Chapter 43 Diagnosis and Treatment of Complex Regional Pain Syndrome
INTRODUCTION
Complex regional pain syndrome (CRPS) is the current terminology that refers to a disease first described during the era of the U.S. Civil War.46 Nomenclature relating to CRPS has varied over the years and included different descriptions, diagnostic studies, and treatments, few of which have been well studied. Adding to the confusion, this disorder has a variable presentation and there is no single test to confirm the diagnosis.7,34,50 CRPS is a clinical diagnosis of exclusion. It is difficult to interpret or gain useful information from the medical literature regarding this disease owing to the vague and varied nature of complaints and the 20 different terms that have been used to denote this syndrome.
CRPS is a chronic pain syndrome that usually follows tissue injury in which the patient experiences severe nondermatomal pain associated with painful responses to nonpainful stimuli, along with changes to the autonomic and trophic systems. The pain is associated with changes in skin color, temperature, sweating, edema, and range of motion. The pain is distinct in that it is out of proportion to the inciting event. This is problematic, because it often causes a delay in diagnosis as physicians believe the patient either is seeking recreational drugs or has a psychological disturbance or some other secondary gain issues.28
By definition, the previously named disease reflex sympathetic dystrophy (RSD) is now referred to as CRPS type I, and the formerly named causalgias are incorporated into the definition of CRPS type II. The International Association for the Study of Pain established the initial definition of CRPS to help eliminate confusion. This definition was later subdivided by Merskey and Bogduk into types I and II.45 The pain generator is described as being either sympathetically maintained pain (SMP) or sympathetically independent pain (SIP), depending on whether or not the sympathetic nervous system is involved.70
CRPS type I is defined as having
TABLE 43-1 Diagnostic Criteria of the International Association for the Study of Pain for Complex Regional Pain Syndrome Type I and Type II
| Type of CRPS | Diagnostic Criteria |
|---|---|
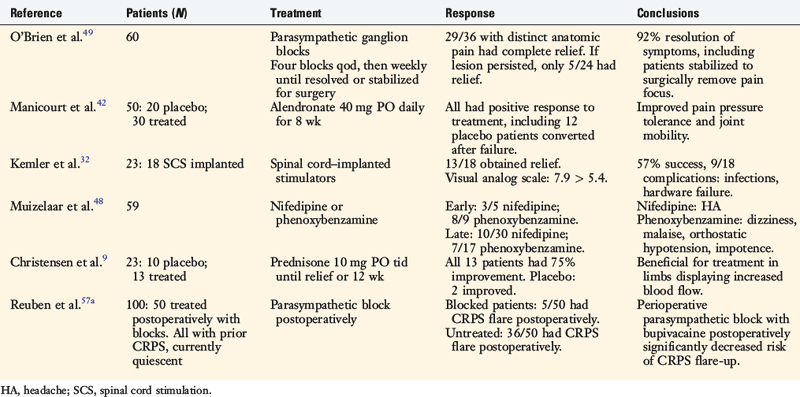
| Type I (reflex sympathetic dystrophy) |
Spontaneous pain or allodynia/hyperalgesia occurs beyond the territory of a single peripheral nerve and is disproportionate to the inciting event.
|
CRPS, complex regional pain syndrome.
Reprinted with permission from Stanton-Hicks, M., Janig, W., Hassenbuch, S., et al.: Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 63:127–133, 1995.
CRPS type II has the same definition as type I with the exception that it is due to injury to a single peripheral nerve. In this respect, the region of disproportionate pain is initially within a dermatome (unlike CRPS type I that follows no dermatomal pattern).45 As the disease progresses, it can spread beyond a single dermatome.
Both CRPS types I and II have components of SMP and SIP.70 In fact, both SMP and SIP will frequently exist in each patient to varying degrees (Fig. 43-1). As is described later, there are periods when the nerve transmissions from the sympathetic nervous system will be interpreted by the body as pain. When this occurs, the normal signals to increase heart rate and blood pressure in response to fight-or-flight conditions not only affect the sympathetic nervous system but also cause pain. This source of pain is SMP. All other pain associated with CRPS is SIP. The only way to determine how much of the patient’s pain is due to SMP and how much is due to SIP is to perform a sympathetic nerve block. The pain relieved is SMP, and the remaining pain is SIP.68
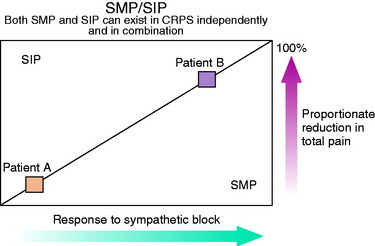
(Reprinted with permission from Janig, W.; Stanton-Hicks, M. [eds.]: Reflex Sympathetic Dystrophy: A Reappraisal Progress in Pain Research and Management, Vol. 6, Seattle, IASP Press, 1996, p. 83.)
Despite the complexity of the definition of CRPS, the hallmark of this disease is pain out of proportion to the inciting event. When this occurs, it is incumbent upon the clinician to be suspicious of CRPS and initiate treatment as soon as possible. Early management yields a more favorable outcome when initiated before the changes of skin color, temperature, and sweating occur.29
ANATOMY
To understand the basis of the pathology of CRPS, the clinician must have a rudimentary understanding of the human nervous system anatomy and function. At the clinical level, a more detailed understanding is not required. The intent is to describe the pathology in a way that makes sense to the clinician and so that it can be explained to patients in a manner that allows them to understand the disease and become active participants in their recovery. It is important to know that of the six predominant pathologic mechanisms that have been proposed to explain CRPS, none completely explains the clinical findings and results of treatment and all have shortcomings explained by other theories.3,7,50
CNS, central nervous system (brain and spinal cord); PNS, peripheral nervous system.
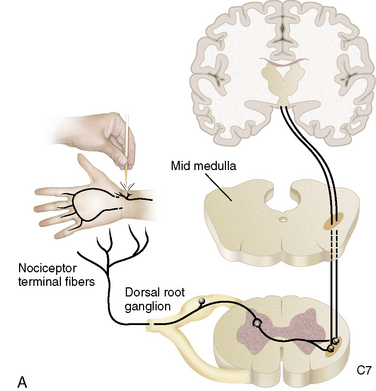
The nervous system consists of somatic and autonomic branches. The somatic system includes the central nervous system (CNS; brain and spinal cord) and the peripheral nervous system (PNS). The dorsal root ganglion is located in the paravertebral region, which contains the sensory nerve cell bodies.21 It is through the dorsal root ganglion that specialized ganglia transmit signals representing pain, pressure, and temperature from sensory nerves to the spinal cord.66
The autonomic nervous system is composed of sympathetic and parasympathetic divisions. Of importance is the sympathetic nervous system, which has cell bodies in the thoracolumbar paravertebral chain ganglions adjacent to the vertebral column. In the cervical region, the most inferior of the paravertebral ganglions is the stellate ganglion, which is believed to supply the majority of the sympathetic innervation to the upper extremities. In the lower extremities, the sympathetic innervation is carried through lumbar paravertebral ganglions.21
The sympathetic nervous system is responsible for the fight-or-flight response. This reaction is mediated by the catecholamine neurotransmitters, epinephrine and norepinephrine. These catecholamines stimulate receptors called α and β receptors. When α receptors are stimulated, the body responds with elevated heart rate and blood pressure, piloerection, and skin vasoconstriction. The β receptors stimulate increased heart rate, muscle vasodilation, and bronchial dilatation.40
In some cases, knee surgery as benign as arthroscopy can injure nerve structures and promote a CRPS type II. In the largest known study of 395,566 arthroscopic procedures, 234 nerve injuries were identified from operative notes.60 In a knee-specific study of 118,590 arthroscopies conducted by the Arthroscopy Association of North America, 63 neurologic complications were noted.60 In the same study, RSD was diagnosed in 44 patients. Both of these studies had a prevalence rate of nerve injury of less than 0.1%.
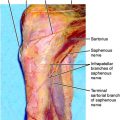
O’Brien and coworkers49 described 60 patients referred for RSD of the knee. Of these, 40 had developed symptoms after knee surgery (24 arthroscopic and 12 arthroplasty procedures). In the authors’ experience, many cases of CRPS begin with symptoms of saphenous neuritis. On the medial side of the knee, the saphenous nerve lies deep to the sartorius muscle. The infrapatellar branch of the saphenous nerve lies inferior to the medial joint line (see Fig. 1-23A). Formation of a neuroma may result from direct injury by the scalpel during anteromedial portal placement. Techniques for avoiding injury to the saphenous nerve and its infrapatellar branch include careful dissection through a 1-cm incision with identification of the nerve, transillumination with use of the arthroscope, and use of a 2-cm posteromedial incision with posterior retraction to deflect the needles anteriorly during inside-out medial meniscal repair.60
PATHOPHYSIOLOGY
Six major theories have been devised to explain the findings and response to treatment of CRPS. Unfortunately, no single unifying theory is available. For that reason, only a few of the current theories are presented in this chapter (Fig. 43-2).
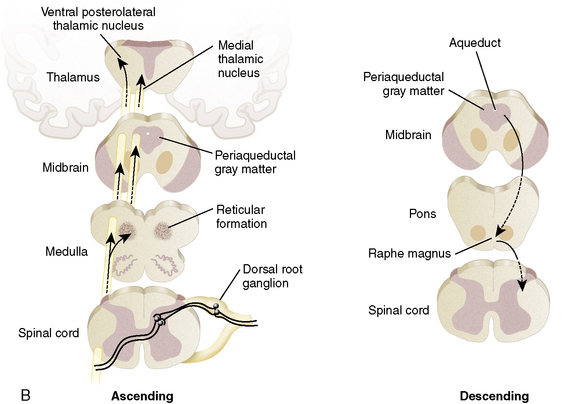
One hypothesis is that a pathologic interaction occurs between the somatic nervous system (in part responsible for sensations) and the autonomic nervous system (responsible for the fight-or-flight mechanism). Normally, these two systems do not directly interact. However, when a pathologic interaction does takes place, it occurs at a newly created abnormal synapse called an ephapse.3 An ephapse may take place in either the PNS or the CNS. The ephapse causes a type of short circuit to occur between the somatic and the autonomic nervous systems. The signals being sent from the CNS to the PNS to control heart rate and other factors are short-circuited to sensory fibers and are misinterpreted as pain signals being sent from the PNS to the CNS.27 The sympathetic nervous system drives the pain and, as a result, increased anxiety (which would normally increase blood pressure and heart rate) amplifies pain.
Another theory focuses on CRPS type II in which a specific nerve has been transected. An explanation for the CRPS findings from such a transection stem from the neuroma that forms as a result of the injury. Within this neuroma, the transected sensory nerve endings develop a catecholamine sensitivity through the up-regulation of α receptors (which respond to epinephrine from the autonomic nervous system).56 In this manner, when the sympathetic nervous system sends signals to increase heart rate, the increased density of α receptors in the sensory nerve endings of the neuroma are stimulated and transmit the pathologic pain signal back to the CNS.3 There is also a theory in which a similar up-regulation of α receptors occurs in the dorsal root ganglion.
Critical Points PATHOPHYSIOLOGY
Theories of the Mechanism of CRPS
The clinical findings of light touch hypersensitivity (allodynia) and cold hypersensitivity (hyperalgesia) are believed to occur through a central sensitization model termed the Raja model.55 In this model, pain receptors (nociceptors) up-regulate their α receptors. Because the body is constantly sending sympathetic signals, these nociceptors are being constantly stimulated, which reduces the CNS threshold for perceiving pain.58 Once again, changes occur in the dorsal root ganglion.22 The mechanoreceptors responsible for light touch sensation and thermoreceptors responsible for cold sensation instead produce an abnormal painful response when they stimulate the sensitized central pain neurons.
In the Raja model, treatment by sympathetic blockade is effective through the production of a temporary inhibition of norepinephrine release. By temporarily blocking the release of catecholamines, the sympathetic block relieves pain. By allowing the CNS to experience relief from pain, the central pain neurons may be desensitized (down-regulation of α receptors), resulting in a long-term improvement in the painful response to light touch.3
The change in regulation of α receptors also explains the change in appearance of the limb over time. Initially, an increase in α receptors causes excess stimulation that presents as a red, warm, and swollen limb (increased sympathetic function). Later, as the body adjusts and down-regulates the expression of α receptors, the same limb will take on a blue, cool, and contracted appearance.3 This can be explained to the patient with the following analogy. The patient controls the home computer (the CNS) and his or her children control the computer speakers (the PNS). If music is playing from the computer and the children like the song, they will turn up the volume to a painful level for the patient (perceived as pain by the CNS). In response to the pain of this loud noise, the patient (CNS) turns down the volume at the computer terminal, thereby decreasing the sound to a reasonable level (and decreasing the pain signal in the body). Unfortunately, by decreasing the strength of the signal from the computer to the speakers, other signals are also being transmitted weaker than normal. So, while the pain is decreased in the body, so is the strength of the sympathetic signal sent to the extremity, changing it from abnormally warm, red, and swollen to abnormally cool, blue, and constricted.
DIAGNOSIS
Clinical Presentation
The clinical presentation of CRPS is variable and comes in different stages with diverse appearances.54 The disease has no predilection for gender, age, or dominant extremity. The common cause is an initiating trauma to an extremity, which can be as obvious as a fracture or as minor as an ankle sprain. It can also occur in response to surgery, arthritis, and inflammatory conditions or reactions.7 In as many as 10% of cases, no initiating trauma can be identified.20
Sensory Disturbances
The hallmark finding of CRPS is pain disproportionate to the inciting event. The disproportionate response includes pain more intense than expected, lasting longer than expected, and expanding beyond the dermatomal region of the extremity. Pain is the body’s response to impending or ongoing cell death. With CRPS, the pain may be generated in the absence of cellular insult.36 Within hours to days after the initial noxious event, the pain becomes diffuse and expands beyond the site of the injury. As the condition progresses, the pain expands beyond dermatomal distributions in a stocking or glove pattern with a preference for distal limb involvement. Patients describe the pain as “burning and shooting” or as “deep, constant, and aching.” A classic finding is allodynia, pain with light touch. This is manifested during the physical examination and described by the patients during the history as the inability to tolerate pressure from bed sheets at night, clothing, and even air currents. The pain may fluctuate in intensity in response to many influencing factors. Aggressive physical therapy that is painful to the patient may worsen the condition. As the weather changes and becomes colder, the condition may worsen. Also, owing to the sympathetic nature of the disease, as patients become excited, their pain may worsen.
Sympathetic Dysfunction
Sympathetic dysfunction is manifested through changes in skin color, swelling, temperature changes, and cold intolerance. Classically, the limb will initially appear red, warm, and dry and progress over time to a mottled, bluish, cool, and moist condition. Note that the sudomotor function is opposite of what is normally expected. A red and swollen limb will normally be moist, and a bluish and cool limb will normally be dry. Side-to-side temperature differences can average 3.5° C in 80% of CPRS patients.77
Patients frequently report swelling that is extra-articular and painful. It is a prominent finding early in the course of the disease that decreases over time. Initially, the skin is edematous but gradually is replaced by skin that is tight, shiny, and lacking in normal skin creases.15
Of particular interest is a corresponding intolerance to cold. This finding is particularly sensitive for diagnosing SMP. Cold intolerance is often first encountered in physical therapy when cryotherapy is used to control swelling and produces a significant painful response.40 In the office, the clinician can use an ice cube test (Fig. 43-3) by applying ice to the sensitive region and asking the patient about the evoked sensations. The abnormal response the clinician seeks is a description of intolerable, burning pain. In the very early phase, the patient may describe the sensation evoked from the ice cube test as decreased cold sensation when compared with the contralateral limb. In a similar manner, cold weather can precipitate a painful flare.40
The natural history of sympathetic dysfunction falls into three stages. These stages do not follow a specific timeline and the patient can go forward and backward through the stages over time or in response to treatment. For this reason, the staging system has little clinical usefulness other than to understand the classic progression of the disease. The patient with CRPS may present with some or all of these symptoms, but may also present only with the symptom of disproportionate pain. The stages are acute, dystrophic, and atrophic.71
The acute stage usually lasts less than 6 months. During this time, the sympathetic system is hyperactive, yielding a limb with red, warm, and dry skin. The dystrophic stage begins after 3 to 9 months when the hyperactive sympathetic system finally diminishes to a period of decreased sympathetic activity. In this stage, the limb is cyanotic or mottled, cool, and moist, with thickening of the nails and coarse hair (owing to decreased blood flow to the skin). When the dystrophic stage persists long enough to produce an overall level of atrophy in the limb, the patient enters the atrophic stage. This stage is characterized by thin, tight, shiny skin, osteoporosis from prolonged decreased weight-bearing, and joint contractures from prolonged decreased range of motion.71
Motor Abnormalities
Motor impairment is a more recently appreciated symptom of CRPS.64 The deficits range from weakness to increased tone and hyperfunction. Even when motor abnormalities are grossly evident, electromyography tests remain normal. This finding suggests that the motor response of CRPS is centrally mediated, most likely at the spinal cord level.77 Initially, weakness can be attributed to disuse and expected muscle atrophy. Some patients develop pseudoparalysis that may extend to limb neglect. When hyperfunction is present, it manifests as action tremors, myoclonus, hyperreflexia, muscle spasm, and voluntary guarding.64 In longstanding illness, dystonia, apraxia, and lack of coordination may be present.77 In 50% of CRPS cases, patients show decreased range of motion, physiologic tremor, and weakness. About 10% of chronic cases will reveal dystonia in the hand or foot of the affected extremity.2
Psychological Issues
The clinician may respond to patients with CRPS and their disproportionate pain by believing they are malingerers or have fictitious disorders or other psychiatric overtones. However, as with many patients with a chronic pain syndrome, comorbid psychiatric disease either develops or promotes the pain syndrome.7,51 It is uncertain whether this is a cause or an effect of the CRPS, but in daily diaries kept by chronic pain patients, investigators found that yesterday’s depressed mood contributed to today’s increased pain, and yesterday’s pain also contributed to today’s depression, anxiety, and anger.20
It has been suggested by some authors that a particular personality type increases the risk for developing CRPS.18 However, this has never been proved.10 It is more likely that the emotional and behavioral disturbances are the result of chronic pain and loss of function. These psychological disturbances have been proved to resolve when the pain resolves.42,47
Objective Tests
Paravertebral Sympathetic Ganglion Blockade
Paravertebral sympathetic ganglion blockade is the best tool for diagnosing SMP-related CRPS and initiating treatment of CRPS. The patient is injected with a local anesthetic under fluoroscopic control. The local anesthetic is injected near the lumbar paravertebral sympathetic ganglion (for lower extremity disease) or the stellate ganglion (for upper extremity disease).40,70,77 Successful block of the ganglion is monitored by documenting vasomotor and sudomotor changes in the extremity. In a technically adequate block, the skin temperature of the extremity will approach core body temperature. In addition, in a stellate ganglion block, Horner’s syndrome (ptosis, miosis, and anhidrosis) will be induced. Relief of pain is due to blocking the SMP portion of CRPS. However, a placebo response as high as 33% has been documented and clinicians should be cautioned not to rely solely on this test for diagnosis.51
Triple-Phase Bone Scan
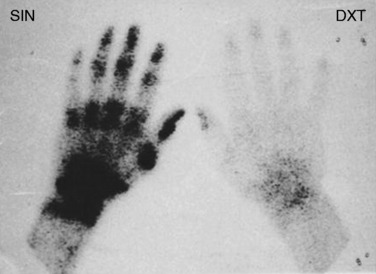
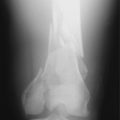
Diagnosis of CRPS based on bone scans is controversial because the test results vary based on the phase of the disease and by successful treatment.25,35,81 The triple-phase bone scan has three phases: arterial, soft tissue, and bone. The radionucleotide tracer injected during a bone scan has increased uptake in CRPS in the second and third phases (soft tissue and bone; Fig. 43-4) of the test.64 This occurs due to the increased blood flow and bone turnover in the acute phase of CRPS. During the acute phase of the disease, the reported sensitivity and specificity of this test range from 60% to 100% and 80% to 98%, respectively.25,26,35 Unfortunately, as the disease progresses to the dystrophic phase, blood flow to the limb decreases and the affected limb will appear cold on bone scan. Finally, successful treatment of a CRPS limb with sympatholysis can result in a paradoxical increase in tracer uptake as the blood flow improves to the affected limb.25,41 Thus, increased uptake to a limb can indicate CRPS in the acute phase or successful treatment of the disease. A normal scan may miss CRPS in the dystrophic phase. This tendency for change in bone scan results over time is also why this test should not be used to track the course of the disease or the success of treatment.25
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a poor test for confirming CRPS. However, it can be useful in ruling out other possible causes of pain and allow the clinician to narrow the differential diagnosis. MRI may also be useful in identifying a possible focus for the pathology that triggered the disease. Radiology literature describes the characteristic MRI findings of CRPS to be thickening of the skin, soft tissue swelling that enhances with contrast, and intra-articular effusions. Chronic changes are muscle atrophy and thinning of the skin.65
Radiographs
Virtually all orthopaedic patients will have radiographs taken of the affected extremity. In the acute phase, no change in radiographic appearance will be noticed. As the disease progresses to the dystrophic and atrophic phases, films will reveal decreased cortical density of the affected extremity when compared with the contralateral limb. The classic radiographic finding for CRPS is Sudeck’s atrophy, a patchy periarticular osteoporosis.67
Differential Spinal and Epidural Blockade
Paravertebral ganglion blocks can be technically challenging to perform. Less accurate, but easier methods to perform are spinal or epidural blocks. The blocks can be used for diagnosis because the nerve fibers respond differently to varying concentrations of local anesthetic. The lowest concentrations of local anesthetic penetrate the least myelinated sympathetic nerve fibers with the goal to relieve the SMP component. At increasing concentrations, the sensory nervous system is blocked, and even higher concentrations block the motor system. When used to test for CRPS, the initial injection is often saline to detect a placebo response. Unfortunately, even at low concentrations, some sensory blockade occurs and can lead to false-positive results when pain is relieved and attributed to SMP rather than generalized sensory blockade.40
Phentolamine Challenge
Phentolamine is a nonspecific α-antagonist with a short half-life of 17 minutes. When administered, any pain relieved from blocking the α receptors of the sympathetic nervous system may be attributed to SMP of CRPS. A study performed in 1991 reported favorable results in using phentolamine to diagnose CRPS; this test is still used today.56 However, a second phentolamine study performed by another group of investigators in 1994 invalidated the results of the 1991 study.61 In the later study, both phentolamine (an α-antagonist) and phenylephrine (an α-agonist) were used in 77 patients in a double-blinded fashion. The results showed no pain relief with phentolamine testing and no worsening of pain with phenylephrine. Of the study group, 22 had pain relief with saline infusions and only 7 had relief with phentolamine. These test results make any use of phentolamine testing questionable.61
Regional Intravenous Sympathetic Blockade
Regional intravenous sympathetic blockade has been used for both diagnosis and treatment. These blocks are performed with either reserpine or bretylium.64 These drugs act at the sympathetic terminals to deplete the stores of norepinephrine and inhibit its reuptake, which may last up to 2 days.8 Upon initial release of norepinephrine, patients with SMP will note a burning pain. Pain relief has been described as lasting from 2 weeks to 6 months. Pain relief less than 5 days favors a diagnosis of SIP.
There are several reasons why these blocks are a poor choice for diagnosing CRPS. First, it is unknown to what degree these medications target sympathetic functions. Second, local anesthetic agents are often injected with reserpine or bretylium, and it is not possible to determine whether the pain relief is from the local anesthetic or a true blockade of the sympathetic nervous system. Third, these injections are painful and poorly tolerated by patients. Finally, this method requires the use of a tourniquet, which is poorly tolerated in patients with CRPS and can actually lead to exacerbation of the condition.64,72
Vasomotor Measurements
Sympathetic dysfunction can be measured by thermography. This test requires extensive setup and monitoring and is not useful clinically if only for cost reasons. Thermography is successful in measuring temperature changes in a limb compared with the contralateral limb and can be depicted using colorized images. Some authors have reported that use of thermography allows for early detection of CRPS.6,30 However, this technique has several drawbacks. Thermography requires a carefully controlled environment that is free of drafts and well controlled for temperature and humidity. It requires a period of 20 minutes to 2 hours for the limb to acclimatize prior to testing. There is no agreement on the amount of temperature difference that constitutes a positive test. If the temperature is set for a difference at 0.4° C, the test is sensitive but poorly specific. If the temperature is set for 1.2° C, the test is specific but not at all sensitive.6 Lastly, even superficial scars may cause thermographic changes.
Sudomotor Measurements
The quantitative sudomotor axon reflex test (QSART) is one method of measuring sweat output. In this test, acetylcholine is administered to the extremities and the sweat output of each extremity is measured and compared.5,62 One criticism of this test is based on theories stating that sudomotor dysfunction is centrally mediated and this test has its mechanism of action at the postganglionic sympathetic neuron. Therefore, the results of this test may not reflect CRPS sympathetic hyperactivity.5
TREATMENT
Many treatments have been proposed since CRPS was first described in 1864, but unfortunately, few are supported by prospective, double-blinded, randomized, clinical trials. A lack of consensus exists regarding the preferred treatment algorithm for CRPS. As stated previously, the placebo response to treatment has been recorded in as high as 33% of patients tested. For this reason, the results of studies that are not randomized should be treated with caution.61
Despite the inability to state with confidence an exact treatment protocol, several principles may be noted. First, treatment should begin as soon as possible, within 3 weeks of the onset of the disease. The long-term prognosis depends upon the time span from the onset of the disease to the initiation of treatment. Authors have postulated that a delay in treatment of more than 6 months is associated with a poor prognosis.80 One study revealed that greater than 80% of patients who had treatment initiated within 1 year of the onset of CRPS had a significant improvement in their symptoms. However, delay of greater than 1 year resulted in some degree of permanent impairment in 50% of the patients.36
CRPS often has a painful focus that perpetuates the SMP. As such, the identification and elimination of this painful focus are critical to alleviating the SMP. Even so, SIP may remain and worsen over time. Eliminating the painful focus in the acute phase is important along with treatment of the SMP to eliminate the pain. In chronic cases with established SMP, elimination of the painful focus late in the course of the disease may be of very little help. Surgery may be poorly tolerated in the CRPS limb, and symptoms are often exacerbated postoperatively if steps are not taken to control perioperative pain.40 However, when a painful focus is identified, surgery may be performed in a manner that minimizes the chance of exacerbating the condition. In most cases, it is worth the risk of worsening the condition to eliminate the root cause of the CRPS symptoms and potentially eliminate the condition.
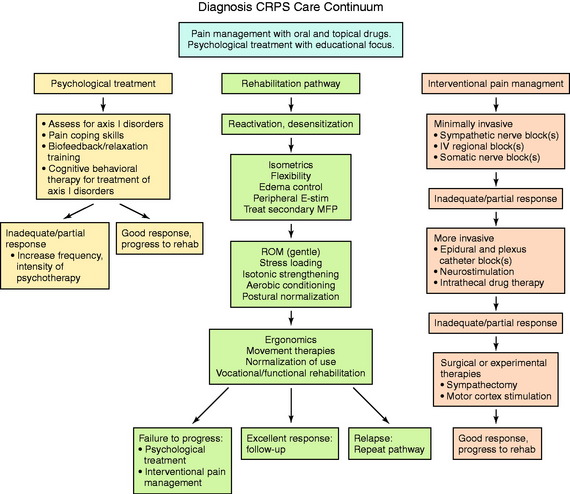
Treatment often requires multiple disciplines. The cooperation of orthopaedists, pain specialists, anesthesiologists, physical therapists, neurologists, psychologists, and primary care physicians is required to provide the most effective care. The following methods are all described in conjunction with physical therapy. With the absence of validated treatment protocols (Table 43-2), individual case-by-case treatment decisions are required to determine the best management for each patient.77
Physical Therapy
Physical therapy is the core treatment for CRPS; all other treatment options are performed in addition to physical and occupational therapy.52 In fact, many drug regimens are intended to improve symptoms to the point at which patients can tolerate effective physical therapy. Several guiding principles have been developed for therapy of CRPS patients. The physical therapist treats pain, swelling, stiffness, and weakness and works toward restoration of function. A consensus panel recommended a four-step approach to progressive return to function.69
Step one is the formation of a patient-therapist alliance. A patient in chronic pain will have many individuals doubting her or his sincerity and desire to return to work. The therapist must remain an advocate for the patient, gaining her or his trust and lending credibility to the treatment regimen. Slow response to treatment and frequent setbacks can be difficult, but confrontations between the patient and the therapist are always counterproductive.69
Step two involves desensitization and mobilization of the affected limb. The significant pain patients experience to light touch (allodynia) must be retrained to be perceived as simple touch and not pain. This is accomplished through vibration, massage, temperature contrast baths, and electrical stimulation.4,23 All therapeutic measures must be done while not inducing significant pain. Initially, light touch will be painful, but as treatment progresses, patients will gradually be able to tolerate greater amounts of stimulation. Patients will have learned that movement of the limb causes pain and will therefore guard by voluntarily restricting range of motion of the affected limb. These movement phobias must be overcome before long-term sequelae develop. Active and active-assist range of motion exercises are preferred. Forced passive motion is too aggressive, painful, and counterproductive to treatment.23 Active motion, elevation, massage, and compression will help control edema.
Step three involves strengthening and stress-loading the limb. Initially, strengthening is performed isometrically to minimize painful motion. Over time, the patient is advanced to isotonic strengthening. Stress-loading helps prevent disuse and restore function. Loads are gradually increased from warm-water exercises to full weight-bearing activities. Gait retraining may be required as patients progress to full weight bearing.69
The fourth and final step is to return the patient to normal function. This is accomplished through work hardening and vocational rehabilitation programs. Eventually, either the patient returns to full duty or work restrictions are implemented as outlined by a functional capacity evaluation.69
Medications
Many medications have been tested for efficacy in chronic pain conditions such as peripheral neuropathy, but few have been tested to treat CRPS.69 Of the medications presented later, only the steroids, bisphosphonates, vitamin C, and adrenergic active drugs have been tested in randomized, controlled trials. Of the remaining, some have been tested in neuropathic pain conditions such as diabetic peripheral neuropathy, some have been tested in a CRPS setting without randomization, and others simply have anecdotal support.61 The following list of oral medications is loosely organized by potential effectiveness.
Corticosteroids
Corticosteroids are most effective early in the course of the disease when the limb is red and swollen.9,34 A prednisolone (Medrol) Dosepak, or other equivalent steroid dosage, is most useful early in the course of the disease process. If the patient has a positive but incomplete response to an initial course of oral steroids, a second course of treatment may be beneficial. In a randomized, controlled trial, Christensen and associates9 found that 30 mg/day of prednisone was significantly better than a placebo in relieving symptoms. It may be necessary to extend the duration of the steroid treatment.
Bisphosphonates
Treatment of CRPS with bisphosphonates has been studied only recently, and therefore, widespread use has not been implemented. Bisphosphonates inhibit osteoclast activity, thereby decreasing bone resorption. These medications may also act on prostaglandins, decreasing pain and swelling as a result. In one large study involving 118 patients, those treated with bisphosphonates had statistically significant improvements compared with those treated with a placebo.63 Another study revealed that intravenous alendronate was successful in improving function and increasing the bone density in the affected limb. The bone density in the unaffected limb remained unchanged.1
A 2004 study by Manicourt and colleagues42 focused on oral alendronate (Fosamax) in a double-blind, placebo-controlled trial. Forty patients with post-traumatic CRPS type I of the lower extremity were divided into two treatment groups. All patients had entered the dystrophic phase and had been treated with various analgesics and anti-inflammatories for 7 to 8 months. All patients continued with physical therapy, but one group received alendronate 40 mg daily for 8 weeks and the other received a placebo. Improvement was seen as early as 4 weeks after alendronate was initiated and continued after discontinuation of the medication. All patients exhibited improved pain levels, pressure tolerance, and joint mobility. Pain scores on visual analog scales of the alendronate group were decreased to one third of those of the placebo group (60% improvement). Pressure tolerance of the alendronate group was twice that of the placebo group. Of note, all postsurgical cases were from arthroscopy to treat meniscal tears. Several studies have demonstrated that post-traumatic CRPS type I responds better to alendronate than CRPS for any other reason. The mechanism by which alendronate affects CRPS remains uncertain.42
Membrane Stabilizers
Of the numerous drugs in this category, only gabapentin (Neurontin) and pregabalin (Lyrica) have shown promise in relieving burning neuropathic pain and also in improving sleep.44 To date, only short-term results have been reported. The mechanisms of action are poorly understood for both medications in regard to neuropathic pain. It is possible that the drugs act at multiple sites and modify calcium currents that cause the transmission cell to decrease its firing. In one pilot study, Neurontin was successful in decreasing both allodynia and hyperalgesia (pain with light touch and cold), when administered at 2400 mg/day.22 It is important to start with smaller doses such as 300 mg three times a day and gradually over weeks increase the dosage, observing for side effects. Both medications have been shown effective in decreasing allodynia in rats.16
Ketamine has been recently described in the literature as effective in treating allodynia in patients with postherpetic neuralgia, chronic post-traumatic pain, and chronic neuropathic pain. Ketamine is thought to be effective as an antagonist of the N-methyl-d-aspartate (NMDA) receptor, which may play a role in the central sensitization responsible for the induction and maintenance of neuropathic pain.22 A study of 33 patients in which subanesthetic doses of ketamine was administered resulted in relief of pain in 25 of 33 patients. These results improved to a 100% response rate with up to three repeated treatments. This pain relief lasted from 3 months to 3 years.12 Additional studies are required, but these findings are encouraging.
Antidepressants
Selective serotonin reuptake inhibitors are ineffective at treating neuropathic pain.69 However, the norepinephrine uptake inhibitor amitriptyline (Elavil) has been shown to provide relief from burning pain. In addition, this medication has the advantage of also treating mood and anxiety disorders that may develop in patients with a chronic pain condition. It is necessary to use an initial 4- to 6-week course of therapy, because it takes this amount of time for peak effects to be generated. In addition, amitriptyline has sedating properties that can help improve sleep. Side effects are sedation, dry mouth, dry eyes, constipation, and urinary retention.33
Nonsteroidal Anti-inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) may be useful in a fashion similar to that of corticosteroids. During the acute phase of CRPS when the limb has an inflamed appearance, NSAIDs may help decrease concomitant arthritis, tendinitis, or other inflammatory conditions that may represent the painful focus of CRPS.69 However, NSAIDs are ineffective at treating SMP.34 The mechanism of action of these agents is inhibition of cyclooxygenase, which decreases the production of prostaglandins.
Calcium Channel Blockers
Nifedipine (Procardia) in doses of 10 to 20 mg three times per day has been shown to decrease pain and improve signs of sympathetic dysfunction. These medications act by decreasing arteriole-venous shunting, minimizing segmental ischemia, and decreasing pain in an atrophic extremity.36 In the authors’ experience, these have proved most useful in patients who have a CRPS flare in response to cold weather changes. Side effects include headaches and orthostatic hypertension.48
Adrenergic Active Agents
Topical clonidine (Catapres) patches may help decrease hyperalgesia. The patches act as an agonist to α receptors and inhibit the release of norepinephrine.75 Besides transdermal patches, these agents are available in oral form and may be administered in epidural injections for the treatment of CRPS. Studies are required to determine the efficacy and safety of this treatment option.34
Other oral α blockers may work in theory, but they have significant side effects. Prazosin, terazosin, phenoxybenzamine, and reserpine all block α receptors, interrupting sympathetic outflow.69 The long-term efficacy of these medications is unknown, and their cardiovascular risk profiles limit their use.
Capsaicin
Capsaicin is the component of hot peppers that produces a spicy taste. When used topically, this drug depletes substance P from sensory neurons, causing an initial burning sensation. When used on a scheduled daily basis, capsaicin may help desensitize the affected area. However, this effect is reversed within 2 to 4 weeks of discontinuing its use.24 The cream is administered in concentrations of 0.025% and 0.075% and can be found in many over-the-counter remedies.
Vitamin C
Zollinger and coworkers79 found vitamin C, taken at 500 mg/day for 50 days, to be effective in reducing the prevalence of CRPS in wrist fractures. The authors conducted a randomized, double-blinded, placebo-controlled study in which 416 patients with 427 wrist fractures were randomly divided into placebo, 200 mg vitamin C, 500 mg vitamin C, or 1500 mg vitamin C treatment groups. The investigators found the incidence of CRPS in the placebo group to be 10%. In the vitamin C category, the 500-mg dose lowered the incidence to 1.8%. The use of 1500 mg lowered the incidence to 1.7%, which was not statistically different from the 500-mg dose. This study revealed that vitamin C prevents the onset of CRPS. The mechanism is unknown. This is a preventive treatment only. It is not known whether it can effectively treat established CRPS.
Calcitonin
Calcitonin is frequently used to slow the advance of osteoporosis and has been tested with mixed results. One study revealed its effectiveness in mildly decreasing pain and increasing function in CRPS limbs.61 However, another study found no significant difference between calcitonin and a placebo in 40 patients treated for 3 months.61
Opiates/Benzodiazepines
There is no doubt that opiates are effective in treating pain. The implementation of these drugs in CRPS patients is controversial because long-term use in chronic conditions leads to habituation and dependency. In addition, pain control after any future surgery becomes difficult to manage. As a matter of course, orthopaedists should avoid using narcotics in chronic pain conditions and explain the disadvantages to patients. There are times when it may be necessary to use narcotics, such as when patients present already addicted, and in those instances, it is best to provide a pain-management referral and have only one physician dispensing the narcotics.66 A non-narcotic choice is available in the form of Tramadol. This agent has its action on serotonin, norepinephrine, and μ-opioid receptors and has been proved clinically useful in two randomized, controlled trials to decrease pain associated with peripheral neuropathy.61
Psychotherapy
Most patients early in the disease have no clinical anxiety or depression. However, as this chronic pain condition persists, patients become anxious that the syndrome will not resolve and living with daily pain leads to depression. After 6 months, nearly all patients demonstrate some degree of depression due to sleep deprivation, chronic pain, and anxiety. Psychological counseling can be beneficial to learn coping mechanisms and stress management.19 Entering group therapy can also help patients vent anger and share experiences with other individuals who can truly understand their emotions.69
Patients with CRPS and refractory clinical depression may benefit from electroconvulsive therapy (ECT). The mechanism of action of ECT is not understood, but this treatment is well known to treat chronic refractory depression. In patients who had both CRPS and depression, use of ECT has relieved both the depression and the chronic pain.43 Despite this, its use is only considered when the patient has clinical depression unresponsive to other treatments.
Paravertebral Sympathetic Chain Ganglion Blockade
Paravertebral sympathetic chain ganglion blocks have been shown to be significantly better than placebo in treating CRPS. Upper extremity disease requires a stellate ganglion block, whereas lower extremity disease requires lumbar paravertebral sympathetic ganglion blockade.37,76 Treatment within 6 months of onset of the disease is associated with a better prognosis.76 A successful block will create color and temperature changes within the limb. In stellate ganglion blocks, Horner’s syndrome (ptosis, miosis, and anhidrosis) will be induced. These are the only means of determining clinically whether the block was performed correctly. As many as 10 to 15 blocks may be required for complete resolution of symptoms, but patients should experience longer and longer periods of relief between each block.78 In addition, when it is necessary to operate on a limb with CRPS, use of a perioperative sympathetic ganglion block can significantly reduce the recurrence rate of the disease. A treatment protocol devised by O’Brien and coworkers49 had a 92% success rate using paravertebral ganglion blocks. This protocol employed a block every other day for 8 days for a total of four blocks. The frequency was then reduced to one block weekly until the patient became asymptomatic, reached a plateau, or stabilized to undergo surgery for a surgically correctable lesion.
Continuous cervical or lumbar epidural infusion can be administered with bupivacaine (Marcaine) or fentanyl, can be used to treat CRPS, and is the anesthesia of choice when operating on a CRPS limb.38 The drug concentration can be titrated to allow motor function to remain intact while still blocking sympathetic and sensory input. This infusion is continued for 2 to 5 days postoperatively to minimize the chance of CRPS recurrence.38 In the postoperative period, the block is gradually reduced until it is discontinued. If, during this weaning period, patients begin to experience sympathetic pain, the dosage is increased until the pain is relieved and weaning begins again the following day. During this treatment phase, it is critical to think of these patients as new spinal cord injuries and protect them from decubitus ulcers. Patients should log roll every 2 hours, use heel cushions, be placed on an air mattress, and be helped out of bed frequently during the day. Sequential compression devices and antiembolism stockings are required to prevent deep vein thrombosis. Anticoagulant medications are contraindicated in patients with indwelling epidural catheters. A Foley catheter may be required if the patient is unable to void. A history of peripheral venous disease, blood clots, or high-risk factors (diabetes, obesity) contraindicate this form of treatment. Lower extremity ultrasound for venous clots is used as indicated, and the patient is examined every day for signs of a lower extremity venous clot condition (tibial edema, calf or inner thigh compression pain, Homan’s test).
Surgical Sympathectomy
Surgical sympathectomy has been used in chronic cases of SMP. If regional sympathetic blockade provides relief, surgical sympathectomy can be considered. Initial pain relief may be significant, but symptoms tend to recur over the next 2 to 5 years.53 This is believed to occur owing to incomplete surgical removal of all sympathetic innervation to the extremity. Collateral reinnervation can occur, but bilateral sympathectomy may cause bowel, bladder, or sexual dysfunction.14
It is possible to disrupt the sympathetic chain ganglion by treatments other than surgery. Ablation with radiofrequency devices and caustic chemicals (such as alcohol) have been described, but the region of necrosis may expand beyond the ganglion and long-term results are unknown.59,69 As such, surgical sympathectomy is considered strictly as a last resort.
Neuromodulation
Neuromodulation includes peripheral nerve stimulation and spinal cord stimulation. The treatment methods are believed to be effective by blocking the transmission of pain nerve fibers or causing the release of endogenous opioids.64 Spinal cord stimulation (also called dorsal column stimulation) has been used in chronic pain conditions for years.59 Electrodes are implanted in the epidural space in either the cervical or the lumbar region. Stanton-Hicks and colleagues69 reported that 70% of patients responded favorably to this treatment method. The only randomized, controlled trial was performed by Kemler and coworkers,32 who reported a success rate of only 56%. This disappointing response is further clouded by numerous morbidities of the procedure. Even if the procedure is entirely successful, the patient will require repeat surgery for battery changes. There is also the possibility of infection, electrode displacement, and broken electrodes. The complication rate is between 30% and 64%.74 The best results have been reported in patients with CRPS type II in which a single nerve is affected.
Surgery
Operating on a limb with active or quiescent CRPS should normally be avoided. However, if the painful focus can be identified, appropriate surgical intervention to eliminate this focus is advocated.11 Without this intervention, resolution of the CRPS is unlikely to occur. When possible, the limb should be allowed to “cool down” from acute flares prior to surgery. Tourniquets should be avoided because they can cause a painful flare.73 Exacerbation of CRPS symptoms has been reported to occur in patients when appropriate perioperative pain management has not been utilized.31,73 The likelihood of a painful flare response may be decreased by blocking the sympathetic system during and after the procedure. A continuous epidural infusion for 2 to 5 days after surgery can help prevent a painful flare.38 Regional anesthesia has proved to be more effective than general anesthesia in blocking painful flares. In the authors’ experience, no exacerbation of CRPS symptoms has occurred as long as a sympathetic blockade and other appropriate pain management are used in the perioperative period.
Patellofemoral surgery is associated with a high rate of painful CRPS flares.17 In addition, injury to the saphenous nerve may cause a saphenous neuritis, resulting in CRPS type II.54 The infrapatellar branch of the saphenous nerve can be injured when making the anteromedial portal for knee arthroscopy. To avoid injury, the medial portal incision should be made horizontally and above the joint line. Some authors recommend transilluminating the medial portal site from the lateral portal to visualize the concomitant vein and thereby avoid the nerve.57 If neuritis occurs, surgery to release the entrapped nerve or resect a neuroma has been shown to be effective in treating CRPS type II.29,79
Lankford and Thompson39 recommended that sympathetic blocks be performed and the CRPS process be allowed to “cool down” for a year prior to considering other surgical procedures. Katz and Hungerford31 recommended in postarthroplasty patients with CRPS that surgery to correct mechanical dysfunction be delayed until the CRPS symptoms are under good control. Only then should surgery be performed for aseptic loosening, ligament imbalance, or component malalignment. These authors also recommended that patients undergo a series of sympathetic blocks before the anticipated surgery.
Patients with chronic, unremitting pain may demand an amputation, which, in the authors’ opinion, should not be performed. Dielissen and associates13 reviewed results of 34 amputations of CRPS limbs and reported that only 2 patients experienced complete relief. Eighty-two percent had CRPS symptoms in the residual limb, and many experienced phantom pain or phantom limb symptoms.
Authors’ Preferred Treatment
It is important to take all complaints seriously before discounting a patient’s comments as drug-seeking behavior. By maintaining a high degree of clinical suspicion, the likelihood of early diagnosis of CRPS is greatest. At the time of the initial diagnosis, it is important for a patient to be educated and become participants in his or her recovery. The authors commonly start patients on a Medrol Dosepak and send them to physical therapy for desensitization and active and active-assisted range of motion exercises. If the patient obtains some, but incomplete relief, a second Medrol Dosepak is prescribed. If the patient does not demonstrate complete relief of his or her symptoms after the second Dosepak, Neurontin is prescribed and he or she is referred to an anesthesiologist skilled with administering paravertebral ganglion blocks. Coordinating the blocks and a clinic appointment are very helpful in detecting a possible painful focus. Prior to a block, patients may be able to describe only a global knee pain. After the SMP is blocked, physical examination may localize to the medial joint line or over the saphenous nerve, identifying possible painful foci. Stanton-Hicks68 outlined a possible treatment algorithm, which is depicted in Figure 43-5.
ILLUSTRATIVE CASES
Case 2
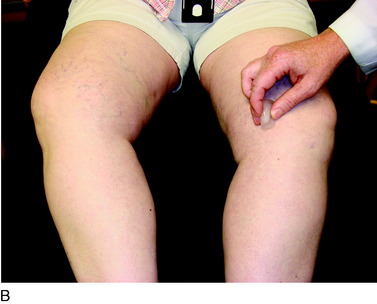
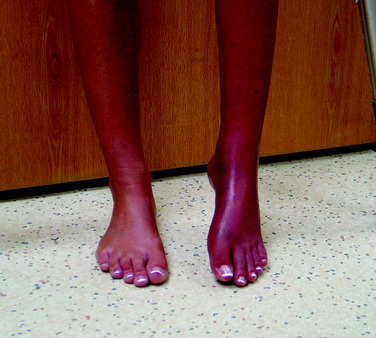
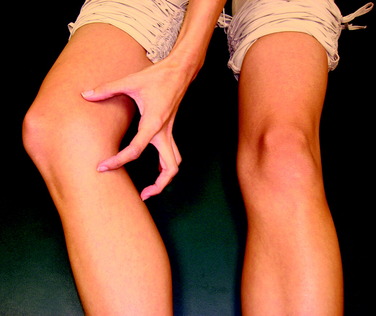
A 28-year-old woman presented for evaluation of left lower extremity pain. She had an acute onset of left-sided weakness, numbness, tingling, and blurred vision of the left eye, which was attributed to transverse myelitis. She had previously been seen by neurologists and pain-management specialists. On evaluation, there was dark discoloration of the limb (Fig. 43-6) and increased sensitivity to light touch from the knee to the foot. A 30° flexion contracture of the knee and ankle equinus were noted. The patient was unable to sleep under sheets owing to the inability to tolerate the pain from foot contact with the sheets. In addition, pulses could not be palpated owing to her inability to tolerate the pressure required. The patient had been previously diagnosed with RSD and underwent three sympathetic blocks and implantation of a spinal cord stimulator. She was taking gabapentin (Neurontin), Lyrica, morphine, and hydrocodone and acetaminophen (Vicodin).
Case 6
A 26-year-old woman diagnosed with a medial meniscus tear by MRI was referred for extreme pain. The patient was taking oxycodone and acetaminophen (Percocet) and celecoxib (Celebrex). Her medial knee and tibia were exquisitely tender to palpation (Fig. 43-7). She held her leg flexed 30° and would not allow physical examination secondary to extreme pain with light touch. She had a mild effusion with no color changes, but her limb was cool to the touch. She reported being unable to tolerate the pressure of her bed sheets on her knee. She was prescribed Neurontin and referred for paraspinal ganglion blocks. Although the Neurontin helped her pain, the patient was prescribed tramadol because she could not tolerate the sedating effects. She underwent 10 blocks, which relieved her pain. She is currently in remission and being treated conservatively for patellar malalignment syndrome. She never underwent arthroscopy at our institution. This case demonstrates the importance of an immediate diagnosis, because the pain could have been mistakenly attributed to the medial meniscus tear.
1 Adami S., Fossaluzza V., Gatti D., et al. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis. 1997;56:201-204.
2 Baron R. Reflex sympathetic dystrophy and causalgia. Suppl Clin Neurophysiol. 2004;57:24-38.
3 Baron R., Levine J.D., Fields H.L. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? Muscle Nerve. 1999;22:678-695.
4 Bengtson K. Physical modalities for complex regional pain syndrome. Hand Clin. 1997;13:443-454.
5 Birklein F., Riedl B., Claus D., Neundorfer B. Pattern of autonomic dysfunction in time course of complex regional pain syndrome. Clin Auton Res. 1998;8:79-85.
6 Bruehl S., Lubenow T.R., Nath H., Ivankovich O. Validation of thermography in the diagnosis of reflex sympathetic dystrophy. Clin J Pain. 1996;12:316-325.
7 Bushnell T.G., Cobo-Castro T. Complex regional pain syndrome: becoming more or less complex? Man Ther. 1999;4:221-228.
8 Campbell J.N., Raja S.N., Meyer R.A., Mackinnon S.E. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89-94.
9 Christensen K., Jensen E.M., Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653-655.
10 Ciccone D.S., Bandilla E.B., Wu W. Psychological dysfunction in patients with reflex sympathetic dystrophy. Pain. 1997;71:323-333.
11 Cooper D.E., DeLee J.C. Reflex sympathetic dystrophy of the knee. J Am Acad Orthop Surg. 1994;2:79-86.
12 Correll G.E., Maleki J., Gracely E.J., et al. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5:263-275.
13 Dielissen P.W., Claassen A.T., Veldman P.H., Goris R.J. Amputation for reflex sympathetic dystrophy. J Bone Joint Surg Br. 1995;77:270-273.
14 Dobritt D.W., Gutowski P. Successful treatment of recurrent reflex sympathetic dystrophy with bilateral lumbar sympathectomies. J Am Osteopath Assoc. 1997;97:533-535.
15 Dzwierzynski W.W., Sanger J.R. Reflex sympathetic dystrophy. Hand Clin. 1994;10:29-44.
16 Field M.J., McCleary S., Hughes J., Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391-398.
17 Finsterbush A., Frankl U., Mann G., Lowe J. Reflex sympathetic dystrophy of the patellofemoral joint. Orthop Rev. 1991;20:877-885.
18 Geertzen J.H., de Bruijn-Kofman A.T., de Bruijn H.P., et al. Stressful life events and psychological dysfunction in complex regional pain syndrome type I. Clin J Pain. 1998;14:143-147.
19 Geertzen J.H.B., de Bruijn H., de Bruijn-Kofman A.T., Arendzen J.H. Reflex sympathetic dystrophy: early treatment and psychological aspects. Arch Phys Med Rehabil. 1994;75:442-446.
20 Grabow T.S., Christo P.J., Raja S.N. Complex regional pain syndrome: diagnostic controversies, psychological dysfunction, and emerging concepts. Adv Psychosom Med. 2004;25:89-101.
21 Gray H., Pick T.P., Howden R., editors. Gray’s Anatomy. New York: Bounty, 1977.
22 Harden R.N. Chronic neuropathic pain. Mechanisms, diagnosis, and treatment. Neurologist. 2005;11:111-122.
23 Hardy M.A., Hardy S.G. Reflex sympathetic dystrophy: the clinician’s perspective. J Hand Ther. 1997;10:137-150.
24 Hautkappe M., Roizen M.F., Toledano A., et al. Review of the effectiveness of capsaicin for painful cutaneous disorders and neural dysfunction. Clin J Pain. 1998;14:97-106.
25 Hoffman J., Phillips W., Blum M., et al. Effect of sympathetic block demonstrated by triple-phase bone scan. J Hand Surg [Am]. 1993;18:860-864.
26 Holder L.E., Michael R.H. The specific scintigraphic pattern of “shin splints in the lower leg”: concise communication. J Nucl Med. 1984;25:865-869.
27 Hooshmand H., editor. Chronic Pain: Reflex Sympathetic Dystrophy. Prevention and Management. Boca Raton, FL: CRC; 1993:123-168.
28 Intenzo C.M., Kim S.M., Capuzzi D.M. The role of nuclear medicine in the evaluation of complex regional pain syndrome type I. Clin Nucl Med. 2005;30:400-407.
29 Jupiter J.B., Seiler J.G.3rd, Zienowicz R. Sympathetic maintained pain (causalgia) associated with a demonstrable peripheral-nerve lesion. Operative treatment. J Bone Joint Surg Am. 1994;76:1376-1384.
30 Karstetter K.W., Sherman R.A. Use of thermography for initial detection of early reflex sympathetic dystrophy. J Am Podiatr Med Assoc. 1991;81:198-205.
31 Katz M.M., Hungerford D.S. Reflex sympathetic dystrophy affecting the knee. J Bone Joint Surg Br. 1987;69:797-803.
32 Kemler M.A., van de Vusse A.C., van den Berg-Loonen E.M., et al. HLA-DQ1 associated with reflex sympathetic dystrophy. Neurology. 1999;53:1350-1351.
33 King S.A. Antidepressants: a valuable adjunct for musculoskeletal pain. J Musculoskel Med. 1995;12:51-57.
34 Kingery W.S. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123-139.
35 Kline S.C., Holder L.E. Segmental reflex sympathetic dystrophy: clinical and scintigraphic criteria. J Hand Surg [Am]. 1993;18:853-859.
36 Koman L.A., Smith B.P., Ekman E.F., Smith T.L. Complex regional pain syndrome. Instr Course Lect. 2005;54:11-20.
37 Kozin F. Reflex sympathetic dystrophy syndrome: a review. Clin Exp Rheumatol. 1992;10:401-409.
38 Ladd A., DeHaven K.E., Thanik J., et al. Reflex sympathetic imbalance: response to epidural blockade. Am J Sports Med. 1989;17:660-668.
39 Lankford L.L., Thompson J.E. Reflex sympathetic dystrophy, upper and lower extremity: diagnosis and management. Instr Course Lect. 1977;26:163-178.
40 Lindenfeld T.N., Bach B.R., Wojtys E.M. Reflex sympathetic dystrophy and pain dysfunction in the lower extremity. J Bone Joint Surg Am. 1996;78:1936-1944.
41 Mailis A., Meindok H., Papagapiou M., Pham D. Case report. Alterations of the three-phase bone scan after sympathectomy. Clin J Pain. 1994;10:146-155.
42 Manicourt D.H., Brasseur J.P., Boutsen Y., et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum. 2004;50:3690-3697.
43 McDaniel W.W. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19:226-229.
44 Mellick G.A., Mellick L.B. Reflex sympathetic dystrophy treated with gabapentin. Arch Phys Med Rehabil. 1997;78:98-105.
45 Merskey H., Bogduk N., editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. International Association for the Study of Pain Press; 1994:40-43.
46 Mitchell S.W., Morehouse G.R., Keen W.W., editors. Gunshot Wounds and Other Injuries of Nerves. Philadelphia: J. B. Lippincott, 1864.
47 Monti D.A., Herring C.L., Schwartzman R.J., Marchese M. Personality assessment of patients with complex regional pain syndrome type I. Clin J Pain. 1998;14:295-302.
48 Muizelaar J.P., Kleyer M., Hertogs I.A., DeLange D.C. Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia): management with the calcium channel blocker nifedipine and/or the alpha-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg. 1997;99:26-30.
49 O’Brien S.J., Ngeow J., Gibney M.A., et al. Reflex sympathetic dystrophy of the knee. Causes, diagnosis, and treatment. Am J Sports Med. 1995;23:655-659.
50 Ochoa J.L. Truths, errors, and lies around “reflex sympathetic dystrophy” and “complex regional pain syndrome. J Neurol. 1999;246:875-879.
51 Ochoa J.L., Verdugo R.J. Reflex sympathetic dystrophy. A common clinical avenue for somatoform expression. Neurol Clin. 1995;13:351-363.
52 Oerlemans H.M., Oostendorp R.A., de Boo T., et al. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil. 2000;81:49-56.
53 Olcott C.T., Eltherington L.G., Wilcosky B.R., et al. Reflex sympathetic dystrophy—the surgeon’s role in management. J Vasc Surg. 1991;14:488-492. discussion 492–495
54 Poehling G.G., Koman L.A., Pollock F.E.J. Reflex sympathetic dystrophy of the knee. In: Sprague N.E.I., editor. Complications in Arthroscopy. New York: Raven; 1989:53-72.
55 Raja S.N., Davis K.D., Campbell J.N. The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg. 1992;8:63-69.
56 Raja S.N., Treede R.D., Davis K.D., Campbell J.N. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691-698.
57 Ramasastry S.S., Dick G.O., Futrell J.W. Anatomy of the saphenous nerve: relevance to saphenous vein stripping. Am Surg. 1987;53:274-277.
57a Reuben S.S., Rosenthal E.A., Steinberg R.B. Surgery on the affected upper extremity of patients with a history of complex regional pain syndrome: a retrospective study of 100 patients. J Hand Surg (Am). 2000;25:1147-1151.
58 Roberts W.J., Foglesong M.E. Spinal recordings suggest that wide-dynamic-range neurons mediate sympathetically maintained pain. Pain. 1988;34:289-304.
59 Rocco A.G. Radiofrequency lumbar sympatholysis. The evolution of a technique for managing sympathetically maintained pain. Reg Anesth. 1995;20:3-12.
60 Rodeo S.A., Forster R.A., Weiland A.J. Neurological complications due to arthroscopy. J Bone Joint Surg Am. 1993;75:917-926.
61 Rowbotham M.C. Pharmacologic management of complex regional pain syndrome. Clin J Pain. 2006;22:425-429.
62 Sandroni P., Low P.A., Ferrer T., et al. Complex regional pain syndrome I (CRPS I): prospective study and laboratory evaluation. Clin J Pain. 1998;14:282-289.
63 Schott G.D. Bisphosphonates for pain relief in reflex sympathetic dystrophy? Lancet. 1997;350(9085):1117.
64 Schurmann M., Gradl G., Andress H.J., et al. Assessment of peripheral sympathetic nervous function for diagnosing early post-traumatic complex regional pain syndrome type I. Pain. 1999;80:149-159.
65 Schweitzer M.E., Mandel S., Schwartzman R.J., et al. Reflex sympathetic dystrophy revisited: MR imaging findings before and after infusion of contrast material. Radiology. 1995:211-214.
66 Simon S.R., editor. Orthopaedic Basic Science. Rosemont: IL: American Academy of Orthopaedic Surgeons, 1994.
67 Sintzoff S., Sintzoff S.Jr., Stallenberg B., Matos C. Imaging in reflex sympathetic dystrophy. Hand Clin. 1997;13:431-442.
68 Stanton-Hicks M. Complex regional pain syndrome: manifestations and the role of neurostimulation in its management. J Pain Symptom Manage. 2006;31(4 suppl):S20-S24.
69 Stanton-Hicks M., Baron R., Boas R., et al. Complex regional pain syndromes: guidelines for therapy. Clin J Pain. 1998;14:155-166.
70 Stanton-Hicks M., Janig W., Hassenbusch S., et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127-133.
71 Steinbrocker O., Argyros T.G. The shoulder-hand syndrome: present status as a diagnostic and therapeutic entity. Med Clin North Am. 1958;42:1533-1553.
72 Veldman P.H., Goris R.J. Surgery on extremities with reflex sympathetic dystrophy. Unfallchirurg. 1995;98:45-48.
73 Veldman P.H.J.M., Goris R.J.A. Shoulder complaints in patients with reflex sympathetic dystrophy of the upper extremity. Arch Phys Med Rehabil. 1995;76:239-242.
74 Verdugo R.J., Ochoa J.L. Sympathetically maintained pain.” I. Phentolamine block questions the concept. Neurology. 1994;44:1003-1010.
75 Walker S.M., Cousins M.J. Complex regional pain syndromes: including “reflex sympathetic dystrophy” and “causalgia. Anaesth Intensive Care. 1997;25:113-125.
76 Wang J.K., Johnson K.A., Ilstrup D.M. Sympathetic blocks for reflex sympathetic dystrophy. Pain. 1985;23:13-17.
77 Wasner G., Backonja M.M., Baron R. Traumatic neuralgias: complex regional pain syndromes (reflex sympathetic dystrophy and causalgia): clinical characteristics, pathophysiological mechanisms and therapy. Neurol Clin. 1998;16:851-868.
78 Wu C.T., Ho S.T., Tsai C.S., et al. Repeated lumbar sympathetic blockade for complex regional pain syndromes type I—a case report. Acta Anaesthesiol Sin. 1998;36:155-158.
79 Zollinger P.E., Tuinebreijer W.E., Breederveld R.S., Kreis R.W. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am. 2007;89:1424-1431.
80 Zyluk A. The reasons for poor response to treatment of posttraumatic reflex sympathetic dystrophy. Acta Orthop Belg. 1998;64:309-313.
81 Zyluk A. The usefulness of quantitative evaluation of three-phase scintigraphy in the diagnosis of post-traumatic reflex sympathetic dystrophy. J Hand Surg [Br]. 1999;24:16-21.