Chapter 180 Diphtheria (Corynebacterium diphtheriae)
Clinical Manifestations
Respiratory Tract Diphtheria
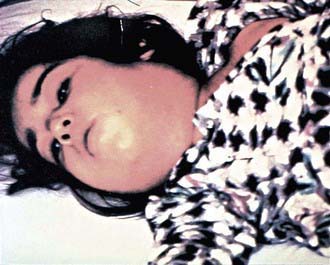
In a classic description of 1,400 cases of diphtheria in California (1954), the primary focus of infection was the tonsils or pharynx (94%), with the nose and larynx the next 2 most common sites. After an average incubation period of 2-4 days, local signs and symptoms of inflammation develop. Infection of the anterior nares is more common among infants and causes serosanguineous, purulent, erosive rhinitis with membrane formation. Shallow ulceration of the external nares and upper lip is characteristic. In tonsillar and pharyngeal diphtheria, sore throat is the universal early symptom: Only half of patients have fever, and fewer have dysphagia, hoarseness, malaise, or headache. Mild pharyngeal injection is followed by unilateral or bilateral tonsillar membrane formation, which can extend to involve the uvula (which may cause toxin-mediated paralysis), soft palate, posterior oropharynx, hypopharynx, or glottic areas (Fig. 180-1). Underlying soft tissue edema and enlarged lymph nodes can cause a bull-neck appearance. The degree of local extension correlates directly with profound prostration, bull-neck appearance, and fatality due to airway compromise or toxin-mediated complications (Fig. 180-2).
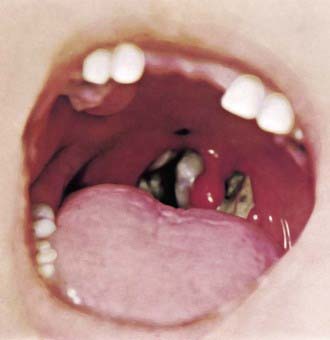
Figure 180-1 Tonsillar diphtheria.
(Courtesy Franklin H. Top, MD, Professor and Head of the Department of Hygiene and Preventive Medicine, State University of Iowa, College of Medicine, Iowa City, IA; and Parke, Davis & Company’s Therapeutic Notes.)
Centers for Disease Control and Prevention. Respiratory diphtheria-like illness caused by toxigenic Corynebacterium ulcerans—Idaho, 2010. MMWR. 2011;60(3):77.
Centers for Disease Control and Prevention. Fatal respiratory diphtheria in a US traveler to Haiti—Pennsylvania, 2003. MMWR Morbid Mortal Wkly Rep. 2004;52:1285-1286.
Pantukosit P, Arpornsuwan M, Sookananta K. A diphtheria outbreak in Buri Ram, Thailand. Southeast Asian J Trop Med Public Health. 2008;39:690-696.
Tiwari TS, Golaz A, Yu DT, et al. Investigations of 2 cases of diphtheria-like illness due to toxigenic Corynebacterium ulcerans. Clin Infect Dis. 2008;46:395-401.
Vitek CR. Diphtheria. Curr Topics Microbiol Immunol. 2006;304:71-94.