenosis in which the relief of an extremely high afterload unmasks the physiology when trying to come off CPB.119 There seems to be a small number of cardiac surgery patients who are prone to development of SAM when they are hypovolemic and hyperdynamic, even though they appear to have otherwise normal ventricles and mitral valves, especially when coming off CPB.120
Hemodynamic changes that decrease the end-systolic volume of the left ventricle increase mitral SAM and its adverse effects. These include hypovolemia, increased myocardial contractility, and decreased afterload. Measures that enlarge the left ventricle will decrease SAM and include volume administration, decreasing myocardial contractility (β-antagonist drugs, e.g., esmolol), and increasing afterload (α-agonist drugs, e.g., phenylephrine). Atrioventricular pacing also has been used effectively in patients with hypertrophic obstructive cardiomyopathy and theoretically could be used to treat SAM in other clinical settings, such as cardiac surgery, in which artificial pacing easily is accomplished. Most patients who experience development of mitral SAM when attempting to discontinue CPB can be successfully managed if the correct diagnosis is made and appropriate interventions are administered (giving volume is probably the most important and helpful maneuver; see SAM Videos 3 through 6 on the website) and inappropriate treatments avoided (milrinone and IABP counterpulsation are especially harmful). Although mitral SAM after mitral valve repair does not cause severe LVOT obstruction and responds to conservative treatment in most patients,121 if severe and persistent despite optimization of hemodynamics, consideration should be given to revision of the repair or valve replacement.
1 Cook D.J. Changing temperature management for cardiopulmonary bypass. Anesth Analg. 1999;88:1254.
2 Nussmeier N.A., Moskowitz G.J., Weiskopf R.B., et al. In vitro anesthetic washin and washout via bubble oxygenators. Anesth Analg. 1988;67:982.
3 Stone J.G., Young W.L., Smith C.R., et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82:344.
4 Grigore A.M., Grocott H.P., Mathew J.P., et al. Neurologic Outcome Research Group of the Duke Heart Center. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94:4.
5 Gerst P.H., Fleming W.H., Malm J.R. Increased susceptibility of the heart to ventricular fibrillation during metabolic acidosis. Circ Res. 1966;19:63.
6 Cingolani H.E., Mattiazzi A.R., Blesa E.S., et al. Contractility in isolated mammalian heart muscle after acid-base changes. Circ Res. 1970;26:269.
7 Kassirer J.P. Serious acid-base disorders. N Engl J Med. 1974;291:773.
8 Bleich H.L., Schwartz W.B. TRIS buffer (THAM). N Engl J Med. 1966;274:782.
9 Jonas R.A. Optimal hematocrit for adult cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2001;15:672.
10 Spiess B.D. Blood transfusion for cardiopulmonary bypass: The need to answer a basic question. J Cardiothorac Vasc Anesth. 2002;16:535.
11 Boyd W.C., Thomas S.J. Pro: Magnesium should be administered to all coronary artery bypass graft surgery patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000;14:339.
12 Grigore A.M., Mathew J.P. Con: Magnesium should not be administered to all coronary artery bypass graft surgery patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000;14:344.
13 DiNardo J.A. Pro: Calcium is routinely indicated during separation from cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:905.
14 Prielipp R., Butterworth J. Con: Calcium is not routinely indicated during separation from cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:908.
15 Tofler O.B. Electrocardiographic changes during profound hypothermia. Br Heart J. 1962;24:265.
16 Schwab R.H., Lewis D.W., Killough J.H., et al. Electrocardiographic changes occurring in rapidly induced deep hypothermia. Am J Med Sci. 1964;248:290.
17 Trevino A., Razi B., Beller B.M. The characteristic electrocardiogram of accidental hypothermia. Arch Intern Med. 1971;127:470.
18 Braunwald E. The hemodynamic significance of atrial systole. Am J Med. 1964;37:665.
19 Konstadt S.N., Reich D.L., Thys D.M., et al. Importance of atrial systole to ventricular filling predicted by transesophageal echocardiography. Anesthesiology. 1990;72:971.
20 England M.R., Gordon G., Salem M., et al. Magnesium administration and dysrhythmias after cardiac surgery. JAMA. 1992;268:2395.
21 Abraham W.T., Hayes D.L. Cardiac resynchronization therapy for heart failure. Circulation. 2003;108:2596.
22 Dubin A.M., Feinstein J.A., Reddy V.M., et al. Electrical resynchronization: A novel therapy for the failing right ventricle. Circulation. 2003;107:2287.
23 Royster R.L., Butterworth J.F., Prough D.S., et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729.
24 Evans G.L., Smulyan H., Eich R.H. Role of peripheral resistance in the control of cardiac output. Am J Cardiol. 1967;20:216.
25 Hansen R.M., Viquerat C.E., Matthay M.A., et al. Poor correlation between pulmonary arterial wedge pressure and left ventricular end-diastolic volume after coronary artery bypass graft surgery. Anesthesiology. 1986;64:764.
26 Entress J.J., Dhamee S., Olund T., et al. Pulmonary artery occlusion pressure is not accurate immediately after cardiopulmonary bypass. J Cardiothorac Anesth. 1990;4:558.
27 Cheung A.T., Savino J.S., Weiss S.J., et al. Echocardiographic and hemodynamic indexes of left ventricular preload in patients with normal and abnormal ventricular function. Anesthesiology. 1994;81:376.
28 Konstadt S.N., Thys D., Mindich B.P., et al. Validation of quantitative intraoperative transesophageal echocardiography. Anesthesiology. 1986;65:418.
29 Abel M.D., Nishimura R.A., Callahan M.J., et al. Evaluation of Intraoperative transesophageal echocardiography. Anesthesiology. 1987;66:64.
30 Hoka S., Okamoto H., Yamaura K., et al. Removal of retained air during cardiac surgery with transesophageal echocardiography and capnography. J Clin Anesth. 1997;9:457.
31 Park K.W. Protamine and protamine reactions. Int Anesthesiol Clin. 2004;42:135.
32 Horrow J.C. Protamine: A review of its toxicity. Anesth Analg. 1985;64:348.
33 Kimmel S.E., Sekeres M., Berlin J.A., et al. Mortality and adverse events after protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg. 2002;94:1402.
34 Murphy D.A., Craver J.M., Jones E.L., et al. Recognition and management of ascending aortic dissection complicating cardiac surgical operations. J Thorac Cardiovasc Surg. 1983;85:247.
35 Branwald E., Kloner R.A. The stunned myocardium: Prolonged postischemic ventricular dysfunction. Circulation. 1982;66:1146.
36 Bolli R. Mechanism of myocardial “stunning”. Circulation. 1990;82:723.
37 Bolli R. Myocardial ‘stunning’ in man. Circulation. 1992;86:1671.
38 Ferrari R., Ceconi C., Cerullo S., et al. Myocardial damage during ischaemia and reperfusion. Eur Heart J. 1993;14:25.
39 Fontes M.L., Hines R.L. Pharmacologic management of perioperative left and right ventricular dysfunction. In: Kaplan J.A., editor. Cardiac anesthesia. Philadelphia: WB Saunders; 1999:1155-1191.
40 Bristow M.R., Ginsburg R., Minobe W., et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205.
41 Givertz M., Hare J., Loh E., et al. Effect of milrinone on hemodynamics and pulmonary vascular resistance. J Am Coll Cardiol. 1986;28:1775.
42 Mentzer R.M., Alegre C., Nolan S.P. The effects of dopamine and isoproterenol on the pulmonary circulation. J Thorac Cardiovasc Surg. 1976;71:807.
43 Cuffe M.S., Califf R.M., Adams K.F.Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541.
44 Stevenson L.W. Inotropic therapy for heart failure. N Engl J Med. 1998;339:1848.
45 Leenen F.H., Chan Y.K., Smith D.L., et al. Epinephrine and left ventricular function in humans: Effects of beta-1 vs nonselective beta-blockade. Clin Pharmacol Ther. 1988;43:519.
46 Butterworth J.F.4th, Prielipp R.C., Royster R.L., et al. Dobutamine increases heart rate more than epinephrine in patients recovering from aortocoronary bypass surgery. J Cardiothorac Vasc Anesth. 1992;6:535.
47 Miura T., Yoshida S., Iimura O., et al. Dobutamine modifies myocardial infarct size through supply-demand balance. Am J Physiol. 1988;254(Pt 2):H855.
48 Kertai M.D., Poldermans D. The utility of dobutamine stress echocardiography for perioperative and long-term cardiac risk assessment. J Cardiothorac Vasc Anesth. 2005;19:520.
49 Frishman W.H., Hotchkiss H. Selective and nonselective dopamine receptor agonists: An innovative approach to cardiovascular disease treatment. Am Heart J. 1996;132:861.
50 Richer M., Robert S., Lebel M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit Care Med. 1996;24:1150.
51 Steen P.A., Tinker J.H., Pluth J.R., et al. Efficacy of dopamine, dobutamine, and epinephrine during emergence from cardiopulmonary bypass in man. Circulation. 1978;57:378.
52 Meadows D., Edwards J.D., Wilkins R.G., et al. Reversal of intractable septic shock with norepinephrine therapy. Crit Care Med. 1988;16:663.
53 Desjars P., Pinaud M., Potel G., et al. A reappraisal of norepinephrine therapy in human septic shock. Crit Care Med. 1987;15:134.
54 Kristof A.S., Magder S. Low vascular resistance state in patients undergoing cardiopulmonary bypass. Crit Care Med. 1999;27:1121.
55 Molloy D.W., Lee K.Y., Jones D., et al. Effects of noradrenaline and isoproterenol on cardiopulmonary function in a canine model of acute pulmonary hypertension. Chest. 1985;88:432.
56 Dupuis J.Y., Bondy R., Cattran C., et al. Amrinone and dobutamine as primary treatment of low-cardiac-output syndrome following coronary artery surgery: A comparison of their effects on hemodynamics and outcome. J Cardiothorac Vasc Anesth. 1992;6:542.
57 Butterworth J.F., Royster R.L., Prielipp R.C., et al. Amrinone in cardiac surgical patients with left ventricular dysfunction. A prospective, randomized placebo-controlled trial. Chest. 1993;104:1660.
58 Gage J., Rutman H., Lucido E., et al. Additive effects of dobutamine and amrinone on myocardial contractility and ventricular performance in patients with severe heart failure. Circulation. 1986;74:367.
59 Royster R.L., Butterworth J.F.4th, Prielipp R.C., et al. Combined inotropic effects of amrinone and epinephrine after cardiopulmonary bypass in humans. Anesth Analg. 1993;77:662.
60 George M., Lehot J.J., Estanove S. Haemodynamic and biological effects of intravenous milrinone in patients with a low-cardiac-output syndrome following cardiac surgery: Multicentre study. Eur J Anaesthesiol Suppl. 1992;5:31.
61 Levy J.H., Bailey J.M., Deeb J.M. Intravenous milrinone in cardiac surgery. Ann Thorac Surg. 2002;73:325.
62 Monrad E.S., Baim D.S., Smith H.S., et al. Effects of milrinone on coronary hemodynamics and myocardial energetics in patients with congestive heart failure. Circulation. 1985;71:972.
63 Feneck R.O. Intravenous milrinone following cardiac surgery. I. Effects of bolus infusion followed by variable dose maintenance infusion. The European Milrinone Multicentre Trial Group. J Cardiothorac Vasc Anesth. 1992;6:554.
64 Bailey J.M., Levy J.H., Kikura M., et al. Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology. 1994;81:616.
65 Butterworth J.F.4th, Hines R.L., Royster R.L., et al. A pharmacokinetic and pharmacodynamic evaluation of milrinone in adults undergoing cardiac surgery. Anesth Analg. 1995;81:783.
66 Kikura M., Sato S. The efficacy of preemptive milrinone or amrinone therapy in patients undergoing coronary artery bypass grafting. Anesth Analg. 2002;94:22.
67 Leien C.V., Banbach D., Thompson M.J., et al. Central and regional hemodynamic effects of intravenous isosorbide dinitrate, nitroglycerin, and nitroprusside in patients with congestive heart failure. Am J Cardiol. 1981;48:115.
68 Lee K.Y. Effects of hydralazine and nitroprusside on cardiopulmonary function when cardiac output is acutely reduced by pulmonary vascular resistance. Circulation. 1983;69:1299.
69 Ziskind Z., Pohoryles L., Mohr R., et al. The effect of low-dose intravenous nitroglycerin on pulmonary hypertension immediately after replacement of a stenotic mitral valve. Circulation. 1985;72:164.
70 Felker J.M. Inotropic therapy for heart failure: An evidence-based approach. Am Heart J. 2001;142:393.
71 Stevenson L.W., Massie B.M., Francis G.S., et al. Optimizing therapy for complex or refractory heart failure. Am Heart J. 1998;135:5293.
72 Gomes W.J., Carvalho A.C., Palma J.H., et al. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg. 1998;39:619-623.
73 Levin M.A., Lin H.M., Castillo J.G., et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120:1664-1671.
74 Treschan T.A., Peters J. The vasopressin system: Physiology and clinical strategies. Anesthesiology. 2006;105:599-612.
75 Landry D.W., Levin H.R., Gallant E.M., et al. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279-1282.
76 Argenziano M., Chen J.M., Choudhri A.F., et al. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998;116:973-980.
77 Masetti P., Murphy S.F., Kouchoukos N.T. Vasopressin therapy for vasoplegic syndrome following cardiopulmonary bypass. J Card Surg. 2002;17:485-489.
78 Garcia-Villalon A.L., Garcia J.L., Fernandez N., et al. Regional differences in the arterial response to vasopressin: Role of endothelial nitric oxide. Br J Pharmacol. 1996;118:1848-1854.
79 Russell J.A. Vasopressin in vasodilatory and septic shock. Curr Opin Crit Care. 2007;13:383-391.
80 Dunser M.W., Mayr A.J., Tur A., et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine-resistant vasodilatory shock: Incidence and risk factors. Crit Care Med. 2003;31:1394-1398.
81 Leone R.J.Jr, Weiss H.R., Scholz P.M. Positive functional effects of milrinone and methylene blue are not additive in control and hypertrophic canine hearts. J Surg Res. 1998;77:23-28.
82 Evora P.R., Ribeiro P.J., de Andrade J.C. Methylene blue administration in SIRS after cardiac operations. Ann Thorac Surg. 1997;63:1212-1213.
83 Shanmugam G. Vasoplegic syndrome—the role of methylene blue. Eur J Cardiothorac Surg. 2005;28:705-710.
84 Maslow A.D., Stearns G., Butala P., et al. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg. 2006;103:2-8.
85 Ozal E., Kuralay E., Yildirim V., et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79:1615-1619.
86 Shanmugam G. Vasoplegic syndrome—the role of methylene blue. Eur J Cardiothorac Surg. 2005;28:705-710.
87 Holland F.W.2nd, Brown P.S., Weintraub B.D., Clark R.E. Cardiopulmonary bypass and thyroid function: A “euthyroid sick syndrome. Ann Thorac Surg. 1991;52:46.
88 Chu S.H., Huang T.S., Hsu R.B., et al. Thyroid hormone changes after cardiovascular surgery and clinical implications. Ann Thorac Surg. 1991;52:791.
89 Salter D.R., Dyke C.M., Wechsler C.S. Triiodothyronine (T3) and cardiovascular therapeutics: A review. J Card Surg. 1991;7:363.
90 Gomberg-Maitland M., Frishman W. Thyroid hormone and cardiovascular disease. Am Heart J. 1998;135:187.
91 Novitzky D., Cooper D.K., Swanepoel A. Ionotropic effect of triiodothyronine (T3) in low cardiac output following cardioplegic arrest and cardiopulmonary bypass: An initial experience in patients undergoing open heart surgery. Eur J Cardiothorac Surg. 1989;3:140.
92 Smith A., Grattan A., Harper M., et al. Coronary revascularization: A procedure in transition from on-pump to off-pump? The role of glucose-insulin-potassium revisited in a randomized, placebo-controlled study. J Cardiothorac Vasc Anesth. 2002;16:413.
93 Van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy and critically ill patients. N Engl J Med. 2001;245:1359.
94 Wallin M., Barr G., Owall A., et al. The influence of glucose-insulin-potassium on GH/IGF-1/IGFBP-1 axis during elective coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2003;17:470.
95 Svedjeholm R., Hallhagen S., Ekroth R., et al. Dopamine and high-dose glucose-insulin-potassium after a cardiac operation: Effects on myocardial metabolism. Ann Thorac Surg. 1991;51:262.
96 Gradinac S., Coleman G., Taegtmeyer H., et al. Improved cardiac function with glucose-insulin-potassium after aortocoronary artery bypass grafting. Ann Thorac Surg. 1989;48:484.
97 Zuurbier C.J., Hoek F.J., van Dijk J., et al. Perioperative hyperinsulinaemic normoglycaemic clamp causes hypolipidaemia after coronary artery surgery. Br J Anaesth. 2008;100:442-450.
98 Lehmann A., Boldt J. New pharmacologic approaches for the perioperative treatment of ischemic cardiogenic shock. J Cardiothorac Vasc Anesth. 2005;19:97.
99 Haikala H., Pollesello P. Calcium sensitivity enhancers. Drugs. 2000;3:1199.
100 Lehmann A., Boldt J., Kirchner J. The role of calcium sensitizers for the treatment of heart failure. Curr Opin Crit Care. 2003;9:337.
101 Remme W., Swedberg K. Task Force for the Diagnosis and Treatment of Heart Failure, European Society of Cardiology: Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527.
102 Sonntag S., Sundberg S., Lehtonen L., et al. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty and acute myocardial ischemia. J Am Coll Cardiol. 2004;43:2177.
103 Morais R. Levosimendan in severe right ventricular failure following mitral valve replacement. J Cardiothorac Vasc Anesth. 2006;20:82-84.
104 Siirila-Waris K., Suojaranta-Ylinen R., Harjola V.P. Levosimendan in cardiac surgery. J Cardiothorac Vasc Anesth. 2005;19:345.
105 Eriksson H.I., Jalonen J.R., Heikkinen L.O., et al. Levosimendan facilitates weaning from cardiopulmonary bypass in patients undergoing coronary artery bypass grafting with impaired left ventricular function. Ann Thorac Surg. 2009;87:448-454.
106 Tritapepe L., De Santis V., Vitale D., et al. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. 2009;102:198-204.
107 Mills R., Hobbs R. Nesiritide in perspective: Evolving approaches to the management of acute decompensated heart failure. Drugs Today. 2003;39:767.
108 Keating G., Goa K. Nesiritide: A review of its use in acute decompensated heart failure. Drugs. 2003;63:47.
109 Zineh I., Schofield R., Johnson J. The evolving role of nesiritide in advanced decompensated heart failure. Pharmacotherapy. 2003;23:1266.
110 Publications Committee for the VMAC Investigators. Intravenous nesiritide versus nitroglycerin for treatment of decompensated congestive heart failure: A randomized, controlled trial. JAMA. 2002;287:1531.
111 Burger A., Horten D., LeJemel T., et al. The effect of nesiritide and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated heart failure. Am Heart J. 2002;144:1102.
112 Mentzer R.M.Jr, Oz M.C., Sladen R.N., et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: The NAPA Trial. J Am Coll Cardiol. 2007;49:716-726.
113 Chen H.H., Sundt T.M., Cook D.J., et al. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: A double-blind placebo-controlled pilot study. Circulation. 2007;116(11 Suppl):I134-I138.
114 Goldstein D., Oz M. Mechanical support for postcardiotomy cardiogenic shock. Semin Thorac Cardiovasc Surg. 2000;12:220.
115 Stone G., Ohman E., Miller M., et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The Benchmark Registry. J Am Coll Cardiol. 2003;41:1940.
116 Craver J., Murrah C. Elective intra-aortic balloon counterpulsation for high-risk off-pump coronary artery bypass operations. Ann Thorac Surg. 2001;71:1220.
117 Babatasi G., Massetti M., Bruno P., et al. Preoperative balloon counterpulsation and off-pump coronary artery surgery for high-risk patients. Cardiovasc Surg. 2003;11:145.
118 Ferguson J., Cohen M., Freedan R., et al. The current practice of intra-aortic balloon counterpulsation: Results from the Benchmark Registry. J Am Coll Cardiol. 2001;38:1456.
119 Cutrone F., Coyle J.P., Novoa R., et al. Severe dynamic left ventricular outflow tract obstruction following aortic valve replacement diagnosed by intraoperative echocardiography. Anesthesiology. 1990;72:563-566.
120 Krenz H.K., Mindich B.P., Guarino T., et al. Sudden development of intraoperative left ventricular outflow obstruction: Differential and mechanism. An Intraoperative two-dimensional echocardiographic study. J Card Surg. 1990;5:93-101.
121 Brown M.L., Abel M.D., Click R.L., et al. Systolic anterior motion after mitral valve repair: Is surgical intervention necessary? J Thorac Cardiovasc Surg. 2007;133:136-143.