Chapter 42 Diagnostic and Operative Hysteroscopy: Polypectomy, Myomectomy, and Endometrial Ablation
INTRODUCTION
Hysteroscopy is an accurate surgical approach for evaluating the uterine cavity and treating a multitude of abnormalities. At its essence, it involves the transcervical placement of a lens and light system into the uterine cavity while using gas or liquid for distension of the cavity. Modern hysteroscopy is performed almost exclusively with the addition of a camera and video monitoring system. Operative hysteroscopy is performed using an electrosurgical resectoscope or a sleeve with an operating channel to accommodate instruments for grasping, biopsying, or cutting with scissors or laser.
HISTORY
Hysteroscopy, first described in the late 1800s, did not find widespread clinical use for more than 100 years. In 1869, Pantaleoni performed the first diagnostic and therapeutic hysteroscopy when he used a modified cystoscope to look into the uterine cavity and cauterize a hemorrhagic growth.1 Early hysteroscopists used a tube to mechanically distend the uterus for visualization. Near the beginning of the 20th century, Dr. Isidor C. Rubin, a New York gynecologist best remembered for Rubin’s test, first used carbon dioxide (CO2) to distend the uterus for hysteroscopy. Around the same time, Professor C. J. Gauss, a German surgeon and descendant of the famous mathematician, first performed hysteroscopy using fluid distension media.2 However, hysteroscopy did not find widespread acceptance for another half-century.3
In the 1970s interest in hysteroscopy renewed, in parallel with the rapid advancement of diagnostic and operative laparoscopy. Probably the most important advances came in the form of improved methods for distending the uterine cavity, including use of viscous and low-density liquid solutions. Around the same time insufflation machines were designed for CO2 and fluid media that utilized high pressure and low flow, rather than the low pressure and high flow used for laparoscopy.4–6 Carbon dioxide was often used for diagnostic hysteroscopy, and fluid media became the standard for operative hysteroscopy. It was found that isotonic fluid was ideal for most operative procedures, whereas nonconductive hypotonic media was required for electrosurgical procedures.
The use of hysteroscopy became widespread in the 1980s with the development of better optics and lighting and the use of video cameras. Operative techniques for various intrauterine pathologic conditions continued to be developed. Most notably among these may be removal of submucous myomas using an enhanced urologic resectoscope,7 removal of uterine septum,8 and endometrial ablation using laser,9 resectoscopic loop,10 or rollerball.11 Today, hysteroscopy has become a standard diagnostic and therapeutic technique performed by most gynecologists.
INDICATIONS
Operative Hysteroscopy
The two most common indications for operative hysteroscopy are (1) directed biopsy of focal lesions and (2) treatment of intrauterine lesions, including endometrial polyps and intracavitary leiomyomas, uterine septa, and cornual tubal occlusion. Removal of uterine septum and treatment of cornual occlusion with cannulation are covered in Chapters 43 and 47.
Less common indications for operative hysteroscopy include retrieval of a “lost” intrauterine device with a retracted tailstring, discussed in Chapter 27. Hysteroscopy has also been used in early pregnancy for chorionic villus sampling. The newest indication is the placement of coils into the intramural section of the tube for permanent sterilization, which is covered in Chapter 28.
BASIC HYSTEROSCOPIC EQUIPMENT AND TECHNIQUES
Distension Media
Carbon Dioxide
Another problem associated with the use of CO2 for hysteroscopy is the rare occurrence of fatal gas embolism. Although a small amount of CO2 is rapidly absorbed and cleared from the body via respiration, an open vessel in the uterus can allow enough gas into the venous system to result in fatal heart block.12 To minimize this risk when CO2 is used for hysteroscopy, it has been recommended that the Trendelenburg position should be avoided, the cervix should not be dilated, and no operative procedures should be performed.
Dextran 70
Dextran 70 is rarely used today as hysteroscopic distension media because of several associated problems.13 From an operational perspective, dextran 70 solutions cause instruments such as graspers and scissors to become permanently inoperable if the solution is allowed to dry on the instruments. This problem can usually be avoided by immediate cleaning shortly after finishing the procedure.
Finally, allergic reactions to dextran 70 have also been reported. The risk of anaphylaxis while using dextran 70 for hysteroscopy has been estimated to be as high as 1 per 1500 cases.14
Low-Viscosity Fluid
Hypotonic nonelectrolyte-containing fluids are required when the unipolar resectoscope is used, and several types are available. The most common fluids used are 5% mannitol, 3% sorbitol, and 1.5% glycine. The theoretical advantage of 5% mannitol is that it is rapidly broken down by the liver into glycogen and is excreted through the kidney, with a half-life of 100 minutes.15
When a fluid deficit of 1,000mL of nonelectrolyte solution is identified, blood should be drawn to determine electrolyte levels, the procedure should be terminated, and consideration should be given to administering diuretics, with close monitoring of electrolytes. Injection of 3 to 4mL of dilute vasopressin (10 units in 50mL saline solution) into the cervix decreases both intraoperative bleeding and intravasation for at least 20 to 30 minutes.16
DIAGNOSTIC HYSTEROSCOPY
In the 1980s, the development of hysteroscopes with smaller diameters (<4mm) allowed the use of the hysteroscope for diagnosis without the need for either cervical dilation or anaesthesia. As a result, office hysteroscopy has become a common procedure, which has been documented to have the advantages of patient acceptability, diagnostic accuracy, and cost- effectiveness.17 Hysteroscopy is particularly useful for identifying focal lesions, which are often missed with endometrial sampling.18
Office Hysteroscopy
A low (<1%) complication rate is noted when office hysteroscopy is performed by skilled physicians. Complications include uterine perforation, infections, excessive bleeding, and complications related to the distension media.19
Technique
A slow and thorough evaluation is important to identify abnormalities of the endocervix, fundus, and tubal ostia. Myomas of the endocervix and lower uterine segment can easily be missed with too rapid insertion of the hysteroscope. Likewise, if the hysteroscope is too quickly placed above an intracavitary lesion, it can likewise be missed.
Lesion Size
The size of lesions cannot be accurately determined hysteroscopically, in contrast to transvaginal ultrasound. This is because the hysteroscopic eyepiece is focused at infinity, thereby making the objects that are closer appear magnified and objects viewed further away smaller.20 This phenomenon can lead to surprises in the operating room, especially when the size of a lesion, particularly submucosal myomas, may be underestimated.
HYSTEROSCOPIC POLYPECTOMY
Polyps are often symptomatic. However, in women with abnormal uterine bleeding, investigation may lead to their detection. Symptoms most often related to uterine polyps include abnormal bleeding, postcoital staining, chronic vaginal discharge, or dysmenorrhea. Polyp-related bleeding is often characterized by increased clotting, intermenstrual or premenstrual spotting, or heavier menstrual flow. There is good evidence that polyps can decrease fertility and that their removal will improve the chances of pregnancy.21
It is obvious that symptomatic endometrial polyps should be removed. However, it is also important to remove asymptomatic polyps, particularly in postmenopausal women.22 Although the vast majority are benign, endometrial cancer and hyperplasia will be found in approximately 2% of endometrial polyps and are associated with coexisting malignancies elsewhere in the endometrium. In one study of more than 1400 polyps, endometrial cancer was found in 27 polyps (1.8%).22 All but one of these women were postmenopausal, and 26% were asymptomatic.
HYSTEROSCOPIC MYOMECTOMY
Leiomyomas are the primary indication for more than 40% of the 650,000 hysterectomies performed annually in the United States.23 Submucosal myomas likely account for 10% to 20% of all myomas. Many of these can be removed by operative hysteroscopy. In addition to preserving fertility in many cases, a hysteroscopic approach is associated with a shorter recovery period, lower complication rate, and lower cost than hysterectomy.
For a successful surgical outcome, it is important to identify preoperatively the size, number, location, and depth of intramural extension of uterine myoma. Myoma size, number, and location are determinants of complete resectability, the number of surgical procedures necessary for complete resection, the duration of surgery, and the potential complications from fluid overload.24
Numerous studies have demonstrated that preoperative saline infusion sonohysterography gives more information than hysteroscopy in respect of myomas. Chapter 30 reviews the topic of ultrasonography and sonohysterography in detail.
Hysteroscopic Classification of Myomas
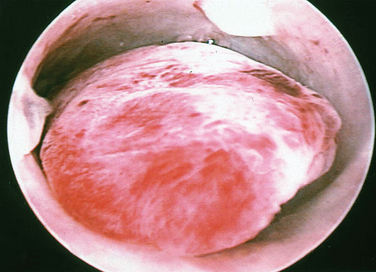
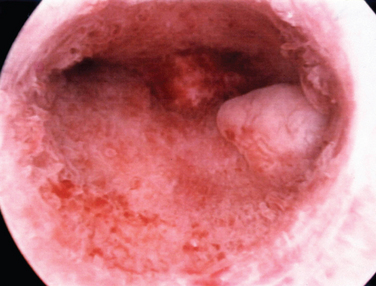
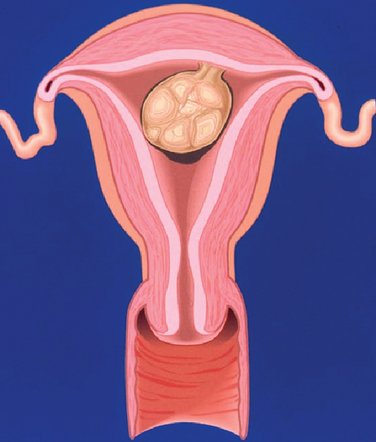
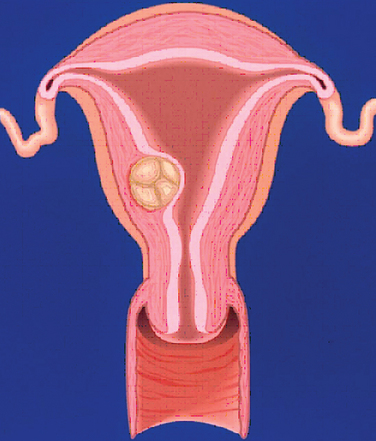
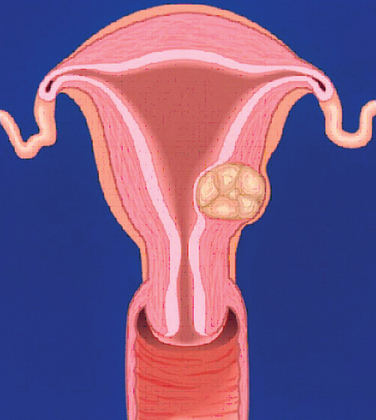
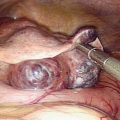
A classification system is important to most accurately determine the appropriate method for performing myomectomy and counseling the patient on risk and prognosis. The European Society of Hysteroscopy classification system is based on myoma location and the amount of myoma protruding or encroaching on the endometrial cavity.25 In this system, Type 0 myomas are pedunculated, with the myoma lying completely within the endometrial cavity (Fig. 42-1). Type I myomas are described as sessile, with less than 50% intramural extension (Fig. 42-2). Finally, Type II myomas are submucosal in location, with more than 50% intramural extension. These include transmural myomas, which extend from the submucosal to the serosal edge. When viewed hysteroscopically, Type II myomas appear as a “bulge” into the endometrial cavity. Multiple myomas such as those in Figure 42-3 are not placed within this classification. They should not accessed by hysteroscopy.
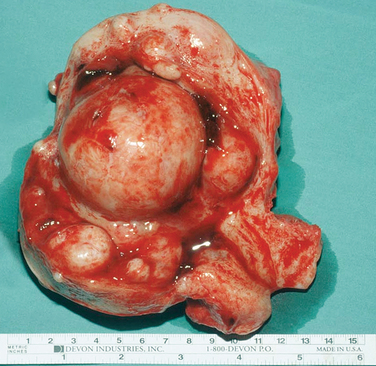
Figure 42-3 Extirpated uterus showing a large (6 cm) intracavitary myoma with multiple intramural and submucous myomas.
This system was originally designed to classify myomas exclusively on hysteroscopic appearance. However, this approach has significant limitations. During hysteroscopy, myomas can be compressed and recede into the myometrium as a result of the pressure of the distension media, thereby preventing full visualization of the myoma. For this reason, preoperative evaluation with ultrasonography is required to accurately determine how many myomas are present and how deeply the myomas penetrate the myometrium.
An ultrasonographic classification system has been developed for intramural myomas that corresponds in part to the hysteroscopic classification and in part to the hysterosalpingography data26 (Table 42-1).
Table 42-1 Hysteroscopic and Sonohysterographic Classification System for Myomas Encroaching Upon the Endometrial Cavity
| Hysteroscopic Type25 | Sonohysterographic Class26 | Description |
|---|---|---|
| Type 0 | Class 1 | Pedunculated myomas, where 100% of the myoma lies within the endometrial cavity with no intramural extension |
| Type I | Class 2 | Sessile myomas, with <50% intramural extension |
| Type II | Class 3 | Submucous myomas, with >50% intramural extension |
Surgical Approach According to Stage
The degree of surgical difficulty and thus the risk to the patient is related to the depth of penetration and size of the myomas. Pedunculated hysteroscopic Type 0 or sonohysterographic Class 1 myomas up to 3cm in dimeter can usually be easily removed hysteroscopically. Larger hysteroscopic Type 0 myomas (>3cm) and hysteroscopic Type I (Class 2 on sonohysterography) myomas can be approached hysteroscopically. However, the risk of fluid intravasation increases as a result of increased surgical time and the opening of myometrial venous channels during resection. Operative hysteroscopy is made more difficult by limited space within the uterus and poor visibility due to an inability to further distend the uterine media and the large amount of myoma “chips” that accumulate within the endometrial cavity. Often, incomplete removal of larger myomas requires two or more separate operative procedures. Only the most experienced hysteroscopist would attempt a hysteroscopic resection of an intracavitary myoma 5cm or larger. Myomas that are large and multiple (see Fig. 42-3) should not be excised by hysteroscopy.
Patient Selection
The ideal patient for hysteroscopic myomectomy should meet the following criteria:
Preoperative Medications
Cervical Preparation
Use of a laminaria or misoprostol is recommended to facilitate dilation. Additionally cervical softening agents decrease the risk of cervical tears, decrease the risk of uterine perforation, and decrease the use of unnecessary force to achieve cervical dilation. The most common complications associated with operative hysteroscopic surgery is cervical laceration (Table 42-2).
Table 42-2 Risk of Complications Associated with Endometrial Ablation and Resection13,14
| Complication | Rate |
|---|---|
| Intraoperative | |
| Cervical lacerations | 4% |
| Uterine perforation | 1.4% |
| Bowel/bladder/vessel injury | Rare |
| Fluid overload | Rare |
| Postoperative | |
| Infection | 0.4% |
| Hematometra | 1–2% |
| Delayed | |
| Bleeding requiring further surgery | 10% |
| Subsequent endometrial cancer | Rare |
Misoprostol can be administered vaginally or orally to soften the cervix, although this indication is not approved by the Food and Drug Administration (FDA). Vaginal misoprostol (200 to 400μg) given 8 to 12 hours before surgery reduces the need for cervical dilation, decreases cervical complications, and reduces operative time in comparison with controls.27 Alternatively, oral misoprostol (400μg) can be given 12 and 24 hours before surgery to soften the cervix and make dilation easier.28 Side effects associated with misoprostol include lower abdominal pain and small amounts of vaginal bleeding.
Technique of Hysteroscopic Myomectomy
In 1978, Neuwirth first reported the use of the monopolar urologic resectoscope for resection of submucosal myomas.29 Hysteroscopic wire-loop resection still remains the most popular method of removing myomas, but newer techniques are emerging.
Performing operative hysteroscopic myomectomy requires four essential components:
Vasopressin
A dilute solution of vasopressin (20mL vasopressin mixed in 100mL normal saline solution) can also be injected circumferentially in 5-mL aliquots into the cervical stroma at the 12, 3, 6, and 9 o’clock position of the cervix. This use of vasopressin is not FDA approved. Vasopressin has been shown to decrease bleeding and the intravascular absorption of distension fluids, apparently as a result of vasoconstriction.30,31 Repeat injections can be administered every 45 to 60 minutes as needed. Close monitoring of blood pressure, heart rate, and arrhythmias is necessary.
Uterine Distension
The fluid deficit should be continuously calculated, preferably with an automated system. When electrolyte-free solutions are being used (i.e., glycine, sorbitol, or mannitol), the procedure should be discontinued when a fluid deficit of 1000mL is reached and serum electrolyte levels should be promptly obtained. When electrolyte solutions are used (e.g., saline) the procedure should be discontinued when a fluid deficit of 1500 to 2000mL is reached and a diuretic administered to decrease the risk of pulmonary edema.
Special Techniques
Prostaglandin F2α Analogue
Despite evaluation, some myomas are technically challenging to remove or the surrounding pseudocapsule is not well demarcated. Myomas with a significant intramural location require the most experienced hysteroscopic expertise to remove. In recent studies carboprost, a methyl analogue of prostaglandin F2α, showed promise in facilitating enucleation of myomas. This is not an FDA-approved use of the drug. A recent report describes the successful use of carboprost in 10 women to facilitate hysteroscopic myomectomy.32 Carboprost led to contraction of the myometrium and extrusion of the unresectable intramural component into the endometrium, facilitating hysteroscopic resection.
ENDOMETRIAL ABLATION
Preoperative Evaluation
A thorough preoperative evaluation is imperative because many serious diseases can present as abnormal uterine bleeding. The investigation and management of abnormal uterine bleeding is described in Chapter 21. A brief overview is given here to highlight the importance of a proper evaluation before a surgical procedure.
The evaluation begins with a detailed history, physical examination, and ultrasonographic imaging. Important laboratory tests for evaluation of women with menorrhagia include a pregnancy test, thyrotropin level, and complete blood count with platelet count. Many clinicians recommend a platelet function screen or a von Willebrand ristocetin cofactor. If the platelet function screen is abnormal, testing for von Willebrand’s disease is indicated. A recent study reported 47% of reproductive-age women with menstrual dysfunction were found to have a hemostatic abnormality such as platelet dysfunction, von Willebrand’s disease, or coagulation factor deficiencies.33
Risks
Complications of endometrial ablation are uncommon, and many are even less common with the newer ablative techniques compared to hysteroscopic techniques. The interoperative risks include fluid overload, uterine perforation, cervical lacerations, and bowel injury (see Table 42-2). These complications are more common in women undergoing traditional hysteroscopic ablation and resection techniques.
Endometrial Ablation Techniques
First-Generation Hysteroscopic Endometrial Ablation
In the early 1980s, endometrial ablation was established as an alternative to hysterectomy for treatment of menstrual dysfunction in women with intractable menorrhagia. Mimicking the physiologic effect of Asherman’s syndrome, the ultimate goals of endometrial ablation were to create severe endometrial scarification and secondary amenorrhea. Initially, Goldrath and colleagues reported laser endometrial ablation using the Nd:YAG laser fiber through the operating port of a hysteroscope. However, this method waned in popularity due to costs and riks of complications in less experienced hands.9
Endomyometrial resection utilizing a resectoscope was first reported by DeCherney and Polan in 1983.34 This technique utilizes unipolar electrocautery and is performed with hypotonic nonelectrolyte-containing distension media. This technique was the forerunner of hysteroscopic rollerball endometrial ablation, which has become the “gold standard” to which all emerging endometrial ablation technology is compared. This technique, which also uses a resectoscope, was popularized by Vancaillie in 1989.11 Each of these devices destroys the basalis layer of the endometrium and is designed to result in hypomenorrhea or amenorrhea.
Technique of Hysteroscopic Rollerball Endometrial Ablation
Rollerball Electrode
The depth that the endometrium is coagulated depends on tissue and equipment factors. Tissue-related factors include endometrial thickness and variations in tissue impedance related to the number of previous passes. Equipment-related factors include the diameter and shape of the electrode, waveform and power utilized, cleanliness of the electrode, time of exposure of the electrode to the endometrium, pressure against tissue, and speed of electrode. Several varieties and shapes of electrodes are available to perform hysteroscopic endometrial ablation, including ball, barrel, ellipsoid, and large caliber loops.
Technique of Endomyometrial Resection
The technique of endomyometrial resection was pioneered by DeCherney and colleagues.10 The 90-degree wire loop, generally 3 to 4mm deep, is buried into the endometrium, and using 60 to 80 watts of pure cutting current, is advanced under direct view to the lower uterine segment. The endomyometrial junction is shaved off, creating crescent-shaped tissue fragments.
Causes of Endometrial Ablation Failure
Hysteroscopic endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 60% of patients undergoing endometrial ablation with roller-ball techniques will achieve amenorrhea, 65% to 70% will become hypomenorrheic, and 5% to 10% will fail. Approximately 10% of patients treated by endometrial ablation will require a subsequent operation.35 Women receiving appropriate preoperative counseling may find this attractive in treating menstrual disorders.
GLOBAL ENDOMETRIAL ABLATION TECHNIQUES
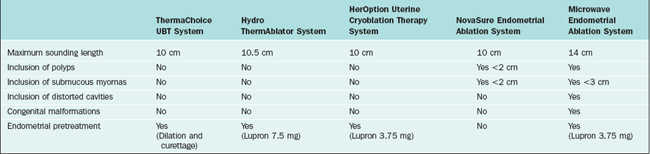
Table 42-3 lists the different devices and the specific patient characteristics. In theory, all these devices can be used in an office setting.
ThermaChoice UBT System
Although not originally evaluated for use with myomas, case series have demonstrated efficacy with their presence.36 These data showed a significant and statistically similar reduction in menstrual blood flow and increase in hemoglobin values with no intraoperative complications.
HerOption Uterine Cryoblation Therapy System
The second global endometrial ablation technology approved by the FDA in April 2001 was the HerOption Uterine Cryoblation Therapy System. This technology uses a cryoprobe and proprietary compressed gas mixture that freezes the endometrium to below 100°C, thereby destroying the endometrial lining. It creates the largest depth of necrosis (9 to12mm) of the second-generation devices. In the FDA trial, patients were pretreated with a single dose of leuprolide acetate (3.75mg intramuscularly). The unit consists of a console, a cryoprobe, and disposable sheath that fits over the cryoprobe. FDA guidelines require continuous transabdominal ultrasound guidance to monitor progression of the ice ball created during the procedure.
Microwave Endometrial Ablation System
During the initial 5 seconds of applicator placement, the temperature rise gate (TRG) calculates intrauterine temperature increase. If the rise in temperature is normal, the procedure is continued; otherwise, the procedure is aborted spontaneously. An abnormal TRG signals possible false passage or applicator perforation. The applicator is then placed near the fundus and moved from side to side until the temperature reaches 70°C. After fundal warming occurs, treatment of the remaining uterine cavity is undertaken by a series of movements of the applicator from side to side from the cornua to midendometrial body, until the endocervix is reached. A built-in computer link allows the physician to monitor the overall treatment. Total treatment time is usually 2 to 5 minutes.
1 Schenk LM, Coddington CC3rd. Laparoscopy and hysteroscopy. Obstet Gynecol Clin North Am. 1999;26:1-22.
2 Gauss CJ. Hysteroskopie. Arch Gynaekol. 1928;133:18-24.
3 Neuwirth RS. Hysteroscopy and gynecology: Past, present, and future. J Am Assoc Gynecol. 2001;8:193-198.
4 Edstrom K, Fernstrom I. The diagnostic possibilities of a modified hysteroscopic technique. Acta Obstet Gynecol Scand. 1970;79:327-330.
5 Lindemann HJ. Eine neue Untersuchungsmethode fur die Hysteroskopie. Endoscopy. 1971;4:194-197.
6 Quinones R, Alvaredo-Duran A, Aznar-Ramos R. Tubal catheterization: Application of a new technique. Am J Obstet Gynecol. 1972;114:164-169.
7 Neuwirth RS, Amin HK. Excision of submucous fibroids with hysteroscopic control. Am J Obstet Gynecol. 1976;126:95-99.
8 Daly DC, Tohan N, Walters C, Riddick DH. Hysteroscopic resection of the uterine septum in the presence of a septate cervix. Fertil Steril. 1983;39:560-563.
9 Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium in the treatment of menorrhagia. Am J Obstet Gynecol. 1981;140:14-19.
10 DeCherney AH, Diamond MP, Lavy G, Polan ML. Endometrial ablation for intractable uterine bleeding: Hysteroscopic resection. Obstet Gynecol. 1987;70:668-670.
11 Vancaillie TG. Electrocoagulation of the endometrium with the ball-end resectosope. Obstet Gynecol. 1989;74:425-427.
12 Loffer FD. Complications of hysteroscopy—their cause, prevention, and correction. J Am Assoc Gynecol Laparosc. 1995;3:11-26.
13 Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
14 Ahmed N, Falcone T, Tulandi T, Houle G. Anaphylactic reaction because of intrauterine 32% dextran-70 instillation. Fertil Steril. 1991;55:1014-1016.
15 Marlow JL. Media and delivery systems. Obstet Gynecol Clin North Am. 1995;22:409-422.
16 Corson SL, Brooks PG, Serden SP, et al. Effects of vasopressin administration during hysteroscopic surgery. J Reprod Med. 1994;39:419-423.
17 Bradley L, Widrich T. State-of-the-art flexible hysteroscopy for office gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
18 Nagele F, O’Connor H, Davies A, et al. 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol. 1996;88:87-92.
19 Serden SP. Diagnostic hysteroscopy to evaluate the cause of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:277-286.
20 Apgar B, Dewitt D. Diagnostic hysteroscopy. Am Family Phys. 1999;46:19S-26S.
21 Perez-Medina T, Bajo-Arenas J, Salazar F, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: A prospective, randomized study. Hum Reprod. 2005;20:1632-1635.
22 Martin-Ondarza C, Gil-Moreno A, Torres-Cuesta L, et al. Endometrial cancer in polyps: A clinical study of 27 cases. Eur J Gynaecol Oncol. 2005;26:55-58.
23 Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
24 Emanuel MH, Verdel MJ, Wamsteker K. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: Clinical implications. Am J Obstet Gynecol. 1995;172:547-552.
25 Wamsteker K, de Kruif J. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: Results regarding the degree of intramural extension. Obstet Gynecol. 1993;82:736-740.
26 Bradley LD, Falcone T, Magen AB. Radiographic/imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:245-276.
27 Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: A randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
28 Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: A double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
29 Neuwirth RS. A new technique for and additional experience with hysteroscopic resection of submucous fibroids. Am J Obstet Gynecol. 1978;131:91-94.
30 Phillips DR, Nathanson HG, Milim SJ, et al. The effect of dilute vasopressin solution on blood loss during operative hysteroscopy: A randomized controlled trial. Obstet Gynecol. 1996;88:761-766.
31 Goldenberg M, Zolti M, Bider D, et al. The effect of intracervical vasopressin on the systemic absorption of glycine during hysteroscopic endometrial ablation. Obstet Gynecol. 1996;87:1025-1029.
32 Indman PD. Use of carboprost to facilitate hysteroscopic resection of submucous myomas. J Am Assoc Gynecol Laparosc. 2004;11:68-72.
33 Philipp CS, Faiz A, Dowling N, et al. Age and the prevalence of bleeding disorders in women with menorrhagia. Obstet Gynecol. 2005;105:61-66.
34 DeCherney A, Polan ML. Hysteroscopic mangement of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-397.
35 Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54:251-262.
36 Soysal ME, Soysal SK, Vicdan K. Thermal balloon ablation in myoma-induced menorrhagia under local anesthesia. Gynecol Obstet Invest. 2001;51:128-133.