Chapter 89 Diagnostic Aspects of Implantable Devices
Monitoring of Atrial Fibrillation
Atrial tachycardias and atrial fibrillation (AT/AF) are significant components of the global burden of cardiovascular disease and their incidence is increasing rapidly because of the aging of the population; projections indicate that the prevalence of AF could exceed 12 million in the United States by 2050.1 These conditions occur concomitantly with bradycardias, supraventricular tachycardias, and ventricular tachyarrhythmias and are frequently observed in populations with these conditions when an implantable device is prescribed therapeutically.
Atrial tachyarrhythmias result in poor quality of life for patients, as they may cause symptoms of tiredness, palpitations, and dizziness. However, in many patients, AT/AF episodes can be completely asymptomatic.2,3 Whether it is symptomatic or asymptomatic, AT/AF also increases the risk of stroke in the presence of clinical risk factors, and it is estimated that 1 out of every 6 strokes occurs in patients with AT/AF.4 It leads to more hospital admissions than any other arrhythmia and increases mortality rates.5,6 Management of this arrhythmia with pharmacologic agents, ablation, and implantable devices requires accurate assessment of the arrhythmia at baseline and after treatment. Regardless of the AF treatment strategy chosen, diagnostics from implantable devices can provide valuable information to help evaluate treatment efficacy and guide clinical decisions. Other important clinical imperatives for monitoring include the following:
Symptoms Versus External Recorders
Monitoring AT/AF on the basis of patient symptoms is appealing because it is relatively inexpensive and has clinical relevance. However, studies have shown that the vast majority of AT/AF episodes are asymptomatic and that most symptoms attributed to AT/AF are not actually associated with arrhythmia.2,3,8,9 Therefore, even if supplemental event recorders are used to verify the presence of an arrhythmia during symptoms, most atrial arrhythmias will escape detection because of lack of symptoms. Since the risk of stroke is similar among patients with symptomatic and asymptomatic AT/AF, current anticoagulation guidelines do not differentiate on the basis of symptoms,10 which emphasizes the importance of identifying asymptomatic AT/AF.10–12
Because of the highly insensitive and nonspecific nature of patient symptoms, external devices were developed to monitor asymptomatic episodes of AT/AF. External devices such as Holter monitors are commonly used to continuously record cardiac data for 24 or 48 hours. Newer systems, such as mobile cardiac outpatient telemetry, are capable of monitoring patients continuously for up to 30 days and have been shown to increase the yield for arrhythmia detection compared with a single 24-hour Holter monitor.13,14 These systems have the benefits of moderate cost and being a noninvasive procedure. However, these external devices often are bulky and interfere with daily activities such as showering. In addition, the patch electrodes can cause skin irritation with prolonged use. Consequently, patient compliance with such systems often is quite low.8,13 Furthermore, since external monitoring can only be performed intermittently over time and for relatively brief durations, the likelihood of missing paroxysmal episodes of asymptomatic AT/AF episodes is quite high.
Implantable Monitors
Therapy Devices
Devices such as implantable pulse generators, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices are capable of monitoring arrhythmias continuously over the lifetime of the device with a high sensitivity as well as a high specificity for AT/AF detection.15,16 Although these devices require an invasive implantation procedure, patient compliance is generally not an issue, and these devices rarely interfere with normal daily activities. Patients can communicate with many modern implantable devices via an external activator to record the occurrence of symptoms in the memory of the device. This symptom information can be retrieved later by the clinician when the device is interrogated via telemetry. However, the monitoring capabilities afforded by these sophisticated devices are available only to those AF patients with comorbid conditions requiring device therapy.
Subcutaneous Monitors
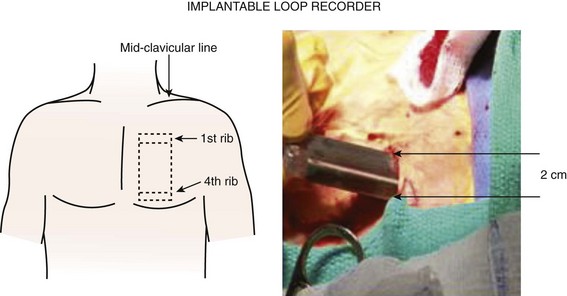
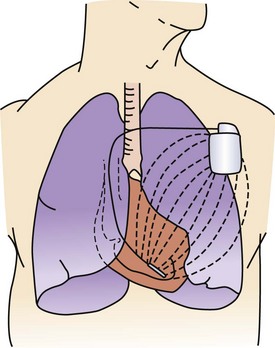
One strategy to address the need for continuous monitoring in a broader population of patients with AF is to use small implantable devices that are capable of monitoring atrial arrhythmias continuously over the lifetime of the device with a high sensitivity and a high specificity for AT/AF detection.17 These devices, about the size of a cigarette lighter, do not have intracardiac leads, as the bipolar recording electrodes are generally located on the surface of the device itself. These monitoring devices are placed subcutaneously, are relatively small in size (3 to 5 cm), and are typically implanted in the left pectoral region in the precordial area, though axillary implants have been described (Figure 89-1, A). Implantation parallel to and in an intercostal space is most desirable for avoidance of local erosion and aesthetics. A local anesthetic agent is infiltrated at the site of entry and in the adjoining subcutaneous tissue where the device pocket will be made. Typically, a small (1 to 2 cm) vertical or horizontal incision is made at one end of the pocket, and the pocket created by blunt dissection (Figure 89-1, B). The device is placed and the electrogram quality assessed using a hand-held wand or wireless connection by the device programmer. If clear electrogram quality and satisfactory amplitude are obtained, the pocket can be closed. If not, device repositioning is needed so that the surface electrodes have an appropriate vector to obtain quality recordings. Although a small skin incision is required to implant these devices, patient compliance is not an issue, as these devices rarely interfere with normal daily activities. Until recently, subcutaneous monitoring devices had only been available for the diagnosis of syncope, but they have now become available for the monitoring of atrial arrhythmias as well. Patients are able to record the occurrence of symptoms in the memory of the device with an external activator, and these data can be retrieved later by the clinician.
Comparison of Monitoring Methods
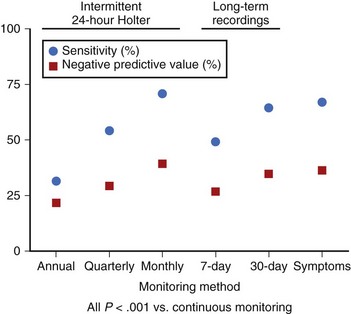
Methods of identifying patients with AT/AF through their symptoms, intermittent external monitoring, and continuous monitoring with implantable devices have been compared in a recent study.18 Symptom-based and intermittent external monitoring methods were shown to have significantly lower sensitivity (range, 31% to 71%) and negative predictive value (range, 21% to 39%) for identification of patients with AT/AF (Figure 89-2) and underestimated AT/AF burden, compared with continuous monitoring. The reported efficacy of clinical procedures such as pulmonary vein ablation for the treatment of AT/AF can vary greatly, depending on how the arrhythmia is monitored. One study reported a success rate of 70% on the basis of symptoms alone and only 50% when intermittent external monitoring was also considered.8 These results were achieved despite the fact that 47% of the patients did not complete the external monitoring protocol and compliance with the monitoring schedule was only 42%. Such results highlight the clinical need for continuous monitoring that does not rely on patient symptoms or patient compliance.
Rhythm Control
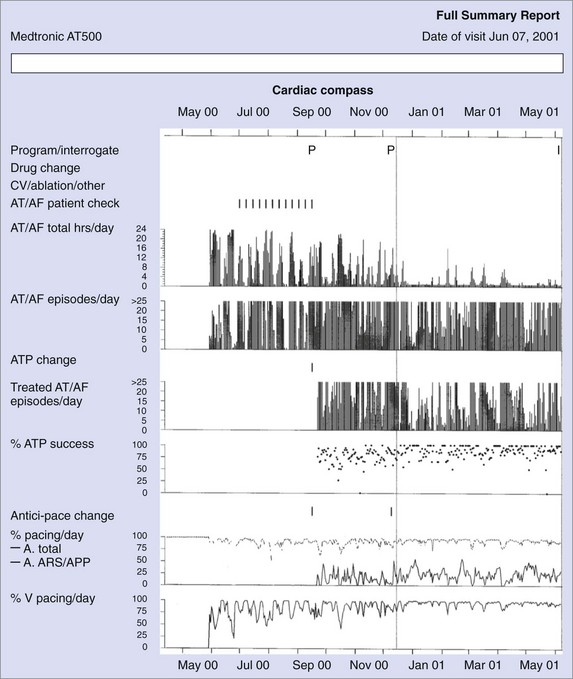
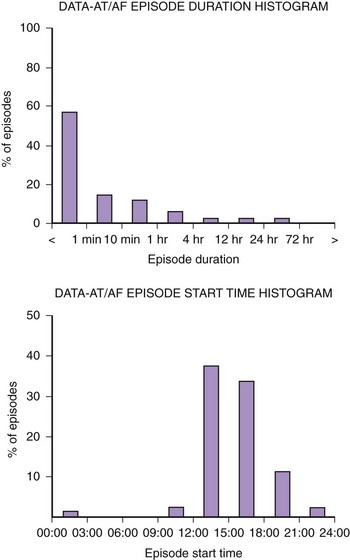
Figure 89-3 shows diagnostic information that can be used to evaluate the efficacy of rhythm control. The total hours of AT/AF per day (AT/AF burden) can be continuously monitored over a period of 14 months before the data get overwritten. Other diagnostics that can provide additional rhythm control clarity are an AT/AF episode duration histogram, episode start time histogram (Figure 89-4), and the duration of the longest episode.
Pulmonary Vein Ablation
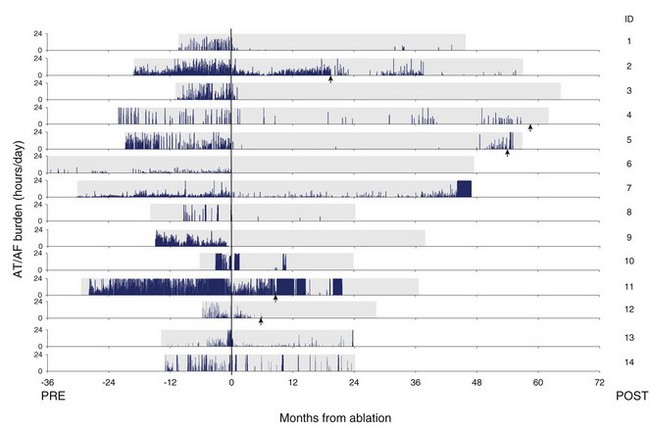
Figure 89-5 shows a series of patients who were treated for AT/AF by undergoing a pulmonary vein ablation procedure anywhere from 6 to 36 months after device implantation. These patients had a pacemaker implanted for their bradycardia, and the device was subsequently used to monitor the effectiveness of the ablation procedure. Patient data are aligned such that the date of ablation corresponds with month 0. The amount of AT/AF experienced each day is plotted over the follow-up period and shown in blue. Despite an improvement in symptoms and quality of life in all patients after the ablation, many still experienced significant amounts of AT/AF.19 This example also illustrates that after an ablation procedure, patients can go for many months without an AT/AF episode and subsequently experience recurrences that would be difficult to record with intermittent monitoring methods. This long-term trending information enabled the physicians conducting the study to perform additional ablation procedures (indicated by the red arrows) in select patients who did not respond completely to the initial procedure.
Antiarrhythmic Drugs and Cardiac Pacing
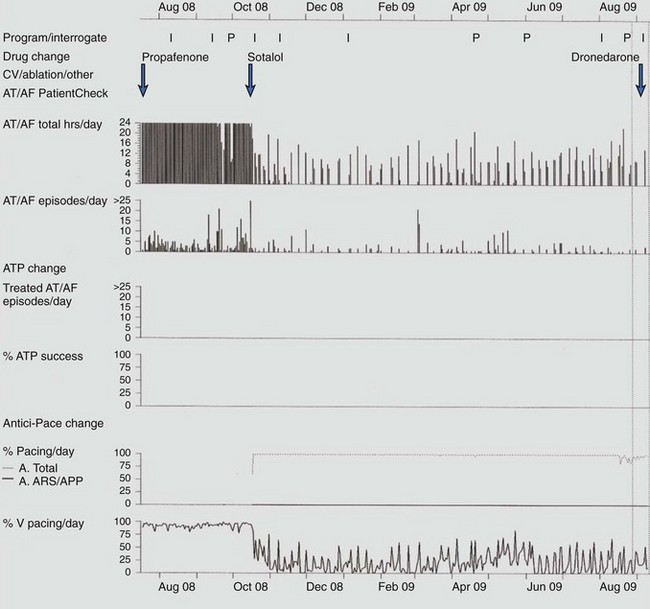
Response to antiarrhythmic drug therapy can be assessed by device-based diagnostic data. Figure 89-6 shows an example of change in AT/AF burden, with conversion of persistent AF to paroxysmal AF, after the initiation of sotalol. The patient continued to have symptomatic paroxysmal AF and was then transitioned to dronedarone, which resulted in the resolution of symptomatic paroxysmal AF.
Recurrence Patterns
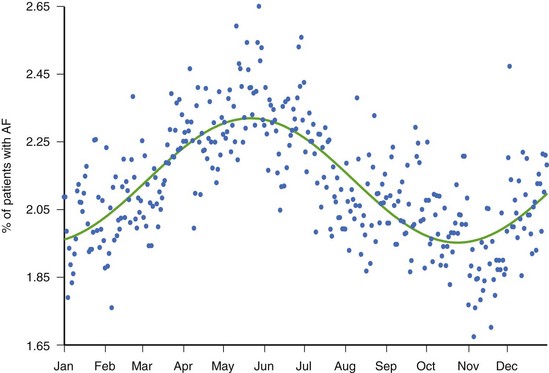
While there is clinical value in knowing whether or not a patient has experienced AT/AF and the percentage of time that has been spent in AT/AF, understanding the pattern of these AT/AF recurrences may be of additional value. The patient data in Figure 89-7 show a very consistent recurrence pattern that persists for the entire 14-month period covered by device diagnostics. On closer examination, it was found that this patient experienced AT/AF predominantly on weekends, with episodes generally initiating on Fridays or Saturdays and typically terminating on Sundays or Mondays. With intermittent arrhythmia monitoring, it might have been erroneously concluded that this patient was either always in AT/AF or always in sinus rhythm, depending on the day(s) selected for monitoring. With continuous monitoring, a more complete picture of the patient’s arrhythmia can be obtained, which may provide additional insight into the patient’s disease and lead to different treatment strategies. In addition to providing more complete information on individual patients, device diagnostics can inform us on the nature of atrial arrhythmias across entire populations. Figure 89-8 shows the percentage of patients with implantable devices (pulse generator, ICD, and CRT) who experienced at least 5 minutes of AT/AF over each day of the year.20 A clear pattern is seen, with a peak incidence in the month of May and a minimum incidence 6 months later in November. While the underlying cause of this cyclic variation is not fully understood, it is interesting to note that the occurrence of stroke has been shown to exhibit a similar recurrence pattern.21
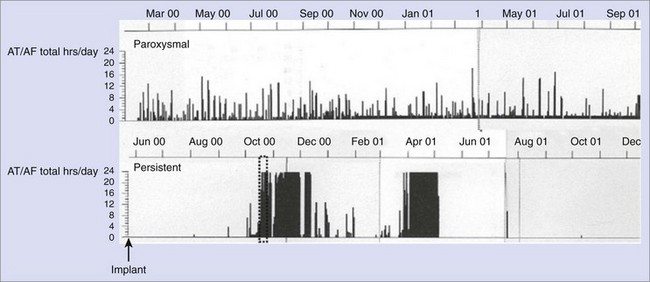
Transition from Paroxysmal Atrial Fibrillation to Persistent Atrial Fibrillation
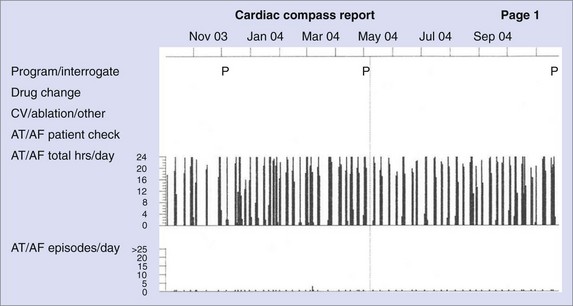
AT/AF recurrence patterns are not static and may change over time as the disease progresses or as new treatments are applied. Device diagnostics have shown that the transition from paroxysmal AT/AF to more persistent forms of AT/AF can occur relatively abruptly (Figure 89-9).22,23 This finding is consistent with changes in the substrate as opposed to an increase in atrial premature beat triggers or paroxysmal AT/AF episodes. This monitoring approach shows the progression of persistent AF events as well as promotes understanding of the disease state.23 Device diagnostics can heighten the awareness of this sudden change in the arrhythmic state so that a change in treatment strategy can be implemented in a timely manner.
Rate Control
Rate Control Diagnostics
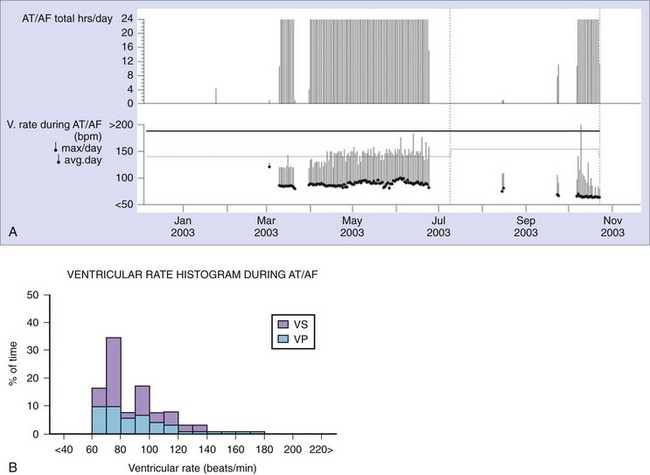
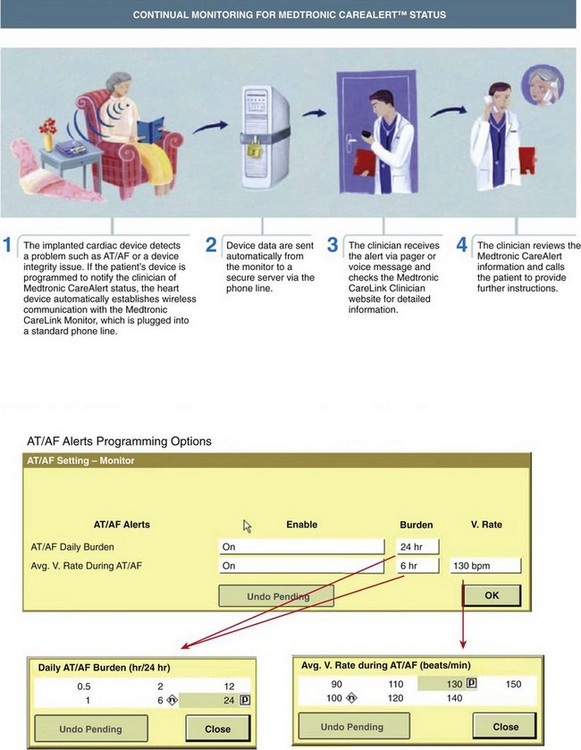
Some implantable devices have the capability to continuously monitor the ventricular rate during AT/AF and to report this information as a daily tabulation of the average and maximum ventricular rates over the past 14 months (Figure 89-10, A) or as a histogram of rates since the previous follow-up visit (Figure 89-10, B). The average ventricular rate during sinus rhythm can also be reported separately for day and night periods. Timely notification of poor rate control may help clinicians prevent the occurrence of symptoms, reduce inappropriate shocks, and ensure continuous CRT therapy in patients with AT/AF. Some newer devices have a wireless alert feature that can notify the clinician if the average ventricular rate exceeds a specified threshold for a programmable period (Figure 89-11).
Anticoagulation
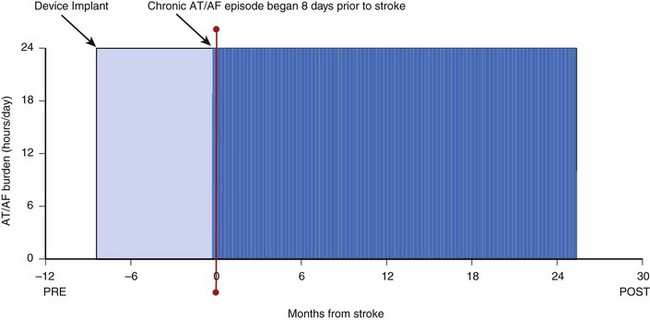
Continuous monitoring of rhythm control is critically important for managing a patient’s anticoagulation regimen. Key questions involve timing of initiation of anticoagulation therapy and timing of safe discontinuation. Current guidelines recommend anticoagulation for patients with risk factors and AT/AF, regardless of the amount of AT/AF or associated symptoms.10 Therefore, it is important to identify patients who experience even relatively brief episodes of AT/AF. Studies have shown that continuous monitoring via implantable devices is particularly well suited to this purpose.18 The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study demonstrated a high risk of stroke in patients who discontinued anticoagulation because it was thought that rhythm control was adequate on the basis of symptoms and infrequent monitoring.7 Studies are in progress to assess the risk of manifest stroke or cryogenic stroke in populations with AF using these devices. The example in Figure 89-5 also shows that patients can go for many months without an AT/AF episode only to experience recurrences that would be difficult to capture with intermittent monitoring methods. Key to timely anticoagulation management may be the early notification of “new-onset” AT/AF (i.e., a patient’s first episode of AT/AF). With most implantable devices, the clinician does not become aware of the presence of new-onset AT/AF until the patient’s device is interrogated in the clinic setting. This could cause a delay of up to 6 months from the time of the first AT/AF occurrence to the first opportunity to treat it. Some newer devices with wireless alert capabilities can automatically send a notification to clinicians when a programmable AT/AF burden threshold is exceeded on a given day (see Figure 89-11). This early notification could enable physicians to intervene sooner and initiate an anticoagulation regimen, thus reducing the risk of stroke in patients who were unaware of their atrial arrhythmias. Figure 89-12 shows data from a patient without a history of AF and no device-recorded AF in the first 8 months following implantation. However, the patient then abruptly went into an episode of persistent AF and suffered an ischemic stroke 8 days after the onset of AF. While this device did not have the capability to notify the physician or the patient as to the presence of AF, the time (8 days) would have been sufficient to implement a treatment strategy to prevent this event had there been awareness of AF. As mentioned before, some modern implantable devices have the capability to generate wireless notifications when prolonged AF episodes occur. Another study showed that approximately 30% of patients with a prior history of thromboembolism and no history of AF were identified as having newly detected AF (NDAF) via continuous monitoring with implantable devices.25 While AF occurred on fewer than 10% of follow-up days in 72% of patients with NDAF, it also persisted for at least 6 hours on at least a day in the majority of patients with NDAF. This highlights not only the difficulty of detecting AF with traditional methods but also the importance of identifying AF in this population. In fact, 62% of patients with NDAF were identified beyond the initial 21 days of the study, which suggests the value of long-term arrhythmia monitoring in this population.
Burden Thresholds for Stroke
While the exact relationship between AT/AF burden and stroke risk is not fully understood, data from implantable devices can be used to gain a better understanding. Several earlier studies have examined the relationship between AF burden and outcome in patients with devices. A substudy of the Mode Selection Trial (MOST) reported that the composite endpoint of non-fatal stroke and death was significantly higher in patients having at least 5-minute episodes of high-rate atrial arrhythmias.26 A study by Italian investigators showed that atrial arrhythmias lasting longer than 24 hours increased the risk of thromboembolic events threefold compared with those having shorter duration or no episodes.27 The TRENDS study reported that an arrhythmia burden of 5.5 hours or more on any of the past 30 days appeared to double the risk of thromboembolic events compared with no burden.28 Taken together, these studies suggest that quantitative AT/AF burden detected by implanted devices is a risk factor for thromboembolism and that the burden threshold for increased risk may be relatively low.
Potential Role of Device Monitoring in Heart Failure Therapy
Long-term management of patients with congestive heart failure is a growing burden on health care systems. In addition, signs and symptoms within this population are often poorly correlated with actual disease status.29,30 Swan-Ganz catheterization and echocardiography are costly and not well suited for repeated serial measurement. Recently, considerable investigation has focused on the development of alternative methods of assessing the disease status in heart failure. Implantable hemodynamic sensors offer a potential surrogate for serial invasive catheterizations in tailoring and titrating medical therapy. Furthermore, continuous monitoring of hemodynamic measurements might provide unique insights into pathophysiological mechanisms and chronic responses to therapeutic regimens.
Pressure Monitoring System
Right-Sided Pressure Monitoring
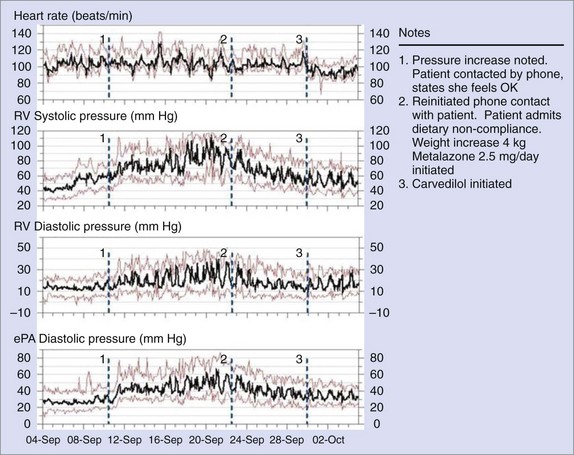
A totally implantable hemodynamic monitor (IHM) system consists of a pacemaker-like device that processes and stores information and a transvenous lead incorporating a high-fidelity pressure sensor near its tip.31 The implantation procedure is similar to that of a single-chamber pacemaker system. The pressure sensing lead is positioned in the right ventricular outflow tract or in the high right ventricular septum in an area of high blood flow. In addition to right ventricular systolic and diastolic pressures and estimated pulmonary arterial diastolic pressure, the IHM also measures and stores peak positive and negative dP/dt, heart rate, patient activity, right ventricular pre-ejection and systolic time intervals, and body temperature. A strong correlation (r = 0.84) has been demonstrated between actual pulmonary artery pressures and the pulmonary arterial diastolic pressure estimates under a variety of physiological conditions.32–35 The implantable system continuously monitors and stores hemodynamic information that can be accessed remotely via the Internet. The Web interface automatically processes and concatenates new data received from the device and displays visual trends over time (Figure 89-13).
Numerous clinical studies have demonstrated the safety as well as the accuracy of the implantable hemodynamic monitoring system. The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial randomized 274 NYHA class III to IV patients to a Chronicle-guided management group (n = 134) or to a control group (n = 140) and monitored them for 6 months.36,37 The study demonstrated that the IHM was safe and had the ability to reduce the rate of heart failure-related events. However, the observed 21% reduction in heart failure events was not statistically significant. Retrospective analyses from the COMPASS-HF study provided new insights into the pathophysiology of the transition from stable, compensated HF to the decompensated state in subjects with left ventricular ejection fraction less than 35% and among HF patients with preserved left ventricular ejection fraction. The currently ongoing Reducing Events in Patients with Chronic Heart Failure (REDUCEhf) trial will prospectively test the hypothesis that the ambulatory hemodynamic monitoring can, indeed, reduce the rate of heart failure–related hospitalization.38
Left-Sided Pressure Monitoring
In addition to right-sided cardiac pressure monitoring, an implantable device that measures left atrial pressure directly is currently being tested in clinical trials.39 The implanted system consists of a hand-held patient-activated monitor that stimulates a passive subcutaneous antenna coil. A lead connects the coil to the sensor module that is anchored in the left atrium from the right side via the fossa ovalis through a femoral venous approach. An initial clinical trial of eight patients determined that “monitoring of direct left atrial pressure with a new implantable device was well tolerated, feasible, and accurate at a short-term follow-up.”40 A larger randomized clinical trial is under way at the time of this writing.
Intrathoracic Impedance Monitoring
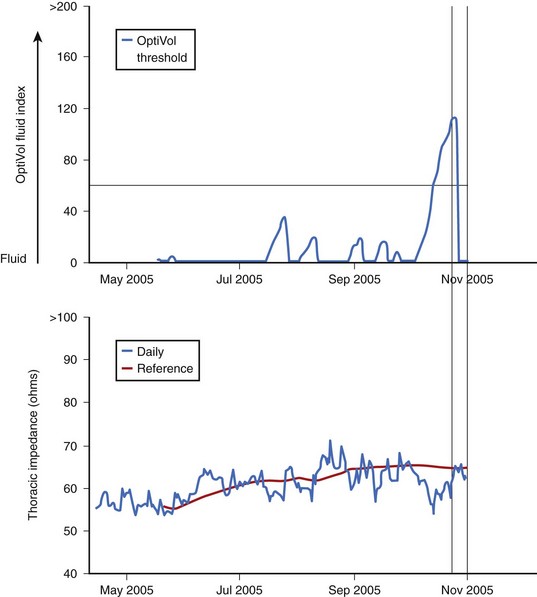
The correlation between changes in biologic impedance and physiological parameters, such as respiration rate and cardiac hemodynamics, has been the subject of scientific investigation for decades.41–43 Recently, the theories behind impedance monitoring of hemodynamic events have been applied to implantable devices. The recent MidHeft trial provided the first clinical evidence that daily monitoring of intrathoracic impedance measured between the right ventricular defibrillation coil and the devise case (Figure 89-14) could provide a clinically useful tool for monitoring the onset of decompensation in acute heart failure.44 Device-recorded daily impedance data from this nonrandomized, double-blind prospective trial (n = 33) were used to develop and validate an algorithm to detect acute pulmonary fluid accumulation based on day-to-day changes in the actual recorded daily intrathoracic impedance. The algorithm automatically calculates a dynamic reference impedance on the basis of trends in the measured daily intrathoracic impedance. Differences between the measured daily impedance and the calculated reference impedance are used to increase or reset a “fluid index” (Figure 89-15). Results from the trial validation set indicated that the fluid index crossed a predetermined fluid index threshold (60 ohm-days) prior to hospitalization in over 77% of the events. The changes in the calculated fluid index occurred, on average, 15 days prior to symptom onset. The rate of fluid index threshold crossings that were not immediately associated with hospitalization was 1.5 events per patient per year.44
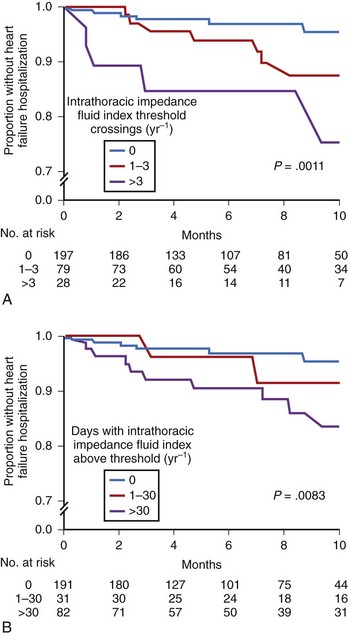
Several recent investigations have further validated the clinical usefulness of chronic heart failure monitoring via intrathoracic impedance. Vollmann and colleagues performed a nonrandomized, multiple-center investigation of 372 patients with CRT-D with intrathoracic impedance monitoring.45 Most subjects were also alerted to fluid index threshold crossings by an audible alert tone transmitted by the device. The results indicated an adjusted sensitivity of 60% and positive predictive value of 60% for various clinically relevant events associated with heart failure. In another study, Ypenburg and colleagues monitored 115 subjects with CRT-D with intrathoracic impedance monitoring over 9 months.46 The sensitivity and specificity of the fluid index reportedly depended strongly on the programmed detection threshold. The authors concluded that optimal algorithm performance may require individualized optimization of the threshold value for the particular patient. Small and colleagues also recently investigated intrathoracic impedance monitoring in 326 patients with CRT-D with no audible alert, since the alert is currently unavailable within the United States.47 The analysis included univariate and multivariate linear regressions of changes in the fluid index as well as other device-recorded diagnostic parameters. The results indicated that each intrathoracic impedance fluid index threshold crossing was associated with a 35% increased probability of hospitalization for heart failure within the same year as the crossing (Figure 89-16). Additional trials have also shown that acute changes in intrathoracic impedance are correlated with both weight and brain natriuretic peptide levels.48–50 All the data from these trials have confirmed that intrathoracic impedance monitoring can provide a useful clinical tool to help manage patients with congestive heart failure.
Peak Endocardial Acceleration
The relative motion of the right ventricular wall, including the septum, can be detected by measuring the peak endocardial acceleration (PEA) by using a sensor integrated into a ventricular pacing lead.51–53 Bordachar et al have correlated this index with LV dP/dT.51 A recent multiple-center observational trial of 15 patients concluded that increases in PEA amplitude were associated with pharmacologic inotropic stimulation and were paralleled by changes in RV dP/dtmax, suggesting that PEA is an index of myocardial contractility.53 Indeed, algorithms to use closed-loop feedback from a lead-based accelerometer to control device therapies, including pacing rate and CRT therapy, have been developed. However, some debate whether the PEA signal is independent of heart sounds still continues, since these two signals are strongly temporally correlated.54 Likewise, the inclusion of additional sensors on ventricular pacing leads may have an uncertain impact on long-term pacing lead reliability.
Heart Rate Variability and Day/Night Heart Rate
Most implantable devices are able to monitor both paced and intrinsic ventricular cycle lengths and hence changes in both day and night heart rates as well as heart rate variability. Such diagnostic data may provide insight into the function of the autonomic nervous system. Recently, Adamson and colleagues reported reductions in device-measured indices of heart rate variability prior to episodes of acute heart failure decompensation.55 Similarly, the OptiVol Fluid InSynch Sentry Registry (OFISSER) clinical trial results demonstrated that increased night heart rates were associated with decompensation in acute heart failure in patients receiving CRT-D therapy.47 However, diagnostic parameters based on heart rate have important limitations. Heart rate variability parameters assume that the rate is controlled by the sinus node. Thus, if the subject is in AF, or if the device is controlling the atrial rate by frequent atrial pacing, then the diagnostic information will not be available. This scenario may represent a significant proportion of time for some patients with implantable devices, especially those who are pacemaker dependent.
Clinical Adoption and Remote Monitoring
Many devices may automatically transmit all sensor-derived parameter trends from the patient’s home directly to the clinic via a bedside monitoring/telemetry device. This device may be programmed by the clinic to transmit automatically on a preset schedule. Alternatively, diagnostic data transmission can also be triggered on the basis of detected clinical events. For example, some devices can be programmed to alert the clinic directly if the patient experiences new-onset atrial tachyarrhythmias or if the ventricular rate during a sustained atrial tachyarrhythmia exceeds a pre-programmed threshold. Such remote monitoring “care alerts” may be quite useful to monitor rate and rhythm control strategies. Also, remote monitoring of heart failure parameters was recommended in at least one published guideline.56 Such remote monitoring capabilities also offer the potential to transmit non–device-recorded information automatically, such as weight, blood pressure, and associated symptoms to the managing clinic.
Integrated Diagnostics
The availability of useful physiological clinical parameters derived from implantable device–based sensors is likely to increase. Future devices may include additional sensors to track respiration rate, minute ventilation, sleep apnea, tissue perfusion, cardiac output, acute ischemia, electrical alternans, and heart rate turbulence. Indeed, some recently released devices already contain some of these fascinating capabilities. The development of practical chemical sensors is also feasible. For example, some external glucose pumps for the chronic management of diabetes also contain chronic glucose monitoring sensors.57 The ability of such chemical sensors to augment other device monitoring capabilities for heart failure or other risks will require additional investigation.
Adamson PB, Smith AL, Abraham WT, et al. InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators: Continuous autonomic assessment in patients with symptomatic heart failure: Prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389-2394.
Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: Crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241-248.
Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073-1079.
Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS Study. Circulation Arrhythmia Electrophysiol. 2009;2(5):474-480.
Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614-1619.
Israel CW, Gronefeld G, Ehrlich JR, et al. Long-term risk of recurrent atrial fibrillation as documented by implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47-52.
Martinek M, Aichinger J, Nesser HJ, et al. New insights into long-term follow-up of atrial fibrillation ablation: Full disclosure by an implantable pacemaker device. J Cardiovasc Electrophysiol. 2007;18:818-823.
Padeletti L, Santini M, Boriani G, et al. Temporal variability of atrial tachyarrhythmia burden in bradycardia-tachycardia syndrome patients. Eur Heart J. 2005;26(2):165-172.
Saksena S, Hettrick DA, Koehler KL, et al. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884-892.
Reiffel JA, Schwarzberg R, Murry M. Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour Holter monitors for arrhythmia detection. Am J Cardiol. 2005;95:1055-1059.
Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: Initial experience with a new permanent implantable device. Circulation. 2007;18(116):2952-2959.
Small R, Wickemeyer W, Germany R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: Clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15(6):475-481.
Strickberger SA, Ip J, Saksena S, et al. Relationship between atrial tachycardias and symptoms. Heart Rhythm. 2005;2:125-131.
Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
Vogel M, Schmidt MR, Kristiansen SB, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: Comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002;105:1693-1699.
Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841-848.
Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445-1452.
1 Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
2 Strickberger SA, Ip J, Saksena S, et al. Relationship between atrial tachycardias and symptoms. Heart Rhythm. 2005;2:125-131.
3 Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224-227.
4 Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: A decade of progress in stroke prevention. Ann Intern Med. 1999;(131):688-695.
5 Bialy D, Lehmann MH, Schumacher DN, et al. Hospitalization for arrhythmias in the United States: Importance of atrial fibrillation. J Am Coll Cardiol. 1992;19:41A.
6 Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: The Framingham Study. Am Heart J. 1983;106:389-396.
7 Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
8 Vasamreddy CR, Dalal D, Dong J, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:134-139.
9 Quirino G, Giammaria M, Corbucci G, Pistelli P, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: Relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32:91-98.
10 Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. Eur Heart J. 2001;22:1852-1923.
11 Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: Crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241-248.
12 Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: Prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369-382.
13 Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18(3):241-247.
14 Reiffel JA, Schwarzberg R, Murry M. Comparison of autotriggered memory loop recorders versus standard loop recorders versus 24-hour Holter monitors for arrhythmia detection. Am J Cardiol. 2005;95:1055-1059.
15 Israel CW, Hugl B, Unterberg C, et al. Pace-termination and pacing for prevention of atrial tachyarrhythmias: Results from a multicenter study with an implantable device for atrial therapy. J Cardiovasc Electrophysiol. 2001;12:1121-1128.
16 Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27:983-992.
17 Sarkar S, Ritscher D, Mehra R. A detector for a chronic implantable atrial tachyarrhythmia monitor. IEEE Trans Biomed Eng. 2008;55:1219-1224.
18 Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445-1452.
19 Martinek M, Aichinger J, Nesser HJ, et al. New insights into long-term follow-up of atrial fibrillation ablation: Full disclosure by an implantable pacemaker device. J Cardiovasc Electrophysiol. 2007;18:818-823.
20 Ziegler PD, Glotzer TV, Daoud EG, et al. Temporal trends from the TRENDS study: Incidence of atrial fibrillation exhibits annual, but not weekly or monthly, variation. Heart Rhythm. 2009;6:S219.
21 Oberg AL, Ferguson JA, McIntyre LM, Horner RD. Incidence of stroke and season of the year: Evidence of an association. Am J Epidemiol. 2000;152:558-564.
22 Saksena S, Hettrick DA, Koehler JL, et al. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884-892.
23 Nagarakanti R, Saksena S, Hettrick D, et al. Progression of new onset to established persistent atrial fibrillation: An implantable device-based analysis with implications for clinical classification of persistent atrial fibrillation. J Interv Cardiol Electrophysiol. 2011;32:7-16.
24 Knight BP, Desai A, Coman J, et al. Long-term retention of cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44(1):72-77.
25 Ziegler PD, Glotzer TV, Daoud EG, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a prior history of stroke. Stroke. 2010;41:256-260.
26 Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614-1619.
27 Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913-1920.
28 Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS Study. Circulation Arrhythmia Electrophysiol. 2009;2(5):474-480.
29 Wilson JR, Hanamanthu S, Chomsky DB, Davis SF. Relationship between exertional symptoms and functional capacity in patients with heart failure. J Am Coll Cardiol. 1999;33:1943-1947.
30 Androne AS, Hryniewicz K, Hudaihed A, et al. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93:1254-1259.
31 Bennett T, Kjellstrom B, Taepke R, Ryden L. Development of implantable devices for continuous ambulatory monitoring of central hemodynamic values in heart failure patients. PACE. 2005;28:573-584.
32 Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure: Clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41(4):565-571.
33 Ohlsson A, Kubo SH, Steinhaus D, et al. Continuous ambulatory monitoring of absolute right ventricular pressure and mixed venous oxygen saturation in patients with heart failure using an implantable haemodynamic monitor: Results of a 1 year multicentre feasibility study. Eur Heart J. 2001;22:942-954.
34 Reynolds DW, Bartelt N, Taepke R, Bennett TD. Measurement of pulmonary artery diastolic pressure from the right ventricle. J Am Coll Cardiol. 1995;25:1176-1182.
35 Steinhaus DM, Lemery R, Bresnehan DR, et al. Initial experience with an implantable hemodynamic monitor. Circulation. 1996;93:745-752.
36 Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: The COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073-1079.
37 Zile MR, Bourge RC, Bennett TD, et al. Application of implantable hemodynamic monitoring in the management of patients with diastolic heart failure: A sub-study of the COMPASS-HF trial. J Card Fail. 2008;14:816-823.
38 Adamson PB, Conti JB, Smith AL, et al. Reducing events in patients with chronic heart failure (REDUCEhf ) study design: Continuous hemodynamic monitoring with an implantable defibrillator. Clin Cardiol. 2007;30:567-575.
39 Walton AS, Krum H. The Heartpod implantable heart failure therapy system. Heart Lung Circ. 2005;14(Suppl 2):S31-S33.
40 Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: Initial experience with a new permanent implantable device. Circulation. 2007;18(116):2952-2959.
41 Baan J, Jong TT, Kerkhof PL, et al. Continuous stroke volume and cardiac output from intra-ventricular dimensions obtained with impedance catheter. Cardiovasc Res. 1981;15(6):328-334.
42 Ellenbogen KA, Wood MA, Shepard RK, et al. Detection and management of an implantable cardioverter defibrillator lead failure: Incidence and clinical implications. J Am Coll Cardiol. 2003;41(1):73-80.
43 Cole CR, Jensen DN, Cho Y, et al. Correlation of impedance minute ventilation with measured minute ventilation in a rate responsive pacemaker. Pacing Clin Electrophysiol. 2001;24(6):989-993.
44 Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841-848.
45 Vollmann D, Nägele H, Schauerte P, et al. European InSync Sentry Observational Study Investigators: Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28(15):1835-1840.
46 Ypenburg C, Bax JJ, van der Wall EE, et al. Intrathoracic impedance monitoring to predict decompensated heart failure. Am J Cardiol. 2007;99(4):554-557.
47 Small R, Wickemeyer W, Germany R, et al. Changes in intrathoracic impedance are associated with subsequent risk of hospitalizations for acute decompensated heart failure: Clinical utility of implanted device monitoring without a patient alert. J Card Fail. 2009;15(6):475-481.
48 Andriulli J, Crossley G, McKenzie J, et al. Acute weight increases are associated with decreased intrathoracic impedance in heart failure patients: Initial results of the IMPEDE HF trial (abstract). J card Fail. 2008;14:S70.
49 Lüthje L, Vollmann D, Drescher T, et al. Intrathoracic impedance monitoring to detect chronic heart failure deterioration: Relationship to changes in NT-proBNP. Eur J Heart Fail. 2007;9:716-722.
50 Matsushita K, Ishikawa T, Sumita S, et al. Daily shock impedance measured by implantable cardioverter defibrillator is useful in the management of congestive heart failure. Circ J. 2006;70(11):1462-1465.
51 Bordachar P, Labrousse L, Ploux S, et al. Validation of a new noninvasive device for the monitoring of peak endocardial acceleration in pigs: Implications for optimization of pacing site and configuration. J Cardiovasc Electrophysiol. 2008;19(7):725-729.
52 Rickards AF, Bombardini T, Corbucci G, Plicchi G. An implantable intracardiac accelerometer for monitoring myocardial contractility. The Multicenter PEA Study Group. Pacing Clin Electrophysiol. 1996;19:2066-2071.
53 Vogel M, Schmidt MR, Kristiansen SB, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: Comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002;105:1693-1699.
54 Tassin A, Kobeissi A, Vitali L, et al. Relationship between amplitude and timing of heart sounds and endocardial acceleration. Pacing Clin Electrophysiol. 2009;32:S101-S104.
55 Adamson PB, Smith AL, Abraham WT, et al. InSync III Model 8042 and Attain OTW Lead Model 4193 Clinical Trial Investigators: Continuous autonomic assessment in patients with symptomatic heart failure: Prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389-2394.
56 Wilkoff BL, Auricchio A, Brugada J, et al. Heart Rhythm Society (HRS); European Heart Rhythm Association (EHRA); American College of Cardiology (ACC); American Heart Association (AHA); European Society of Cardiology (ESC); Heart Failure Association of ESC (HFA); Heart Failure Society of America (HFSA). HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations: Developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace. 2008;10:707-725.
57 Steil GM, Rebrin K, Mastrototaro J, et al. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther. 2003;5:27-31.