17 DIGESTIVE GLANDS
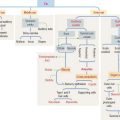
TYPES OF DIGESTIVE GLANDS
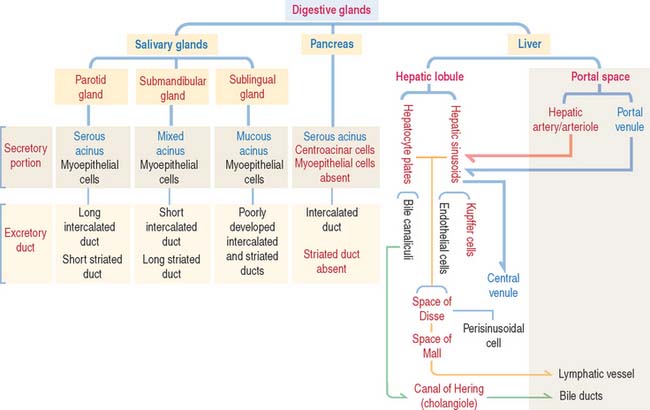
The three major digestive glands are:
The structure and function of the gallbladder are included at the end of the liver section.
Branching duct system of a salivary gland
We initiate the discussion with the general organization of a salivary gland, in particular its branching ducts (see Box 17-A).
Box 17-A Classification of exocrine glands
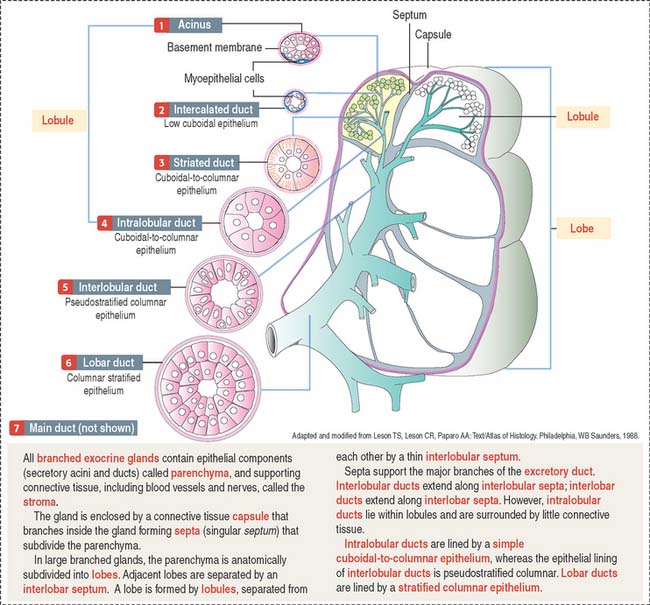
The secretory product of an acinus is drained sequentially by the following (Figures 17-1 and 17-2):
Saliva is the major product of salivary glands
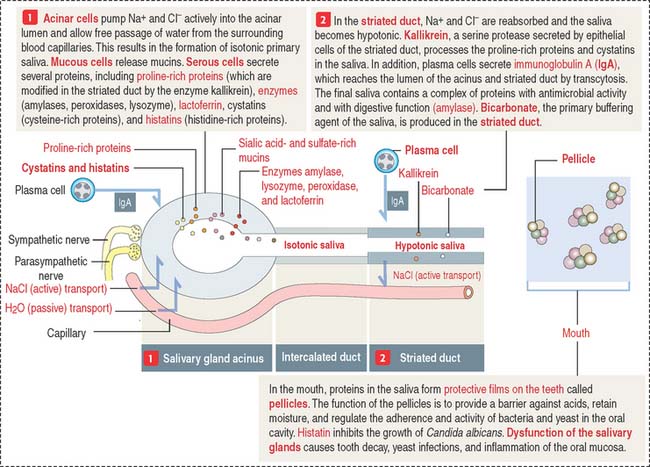
Saliva, amounting to a half-liter daily, contains proteins, glycoproteins (mucus), ions, water, and immunoglobulin A (IgA) (Figure 17-3). The submandibular gland produces about 70% of the saliva. The parotid gland contributes 25% and secretes an amylase-rich saliva. The production of saliva is under the control of the autonomic nervous system. Upon stimulation, the parasympathetic system induces the secretion of a water-rich saliva; the sympathetic system stimulates the release of a protein-rich saliva.
PAROTID GLAND
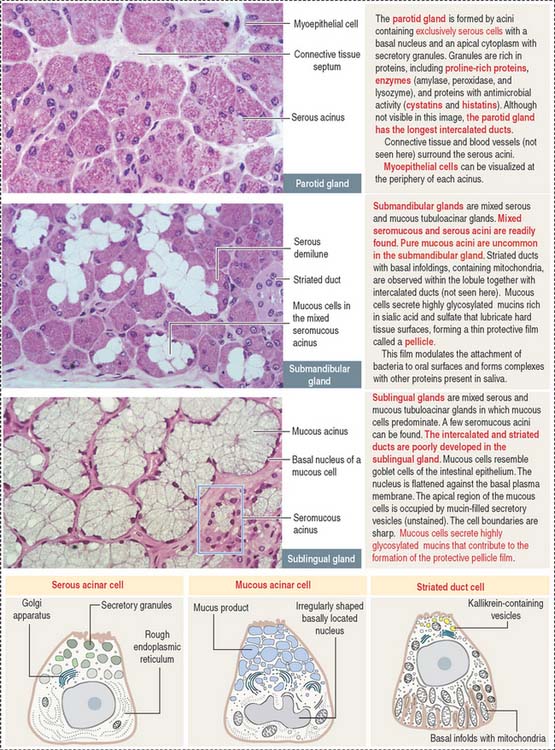
The parotid gland is the largest salivary gland. It is a branched tubuloalveolar gland surrounded by a connective tissue capsule with septa—representing a component of the stroma, the supporting tissue of the gland. Adipose cells are frequently found in the stroma.
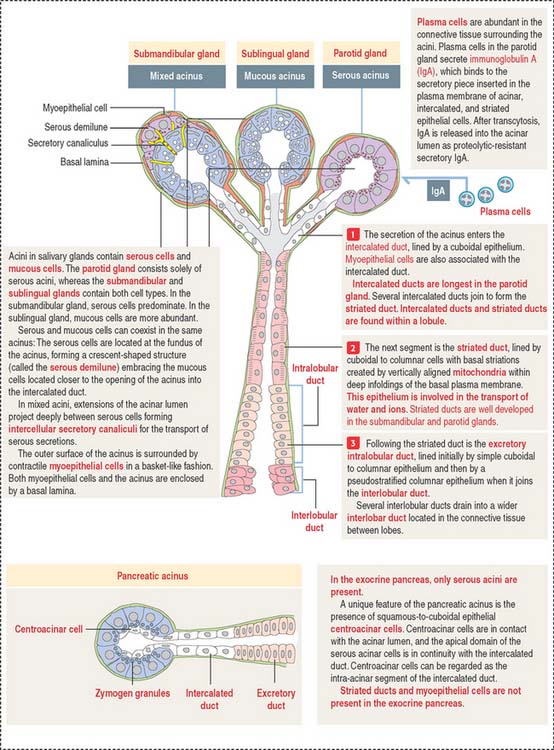
Septa divide the gland into lobes and lobules (see Figure 17-1). Septa also provide support to blood vessels, lymphatics, and nerves gaining access to the acini, the main components of the parenchyma—the functional constituent of the gland. Acini are surrounded by reticular connective tissue, a rich capillary network, plasma cells, and lymphocytes. Acini consist mainly of serous secretory cells and, therefore, are classified as serous acini.
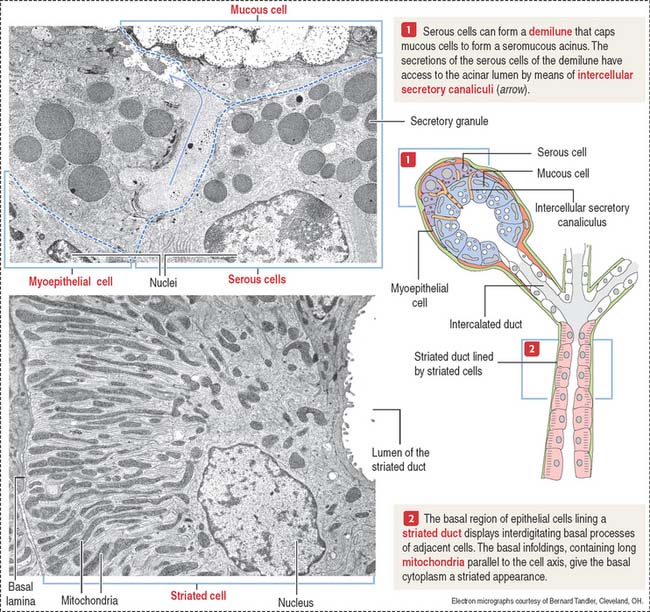
Each serous acinus is lined by pyramidal cells with a basally located nucleus. Similar to all protein-producing cells, a prominent rough endoplasmic reticulum system occupies the cell basal region. Secretory granules are visible in the apical region (Figure 17-4).
The lumen of the acinus collects the secretory products, which are transported by long intercalated ducts to the less abundant striated ducts (Figure 17-5). The secretory product of the serous acini is modified by the secretion of the striated duct and then transported by the oral cavity by a main excretory duct (Stensen’s duct).
SUBMANDIBULAR (SUBMAXILLARY) GLAND
Although both serous and mucous cells are present in the secretory units, the serous cells are the predominant component (see Figure 17-4). Mucous cell–containing acini are capped by serous demilunes. The intercalated ducts are shorter and the striated ducts are longer than those in the parotid gland. Adipocytes are not frequently seen in the submandibular gland.
SUBLINGUAL GLAND
Contrasting with the parotid and submandibular glands, which are surrounded by a dense connective tissue capsule, the sublingual gland does not have a defined capsule. However, connective tissue septa divide the glandular parenchyma into small lobes. The sublingual gland is a branched tubuloalveolar gland with both serous and mucous cells (see Figure 17-4), although most of the secretory units contain mucous cells. The intercalated and striated ducts are poorly developed. Usually each lobe has its own excretory duct that opens beneath the tongue.
EXOCRINE PANCREAS
The pancreas is a combined endocrine and exocrine gland. The endocrine component is the islet of Langerhans and represents about 2% of the pancreas volume. The main function of the endocrine pancreas is the regulation of glucose metabolism by hormones secreted into the bloodstream (see discussion of the islet of Langerhans in Chapter 19, Endocrine System).
The pancreas has structural similarities to the salivary glands: (1) It is surrounded by connective tissue but does not have a capsule proper. (2) Lobules are separated by connective tissue septa containing blood vessels, lymphatics, nerves, and excretory ducts.
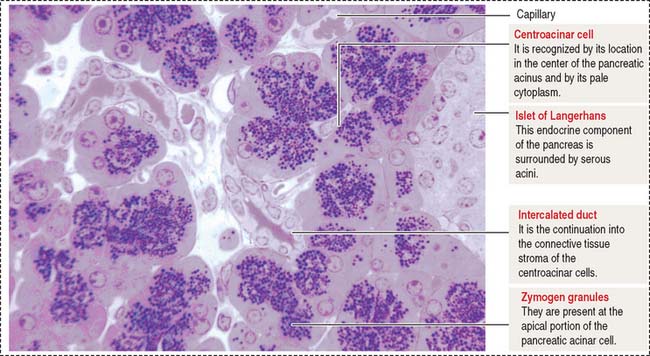
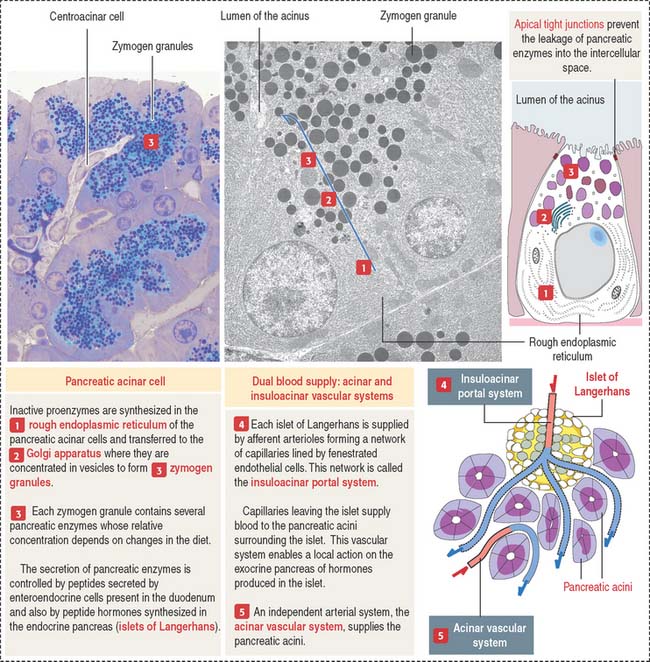
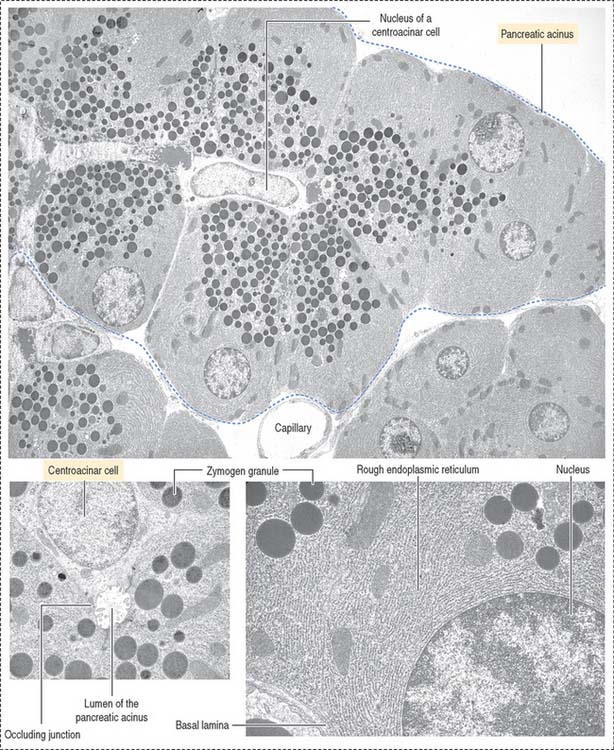
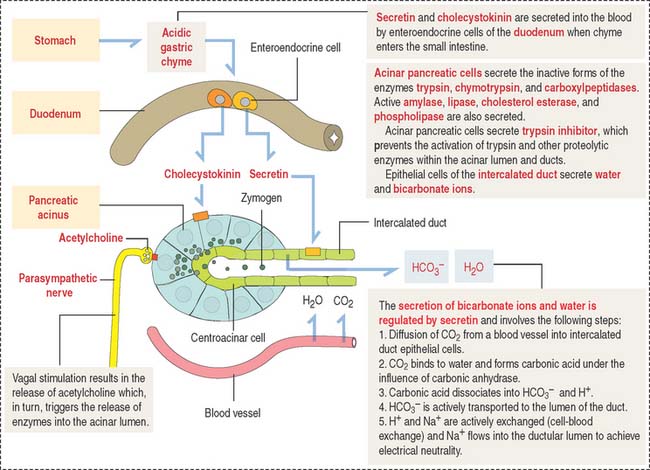
The functional histologic unit of the exocrine pancreas is the acinus (Figures 17-6 to 17-8). The lumen of the acinus is the initiation of the secretory-excretory duct system and contains centroacinar cells that are unique to the pancreas. Centroacinar cells are continuous with the low cuboidal epithelial lining of the intercalated duct. The exocrine pancreas lacks striated ducts and myoepithelial cells. Intercalated ducts converge to form interlobular ducts lined by a columnar epithelium with a few goblet cells and occasional enteroendocrine cells.
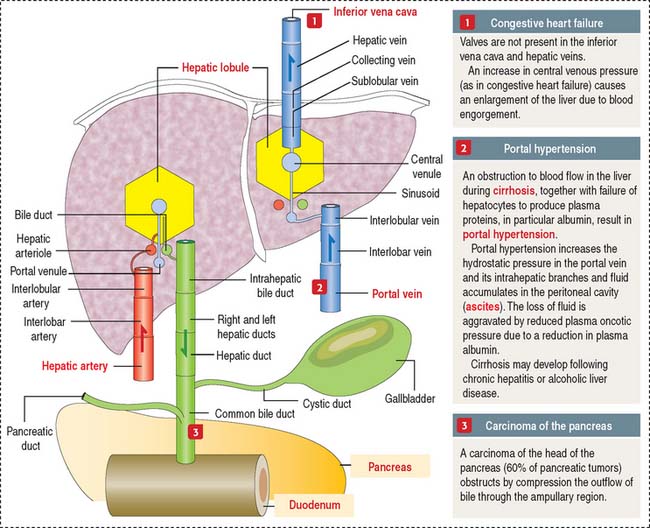
Clinical significance: Carcinoma of the pancreas
The pancreatic duct–bile duct anatomic relationship is of clinical significance in carcinoma of the pancreas localized in the head region, because compression of the bile duct causes obstructive jaundice. The close association of the pancreas with large blood vessels, the extensive and diffuse abdominal drainage to lymph nodes, and the frequent spread of carcinoma cells to the liver via the portal vein are factors contributing to the ineffectiveness of surgical removal of pancreatic tumors.
Functions of the pancreatic acinus
The pancreatic acinus is lined by pyramidal cells joined to each other by apical junctional complexes (see Figure 17-8), which prevent the reflux of secreted products from the ducts into the intercellular spaces. The basal domain of an acinar pancreatic cell is associated with a basal lamina and contains the nucleus and a well-developed rough endoplasmic reticulum. The apical domain displays numerous zymogen granules (see Figure 17-8) and the Golgi apparatus.
The polypeptide hormone cholecystokinin, produced in enteroendocrine cells of the duodenal mucosa, binds to specific receptors of acinar cells and stimulates the release of zymogen (Figure 17-9).
Clinical significance: Acute pancreatitis and cystic fibrosis
Cystic fibrosis is an inherited, autosomal recessive disease affecting the function of mucus-secreting tissues of the respiratory (see Chapter 13, Respiratory System), intestinal, and reproductive systems; the sweat glands of the skin (see Chapter 11, Integumentary System); and the exocrine pancreas in children and young adults. A thick sticky mucus obstructs the duct passages of the airways, pancreatic and biliary ducts, and intestine, followed by bacterial infections and damage of the functional tissues. A large number of patients (85%) have chronic pancreatitis characterized by a loss of acini and dilation of the pancreatic excretory ducts into cysts surrounded by extensive fibrosis (hence the designation cystic fibrosis of the pancreas). Insufficient exocrine pancreatic secretions cause the malabsorption of fat and protein, reflected by bulky and fatty stools (steatorrhea).
The lack of transport of Cl− ions across epithelia is associated with a defective secretion of Na+ ions and water. A genetic defect in the chloride channel protein called cystic fibrosis transmembrane conductance regulator (CFTR) is responsible for cystic fibrosis. The disease is detected by the demonstration of increased concentration of NaCl in sweat. Children with cystic fibrosis “taste salty” after copious sweating.
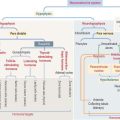
LIVER
The liver, the largest gland in the human body, consists of four poorly defined lobes. The liver is surrounded by a collagen-elastic fiber–containing capsule (of Glisson) and is lined by the peritoneum.
Blood is supplied to the liver by two blood vessels (Figure 17-10): (1) The portal vein (75% to 80% of the afferent blood volume) transports blood from the digestive tract, spleen, and pancreas. (2) The hepatic artery, a branch of the celiac trunk, supplies 20% to 25% of oxygenated blood to the liver by the interlobar artery and interlobular artery pathway before reaching the portal space.
The right and left hepatic bile ducts leave the liver and merge to form the hepatic duct. The hepatic duct becomes the common bile duct soon after giving rise to the cystic duct, a thin tube connecting the bile duct to the gallbladder (see Figure 17-10).
Hepatic lobule
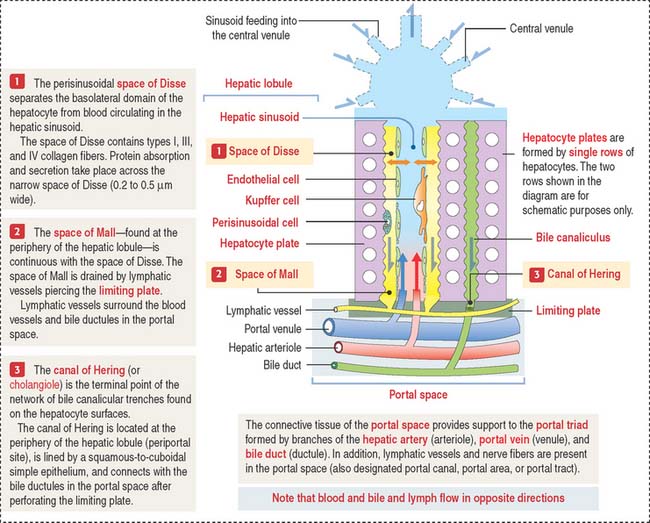
The structural and functional unit of the liver is the hepatic lobule. The hepatic lobule consists of anastomosing plates of hepatocytes limiting blood sinusoidal spaces (see Figure 17-12). A central venule (or vein) in the core of the hepatic lobule collects the sinusoidal blood containing a mixture of blood supplied by branches of the portal vein and the hepatic artery.
Branches of the hepatic artery and portal vein, together with a bile duct, form the classic portal triad found in the portal space surrounding the hexagonal-shaped hepatic lobule (Figure 17-11).
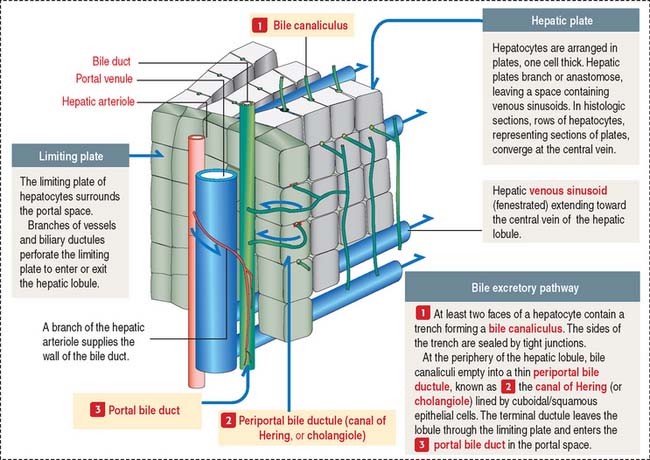
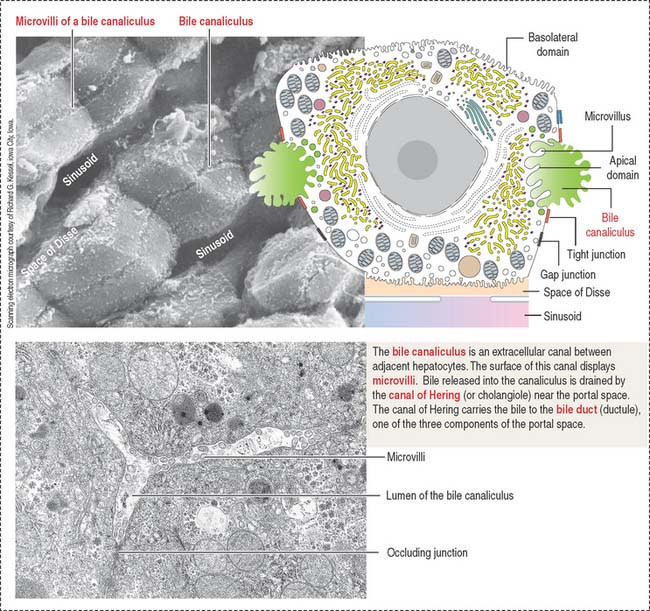
Bile produced in the hepatocytes is secreted into narrow intercellular spaces, the bile canaliculi, located between the apposed surfaces of adjacent hepatocytes.
Bile flows in the opposite direction to the blood. Bile flows from the bile canaliculi into periportal bile ductules (cholangioles, or canals of Hering), and then into the bile ducts (or ductules) of the portal space after crossing the hepatic plate at the periphery of the hepatic lobule (Figure 17-12). Bile ductules converge at the intrahepatic bile ducts.
Functional view of the hepatic lobule
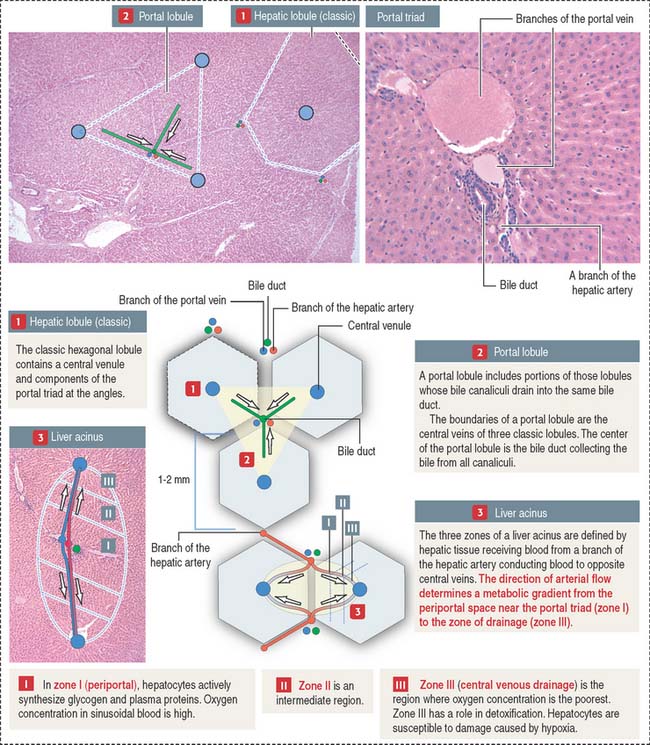
There are three conceptual interpretations of the architecture of the liver lobule (see Figure 17-11): (1) the classic concept of the hepatic lobule, based on structural parameters; (2) the portal lobule concept, based on the bile drainage pathway from adjacent lobules toward the same bile duct; and (3) the liver acinus concept, based on the gradient distribution of oxygen along the venous sinusoids of adjacent lobules.
The classic hepatic lobule is customarily described as a polyhedral structure, usually depicted as a hexagon with a central venule to which blood sinusoids converge (see Figure 17-11).
Components of the portal triad, constituting a branch of the portal vein and hepatic artery and a bile duct, are usually found at the angles of the hexagon. This geometric organization is poorly defined in humans because the limiting perilobular connective tissue is not abundant. However, recognition of the components of the portal triad is helpful in determining the boundaries of the human hepatic lobule.
Functional considerations have modified the classic view and a liver acinus concept has gained ground in pathophysiology. In the liver acinus, the boundaries are determined by a terminal branch of the hepatic artery. The flow of arterial blood within the venous sinusoids creates gradients of oxygen and nutrients classified as zones I, II, and III. Zone I is the richest in oxygen and nutrients. Zone III, closer to the central vein, is oxygen-poor. Zone II is intermediate in oxygen and nutrients (see Figure 17-11).
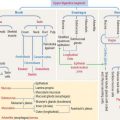
Hepatocyte
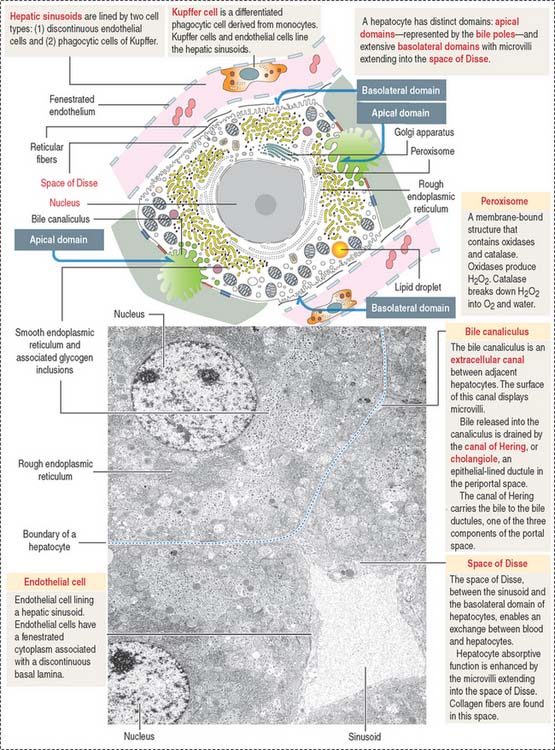
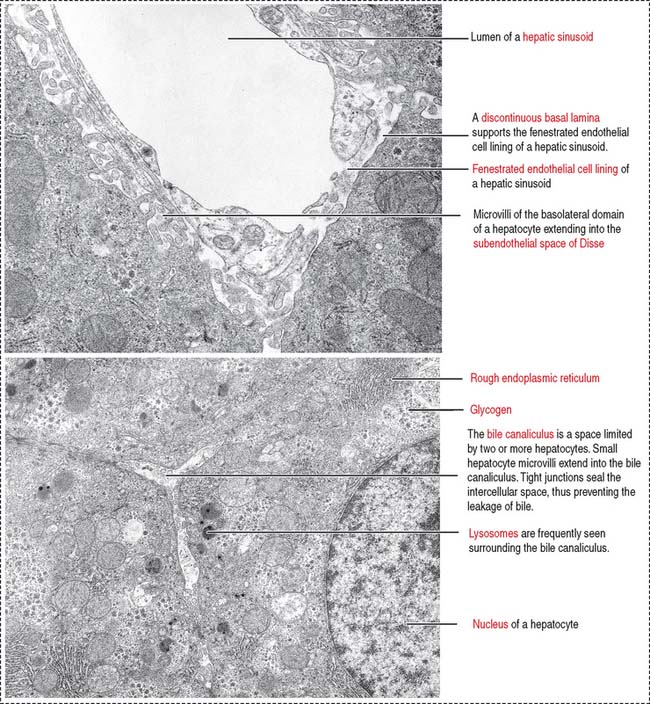
The hepatocyte is the functional exocrine and endocrine cell of the hepatic lobule. Hepatocytes form anastomosing one-cell-thick plates limiting the sinusoidal spaces. The perisinusoidal space of Disse separates the hepatocytes from the blood sinusoidal space (Figure 17-13).
The components of the portal triad, embedded in connective tissue, are separated from the hepatic lobule by a limiting plate of hepatocytes (see Figure 17-12). Blood from the portal vein and hepatic artery flows into the sinusoids and is drained by the central venule. Recall that bile flows in the opposite direction, from the hepatocytes to the bile duct in the portal space (see Figure 17-13).
A hepatocyte has two cellular domains: (1) a basolateral domain and (2) an apical domain (Figures 17-14 to 17-16):
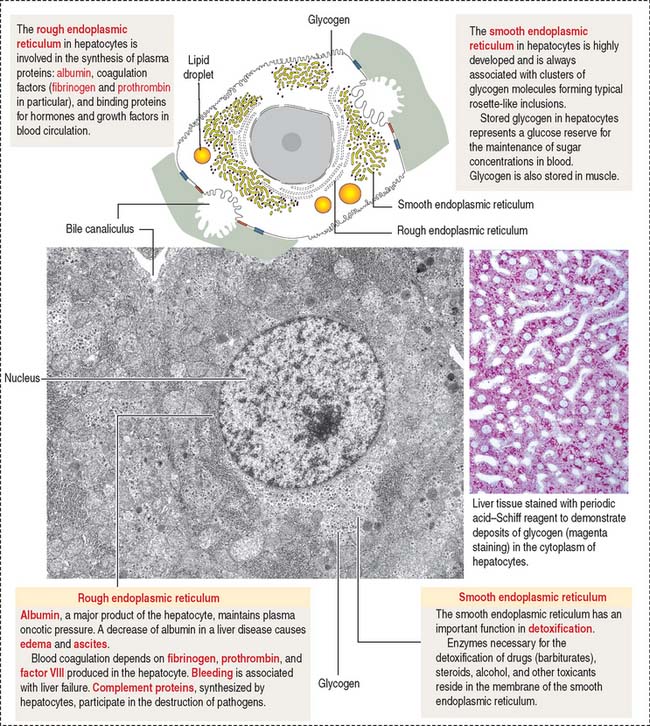
The basolateral domain participates in the absorption of blood-borne substances and in the secretion of plasma proteins (such as albumin, fibrinogen, prothrombin, and coagulation factors V, VII, and IX). Note that hepatocytes synthesize several plasma proteins required for blood clotting (see Chapter 6, Blood and Hematopoiesis). Blood coagulation disorders are associated with liver disease.
The apical domain borders the bile canaliculus, a trenchlike depression lined by microvilli and sealed at the sides by occluding junctions to prevent leakage of bile, the exocrine product of the hepatocyte (see Figure 17-15).
The hepatocyte contains a rough endoplasmic reticulum (see Figure 17-14), involved in the synthesis of plasma proteins, and a highly developed smooth endoplasmic reticulum, associated with the synthesis of glycogen, lipid, and detoxification mechanisms (Figure 17-17).
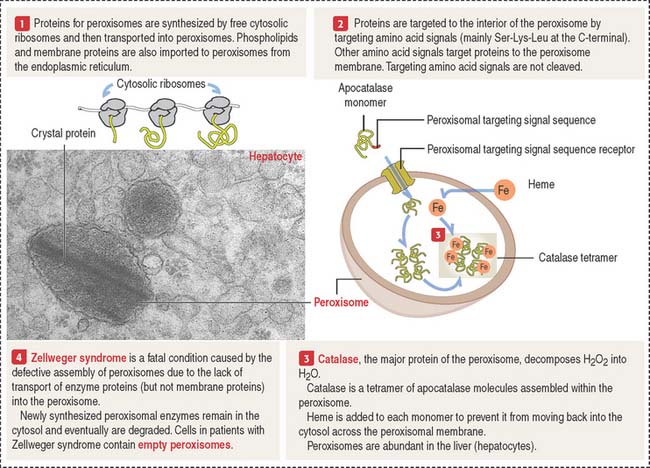
Peroxisomes
Peroxisomes are membrane-bound organelles with a high content of oxidases that generate hydrogen peroxide (Figure 17-18). Because hydrogen peroxide is a toxic metabolite, the enzyme catalase degrades this product into oxygen and water. This catalytic event occurs in hepatocytes and cells of the kidneys.
Peroxisomes derive from preexisting peroxisomes by a budding process. Then, the organelle imports peroxisomal matrix proteins. A peroxisome contains about 50 enzymes involved in various metabolic pathways. The biogenesis of peroxisomes and their role in inherited disorders are outlined in Figure 17-18 and in Chapter 2, Epithelial Glands.
Clinical significance: Liver storage diseases
Wilson’s disease (hepatolenticular degeneration) is a hereditary disorder of copper metabolism in which excessive deposits of copper in liver and brain lysosomes produce chronic hepatitis and cirrhosis.
Clinical significance: Alcoholism and fatty liver (alcoholic steato-hepatitis)
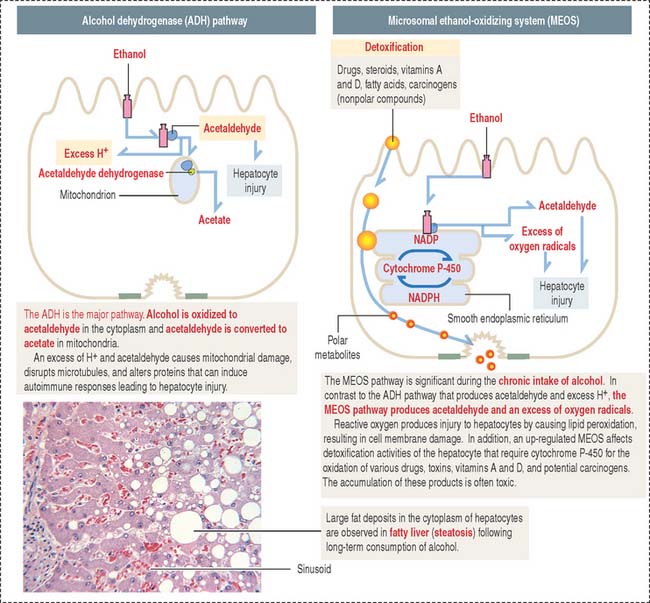
After absorption in the stomach, most ethanol is transported to the liver, where it is metabolized to acetaldehyde and acetate in the hepatocytes. Ethanol is mainly oxidized by alcohol dehydrogenase, an NADH (reduced form of nicotinamide adenine dinucleotide)–dependent enzyme. This mechanism is known as the alcohol dehydrogenase (ADH) pathway. An additional metabolic pathway is the microsomal ethanol-oxidizing system (MEOS), present in the smooth endoplasmic reticulum. The two pathways are summarized in Figure 17-17.
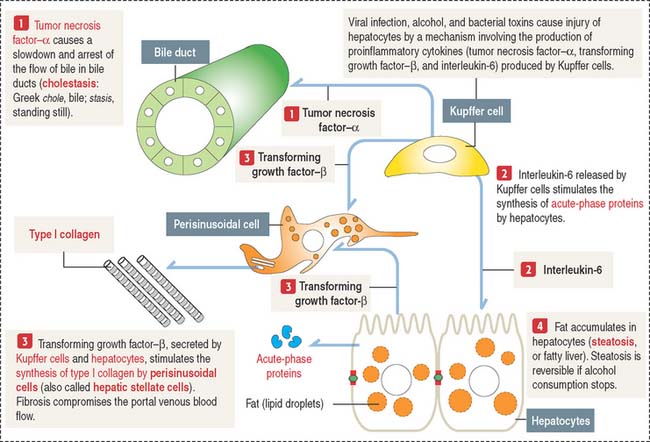
The production of tumor necrosis factor–α (TNF-α) is one of the initial events in liver injury. TNF-α triggers the production of other cytokines. TNF-α, regarded as a proinflammatory cytokine, recruits inflammatory cells that cause hepatocyte injury and promote the production of type I collagen fibers by perisinusoidal cells of Ito (a process known as fibrogenesis) as a healing response.
Injury of hepatocytes results in programmed cell death, or apoptosis, caused by the activation of caspases (see Chapter 3, Cell Signaling). TNF-α participates in a number of inflammatory processes such as in the articular joints (Chapter 5, Osteogenesis) and the extravasation of inflammatory cells (Chapter 10, Immune-Lymphatic System).
Ethanol, viruses, or toxins induce Kupffer cells to synthesize TNF-α as well as transforming growth factor-β (TGF-β) and interleukin-6 (Figure 17-19). TGF-β stimulates the production of type I collagen by perisinusoidal cells, which increase in number. TNF-α acts on biliary ducts to interfere with the flow of bile (cholestasis).
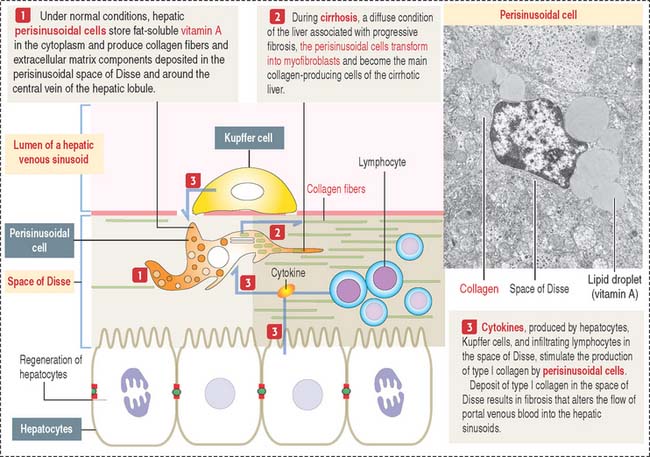
Clinical significance: Perisinusoidal cells
Perisinusoidal cells (of Ito; also called hepatic stellate cells) are found in the space of Disse in proximity to the hepatic sinusoids. These cells are of mesenchymal origin, contain fat, and are involved in (1) the storage and release of retinoids; (2) production and turnover of extracellular matrix; and (3) regulation of blood flow in the sinusoids. Perisinusoidal cells remain in a quiescent, nonproliferative state, but can proliferate when activated by Kupffer cells and hepatocytes. Activation occurs after partial hepatectomy, focal hepatic lesions, and in different conditions that lead to fibrosis (Figure 17-20).
In pathologic conditions, perisinusoidal cells change into collagen-producing cells. In addition to the synthesis and secretion of type I collagen, perisinusoidal cells secrete laminin, proteoglycans, and growth factors. The deposit of collagen and extracellular matrix components increases, leading to a progressive fibrosis of the liver, which is typical of cirrhosis.
TGF-β, produced by Kupffer cells and hepatocytes (see Figures 17-19 and 17-20), stimulates collagen production by perisinusoidal cells. An increased deposit of collagen fibers and extracellular matrix within the space of Disse is followed by a loss of fenestrations and gaps of sinusoidal endothelial cells.
Bile: Mechanism of secretion
Bile is a complex mixture of organic and inorganic substances produced by the hepatocyte, transported by the bile canaliculus, an extracellular canal between adjacent hepatocytes (Figure 17-21). The bile canaliculus defines the apical domain of the hepatocyte. The basolateral domain faces the sinusoidal space. Tight junctions between adjacent hepatocytes seal the biliary canalicular compartment.
Bile has five major functions:
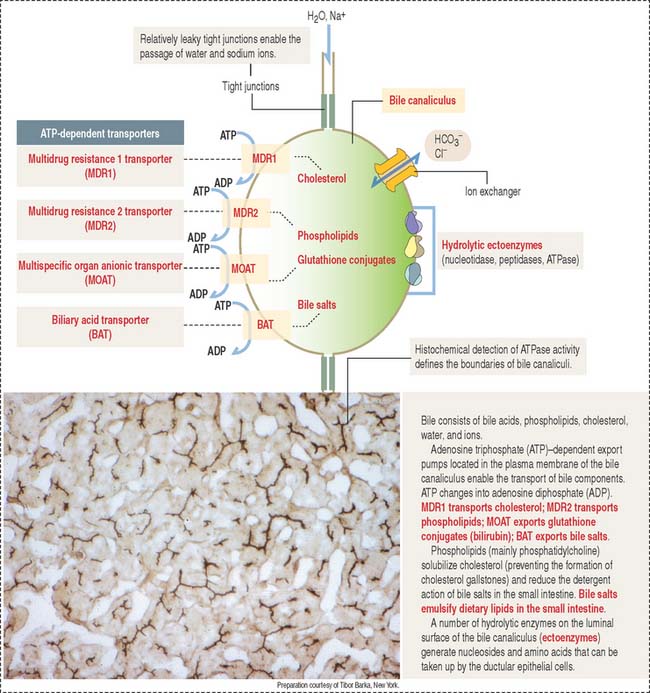
The transport of bile and other organic substances from the hepatocyte to the lumen of the bile canaliculus is an adenosine triphosphate (ATP)–mediated process. Four ATP-dependent transporters, present in the canalicular plasma membrane, participate in transport mechanisms of the bile (Figure 17-22).
These ATP transporters belong to the family of ABC transporters characterized by highly conserved ATP-binding domains, or ATP binding cassettes. The first ABC transporter was discovered as the product of the gene mdr (for multiple drug resistance). The mdr gene is highly expressed in cancer cells and the encoded product, MDR transporter, pumps drugs out of cells, making cancer cells resistant to cancer treatment with chemotherapeutic agents (see Cell Nucleus in Chapter 1, Epithelium).
The secretion of bile acids generates the osmotic gradient necessary for osmotic water flow into the bile canaliculus. In addition, an ion exchanger enables the passage of HCO3− and Cl− ions. Finally, hydrolytic enzymes associated with the plasma membrane (ectoenzymes) of the bile canaliculus and bile duct produce nucleoside and amino acid breakdown products, which are reabsorbed by ductular epithelial cells.
Metabolism of bilirubin
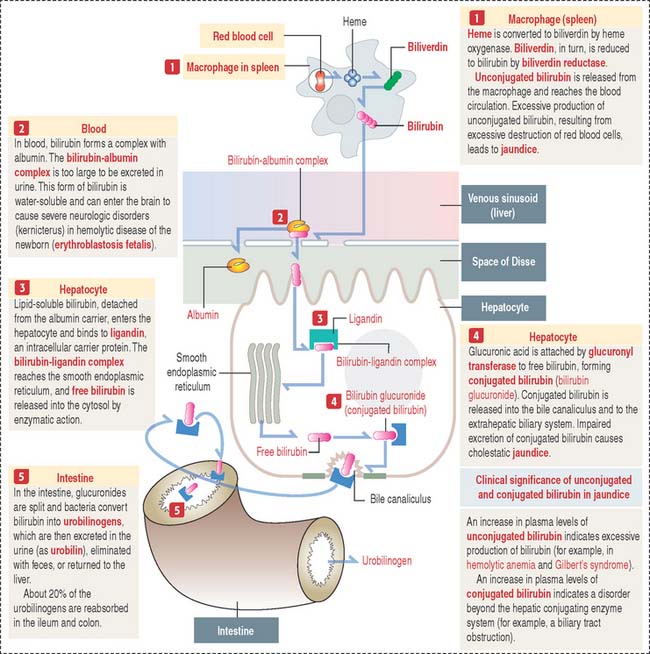
Bilirubin is the end product of heme catabolism and about 85% originates from senescent red blood cells destroyed mainly in the spleen by macrophages (Figure 17-23).
Bilirubin is released into the circulation, where it is bound to albumin and transported to the liver. Unlike albumin-bound bilirubin, free bilirubin is toxic to the brain. Recall from our discussion of erythroblastosis fetalis (see Chapter 6, Blood and Hematopoiesis) that an antibody-induced hemolytic disease in the newborn is caused by blood group incompatibility between the mother and fetus. The hemolytic process results in hyperbilirubinemia caused by elevated amounts of free bilirubin, which causes irreversible damage to the central nervous system (kernicterus).
When albumin-conjugated bilirubin reaches the hepatic sinusoids, the albumin-bilirubin complex dissociates, and bilirubin is transported across the plasma membrane of hepatocytes after binding to a plasma membrane receptor. Inside the hepatocyte, bilirubin binds to ligandin, a protein that prevents bilirubin reflux into the circulation. The bilirubin-ligandin complex is transported to the smooth endoplasmic reticulum, where bilirubin is conjugated to glucuronic acid by the uridine diphosphate (UDP)–glucuronyl transferase system. This reaction results in the formation of a water-soluble bilirubin diglucuronide, which diffuses through the cytosol into the bile canaliculus, where it is secreted into the bile.
Composition of the bile
The human liver produces about 600 mL of bile per day. The bile consists of organic components (such as bile acids, the major component; phospholipids, mainly lecithins; cholesterol; and bile pigments, bilirubin) and inorganic components (predominantly Na+ and Cl− ions).
Bile secreted by the liver is stored in the gallbladder and released into the duodenum during a meal to facilitate the breakdown and absorption of fats (see Figure 16-9 in Chapter 16, Lower Digestive Segment). About 90% of both primary and secondary bile acids is absorbed from the intestinal lumen by enterocytes and transported back to the liver through the portal vein. This process is known as the enterohepatic circulation. The absorption of bile acids by the enterocyte is mediated at the apical plasma membrane by an Na+-dependent transporter protein and released through the basolateral plasma membrane by an Na+-independent anion exchanger.
Bilirubin is not absorbed in the intestine. Bilirubin is reduced to urobilinogen by bacteria in the distal small intestine and colon (see Figure 17-23). Urobilinogen is partially secreted in the feces, part returns to the liver through the portal vein, and some is excreted in urine as urobilin, the oxidized form of urobilinogen.
Clinical significance: Hyperbilirubinemia
Gilbert’s syndrome is the most common inborn error of metabolism causing moderate hyperbilirubinemia. Elevated levels of unconjugated bilirubin, with no serious health consequences, are detected in the bloodstream. The cause is the reduced activity of the enzyme glucuronyl transferase, which conjugates bilirubin (see Figure 17-23).
GALLBLADDER
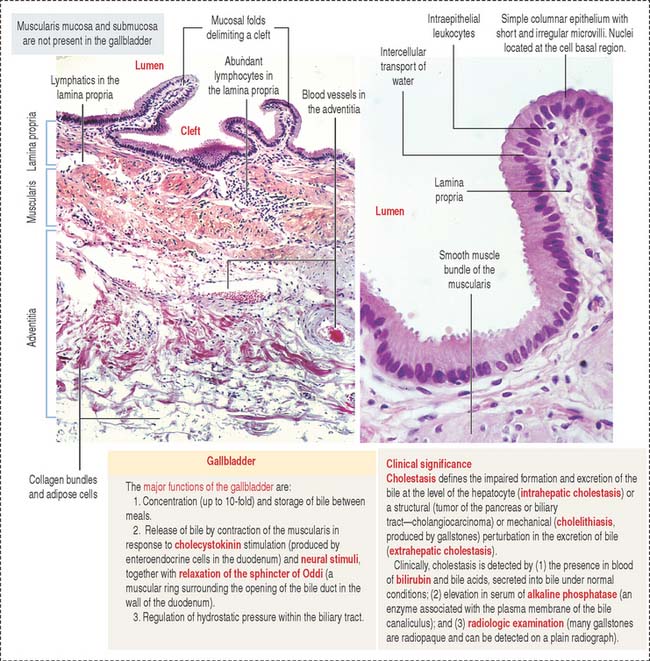
The wall of the gallbladder consists of a mucosa, a muscularis, and an adventitia (Figure 17-24). The portion of the gallbladder that does not face the liver is covered by the peritoneum.
Digestive Glands
Intralobular ducts converge to form an interlobular duct (found between lobules; lined by a pseudostratified columnar epithelium). Interlobular ducts converge to form lobar ducts (lined by stratified columnar epithelium). Lobar ducts join the main duct, which displays a stratified squamous epithelium near its opening in the oral cavity. Connective tissue septa provide support to the branching duct system. Blood vessels, lymphatics, and nerves are found along the ducts.